Abstract
Background
Conventional MRI is the standard method to diagnose recurrence of brain metastases after radiation. However, following radiation therapy, reactive transient blood–brain barrier alterations with consecutive contrast enhancement can mimic brain metastasis recurrence. Recent studies have suggested that O-(2-18F-fluoroethyl)-L-tyrosine (FET) PET improves the correct differentiation of brain metastasis recurrence from radiation injury. Based on published evidence and clinical expert opinion, we analyzed effectiveness and cost-effectiveness of the use of FET PET in addition to MRI compared with MRI alone for the diagnosis of recurrent brain metastases.
Methods
A decision-tree model was designed to compare the 2 diagnostic strategies from the perspective of the German Statutory Health Insurance (SHI) system. Effectiveness was defined as correct diagnosis of recurrent brain metastasis and was compared between FET PET with MRI and MRI alone. Costs were calculated for a baseline scenario and for a more expensive scenario. Robustness of the results was tested using sensitivity analyses.
Results
Compared with MRI alone, FET PET in combination with MRI increases the rate of correct diagnoses by 42% (number needed to diagnose of 3) with an incremental cost-effectiveness ratio of €2821 (baseline scenario) and €4014 (more expensive scenario) per correct diagnosis. The sensitivity analyses confirmed the robustness of the results.
Conclusions
The model suggests that the additional use of FET PET with conventional MRI for the diagnosis of recurrent brain metastases may be cost-effective. Integration of FET PET has the potential to avoid overtreatment with corresponding costs as well as unnecessary side effects.
Keywords: 18F-FET PET, cost-effectiveness analysis, decision tree model, recurrent brain metastases
Importance of the study
For management of brain metastases, the differentiation of radiation-induced necrosis versus recurrent tumor is of utmost importance. Contrast-enhanced MRI, the current standard method, is often inconclusive if the blood–brain barrier is altered because consecutive contrast enhancement can mimic brain metastasis recurrence. Studies have suggested that FET PET improves the correct differentiation of brain metastasis recurrence from radiation injury.
However, the inclusion of FET PET in the management of patients with brain metastasis will lead to an increase in additional diagnostic costs that have to be balanced with potentially reduced treatment costs as a result of more accurate therapy planning and additional benefits for the patients. Therefore, we analyzed effectiveness and cost-effectiveness of the integration of FET PET in the management of recurrent brain metastases. We found that it may be cost-effective and has the potential to avoid overtreatment with corresponding costs as well as unnecessary side effects.
Extracranial tumors such as malignant melanoma, lung cancer, breast cancer, and renal cancer have a high risk for the development of brain metastases. As a result, annually about 24%–45% of all patients with these tumors develop brain metastases.1 Among the various possible treatment options, particularly stereotactic radiosurgery or whole-brain radiotherapy is frequently used. Currently, the method of choice for follow-up after radiotherapy is conventional contrast-enhanced MRI. Radiation injury of the brain (eg, radiation necrosis), expressing itself by an enlarging, contrast-enhancing lesion, may occur in a small percentage of patients treated with whole-brain radiotherapy and in up to 30%–35% of patients treated with stereotactic radiosurgery.2–4 Distinguishing radiation injury from recurrent brain metastasis is challenging by conventional MRI alone.5 For example, following radiation therapy, reactive transient blood–brain barrier (BBB) alterations with consecutive contrast enhancement can mimic brain metastasis recurrence or even nonresponse to treatment. This may result in unnecessary overtreatment.6
Thus, a diagnostic procedure is needed that reliably differentiates between radiation-induced changes and recurrent brain metastasis, especially in areas with BBB disruption as shown by contrast enhancement on MRI. Amino acid tracers for PET such as O-[2-18F-fluoroethyl]-L-tyrosine (FET) are transported via the system L amino acid transporter and taken up into tumors predominantly by its subtype LAT 1.7 It has been shown that FET uptake is not significantly affected by alterations of the BBB (ie, a disruption of the BBB per se does not lead to increased tracer uptake).8 Moreover, FET shows relatively low uptake in healthy brain parenchyma, which results in high tumor-to-background contrast.9
Previous work of our group suggested that the use of the amino acid tracer FET may contribute significantly to the management of patients with brain metastasis.10,11 It could be demonstrated that the combined evaluation of tumor-to-brain ratios and dynamic parameters of FET uptake can differentiate recurrent brain metastasis from radiation injury with high diagnostic accuracy. These results are in line with other studies using amino acid PET tracers with similar properties, such as L-[methyl-11C] methionine (MET)12,13 or 6-[18F]-fluoro-L-3,4-dihydroxyphenylalanine (F-DOPA).14,15
However, the inclusion of FET PET in the management of patients with brain metastasis will lead to an increase in additional diagnostic costs that have to be balanced with potentially reduced treatment costs as a result of more accurate therapy planning and additional benefits for the patients. Therefore, we developed a decision-tree model to evaluate the effectiveness and cost-effectiveness of FET PET in addition to conventional MRI for the differential diagnosis of recurrent brain metastasis and radiation injury, thereby focusing on possible false positive MRI results. The analysis was performed from the perspective of the Statutory Health Insurance (SHI) system in Germany. To the best of our knowledge, this is the first study that evaluates effectiveness and cost-effectiveness of FET PET in addition to conventional MR imaging for this particular indication in patients with brain metastasis.
Materials and Methods
Calculation of Effectiveness of FET PET
Input data
Our analysis on the use of FET PET for differentiation of brain metastasis recurrence from radiation injury after radiotherapy is based on the study by Ceccon et al.10 This study, which included data of a pilot study,11 is currently the largest study addressing this subject. It comprises 62 patients (mean age, 55 ± 11 y) with single or multiple contrast-enhancing brain lesions (n = 76) on conventional T1-weighted images pre- and post-intravenous application of gadolinium-containing contrast agent after radiotherapy or radiosurgery of brain metastases which were investigated additionally with FET PET. Diagnoses (radiation injury vs brain metastasis recurrence) were confirmed either histologically in 34% of the lesions or by clinical follow-up (median follow-up period, 16 mo).10 Since no other data have been published on the use of FET PET for this indication, the analysis is based on this patient cohort as described below.
Decision-tree model
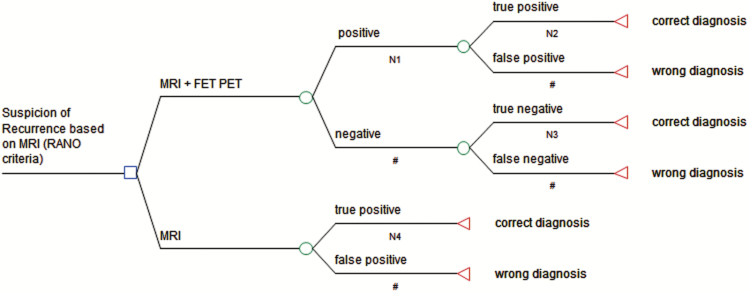
The construction of the decision-tree model was performed using TreeAge Pro 2013 software. The structure of the model is based on other models evaluating the cost-effectiveness of amino acid PET (14). The decision-tree model describes the clinical situation of patients with brain metastases after initial treatment in which MRI findings were suggestive for brain metastasis recurrence or progression (Figure 1). MRI findings were evaluated according to Response Assessment in Neuro-Oncology (RANO) criteria for brain metastasis recurrence.16 According to these criteria, recurrent disease is assumed if a new contrast-enhancing lesion appears at exactly the same site as the treated metastasis after initial complete response. Recurrent disease is also assumed if (i) the treated brain metastasis during follow-up shows an increase in size of >20% in the pretreated volume on contrast-enhanced T1-weighted MR images, and (ii) patients develop new neurological deficits or an exacerbation of existing neurological symptoms that prompts a change in treatment. Following these criteria, the MRI scans were evaluated as positive for recurrent brain metastasis. Thus, only positive MRI scans were considered for the analysis. The readers of FET PET were aware of the positive MRI but were blinded to all other clinical information. The decision-tree model compares the strategy to perform FET PET in addition to conventional contrast-enhanced MRI with the strategy to proceed based on the MRI alone.
Fig. 1.
The decision-tree model for assessment of the effectiveness of additional FET PET for the differential diagnosis of local recurrent brain metastasis versus radiation injury after radiotherapy. The model includes the 2 alternative strategies of using MRI alone or in addition with FET PET. The probability of the correct diagnostic assessment is defined as outcome. O = chance node; ◄ = termination node, # corresponding likelihood (1 − n).
We defined the probability of a correct diagnosis as the primary outcome of our model (Figure 1). This appears as an appropriate surrogate, since it strongly influences the decision making of treatment planning and monitoring. A false diagnosis of brain metastasis recurrence will lead to a potentially premature aggressive treatment with the risk of unnecessary serious side effects and avoidable costs for an unnecessary treatment.
In Figure 1, the square (■) indicates the decision between 2 alternative strategies. The circle (○) symbolizes chance nodes with 2 complementary likelihoods. The lower branch describes the diagnostic strategy to use MRI alone and not to perform additional diagnostic FET PET. Since the model assumes a positive MRI scan for all patients as described above, the first chance node of this branch (MRI positive) represents the likelihood of true positive and false positive diagnoses. To obtain these values, the data of the MRI scans used in the study by Ceccon et al10 were reassessed according to the above-mentioned criteria.
The upper branch refers to the diagnostic strategy to complement the use of MRI with FET PET (Figure 1). The first chance node of the upper branch (MRI positive + PET) shows the likelihood of positive and negative FET PET scans. These likelihoods were calculated based on the combined analysis of mean tumor-to-brain ratio (TBRmean) and the slope of the time-activity curve (TAC slope) as reported by Ceccon et al.10 The FET PET is considered positive (diagnosis of recurrent brain metastasis) if a TBRmean >1.95 in combination with a TAC slope <0.37 standardized uptake value per hour is present; otherwise, it is considered negative (diagnosis of radiation injury). The following chance nodes (positive and negative) represent the likelihood of true positive and false positive as well as true negative and false negative results.
Calculation of the Costs
Usually, the German SHI does not cover the costs for FET PET. Therefore, these costs were calculated referring to the “Medical Fee Schedule for Care Outside the Statutory Health Insurance Scheme” (http://www.e-bis.de/goae/defaultFrame.htm).17 Only the additional costs for the FET PET scan were taken into account, since conventional MRI is performed for every patient in both strategies. Indirect costs due to productivity losses were not included, since they are irrelevant for the SHI perspective. The cost-effectiveness of FET PET was evaluated by calculating an incremental cost-effectiveness ratio (ICER).18
A more detailed explanation of the calculation was described previously.19 Briefly, to reflect different levels of complexity of patient care, we considered a baseline scenario and an adjusted scenario. The baseline scenario reflects reimbursement of a standard case. The adjusted scenario has a higher reimbursement that allows adjusting for various factors such as the difficulty of the procedure or the qualification of the health personnel. The following costs were included for both scenarios: detailed patient consultation €8.74 (€20.10), report on diagnostic findings €7.58 (€17.43), intravenous injection €4.08 (€9.38), whole-body tumor scintigraphy €131.15 (€236.07), and PET with quantitative analysis €417.15 (€786.89). They refer to the codes 3, 75, 253, 5431, and 5489 of the “Medical Fee Schedule for Care Outside the Statutory Health Insurance Scheme.” Additionally, the costs for the radioactive tracer were estimated. Whereas some institutions produce the tracers on-site, others receive 18F-FET PET via commercial enterprises. In order to represent the range of costs, we calculated the mean of the price of 2 German enterprises and 1 on-site facility. Adding value-added tax of 19%, the cost for the tracer was assumed to be €616.
Based on this cost, the baseline scenario for a FET PET resulted in a total cost of €1185 and the adjusted scenario in a total cost of €1686.19
Finally, we calculated the follow-up costs of a false positive MRI. The precise amount of these costs is difficult to calculate, since there are no published data. According to current guidelines we assumed that in most cases a positive MRI will result in a stereotactic biopsy in order to confirm the diagnosis.20 This would incur additional costs for the diagnostic procedure, including prolonged hospital stay as well as treatment costs related to possible side effects. In Germany, costs of hospitalization are reimbursed by lump compensation (German Diagnosis Related Groups, www.g-drg.de). For the calculations, we applied the procedure “stereotactic biopsy on intracranial tissue for one up to five biopsies” (OPS 1–511.00) with the main diagnosis brain metastasis (C79.3). For this analysis, hospital costs were calculated based on data from the most populated state of Germany (North Rhine–Westphalia). Assuming a hospital stay of 3 days, this would result in costs of €6232. In addition, treatment of side effects might increase the total costs in 0.7%–9.6% of the interventions.21 However, since these side effects are rare and highly variable for different institutions, we did not include them in the calculation.
Thus, to evaluate the follow-up costs of a false positive MRI with regard to the costs of FET PET we included them in the analyses as additional costs for false positive MRI and false positive FET PET combined with false positive MRI.
Sensitivity Analyses
In order to test the robustness of the results, we calculated deterministic and probabilistic sensitivity analyses. For input data, we relied on studies using other amino acid tracers with similar properties, such as MET12,13 and F-DOPA.14,15 In these studies, a similar diagnostic performance of these tracers has been reported.
Deterministic one-way sensitivity analyses were conducted for the effectiveness values of the decision-tree model to calculate the impact of their uncertainty. For each variable, 4 intervals were chosen, which were derived from the above-mentioned papers. In order to represent the whole range of values from the literature, we selected the highest and the lowest value reported in the papers as high and low values of the one-way sensitivity analyses. Since Cicone et al14 and Terakawa et al12 did not report these values, we calculated them based on their papers. In the other studies the values were reported (Table 1).
Table 1.
Values for the one-way deterministic sensitivity analyses
| Likelihoods | Variable | Tsuyuguchi et al | Lizarraga et al | Cicone et al * | Terakawa et al * |
|---|---|---|---|---|---|
| Positive FET | N1 | 0.52 | 0.41 | 0.43 | 0.48 |
| True positive FET | N2 | 0.82 | 0.77 | 0.89 | 0.7 |
| True negative FET | N3 | 1 | 0.88 | 0.95 | 0.83 |
| True positive MRI | N4 | 0.43 | 0.39 | 0.43 | ** |
| Number of lesions | 21 | 83 | 46 | 56 | |
| Amino acid | MET | F-DOPA | F-DOPA | MET |
The high and the low values were determined by the highest and lowest value derived from the papers (marked in bold). For each variable, 4 intervals were chosen. The variables refer to the decision-tree model (Figure 1).
The values were calculated by us based on the paper
Not reported.
Probabilistic sensitivity analyses were performed using second-order Monte Carlo simulations with 10000 samples.22 To apply this method, we attributed beta distributions to the effectiveness variables of the decision tree. The beta distributions were defined by mean and standard deviation. The standard deviation was estimated based on the above-mentioned papers (Table 2). The costs were modeled by a gamma distribution with the mean of the baseline scenario and a conservative standard deviation of 50%. Moreover, the distributions were checked for plausibility by an interdisciplinary team of experts in the field, including nuclear medicine (K.J.L.), neuro-oncology (N.G.), neuroradiology (E.H., M.W.), and radiation oncology (M.K.).
Table 2.
Values for the decision tree model (Figure 1) and the Monte Carlo statistics
| Likelihoods | Variable | Value | STDW | Distribution |
|---|---|---|---|---|
| Positive FET | N1 | 0.49 | 0.05 | beta |
| True positive FET | N2 | 0.91 | 0.08 | beta |
| True negative FET | N3 | 0.88 | 0.08 | beta |
| True positive MRI | N4 | 0.47 | 0.24 | beta |
The values for the variables are derived from Ceccon et al, 2016. The values for the standard deviation (STDW) were estimated based on the papers by Tsuyuguchi et al, 2003 and Terakawa et al, 2008 applying methionine, as well as Cicone et al, 2015 and Lizarraga et al, 2014 using F-DOPA.
Based on the results from the deterministic and the probabilistic sensitivity analyses, we calculated worst-case scenarios with regard to costs and effectiveness (ie, highest costs, lowest effectiveness for the combination of MRI and FET PET).
Results
Decision-tree Model
The decision tree revealed that the additional use of FET PET increased the rate of a correct diagnosis by 42% compared with MRI alone (likelihood of 89% vs likelihood of 47%). In order to avoid one false diagnosis, 3 patients have to be diagnosed with FET PET (number needed to diagnose: 1/0.42 = 2.38). For the baseline scenario, this results in an ICER of €1185/0.42 = €2821 (adjusted scenario: €1686/0.42 = €4014).
Sensitivity Analyses
Strategy MRI in combination with PET
The one-way sensitivity analyses for N1 resulted in likelihoods in the range of 0.89–0.9 for the combination of MRI and PET; for N2, 0.79–0.88; and for N3, 0.87–0.96, respectively.
Strategy MRI alone
For N4, likelihoods were between 0.39 and 0.43.
Thus, a worst-case scenario for the strategy of MRI in combination with PET would lead to a likelihood of 0.79 for a correct diagnosis, whereas a best-case scenario for the strategy of MRI alone would lead only to a likelihood of 0.43. This means that 4 patients have to be diagnosed with FET PET in order to avoid one false diagnosis (number needed to diagnose, 1/0.36 = 4), resulting in an ICER of €1185/0.36 = €3293 (adjusted scenario: €1686/0.36 = €4683).
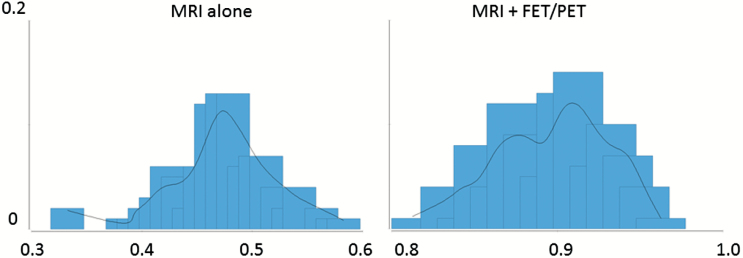
The Monte Carlo statistics for MRI in combination with PET as well as for MRI alone are shown in Table 3 as well as Figures 2 and 3. Assuming a best-case scenario for the strategy of MRI alone (results from the 97.5th percentile) and a worst-case scenario for MRI in combination with PET (results from the 2.5th percentile), the incremental effectiveness would still result in a likelihood of 25%. This means that 4 patients have to be diagnosed by FET PET in order to avoid one false diagnosis (number needed to diagnose, 1/0.25 = 4).
Table 3.
Statistics resulting from the Monte Carlo simulation (10000 samples) for effectiveness and cost of FET PET for diagnosis of recurrent brain metastases
| MRI Alone | MRI + PET | IE | Cost [€] | |
|---|---|---|---|---|
| Mean | 0.47 | 0.89 | 0.42 | 1182.43 |
| SD | 0.04 | 0.03 | 585.86 | |
| Minimum | 0.33 | 0.81 | 0.48 | 48.33 |
| 2.50% | 0.38 | 0.83 | 0.45 | 329.62 |
| 10% | 0.42 | 0.85 | 0.43 | 520.7 |
| Median | 0.47 | 0.9 | 0.43 | 1085.59 |
| 90% | 0.53 | 0.94 | 0.41 | 1965.59 |
| 97.50% | 0.56 | 0.95 | 0.39 | 2570.81 |
| Maximum | 0.58 | 0.96 | 0.38 | 4484.82 |
The 2 center columns represent the 2 alternative diagnostic strategies applying MRI alone or MRI combined with FET PET. The values show the probability of obtaining a correct diagnosis (rounded to 2 decimal places). IE = incremental effectiveness (ie, the benefit that results by adding FET PET to MRI). The right column shows the cost of adding FET PET. SD = standard deviation.
Fig. 2.
The figure illustrates the distribution of the results from the Monte Carlo statistics with regard to the effectiveness of an additional FET PET (MRI in combination with FET PET) compared with MRI alone. The x-axis depicts the likelihood of a correct diagnosis as outcome. The y-axis depicts the likelihood that this outcome is reached.
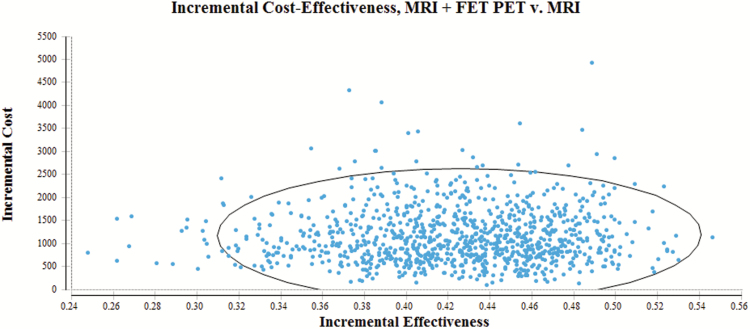
Fig. 3.
Distribution of results from Monte Carlo simulation (dots) with regard to incremental cost-effectiveness of an additional FET PET (MRI in combination with FET PET) compared with MRI alone. Within the circle are 95% of the values. The x-axis depicts the increase in likelihood of a correct diagnosis as outcome (incremental effectiveness). The y-axis depicts the increase in cost [€] (incremental cost).
Considering the cost with regard to the worst-case scenario (results from the 97.5th percentile), the ICER would be €2570/0.25 = €10280.
The calculation considering follow-up costs of false positive results leads to costs of €1460 (FET PET) and €3303 (MRI). The Monte Carlo simulations resulted in median costs of €1451 for FET PET and €3303 for MRI.
Discussion
This study evaluated the effectiveness and cost-effectiveness of additional FET PET for the differentiation of brain metastasis recurrence from radiation injury after radiotherapy. Compared with MRI alone, FET PET increases the rate of correct diagnoses by 42% (ie, 3 patients have to be diagnosed by FET PET to avoid one false diagnosis). This leads to ICERs of €2821 (baseline scenario) and €4014 (adjusted reimbursement rate scenario). The probabilistic sensitivity analyses confirmed the robustness of the results (ie, the cost-effectiveness ratio remains below a threshold of €12000 even if a worst-case scenario and the highest cost were assumed.
It might be argued that cost-effectiveness can only be evaluated with regard to direct patient-related benefits such as survival or increase in health-related quality of life. However, up to now there are no published studies using direct patient-related outcomes. Furthermore, these criteria might not be appropriate for diagnostic tests, since it is often difficult to disentangle the specific benefit of a diagnostic procedure from the complex chain of diagnostic and therapeutic interventions comprised by the totality of clinical care.23
For our decision-tree model, the probability of a correct diagnosis appears to be an appropriate surrogate, as further therapy planning in patients with recurrent brain metastases is based on it. Based on the input data used for the model, conventional MRI alone is an insufficient option, since the likelihood of a correct diagnosis is about or even below 50% and therefore does not permit a reliable diagnosis. Because patients with recurrent brain metastases require chemotherapy or radiation, a wrong diagnosis may lead to premature aggressive treatment with the risk of serious side effects, reduced survival, and a decrease in health-related quality of life.
The additional costs of FET PET have to be counterbalanced with the costs caused by a wrong diagnosis applying MRI alone. This includes the 2 possibilities of false positive and false negative MRI results. In this paper we focused on possible false positive MRI results, since this is the main indication for FET PET. The sensitivity of MRI is already high and due to the lower spatial resolution of PET technology it seems unlikely that a combined reading of FET PET and MRI will significantly improve the sensitivity. Thus, additional FET PET seems unlikely to be cost-effective if the MRI is considered negative.
However, false positive MRI results are likely to incur additional costs. Because the precise amount of these costs is difficult to calculate due to lack of data, we performed a rough calculation that resulted in costs of €1460 for combined MRI FET PET and €3303 for MRI alone, indicating the superior cost-effectiveness of combined MRI/FET PET. The calculation is based on the assumption that a positive MRI will result in a stereotactic biopsy to confirm the diagnosis. However, it has to be noted that biopsy is unlikely to be performed in all patients, since, for instance, some patients might refuse a biopsy or may be in a severe clinical state that does not permit a biopsy. Thus, studies are needed that directly address the follow-up costs in various clinical settings.
Moreover, the costs for FET PET have to be considered with regard to the total costs for patient care and the costs for possible treatments that might be avoided. Due to highly individualized treatment strategies and the vast range of different types of cancer that may lead to brain metastases, this is difficult to estimate. Analyzing 3 large administrative databases in the USA, in patients with non–small cell lung cancer, Guerin et al24 found an average total monthly cost of $22645. Moreover, Ray et al25 reported a total monthly cost of $23426 for malignant melanoma, of $19708 for breast cancer, and of $17007 for lung cancer. Both analyses are based on data from the USA. Although these costs may not precisely reflect treatment costs in European countries, a similar cost level has to be assumed. Thus, FET PET can be considered a small percentage of total treatment costs with the potential for cost reduction if unnecessary treatments can be avoided.
For the sensitivity analyses, we relied on studies using other amino acid tracers, such as MET and F-DOPA. This is justified by similar properties of the amino acid tracers. Similar to FET, they show high radiotracer uptake with low background signal in brain metastases and their uptake represents an increased expression of amino acid transporters on tumor cells, namely the amino transport system L.26,27 Moreover, a direct comparison of FET and MET showed a high correlation between their uptake in normal cortex and tumor tissue (brain gliomas and metastases).28 To date there are no direct comparisons of FET versus F-DOPA for brain metastases; however, using the tracers for high-grade gliomas, there were no significant differences in uptake pattern for FET and F-DOPA. Also with regard to tumor delineation, both tracers performed equally well.29 Thus, a similar performance for the diagnosis of recurrent brain metastases may be assumed.
Our results are in line with other cost-effectiveness analyses evaluating the use of FET PET in patients with gliomas for planning neurosurgical resection, therapy monitoring, and definition of biopsy target.17,19,30 It should be noted that FET PET cannot replace MRI as a standard tool in the diagnosis of brain tumors, but it may provide cost-effective complementary information with vital implications for the patients’ management.
An important limitation of our study has to be considered. The clinical data applied in the decision-tree model could only be derived from one study relying on longitudinal within-group comparisons. The retrospective character of this study may lead to biased results.
It has to be noted that many patients suffered from multiple lesions, and therefore biopsy was available for only 34% of all lesions, while almost two-thirds of the lesions were evaluated using RANO criteria within clinical follow-up. To account for possible differences, a separate analysis of the patients with histological confirmation of diagnosis was performed. The analysis using TBRmean and TAC slope revealed similar diagnostic accuracy for the subgroup of histologically confirmed lesions as for the whole group.10
Moreover, we performed sensitivity analyses relying on data from studies on PET tracers with similar properties and the estimations of clinical experts in the field. However, ultimately, additional studies with prospective designs are needed to confirm the results.
In addition, transient posttreatment enhancement has to be distinguished from radiation injury. However, in our sample the mean time interval between radiation therapy and MRI was 14 months. Transient posttreatment enhancement is most likely seen in the first 3 months after radiation.31 Therefore, we do not expect that this might influence our analysis.
Conclusion
The model delivers evidence that additional FET PET may be an effective and cost-effective tool for the differentiation of brain metastasis recurrence from radiation injury after radiotherapy. Additional studies, ideally randomized controlled trials, are needed to confirm the results, particularly the expected additional benefit.
Funding
The research was not supported by any funding.
Conflict of interest statement. The authors state that there are no conflicts of interest.
References
- 1. Barnholtz-Sloan JS, Yu C, Sloan AE, et al. A nomogram for individualized estimation of survival among patients with brain metastasis. Neuro Oncol. 2012;14(7):910–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lawrence YR, Li XA, el Naqa I, et al. Radiation dose-volume effects in the brain. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S20–S27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Le Rhun E, Dhermain F, Vogin G, Reyns N, Metellus P. Radionecrosis after stereotactic radiotherapy for brain metastases. Expert Rev Neurother. 2016;16(8):903–914. [DOI] [PubMed] [Google Scholar]
- 4. Wiggenraad R, Verbeek-de Kanter A, Kal HB, Taphoorn M, Vissers T, Struikmans H. Dose-effect relation in stereotactic radiotherapy for brain metastases. A systematic review. Radiother Oncol. 2011;98(3):292–297. [DOI] [PubMed] [Google Scholar]
- 5. Stockham AL, Tievsky AL, Koyfman SA, et al. Conventional MRI does not reliably distinguish radiation necrosis from tumor recurrence after stereotactic radiosurgery. J Neurooncol. 2012;109(1):149–158. [DOI] [PubMed] [Google Scholar]
- 6. Wick W, Wick A, Weiler M, Weller M. Patterns of progression in malignant glioma following anti-VEGF therapy: perceptions and evidence. Curr Neurol Neurosci Rep. 2011;11(3):305–312. [DOI] [PubMed] [Google Scholar]
- 7. Habermeier A, Graf J, Sandhöfer BF, Boissel JP, Roesch F, Closs EI. System L amino acid transporter LAT1 accumulates O-(2-fluoroethyl)-L-tyrosine (FET). Amino Acids. 2015;47(2):335–344. [DOI] [PubMed] [Google Scholar]
- 8. Langen KJ, Galldiks N. Reply to “[18F]-fluoro-ethyl-L-tyrosine PET: a valuable diagnostic tool in neuro-oncology, but not all that glitters is glioma” by Hutterer et al. Neuro Oncol. 2013;15(7):816–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Galldiks N, Langen KJ, Pope WB. From the clinician’s point of view—what is the status quo of positron emission tomography in patients with brain tumors? Neuro Oncol. 2015;17(11):1434–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ceccon G, Lohmann P, Stoffels G, et al. Dynamic O-(2-18F-fluoroethyl)-L-tyrosine positron emission tomography differentiates brain metastasis recurrence from radiation injury after radiotherapy. Neuro Oncol. 2016;pii: now149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Galldiks N, Stoffels G, Filss CP, et al. Role of O-(2-(18)F-fluoroethyl)-L-tyrosine PET for differentiation of local recurrent brain metastasis from radiation necrosis. J Nucl Med. 2012;53(9):1367–1374. [DOI] [PubMed] [Google Scholar]
- 12. Terakawa Y, Tsuyuguchi N, Iwai Y, et al. Diagnostic accuracy of 11C-methionine PET for differentiation of recurrent brain tumors from radiation necrosis after radiotherapy. J Nucl Med. 2008;49(5):694–699. [DOI] [PubMed] [Google Scholar]
- 13. Tsuyuguchi N, Sunada I, Iwai Y, et al. Methionine positron emission tomography of recurrent metastatic brain tumor and radiation necrosis after stereotactic radiosurgery: is a differential diagnosis possible? J Neurosurg. 2003;98(5):1056–1064. [DOI] [PubMed] [Google Scholar]
- 14. Cicone F, Minniti G, Romano A, et al. Accuracy of F-DOPA PET and perfusion-MRI for differentiating radionecrotic from progressive brain metastases after radiosurgery. Eur J Nucl Med Mol Imaging. 2015;42(1):103–111. [DOI] [PubMed] [Google Scholar]
- 15. Lizarraga KJ, Allen-Auerbach M, Czernin J, et al. (18)F-FDOPA PET for differentiating recurrent or progressive brain metastatic tumors from late or delayed radiation injury after radiation treatment. J Nucl Med. 2014;55(1):30–36. [DOI] [PubMed] [Google Scholar]
- 16. Lin NU, Lee EQ, Aoyama H, et al. ; Response Assessment in Neuro-Oncology (RANO) group. Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol. 2015;16(6):e270–e278. [DOI] [PubMed] [Google Scholar]
- 17. Heinzel A, Müller D, Langen KJ, et al. The use of O-(2-18F-fluoroethyl)-L-tyrosine PET for treatment management of bevacizumab and irinotecan in patients with recurrent high-grade glioma: a cost-effectiveness analysis. J Nucl Med. 2013;54(8):1217–1222. [DOI] [PubMed] [Google Scholar]
- 18. Karlsson G, Johannesson M. The decision rules of cost-effectiveness analysis. Pharmacoeconomics. 1996;9(2):113–120. [DOI] [PubMed] [Google Scholar]
- 19. Heinzel A, Stock S, Langen KJ, Müller D. Cost-effectiveness analysis of FET PET-guided target selection for the diagnosis of gliomas. Eur J Nucl Med Mol Imaging. 2012;39(7):1089–1096. [DOI] [PubMed] [Google Scholar]
- 20. Kocher M, Wittig A, Piroth MD, et al. Stereotactic radiosurgery for treatment of brain metastases. A report of the DEGRO working group on stereotactic radiotherapy. Strahlenther Onkol. 2014;190(6):521–532. [DOI] [PubMed] [Google Scholar]
- 21. Livermore LJ, Ma R, Bojanic S, Pereira EA. Yield and complications of frame-based and frameless stereotactic brain biopsy – the value of intra-operative histological analysis. Br J Neurosurg. 2014;28(5):637–644. [DOI] [PubMed] [Google Scholar]
- 22. Doubilet P, Begg CB, Weinstein MC, Braun P, McNeil BJ. Probabilistic sensitivity analysis using Monte Carlo simulation. A practical approach. Med Decis Making. 1985;5(2):157–177. [DOI] [PubMed] [Google Scholar]
- 23. Hillman BJ, Frank RA, Abraham BC. The medical imaging & technology alliance conference on research endpoints appropriate for medicare coverage of new PET radiopharmaceuticals. J Nucl Med. 2013;54(9):1675–1679. [DOI] [PubMed] [Google Scholar]
- 24. Guérin A, Sasane M, Zhang J, et al. Brain metastases in patients with ALK+ non-small cell lung cancer: clinical symptoms, treatment patterns and economic burden. J Med Econ. 2015;18(4):312–322. [DOI] [PubMed] [Google Scholar]
- 25. Ray S, Dacosta-Byfield S, Ganguli A, Bonthapally V, Teitelbaum A. Comparative analysis of survival, treatment, cost and resource use among patients newly diagnosed with brain metastasis by initial primary cancer. J Neurooncol. 2013;114(1):117–125. [DOI] [PubMed] [Google Scholar]
- 26. Langen KJ, Bröer S. Molecular transport mechanisms of radiolabeled amino acids for PET and SPECT. J Nucl Med. 2004;45(9):1435–1436. [PubMed] [Google Scholar]
- 27. Youland RS, Kitange GJ, Peterson TE, et al. The role of LAT1 in (18)F-DOPA uptake in malignant gliomas. J Neurooncol. 2013;111(1):11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Grosu AL, Astner ST, Riedel E, et al. An interindividual comparison of O-(2-[18F]fluoroethyl)-L-tyrosine (FET)- and L-[methyl-11C]methionine (MET)-PET in patients with brain gliomas and metastases. Int J Radiat Oncol Biol Phys. 2011;81(4):1049–1058. [DOI] [PubMed] [Google Scholar]
- 29. Lapa C, Linsenmann T, Monoranu CM, et al. Comparison of the amino acid tracers 18F-FET and 18F-DOPA in high-grade glioma patients. J Nucl Med. 2014;55(10):1611–1616. [DOI] [PubMed] [Google Scholar]
- 30. Nikolaus S, Hautzel H, Heinzel A, Müller HW. Key players in major and bipolar depression – a retrospective analysis of in vivo imaging studies. Behav Brain Res. 2012;232(2):358–390. [DOI] [PubMed] [Google Scholar]
- 31. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: Response Assessment in Neuro-Oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]