Abstract
One of the biggest challenges in studying how genes work is understanding their effect on the physiology and anatomy of the body. Existing tools try to address this using indirect features, such as expression levels and biochemical pathways. Here, we present Gene ORGANizer (geneorganizer.huji.ac.il), a phenotype-based tool that directly links human genes to the body parts they affect. It is built upon an exhaustive curated database that links >7000 genes to ∼150 anatomical parts using >150 000 gene-organ associations. The tool offers user-friendly platforms to analyze the anatomical effects of individual genes, and identify trends within groups of genes. We demonstrate how Gene ORGANizer can be used to make new discoveries, showing that chromosome X is enriched with genes affecting facial features, that positive selection targets genes with more constrained phenotypic effects, and more. We expect Gene ORGANizer to be useful in a variety of evolutionary, medical and molecular studies aimed at understanding the phenotypic effects of genes.
INTRODUCTION
Many high-throughput methods such as whole-genome sequencing, expression microarrays, RNA-seq, and whole-genome methylation mapping produce genome-wide data whose analyses produce long lists of genes of interest. These lists typically include genes that share a certain trait such as being bound by the same transcription factor, being differentially methylated between two samples, having high conservation levels, or being differentially expressed following a treatment. Such lists have become a common product of biological research, but understanding how they affect the biology of an organism at the physiological and anatomical level remains a challenging task (1).
Dozens of tools have been developed to address this challenge, providing researchers with powerful means to tease out biological processes and functions that are associated with the genes they investigate (1,2,3). For example, a popular tool is DAVID (2,4), where genes can be analyzed for shared Gene Ontology (GO) terms, disease associations, expression patterns and biochemical pathways. The strategy adopted by many of these tools, e.g. Human Phenotype Ontology (HPO)(5), DisGeNet (6), PhenGenl (7), PhenomicDB (8) and Organ System Heterogeneity DB (9), is to focus on the phenotypic effects of genes. Thus, these tools usually harbor databases (DBs) for gene–phenotype associations. However, genes in these DBs are linked either to diseases (e.g. ‘primary ciliary dyskinesia’), or to the phenotypes of a disease (e.g. ‘peripheral traction retinal detachment’), but not directly to organs (e.g. ‘eye’). Tools such as OMIM (10), Organ System Heterogeneity DB (9) and BRITE (11) do offer some direct links between genes and organs, but include only a limited number of organs and systems (33 in OMIM, 26 in Organ System Heterogeneity DB, 12 in BRITE), and lack platforms to efficiently and statistically analyze these data.
Another approach for linking genes to body parts is based on expression rather than phenotype, where mRNA levels are used to determine in which tissues and cell types genes are active. For example, Tissues (12) is an integrative tool for gene expression analyses, integrating transcriptomics, proteomics, text mining and manual curation and Expression Atlas (13) is a tool allowing analyses of gene expression in different cell types, diseases and developmental stages, based on comprehensive RNA-seq and microarray data. While very useful in many cases, expression-based analysis is an indirect approach that suffers from a number of drawbacks. First, the repertoire of expression datasets is limited, with a strong bias towards certain organs and tissues (e.g. brain, blood and skin), whereas many other body parts are rare or completely absent (e.g. bone, face, larynx, urethra, teeth, fingers and spinal cord). Second, samples used for expression analyses are usually obtained from specific developmental stages, taken postmortem, and extracted from particular parts of the organ. Thus, the data collected rarely capture the entire temporal and structural variation of organs. Third, expression analyses generally focus on specific cell types or tissues (e.g. cardiomyocytes), rather than on whole organs (e.g. heart), systems (e.g. the cardiovascular), or anatomical regions (e.g. the thorax), hence providing partial or skewed information on how whole organs are affected. Finally, gene expression does not directly translate into an observable phenotype. This limited correspondence between expression and phenotype stems from several reasons: (a) The correlation between mRNA levels and protein levels is generally low, reported to be <0.5 (14,15,16,17). (b) Expression assays, especially if done in low coverage, might miss lowly expressed genes. However, these genes tend to be more medically relevant and underlie organ-specific phenotypes (18). (c) The activity of a gene is not necessarily limited to the tissue in which it is expressed. For example, expression of a gene in the endocrine system would often have phenotypic consequences in other tissues, due to its secretory function.
Thus, despite the plethora of tools designed for the analysis of gene lists, direct association of genes to body parts is largely unavailable. Today, researchers who seek to link genes to the organs they affect are left with two main options: either to use gene expression DBs, which do not provide a direct phenotype-based association, or to conduct a manual review of the literature and free text DBs such as OMIM (10), Gene Cards (19) and GenBank (20), which are not constructed for gene list analyses.
Gene ORGANizer was developed to fill this gap. We have constructed a comprehensive fully curated DB, consisting of >150 000 gene-body part associations, and covering over 7000 human genes. The body parts are divided into four levels of hierarchy: body systems (e.g. cardiovascular, hereinafter systems), anatomical regions (e.g. thorax, hereinafter regions), organs (e.g. heart) and germ layers (e.g. mesoderm). On top of this DB, we have created a web platform that allows users to browse for a specific gene, as well as to analyze gene lists in order to test whether they are enriched or depleted with certain body parts.
MATERIALS AND METHODS
Backend database
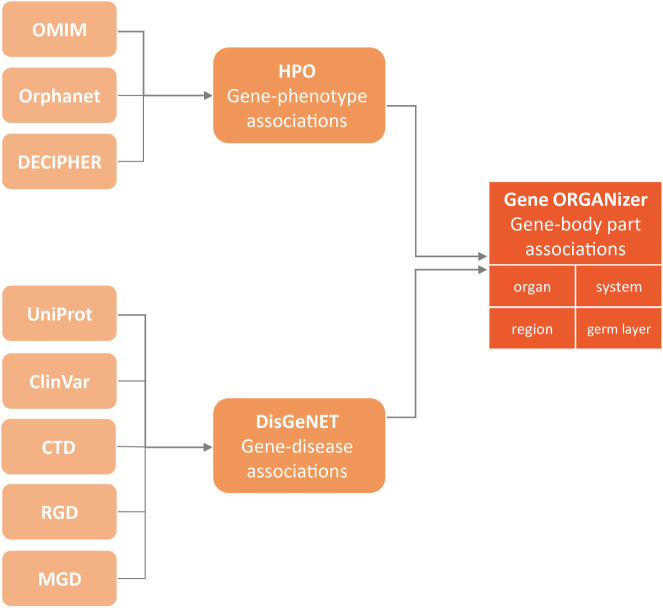
In non-human organisms phenotypes can be directly observed using various genetic manipulations such as knockout or knockdown. In humans, however, the principal way to associate genes to phenotypes is through observed diseases. To construct the Gene ORGANizer DB, we used two of the largest DBs for gene-disease and gene-phenotype associations in human: Human Phenotype Ontology (HPO) (5) and DisGeNET (6). HPO integrates data from three highly-curated sources: OMIM (10), Orphanet (21) and DECIPHER (22). DisGeNET integrates data from UniProt (23), The Comparative Toxicogenomics Database (CTD) (24) and ClinVar (25), as well as from non-human sources, such as CTD mouse (24), CTD rat (24), The Mouse Genome Database (MGD) (26) and The Rat Genome Database (RGD) (27). DisGeNET also includes annotations based on literature text mining, which we do not use for Gene ORGANizer, as they are not curated. Together, these DBs link 7132 human genes to diseases and phenotypes (see online Methods).
We have built our tool based on the entire HPO DB and the curated portion of DisGeNET, which together comprise over 150 000 gene–phenotype and gene–disease associations. We developed a protocol to translate these data into associations between genes and the anatomical parts in which the phenotype is observed (Figure 1). For example, one of the phenotypes caused by mutations in the HOXA2 gene is microtia—the underdevelopment of the outer ear (OMIM ID: 612290) (28). We have used this association to link HOXA2 to the following body parts: the outer ear, the ear, the head, the integumentary system, the head and neck region and the ectoderm germ layer (see online Methods for a complete description of the annotation protocol). Overall, we have linked genes to 146 body parts, divided into four anatomical hierarchies: (a) three germ layers (endoderm, mesoderm and ectoderm); (b) six regions (head and neck, thorax, abdomen, pelvis, limbs and non-specific); (c) twelve systems (digestive, nervous, reproductive, endocrine, skeletal muscle, skeleton, lymphatic, cardiovascular, immune, urinary, respiratory and integumentary) and (d) 125 organs and sub-organs (Supplementary Table S1). The entire Gene ORGANizer DB can be downloaded from the website's downloads page.
Figure 1.
Sources of the Gene ORGANizer database. Sources of associations that comprise the Gene ORGANizer DB. Associations in Gene ORGANizer are divided into four levels of hierarchy: organ (e.g. stomach), system (e.g. digestive), region (e.g. abdomen) and germ layer (e.g. endoderm).
Using Gene ORGANizer
Gene ORGANizer was designed to provide researchers with the ability to analyze the phenotypic effects of genes and to understand the shared impact of groups of genes. The tool consists of two platforms: Browse and ORGANize. Browse allows users to see all of the body parts affected by a single gene of interest. ORGANize is designed to test which body parts, if at all, are over- or under-represented in a gene list. In both platforms, the user can base the analysis on either the typical phenotypes associated with a gene (defined as those that appear in >50% of sick individuals), or on its typical+non-typical phenotypes (i.e. any frequency). Additionally, the user can choose between confident associations (i.e. inferred from data on humans), and confident+tentative ones (inferred also from additional data on mouse and rat).
The output in both Browse and ORGANize comes in two forms: a color-coded body map and a table. The table contains all information whereas the body map visualizes most of it (125 out of the 146 body parts). Non-localized body parts (e.g. blood) or very small parts (e.g. sweat gland) do not appear in the body map and are represented only in the table. In the Browse option, the table and body map simply present the body parts that are phenotypically affected by the gene of interest, colored by the type of association (confident or tentative; typical or non-typical). Hovering over a body part in the table allows the user to see the phenotypes and diseases that are behind the gene–body part association. In the ORGANize option, the body map represents an interactive heat map, where significantly enriched or depleted body parts are colored based on the level of their enrichment or depletion. Non-significant body parts remain in their original gray color.
The enrichment and depletion tests within a gene list are carried out against a list of background genes. By default, the background consists of all genes that are linked to body parts in our DB. This background assures that even if certain anatomical parts are over-represented in the ontology (because some phenotypes are easier to detect, or some diseases are more studied), it would not bias the results (2). Gene ORGANizer also allows users to enter their own background list. User-specified backgrounds are useful in cases where the initial pool of genes from which the gene list was derived contains an inherent bias. For example, in a list of genes that were found to be differentially regulated based on a microarray experiment, the background should comprise only genes that are represented on that microarray.
Controlling for potential biases
To investigate potential biases in our DB, we ran Gene ORGANizer on random lists of 100, 500 and 1000 genes, and tested how many significantly enriched or depleted body parts are reported for different types of associations—confident, confident+tentative, typical and typical+non-typical. We repeated this procedure 1000 times and found that significantly enriched/depleted body parts were rarely observed. For example, for lists of 100 genes, only 0.5% of the confident typical+non typical iterations returned significant organs (FDR < 0.05), 4.2% for 500 genes and 3.8% for 1000 genes (Supplementary Table S2).
To evaluate whether inter-dependencies between genes may inflate our rate of false discoveries, a phenomenon that has been documented in gene set analyses of expression data (29), we performed simulations that account for such dependencies (see online Methods for details). We show that our false discovery rates are well <0.05, except for unrealistically high levels of dependency between genes (Supplementary Figure S1).
To further assess the level of accuracy in of our DB, we compared Gene ORGANizer to the OMIM organ annotations, which links disease to 33 of our 125 organs (10). Comparing the two, we found that <1% of our annotations were not in accordance with OMIM.
As a positive control we used housekeeping genes, which are genes that participate in basic cellular functions and are thus ubiquitously active and affect many anatomical parts (30). On average, each housekeeping gene is expected to be linked to more organs than in the genomic background. In this case, Gene ORGANizer will produce substantially more enriched body parts than expected by chance. We ran Gene ORGANizer on 3804 housekeeping genes (30) and reassuringly, found that most systems (7 out of 12) and regions (5 out of 6) were significantly enriched, as well as 32 organs (Supplementary Table S3). Such high numbers of significant body parts are rarely observed at random (P = 0.001 for systems, P = 0.015 for regions, P = 0.003 for organs, randomization test of 3804 genes).
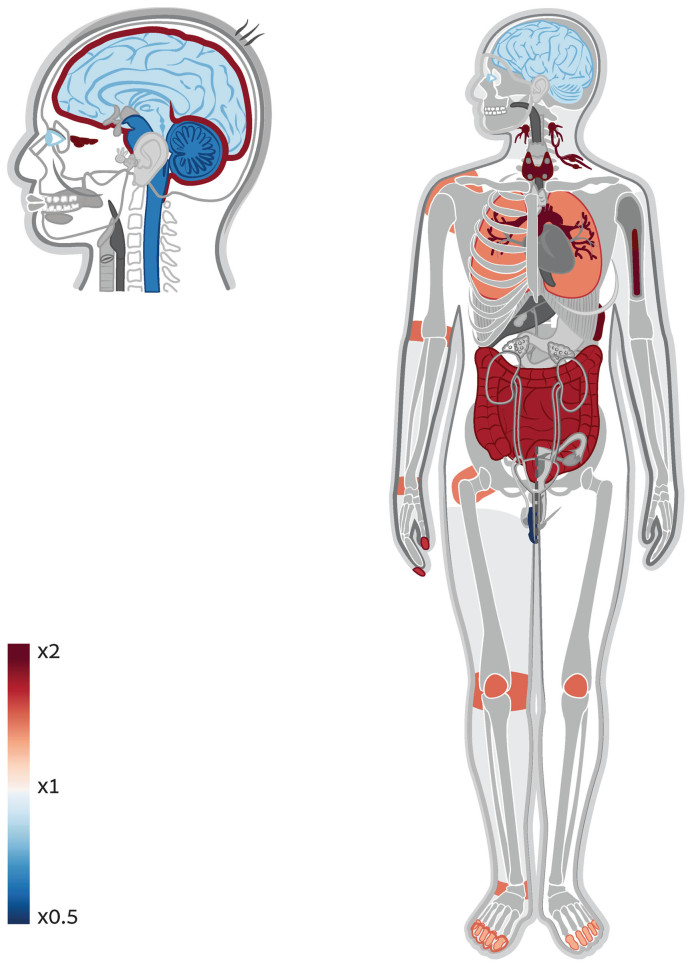
As another positive control, we extracted from the Kyoto Encyclopedia of Genes and Genomes (KEGG) (11) genes that are part of biochemical pathways linked to specific body systems. We did this for all body systems represented in KEGG, namely the circulatory, immune, endocrine, digestive and nervous systems, and demonstrated how in each case, Gene ORGANizer identified the relevant body parts as significantly enriched (Supplementary Table S4). Within the genes in KEGG that are associated with the circulatory system, Gene ORGANizer identified that the most enriched organs are the heart valve (x2.17, FDR = 2 × 10−7), red blood cells (×1.69, FDR = 0.009) and the heart (×1.50, FDR = 5 × 10−4, Supplementary Figure S2). Within immune-related genes, the most enriched systems were the lymphatic (×2.78, FDR < 10−15) and immune (×1.75, FDR = 8 × 10−11) and the most enriched organs were the sinuses (×5.14, FDR = 5 × 10−8), lymph nodes (×4.89, FDR < 10−15), and bone marrow (×4.08, FDR < 10−15, Figure 2). The sinuses probably appear in this list due to the elevated activity of lymphocytes within them, and the systemic link between the mucosal immune system and susceptibility to infections (31). Interestingly, additional characteristics of the immune system can be detected in these results. For example, the brain is significantly depleted, corresponding to the lack of lymphatic drainage system in the brain. However, the meninges is found to be significantly enriched, in accordance with the recent discovery that some lymphatic vasculature exists in the central nervous system in the form of lymphatic vessels in the tissues that surround the brain (32). Within endocrine-related genes, the endocrine system was the most enriched (×1.58, FDR = 3 × 10−4). See Online Methods for additional validations.
Figure 2.
Gene ORGANizer detects enrichment of immune-related organs within immune-related genes. A body and head map of enrichment and depletion of organs across immune-related genes. As a positive control, we extracted from the Kyoto Encyclopedia of Genes and Genomes (KEGG) (11) genes that are associated with specific systems. Genes that are involved in immune response were run in ORGANize and the most enriched body parts were those that are associated with immune response.
RESULTS AND DISCUSSION
Chromosome X is enriched with genes affecting facial features
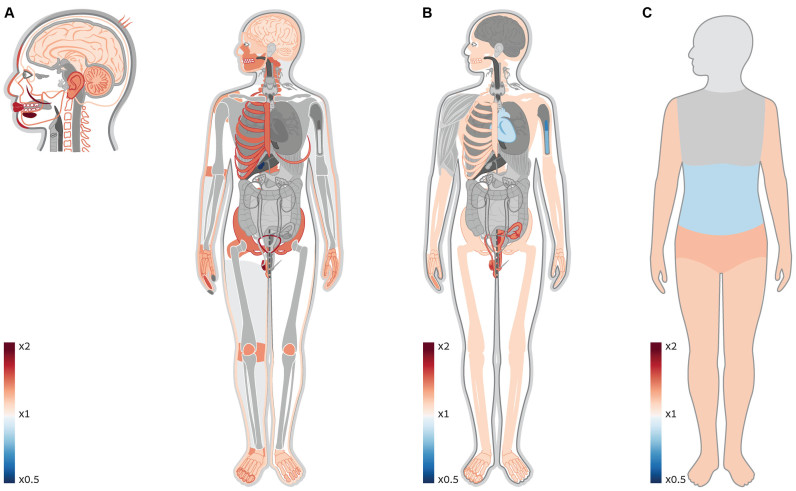
Sex chromosomes have always been of special interest because of their distinctive evolutionary history and means of inheritance, which result in unique selection regime and disease manifestation (33,34,35,36,37,38). The high occurrence of mental disorders in males drove researchers to look into chromosome X and investigate its link to the brain. Indeed, manual inspection of the OMIM DB has shown that chromosome X has >3-fold enrichment in genes associated with mental retardation, raising the hypothesis that there is an over-representation of brain-related genes on chromosome X (37). Other studies have shown that chromosome X is enriched with reproduction-related genes, and in particular with genes that are expressed in the testes (38). As only one body system was investigated in each of these studies, it was impossible to put these findings in a larger context of the entire body and see how these enrichments scale up compared to other body parts, and if they are unique. Using Gene ORGANizer, not only do we validate the enrichment of brain- and reproduction-related genes within chromosome X, but interestingly, we observe a stronger trend that could not have been detected with current tools and DBs. The brain and testes are only two out of 45 organs that are significantly enriched within this chromosome. Almost half of them, including the most enriched ones, are parts of the face (e.g. the mouth, cheeks, lips, chin, teeth, forehead, nose, hair, jaws and outer ear, FDR < 0.05, Figure 3). In fact, aside from the eyes, all facial parts are significantly enriched within X-linked genes. We also show that it is not only testes-related genes that are enriched within chromosome X, but most organs of the urogenital system. Finally, we detect over-representation of many parts of the skeletal system, including the rib cage, pelvis, joints, limb extremities, spinal column and skull.
Figure 3.
Genes affecting the face, the brain, and the urogenital and skeletal systems are over-represented on chromosome X. (A) A heat map of enriched and depleted organs within X-linked genes. Gene ORGANizer detects significant enrichment of the brain and testes within these genes, confirming previous claims. A more pronounced trend is the over-representation of different facial features, including all parts of the face except the eyes. Many parts of the urogenital and skeletal systems are enriched as well. (B) A heat map of enriched and depleted systems within X-linked genes. The reproductive and the skeletal systems are significantly enriched (×1.38 and ×1.12, FDR = 3 × 10−5 and 0.022, respectively). The immune and the cardiovascular systems are significantly depleted (×0.74 and ×0.87, FDR = 0.002 and 0.032, respectively). (C) A heat map of enriched and depleted body regions within X-linked genes. The regions of the pelvis and limbs are significantly over-represented (×1.22 and ×1.16, FDR = 5 × 10−4 and 0.003, respectively). The abdominal region is significantly depleted (×0.84, FDR = 0.008).
As a negative control, we applied Gene ORGANizer to chromosome 16, which resembles chromosome X in both size and number of genes. We found that the genes on chromosome 16 are not enriched with any body part (Supplementary Table S5). More generally, repeating the analysis for all other autosomal chromosomes revealed that their genes rarely show any significant association with specific body parts. The only chromosomes that showed any over-representation were chromosomes 9, 14 and 17, albeit to a much lesser extent compared to chromosome X, both in the number of enriched body parts and in the levels of enrichment (Supplementary Table S5). This suggests that chromosome X likely experiences a unique regime of selection leading to preferential representation of genes that affect the brain, the urogenital and skeletal systems, and above all—facial features.
A possible explanation for these observations is that being hemizygous in males, genes on chromosome X experience stronger and sex-specific selection compared to autosomal genes. This is because a newly emerged recessive allele on chromosome X will be expressed in males, but not in females. With this process in mind, Rice suggested in 1984 that genes on chromosome X play an important role in sexually dimorphic traits and in sexual selection (34). In fact, based on Rice's hypothesis, it is predicted that with time, sexually selected and sexually dimorphic genes will preferentially move, through chromosomal translocation, to chromosome X. Alternatively, this hypothesis predicts that X-linked genes will evolve sexually dimorphic function, and that they will be sexually selected for more often (34). Indeed, it was shown later that chromosome X is highly enriched for genes that control sexually selected and sexually dimorphic traits (35,39). Therefore, a possible explanation for our observations is that some of these organs are targets of sexual selection, and that their sexually dimorphic nature (such as in the case of the face, a classic sexually divergent (40,41) and sexually selected organ (42)), was evolutionary advantageous.
These results emphasize the importance of Gene ORGANizer as a tool to investigate gene function outside the scope of gene expression data. Expression databases rarely provide information for body parts such as the face, and thus, they are restricted in the range of anatomical parts for which they can provide inference. This could explain how the most pronounced trend on chromosome X has not been detected to date.
Imprinted genes tend to affect the same organs
Imprinted genes are genes that are transcribed only from one of the chromosomes—either the maternal or the paternal. This asymmetric silencing is achieved through DNA methylation of one of the alleles. This phenomenon evolved independently in plants and mammals, and its evolutionary role is still debated (43). Aberrant imprinting, where both or none of the alleles are transcribed, results in a variety of abnormalities. Previous studies have shown that human imprinted genes within the same locus show similar temporal patterns of expression (43). Concerted upregulation of imprinted genes from different loci has been identified as well (43). Furthermore, imprinted genes have been shown to participate in similar biochemical pathways (43). These observations suggest an intricate network of co-regulation of imprinted genes. However, the extent to which this phenomenon affects specific organs, and its phenotypic consequences are still to be determined (43). To test this, we ran a list of 37 high-confidence imprinted genes (44) in Gene ORGANizer. We used only typical annotations in order to examine only the most common effects of these genes. We found that the endocrine system is the most enriched system within imprinted genes, with an over-representation of ×3.21 (FDR = 0.018, Supplementary Table S6). This suggests that much of the reported role of imprinted genes in the regulation of development and growth (43) is executed through the endocrine system. Importantly, we show that organs previously hypothesized to be particularly influenced by imprinted genes (e.g. the brain (45) and reproductive organs (46)) are not significantly enriched within these genes, compared to the rest of the genome. This emphasizes the importance of Gene ORGANizer as a tool that enables researchers to analyze associations with organs in a genome-wide context.
Positively selected genes in hominids affect less organs
In order to understand natural selection in a wide context, it is crucial to examine its dynamics across many species. A recent study investigated patterns of natural selection across all extant Hominidae species (great apes, including humans) (47). This study identified hundreds of genes that likely went through positive selection in each lineage. Although most signatures of positive selection are species-specific, we found shared phenotypic effects within these genes. Taking together the top 200 genes with the strongest signs of positive selection in each lineage (1581 unique genes in total), we found that 26 organs and 3 systems are significantly depleted (Supplementary Figure S3, Supplementary Table S7). The only organs that show a limited degree of enrichment (albeit not significant) are related to the nervous system, in accordance with the GO annotation-based analyses in the original study (47). Such across-the-board depletion suggests a more general possibility: these genes tend to affect less organs than expected by random. Indeed, we found that positively selected genes along hominid lineages affect on average ∼5 organs less than random genes (29.4 compared to 34.5, P = 0.006, randomization test). This is also supported by the observation that some of the most depleted organs have ubiquitous functions that affect many aspects of the physiology (e.g. the parathyroid, hypothalamus, thymus and thyroid). These results suggest an intriguing possibility that positive selection tends to occur in genes with narrower and more organ-specific functions.
Although the Gene ORGANizer DB is based mostly on human phenotypes, these associations probably hold to a large extent in other species. By converting a list of gene IDs from a non-human organism to human gene IDs, or by entering gene symbols, which are mostly shared between species, researchers can use our tool to analyze gene function in non-human organisms. In order to test this, we ran in Gene ORGANizer a list of 117 genes that show signals of convergent evolution in bats and dolphins (48). As these mammals independently evolved echolocation, we expected this list to be enriched for genes that affect echolocation-related organs, such as the inner and middle ear. Indeed, we find these organs to be significantly enriched (×3.60 and ×2.42, P = 0.001 and 0.003, respectively). We also ran Gene ORGANizer on genes where signals of positive selection were detected in the gibbon genome (49). Possibly reflecting the exceptional arboreal locomotion of gibbons and their unique skeletal structure, we show how all subcranial bones and joints are significantly over-represented. We also find enrichment in organs related to the digestive, cardiovascular and nervous system (FDR < 0.05, Supplementary Table S8). When researchers first came to analyze the gibbon genome and assign such genomic regions with functional meaning, they were limited to the use of tools that were mainly designed for molecular- and pathway-level analyses (49). Using Gene ORGANizer, we show how higher level anatomical analysis could be easily performed, and how this could provide researchers with novel results in both human and non-human genomes.
We presented here the Gene ORGANizer DB and tool for the phenotypic analyses of gene–organ associations. We trust that Gene ORGANizer could be useful in nearly any genome-wide study where questions related to anatomy are raised, whether from an evolutionary, medical or biochemical perspective.
Supplementary Material
ACKNOWLEDGEMENTS
We thank The Human Phenotype Ontology Consortium (HPO) and DisGeNet for their comprehensive data, and Shiran Bar, Ido Sagi, Sagiv Shifman, Michal Linial and Benny Yakir for their advice and ideas.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Israel Science Foundation FIRST individual grant [ISF 1430/13]. Funding for open access charge: Israel Science Foundation FIRST individual grant [1430/13].
Conflict of interest statement. None declared.
REFERENCES
- 1. Brookes A.J., Robinson P.N.. Human genotype-phenotype databases: aims, challenges and opportunities. Nat. Rev. Genet. 2015; 16:702–715. [DOI] [PubMed] [Google Scholar]
- 2. Huang D.W., Sherman B.T., Lempicki R.A.. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009; 37:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Papatheodorou I., Oellrich A., Smedley D.. Linking gene expression to phenotypes via pathway information. J. Biomed. Semantics. 2015; 6:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang D.W., Lempicki R.A, Sherman B.T.. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009; 4:44–57. [DOI] [PubMed] [Google Scholar]
- 5. Köhler S., Doelken S.C., Mungall C.J., Bauer S., Firth H.V., Bailleul-Forestier I., Black G.C.M., Brown D.L., Brudno M., Campbell J. et al. . The Human Phenotype Ontology project: linking molecular biology and disease through phenotype data. Nucleic Acids Res. 2014; 42:D966–D974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Piñero J., Queralt-Rosinach N., Bravo A., Deu-Pons J., Bauer-Mehren A., Baron M., Sanz F., Furlong L.I.. DisGeNET: a discovery platform for the dynamical exploration of human diseases and their genes. Database. 2015; 2015:bav028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ramos E.M., Hoffman D., Junkins H.A, Maglott D., Phan L., Sherry S.T., Feolo M., Hindorff L.A. Phenotype-Genotype Integrator (PheGenI): synthesizing genome-wide association study (GWAS) data with existing genomic resources. Eur. J. Hum. Genet. 2014; 22:144–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kahraman A., Avramov A., Nashev L.G., Popov D., Ternes R., Pohlenz H.D., Weiss B.. PhenomicDB: a multi-species genotype/phenotype database for comparative phenomics. Bioinformatics. 2005; 21:418–420. [DOI] [PubMed] [Google Scholar]
- 9. Mannil D., Vogt I., Prinz J., Campillos M.. Organ system heterogeneity DB: a database for the visualization of phenotypes at the organ system level. Nucleic Acids Res. 2015; 43:D900–D906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hamosh A., Scott A.F., Amberger J.S., Bocchini C.A., McKusick V.A.. Online Mendelian Inheritance in Man (OMIM), a knowledgebase of human genes and genetic disorders. Nucleic Acids Res. 2005; 33:D514–D517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kanehisa M., Sato Y., Kawashima M., Furumichi M., Tanabe M.. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016; 44:D457–D462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Santos A., Tsafou K., Stolte C., Pletscher-Frankild S., O’Donoghue S.I., Jensen L.J.. Comprehensive comparison of large-scale tissue expression datasets. PeerJ. 2015; 3:e1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Petryszak R., Burdett T., Fiorelli B., Fonseca N.A., Gonzalez-Porta M., Hastings E., Huber W., Jupp S., Keays M., Kryvych N. et al. . Expression Atlas update—a database of gene and transcript expression from microarray- and sequencing-based functional genomics experiments. Nucleic Acids Res. 2014; 42:D926–D932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vogel C., Marcotte E.M.. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012; 13:227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Khan Z., Ford M.J., Cusanovich D.A., Mitrano A., Pritchard J.K., Gilad Y.. Primate transcript and protein expression levels evolve under compensatory selection pressures. Science. 2013; 342:1100–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maier T., Güell M., Serrano L.. Correlation of mRNA and protein in complex biological samples. FEBS Lett. 2009; 583:3966–3973. [DOI] [PubMed] [Google Scholar]
- 17. de Sousa Abreu R, Penalva L.O., Marcotte E.M., Vogel C.. Global signatures of protein and mRNA expression levels. Mol. Biosyst. 2009; 5:1512–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hao L., Ge X., Wan H., Hu S., Lercher M.J., Yu J., Chen W.-H.. Human functional genetic studies are biased against the medically most relevant primate-specific genes. BMC Evol. Biol. 2010; 10:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rebhan M., Chalifa-Caspi V., Prilusky J., Lancet D.. GeneCards: A novel functional genomics compendium with automated data mining and query reformulation support. Bioinformatics. 1998; 14:656–664. [DOI] [PubMed] [Google Scholar]
- 20. Benson D.A., Cavanaugh M., Clark K., Karsch-Mizrachi I., Lipman D.J., Ostell J., Sayers E.W.. GenBank. Nucleic Acids Res. 2013; 41:D36–D42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weinreich S.S., Mangon R., Sikkens J.J., Teeuw M.E., Cornel M.C.. [Orphanet: a European database for rare diseases]. Ned. Tijdschr. Geneeskd. 2008; 152:518–519. [PubMed] [Google Scholar]
- 22. Bragin E., Chatzimichali E.A., Wright C.F., Hurles M.E., Firth H. V., Bevan A.P., Swaminathan G.J.. DECIPHER: database for the interpretation of phenotype-linked plausibly pathogenic sequence and copy-number variation. Nucleic Acids Res. 2014; 42:D993–D1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Apweiler R., Bairoch A., Wu C.H., Barker W.C., Boeckmann B., Ferro S., Gasteiger E., Huang H., Lopez R., Magrane M. et al. . UniProt: the Universal Protein knowledgebase. Nucleic Acids Res. 2004; 32:D115–D119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Davis A.P., Murphy C.G., Johnson R., Lay J.M., Lennon-Hopkins K., Saraceni-Richards C., Sciaky D., King B.L., Rosenstein M.C., Wiegers T.C. et al. . The comparative toxicogenomics database: update 2013. Nucleic Acids Res. 2013; 41:D1104–D1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Landrum M.J., Lee J.M., Riley G.R., Jang W., Rubinstein W.S., Church D.M., Maglott D.R.. ClinVar: public archive of relationships among sequence variation and human phenotype. Nucleic Acids Res. 2014; 42:D980–D985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Blake J.A., Bult C.J., Eppig J.T., Kadin J.A., Richardson J.E., Mouse Genome Database G.. The Mouse Genome Database: integration of and access to knowledge about the laboratory mouse. Nucleic Acids Res. 2014; 42:D810–D817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Laulederkind S.J.F., Hayman G.T., Wang S.J., Smith J.R., Lowry T.F., Nigam R., Petri V., De Pons J., Dwinell M.R., Shimoyama M. et al. . The Rat Genome Database 2013-data, tools and users. Brief. Bioinform. 2013; 14:520–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alasti F., Sadeghi A., Sanati M.H., Farhadi M., Stollar E., Somers T., Van Camp G.. A mutation in HOXA2 is responsible for autosomal-recessive microtia in an Iranian family. Am. J. Hum. Genet. 2008; 82:982–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goeman J.J., Buhlmann P.. Analyzing gene expression data in terms of gene sets: methodological issues. Bioinformatics. 2007; 23:980–987. [DOI] [PubMed] [Google Scholar]
- 30. Eisenberg E., Levanon E.Y.. Human housekeeping genes, revisited. Trends Genet. 2013; 29:569–574. [DOI] [PubMed] [Google Scholar]
- 31. Janeway C.A., Travers P., Walport M., Shlomchik M.. Immunobiology: the Immune system In health and disease. Immunol. Biol. 2001; 5:Taylor & Francis, Inc; 10.1111/j.1467-2494.1995.tb00120.x. [Google Scholar]
- 32. Louveau A., Smirnov I., Keyes T.J., Eccles J.D., Rouhani S.J., Peske J.D., Derecki N.C., Castle D., Mandell J.W., Lee K.S. et al. . Structural and functional features of central nervous system lymphatic vessels. Nature. 2015; 523:337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ross M.T., Grafham D. V, Coffey A.J., Scherer S., McLay K., Muzny D., Platzer M., Howell G.R., Burrows C., Bird C.P. et al. . The DNA sequence of the human X chromosome. Nature. 2005; 434:325–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rice W.R. Sex chromosomes and the evolution of sexual dimorphism. Evolution. 1984; 38:735–742. [DOI] [PubMed] [Google Scholar]
- 35. Gibson J.R., Chippindale A.K., Rice W.R.. The X chromosome is a hot spot for sexually antagonistic fitness variation. Proc. Biol. Sci. 2002; 269:499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lercher M.J., Urrutia A.O., Hurst L.D.. Evidence that the human X chromosome is enriched for male-specific but not female-specific genes. Mol. Biol. Evol. 2003; 20:1113–1116. [DOI] [PubMed] [Google Scholar]
- 37. Zechner U., Wilda M., Kehrer-Sawatzki H., Vogel W., Fundele R., Hameister H.. A high density of X-linked genes for general cognitive ability: a run-away process shaping human evolution. Trends Genet. 2001; 17:697–701. [DOI] [PubMed] [Google Scholar]
- 38. Saifi G.M., Chandra H.S.. An apparent excess of sex- and reproduction-related genes on the human X chromosome. Proc. Biol. Sci. 1999; 266:203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Reinhold K. Sex linkage among genes controlling sexually selected traits. Behav. Ecol. Sociobiol. 1998; 44:1–7. [Google Scholar]
- 40. Samal A., Subramani V., Marx D.. Analysis of sexual dimorphism in human face. J. Vis. Commun. Image Represent. 2007; 18:453–463. [Google Scholar]
- 41. Rosas A., Bastir M.. Thin-plate spline analysis of allometry and sexual dimorphism in the human craniofacial complex. Am. J. Phys. Anthropol. 2002; 117:236–245. [DOI] [PubMed] [Google Scholar]
- 42. Rhodes G. The evolutionary psychology of facial beauty. Annu. Rev. Psychol. 2006; 57:199–226. [DOI] [PubMed] [Google Scholar]
- 43. Patten M.M., Cowley M., Oakey R.J., Feil R.. Regulatory links between imprinted genes: evolutionary predictions and consequences. Proc. R. Soc. B. 2016; 283:20152760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Morison I.M., Ramsay J.P., Spencer H.G.. A census of mammalian imprinting. Trends Genet. 2005; 21:457–465. [DOI] [PubMed] [Google Scholar]
- 45. Wilkinson L.S., Davies W., Isles A.R.. Genomic imprinting effects on brain development and function. Nat. Rev. Neurosci. 2007; 8:832–843. [DOI] [PubMed] [Google Scholar]
- 46. Faisal M., Kim H., Kim J.. Sexual differences of imprinted genes’ expression levels. Gene. 2014; 533:434–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cagan A, Theunert C., Laayouni H., Santpere G., Pybus M., Casals F., Prüfer K., Navarro A., Marques-Bonet T., Bertranpetit J., Andrés AM.. Natural selection in the great apes. Mol. Biol. Evol. 2016; 33:3268–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Parker J., Tsagkogeorga G., Cotton J.A., Liu Y., Provero P., Stupka E., Rossiter S.J.. Genome-wide signatures of convergent evolution in echolocating mammals. Nature. 2013; 502:228–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Carbone L., Harris R.A., Gnerre S., Veeramah K.R., Lorente-Galdos B., Huddleston J., Meyer T.J., Herrero J., Roos C., Aken B. et al. . Gibbon genome and the fast karyotype evolution of small apes. Nature. 2014; 513:195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.