Ependymoma is the third most common brain tumor in children, which can arise anywhere along the neuroaxis. Although historically considered one entity since the 1920s, recent studies have confirmed that supratentorial and posterior fossa ependymoma are completely different entities, particularly with respect to their cell of origin.1–3 Despite this, past and current clinical trials still consider ependymoma as one morphologically homogeneous entity.
Recent integrative genomic studies have revealed distinct subgroups of pediatric intracranial ependymoma. Particularly there are 2 groups of posterior fossa ependymoma and 2 groups of supratentorial ependymoma.2,4 Within the posterior fossa there are 2 distinct subgroups: posterior fossa A (PFA) is characterized by promoter hypermethylation, younger age distribution, and a very poor prognosis, whereas posterior fossa B (PFB) arises in older children and adolescents and has a good prognosis.5 In the supratentorial compartment, the majority of cases belong to the v-rel avian reticuloendotheliosis viral oncogene homolog A (RELA) group, characterized by the presence of fusions of C11orf95 to RELA that can serve as a reliable diagnostic marker and appears to have a worse prognosis.2 Yes-associated protein 1 (YAP1) fusions define a rare subgroup of supratentorial ependymoma of young children with a good prognosis.4 In the posterior fossa, children under 8 are almost exclusively PFA, while in the supratentorial compartment almost all true ependymomas are within the RELA group.5 As such, more robust clinical risk stratification is required for future clinical design. Although a morphological classification exists dividing tumors into classic and anaplastic histologies, this has essentially no clinical utility due to wide interobserver variability, and there are drastically different ratios of anaplastic to classic depending on the trial cohort and conflicting survivals in different cohorts.6 In the posterior fossa, extent of resection is the most powerful predictor of outcome within PFA; however, robust molecular markers are urgently needed.
Telomere maintenance is one of the hallmarks of cancer and the mechanism securing cancer cell survival in case of cellular crisis. Recently, a large study of 31 tumor types demonstrated that human telomerase reverse transcriptase (hTERT) was expressed in 73% of human cancer. In a third of cases the mechanism of telomerase activation was explained by hTERT promoter mutation, amplification, or chromosomal rearrangement. A majority of remaining hTERT mRNA expressing tumors carried hTERT promoter methylation.7 In ependymoma, hTERT mRNA expression and promoter methylation have been suggested to have prognostic significance specifically in posterior fossa ependymoma; however, it has been unclear if this was simply a reflection of PFA versus PFB.8,9
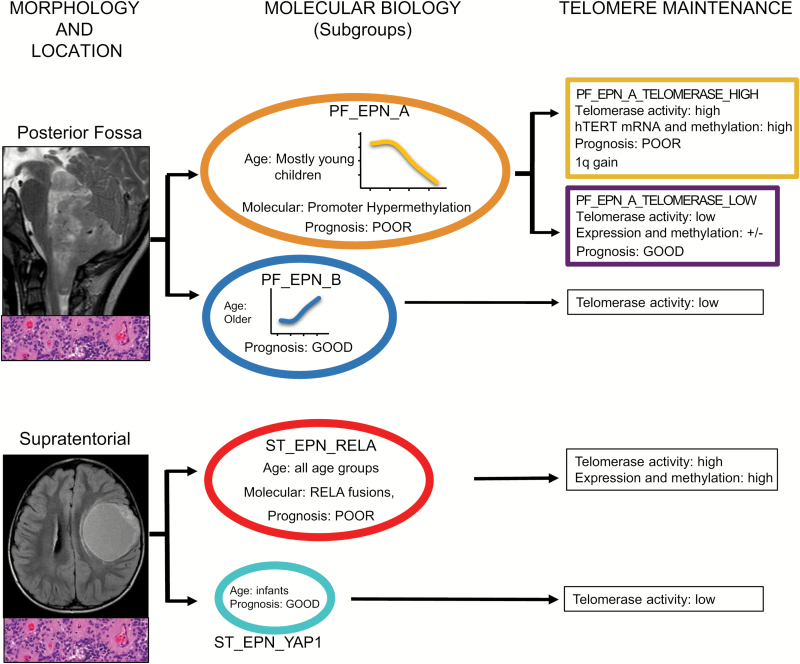
In this issue of Neuro-Oncology, Gojo et al10 report a single center cohort of consecutive frozen ependymomas evaluated using telomeric repeat amplification protocol (TRAP) and a validation cohort for hTERT promoter methylation.4 In a very elegant and comprehensive integrated analysis, the authors explore the relation of hTERT enzymatic activity, mRNA expression, and promoter methylation in ependymoma in a subgroup-dependent manner. This is indeed one of the first studies to evaluate a biomarker within the defined ependymoma subgroups rather than across all anatomical compartments. When restricting their analysis to only PFA ependymoma, they find a clear relationship between elevated hTERT enzymatic activity and a poor prognosis. Five PFBs were also evaluated and found to have low hTERT enzymatic activity. Interestingly, there is a low correlation between hTERT enzymatic activity and hTERT expression or promoter methylation, and hTERT mRNA expression and promoter methylation had no prognostic value within PFA. This clarifies a previous controversy regarding the role of hTERT methylation and expression in the clinical stratification of posterior fossa ependymoma. In addition, hTERT activity was associated with chromosome 1q gain, which was previously described as a poor prognostic marker, and the authors demonstrated the possible mechanism of this association with expression of E26 translocation variant 3. Although limited by small numbers, the RELA group of supratentorial ependymoma had uniformly high levels of hTERT activity, and levels of TERT promoter methylation. Overall it indicates that the TRAP assay evaluating hTERT enzymatic activity might be a promising tool to identify patients with a favorable prognosis in an otherwise poor prognostic subgroup (PFA) (Fig. 1). Although prospective validation of these findings in clinical trial cohorts is crucial, this raises the prospect that within PFA ependymoma, patients can be easily stratified by telomerase activity into PFA-TELOMERASE_HIGH and PFA-TELOMERASE_LOW groups. Evaluation of frozen tissue from cooperative studies integrating hTERT enzymatic activity, molecular subgroup, 1q status, and extent of resection has the potential to provide a robust risk stratification to prioritize patients for novel therapies.
Fig. 1.
Relationship between the molecular subgroups of pediatric ependymoma and their relation to histology, imaging, and hTERT status.
This study represents a step forward in how we approach putative biomarkers of ependymoma, particularly the evaluation of a marker in a subgroup-dependent manner. Despite being distinct tumor entities, supratentorial and posterior fossa ependymoma share similar names for historical reasons, and in 2017 combining these 2 entities into single clinical trials is analogous to primitive neuroectodermal tumor and medulloblastoma being considered a single entity.11 There are some important limitations, however, that need to be considered prior to adopting this approach in a clinical setting. This study evaluated a small cohort of patients with complete resections. Considering the crucial role of surgical resection in the risk stratification of posterior fossa ependymoma, it would be important to evaluate the value of hTERT activity across the entire clinical spectrum of PFA ependymoma.5 Nevertheless, this study not only provides a promising new biomarker within high risk posterior fossa ependymoma, but also suggests that perhaps elevated hTERT enzymatic activity may be responsible for radiation resistance, thus contributing to the poor survival.
Future clinical trials in Europe and possibly in North America will mandate the collection of fresh frozen tissue; therefore, prospective evaluation of the prognostic significance of hTERT activity can be potentially validated. Commercial kits are available to evaluate hTERT activity where small tissue quantities are required and the enzymatic activity is more stable than mRNA, suggesting that it may be a suitable clinical test. Although prospective clinical validation requires prospective evaluation in a molecularly informed cohort, hTERT activity offers a novel and promising tool to risk-stratify patients with PFA ependymoma.
References
- 1. Khatua S, Ramaswamy V, Bouffet E. Current therapy and the evolving molecular landscape of paediatric ependymoma. Eur J Cancer. 2017;70:34–41. [DOI] [PubMed] [Google Scholar]
- 2. Pajtler KW, Mack SC, Ramaswamy V, et al. The current consensus on the clinical management of intracranial ependymoma and its distinct molecular variants. Acta Neuropathol. 2017;133(1):5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Taylor MD, Poppleton H, Fuller C, et al. Radial glia cells are candidate stem cells of ependymoma. Cancer Cell. 2005;8(4):323–335. [DOI] [PubMed] [Google Scholar]
- 4. Pajtler KW, Witt H, Sill M, et al. Molecular classification of ependymal tumors across all CNS compartments, histopathological grades, and age groups. Cancer Cell. 2015;27(5):728–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ramaswamy V, Hielscher T, Mack SC, et al. Therapeutic impact of cytoreductive surgery and irradiation of posterior fossa ependymoma in the molecular era: a retrospective multicohort analysis. J Clin Oncol. 2016;34(21):2468–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ellison DW, Kocak M, Figarella-Branger D, et al. Histopathological grading of pediatric ependymoma: reproducibility and clinical relevance in European trial cohorts. J Negat Results Biomed. 2011;10:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barthel FP, Wei W, Tang M, et al. Systematic analysis of telomere length and somatic alterations in 31 cancer types. Nat Genet. 2017;49(3):349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Castelo-Branco P, Choufani S, Mack S, et al. Methylation of the TERT promoter and risk stratification of childhood brain tumours: an integrative genomic and molecular study. Lancet Oncol. 2013;14(6):534–542. [DOI] [PubMed] [Google Scholar]
- 9. Tabori U, Ma J, Carter M, et al. Human telomere reverse transcriptase expression predicts progression and survival in pediatric intracranial ependymoma. J Clin Oncol. 2006;24(10):1522–1528. [DOI] [PubMed] [Google Scholar]
- 10. Gojo J, Lötsch D, Spiegl-Kreinecker S, et al. Telomerase activation in posterior fossa group A ependymomas is associated with dismal prognosis and chromosome 1q gain. Neuro Oncol. March 24, 2017. doi:10.1093/neuonc/nox027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pomeroy SL, Tamayo P, Gaasenbeek M, et al. Prediction of central nervous system embryonal tumour outcome based on gene expression. Nature. 2002;415(6870):436–442. [DOI] [PubMed] [Google Scholar]