Abstract
While surgical and radiotherapeutic improvements increased life expectancy of meningioma patients, little is known about these patients’ health-related quality of life (HRQoL). Therefore, the objectives of this systematic review were to assess HRQoL in meningioma patients, the methodological quality of the used questionnaires (COSMIN criteria), and the reporting level of patient-reported outcomes (PROs) in the included studies (International Society of Quality of Life Research criteria).
Nineteen articles met our inclusion criteria. HRQoL was measured with 13 different questionnaires, 3 validated in meningioma patients. According to our predefined cutoff, HRQoL data were reported sufficiently in 5 out of 19 studies. Both findings hamper interpretation of the PRO results.
In general, meningioma patients reported clinically worse HRQoL than healthy controls. Although meningioma patients had better HRQoL than glioma patients, this difference was not clinically relevant. Radiotherapy seemed to improve some domains of HRQoL in the short term, while HRQoL decreased to pre-radiotherapy levels in the long term. Tumor resection increased HRQoL, but long-term follow-up showed persistent reduced HRQoL compared with healthy controls. These results suggest an impaired HRQoL in meningioma patients, even years after anti-tumor treatment. Results of this systematic review warrant high quality prospective studies, better instruments to assess HRQoL, and improved level of reporting for this group of patients.
Keywords: health-related quality of life, meningioma, patient-reported outcome, questionnaires, reporting level
Meningiomas are the most prevalent tumors of the central nervous system (36.4%), originating from the arachnoid cap cells,1 with an incidence rate of 7.86 per 100000 population.2 About 90% of meningiomas are benign (World Health Organization [WHO] grade I).3 Depending on the location of the mass, patients may suffer from a wide variety of somatic and psychological symptoms, such as epileptic seizures, visual loss, cognitive symptoms, psychiatric symptoms, and neuropathies.3 In addition, the majority of patients suffer from more general symptoms, such as tiredness, sleep problems, and psychosocial problems. Both the disease-specific and more general symptoms may cause limitations of daily activities and consequently participation restrictions, which are reflected in a deterioration of patients’ health-related quality of life (HRQoL).
During the last 2 decades, new radiation and surgical techniques have improved the treatment of meningioma patients (MP). In modern case series, MP have a near normal 5- and 10-year life expectancy (5-y survival 92%, expected survival 94%; 10-y survival 81%, expected survival 86%) but often suffer from moderate to severe neurological deficits, even 5 years after surgery (67%).4 Parallel to these improvements in therapy and life expectancy, a shift is occurring in treatment objectives; from survival and radical tumor removal to patient performance and HRQoL.5 Indeed, one should now start to measure the net clinical benefit of meningioma therapy.6
HRQoL is a multidimensional concept covering generally valued aspects of life (defined as health or health-related), such as in the physical, social, and psychological domains, as well as disease-specific signs and symptoms caused by the disease and its treatment.7 HRQoL should be patient reported, since doctor-reported and patient-reported HRQoL results differ significantly and patients are thought to be the best source of information on their own HRQoL.8 HRQoL can be measured using generic questionnaires (eg, the Medical Outcomes Study [MOS] 36-Item Short Form Health Survey [SF-36], the EuroQol Five Dimensions [EQ-5D], the Functional Assessment of Cancer Therapy–General [FACT-G], the European Organisation for Research and Treatment of Cancer Core Quality of Life Questionnaire [EORTC QLQ-C30], the M. D. Anderson Symptom Inventory [MDASI])9–14 or disease-specific questionnaires (eg, FACT-Brain [FACT-Br], the EORTC QLQ–Brain Neoplasm [EORTC QLQ-BN20], the MDASI–Brain Tumor [MDASI-BT]).14–16 However, neither in clinical practice nor in research is this done frequently in meningioma patients.
The main objective of this systematic review was to assess HRQoL in meningioma patients. In addition, we assessed the methodological quality of the used HRQoL questionnaires as well as the level of reporting of the patient-reported outcomes (PROs) in the included studies.
Materials and Methods
Search Strategy and Paper Selection
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.17
Search strategy
A literature search was conducted in the following electronic databases: Embase, MEDLINE, Web of Science, CINAHL, PsychInfo, Academic Search Premier, COCHRANE, and ScienceDirect up to October 2015. Search terms used were “meningioma,” “quality of life,” and terms formulated to exclude case reports and studies with animals only (see Supplementary Table 1 for the search strategy in MEDLINE). The search strategy was adapted for the other electronic databases. Reference lists of included articles were scanned for additional studies.
Paper selection
Inclusion criteria were the following: original peer-reviewed articles measuring patient-reported HRQoL in meningioma patients (whole population or reported separately as a subpopulation) using a questionnaire. Both observational and interventional studies, either retrospective or prospective, were included. Exclusion criteria were as follows: articles not in English, case reports (up to 5 patients), reviews, studies with only animals, and studies including a main population of patients younger than 18 years. Two independent reviewers (A.H.Z.N. and M.C.M.P.) screened all titles and abstracts for eligibility. Disagreement was resolved with discussion and consensus, and when discussion failed to lead to consensus, a third researcher mediated (L.D.).
Data Extraction
Information was extracted per included article by 2 independent researchers (A.H.Z.N. and M.C.M.P.) on study design, main inclusion criteria, and subject characteristics: mean age at time of intervention, percentage women, percentage WHO grade I, II, or III tumors, location of tumor and functional status. In addition, when applicable, type of intervention and Simpson Grade were noted. Regarding study outcomes, the timing of HRQoL assessments, the used questionnaire and the HRQoL outcomes (mean and, when reported, the standard deviation) itself were extracted. Data are presented for all studies separately. No meta-analysis was performed due to the small number of studies and heterogeneity of the studies in population (different tumor grades, tumor location), intervention (surgery, radiotherapy, wait-and-scan), and outcomes (different HRQoL questionnaires used).
Assessment of Reporting Level of Included Articles and Quality Assessment of Used Questionnaires
Assessment of reporting level of PROs in the included articles
The level of reporting of the PRO data in the included articles was assessed by 2 researchers independently (A.H.Z.N. and M.C.M.P.) following the criteria for PROs of the International Society of Quality of Life Research (ISOQOL).18 The criteria were adapted for nonrandomized studies and are presented in Supplementary Table 2. A maximum of 16 points could be scored and the predefined cutoff for sufficient reporting was 11/16 points, which is in line with previous work.19
Quality assessment of questionnaires used
Quality of the questionnaires used was assessed by 2 researchers independently (M.C.M.P. and L.D.) using the criteria of the Consensus-based Standards for the selection of health Measurement Instruments (COSMIN).20 In short, the following aspects were evaluated for meningioma patients or patients with other acquired brain injuries: content validity, internal consistency, criterion validity, construct validity, reproducibility, responsiveness, floor and ceiling effects, and interpretability.
Results
Study Characteristics
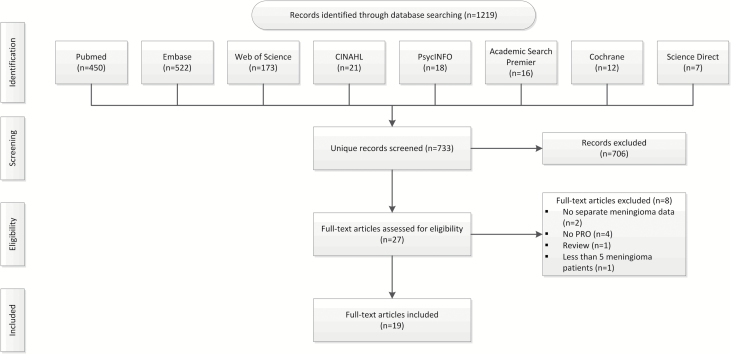
Titles and abstracts of 733 unique articles were screened, resulting in 27 eligible articles. These articles were read full-text and 19 met our inclusion criteria.21–40 Flow diagram of record analysis and article inclusion is depicted in Fig. 1. Study characteristics are presented in Table 1. Of the 19 included articles, 4 studies used a longitudinal21,22,29,38 and 15 a cross-sectional study design.23–28,30–34,36,37,39,40 Six studies included only patients with WHO grade I meningioma,23,30–32,36,38 4 studies also included patients with WHO grade II or III meningioma,21,22,25,29 and 9 studies did not report the WHO grade.24,26–28,33,34,37,39,40 Study population size ranged between 16 and 155 meningioma patients (median 47 patients). Seven studies compared the results of meningioma patients with normative data of healthy controls (HC),21,23,29–32,34 1 study compared results of meningioma patients with normative data of HC and (brain) cancer patients,25 1 study compared meningioma patients with glioma patients (GP),33 and 8 studies presented only results for meningioma patients.22,24,26–28,36–40 Surgery was the primary intervention in 13 studies,21–28,33,34,36–40 of which 2 compared HRQoL results before and after surgery.21,22 Radiotherapy was the primary intervention in 3 studies,29–31 of which 1 compared HRQoL results before and after radiotherapy.29 A wait-and-scan approach was the primary treatment modality in 1 study.32
Fig. 1.
Flow diagram of record analysis and article inclusion.
Table 1.
Characteristics of included studies
| Author (year) | Study Design Regarding PRO Results | Main Inclusion and Exclusion Criteria | Patients and Controls | Age (years) | Gender (% women) | WHO Grade | Location Tumor | Simpson Grade | Functional Status | Primary Intervention | Moment of Measurement |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Miao (2010)21 | Prospective | Meningioma patients histologically confirmed and operated | Meningioma patients: 147 Controls: 96, age-matched |
Meningioma patients median age: 43 (5–77) Controls Median age: 42 (range N/A) |
Meningioma patients: 59% Controls: 67% |
I: 80% II: 7% III: 6% | Parasagittal: 3% Falcine: 16% Convexity: 39% Olfactory groove: 9% Sphenoid ridge: 13% Clivus: 5% Intraventricular: 6% Cerebellum: 4% Other: 4% | 0: 8% I: 18% II: 20% III: 27% IV: 27% V: - | N/A | Surgery | Before and after surgery, not further specified |
| Jakola (2012)22 | Prospective | Meningioma patients, histologically confirmed, aged ≥ 18 y | Meningioma patients: 46 | Meningioma patients mean age: 55 ± 13 | Meningioma Patients: 67% | I: 83% II: 17% | Convexity: 24% Parasagittal or falcine: 33% Supratentorial skull base: 35% Infratentorial: 8% | I-II: 66% III: 17% IV-V: 17% | KPS: 85 ± 11 | Surgery | Before surgery: 1–3 days Short term after surgery: 6 weeks Long term after surgery: 10–58 months |
| Waagemans (2010)23 | Cross- sectional | Meningioma patients: histologically confirmed WHO grade I, without signs of tumor recurrence for at least 1 year after last intervention | Meningioma patients: 89 Controls: 89, age-, sex-, educational level- matched | Meningioma patients mean age: 58 ± 13 Controls mean age: 58 ± 13 | Meningioma patients: 74% Controls: 74% | I: 100% | Convexity: 51% Skull base: 45% Tentorium/falx: 20% Orbital: 7% Olfactory tract: 3% | I: 23% II: 34% III: 13% IV: 24% V: 3% Unknown: 3% | N/A | Surgery | After surgery: at least 1 year after last intervention (mean 3.4 years) |
| Mathiesen (2007)34 | Cross- sectional | Meningioma patients: petroclival tumors, larger than 30mm. | Meningioma patients: 16 | Meningioma patients mean age: 54 (SD N/A) | Meningioma patients: 69% | MIB+ < 2%: 94% 6%>: 6% | Petroclival tumors | I: 4% II: 38% III: 7% IV:52% V: - | N/A | Surgery | Postoperative, at least 1 year after surgery (mean 66 months) |
| Neil-Dwyer (2000)24 Neil- Dwyer (2001)*40 Lang (1999)*39 (same study population and results) | Cross- sectional | Meningioma patients: petroclival tumors arising medial to the 5th cranial nerve | Meningioma patients: 19 (*17) | Meningioma patients age range: 29–63 | Meningioma patients: 79% | N/A | Petroclival tumors | N/A | N/A | Surgery | Postoperative, at least 1 year after surgery |
| Konglund (2012)25 | Cross- sectional | Meningioma patients, ≥60 y, elective surgery |
Meningioma patients: 47 | Meningioma patients median age: 70 (60–84) | Meningioma patients: 65% | I: 94% II: 4% Missing: 2% | Convexity: 44% Skull base: 33% Parasagittal: 11% Tentorial: 9% Intraventricular: 2% | I: 35% II: 39% III: 13% IV: 13% | KPS < 50: 2% 50–70: 9% >70: 89% | Surgery | Postoperative, 6 months after surgery |
| Shin (2013)33 | Cross- sectional | Patients ≥18 y, histologically diagnosed brain tumor: meningioma, glioma and other tumors | Meningioma patients: 107 | All patients: mean age 48 (18–81) | All patients: 57% | N/A | N/A | N/A | All patients: KPS ≤70: 11% >70: 89% | Surgery | Postoperative, not further specified |
| Mohsenipour (2001)26 | Cross- sectional | Meningioma patients, neurosurgical treatment | Meningioma patients: 82 | Meningioma patients: mean age 61 ± 15 | Meningioma patients: 65% | N/A | Convexity: 72% Petrosal: 12% Cerebropontine: 4% Multiple: 4% Spinal: 9% | N/A | N/A | Surgery | Postoperative, not further specified |
| Kalkanis (2000)27 | Cross- sectional | Meningioma patients, undergone craniotomy | Meningioma patients: 155 | Meningioma patients: mean age 59 ± 14 | Meningioma patients: 66% | N/A | N/A | N/A | N/A | Surgery | Postoperative, mean time after surgery 33 mo (0–165) |
| Salo (2002)28 | Cross- sectional | Brain tumor patients, ≥16 y, diagnosed by imaging |
Meningioma patients: 31 | All patients: 49 (20–82) | Meningioma patients: 61% | N/A | Intracranial Left: 46% Right: 35% Bilateral: 14% Undefined: 4% | N/A | N/A | Surgery | Preoperative |
| Henzel (2013)29 | Prospective | Meningioma patients, ≥18 y, ECOG performance status ≥2, KPS≥70%, life expectancy>2 y |
Meningioma patients: 52 | Meningioma patients median age: 57 (40–81) | Meningioma patients: 75% | Known of previous operated 42/52 I: 79% II: 17% III: 5% | Medial wing sphenoid: 56% Petroclival: 15% Tentorial: 6% Petroclival up to sphenoid bone: 12% Falx cerebi: 8% Optic nerve sheath: 2% Olfactory: 2% | N/A | N/A | SRT (42/52 previous surgery) | Before SRT, last day of SRT, thereafter biannually |
| Kangas (2012)30 | Cross- sectional | Meningioma patients, WHO grade I, treated with radiotherapy | Meningioma patients: 70 | Meningioma patients: 57 ± 12 | Meningioma patients: 77% | I: 100% | N/A | N/A | N/A | Radiotherapy | After RT, mean 1.7 y |
| Van Nieuwenhuizen (2007)31 | Cross- sectional | Meningioma patients, WHO grade I | Meningioma patients only surgery: 18 Meningioma patients: surgery and radiotherapy: 18 Healthy controls: 18, age- and sex- matched | Meningioma patients surgery only: 63 ± 12 Meningioma patients surgery and radiotherapy: 63 ± 11 | Meningioma patients surgery only: 84% Meningioma patients surgery and radiotherapy: 89% | I: 100% | N/A | N/A | KPS: Surgery only: 83 ± 20 Surgery and radiotherapy: 71 ± 18 Barthel: Surgery: 17 ± 1 Surgery and radiotherapy: 17 ± 2 |
Surgery with or without RT | Postoperative, at least 1 y after surgery Surgery only: mean 3.3 ± 2.0 years after surgery Surgery and RT: mean 3.3 ± 1.9 y after surgery |
| Van Nieuwenhuizen (2013)32 | Cross- sectional | Meningioma patients: radiologically suspected WHO grade I, who have not received surgery or radiotherapy | Meningioma patients: 21 Controls: 21 | Meningioma patients: 63 ± 14 Controls: 62 ± 14 | Meningioma patients: 81% Controls: 76% | I: 100% | Convexity: 38% Tentorium/Falx: 24% Skull base: 38%: | N/A | KPS 80 (40–100) | No intervention | Pre-operative |
| Bunevicius (2014)37 | Cross- sectional | Adult patients admitted for brain tumor surgery | Meningioma patients: 77 | All patients: 56 ± 15 | All patients: 69% | N/A | N/A | N/A | N/A | Before surgery | Pre-operative |
| Krupp (2009)36 | Cross- sectional | Meningioma patients, supratentorial, WHO grade I, surgically treated | Meningioma patients: 91 | Meningioma patients: 56 ± 10 | Meningioma patients: 66% | I: 100% | Convexity: 46% Sphenoid ridge: 21% Parasagittal, falx: 20% Frontal cranial base: 13% | N/A | N/A | Surgery | Postoperative (mean 15 months, range 10–19 mo) |
| Curey (2012)38 | Prospective | Tuberculum sellae meningioma, surgically treated with the superior interhemispheric approach | Meningioma patients: 20 | Meningioma patients: 59 ± 11 | Meningioma patients: 85% | I: 100% | Tuberculum sellae meningioma | I or II: 95% | N/A | Surgery | Pre-operative and postoperative at 6 mo |
Percentages do not add up to 100% due to rounding KPS: Karnofsky Performance Score; N/A: not assessed or not reported; (S)RT: (stereotactic) radiotherapy.
Data Extraction
Data of the included studies are depicted in Supplementary Table 4; significant and/or clinically relevant results as described in the original articles are presented here.
Meningioma versus normative data healthy controls
In general, meningioma patients reported worse HRQoL compared with healthy controls before surgery. Overall health status was lower (study-specific questionnaire [SSQ]: MP 74 ± 2, HC 91 ± 2, P < .0001; SF-36: MP 53 ± 25, HC 66 ± 21, P = .030),21,32 as were the following subdomains: physical health (SSQ: MP 27 ± 1, HC 37 ± 3, P < .0001),21 patient satisfaction with medical care (SSQ: MP 5 ± 2, HC 7 ± 2, P < .001),21 self-care (SSQ: MP 14 ± 2, HC 20 ± 1, P < .0001),21 and vitality (SF-36: MP 56 ± 19, HC 66 ± 23, P = .043).32 Postoperatively, studies reported both worse and better HRQoL scores in meningioma patients compared with healthy controls. About 3.4 years after surgery, meningioma patients had more role limitations caused by physical problems (SF-36: MP 50, HC 65, P < .05),23 while they had less role limitations 6 months after surgery (SF-36: MP 77, P = .01).25 Compared with healthy controls, meningioma patients still scored worse 6 months after tumor removal on cognitive functioning (EORTC QLQ-C30: MP 79, P = .02), yet better on physical functioning (EORTC QLQ-C30: MP 80, P = .01) and social functioning (EORTC QLQ-C30: MP 84, P < .01).25 Data for healthy controls were not described in this article by Konglund et al.25
Meningioma versus glioma patients and normative data of cancer and brain cancer patients
HRQoL of meningioma patients and glioma or (brain) cancer patients was compared using the EORTC QLQ-C30 and QLQ-BN20 questionnaires. Compared with glioma patients (GP), meningioma patients scored better on cognitive functioning (MP 73 ± 25, GP 64 ± 28, P = .008), social functioning (MP 81 ± 26, GP 64 ± 34, P < .001), physical functioning (MP 75±20, GP 66 ± 29, P = .02), future uncertainty (MP 28 ± 21, GP 39 ± 24, P = .003), motor dysfunction (MP 24 ± 23, GP 34 ± 33, P = .02), and communication deficits (MP 16 ± 23, GP 30 ± 31, P < .001).33 Compared with brain cancer patients (all grades), meningioma patients scored also better on cognitive functioning (MP 79, P = .02) and emotional functioning (MP 82, P = .04), but meningioma patients had more insomnia (MP 28, P = .01).25 Compared with the general cancer population, meningioma patients scored better on the following domains of the EORTC QLQ-C30 and QLQ-BN20: physical functioning (MP 80, P = .01), role functioning (MP 77, P = .02), emotional functioning (MP 82, P = .04), and social functioning (MP 84, P = .03), but worse on cognitive functioning (MP 79, P = .02). Data for HC were not described in this article by Konglund et al.25
HRQoL in meningioma patients before and after intervention
Long-term (10–58 mo postoperative) general HRQoL improved significantly after surgery (EQ-5D: mean improvement 0.09, P = .040; SSQ: preoperative 74 ± 2, postoperative 85 ± 2, P < .0001)22 as well as on the following domains: physical health (SSQ: preoperative 27 ± 1, postoperative 36 ± 2, P < .0001),21 patient satisfaction with medical care (SSQ: preoperative 5 ± 2, postoperative 7 ± 1, P = .01),21 self-care (SSQ: preoperative 14 ± 2, postoperative 16 ± 3, P = .04),21 and olfactory function (impact of surgery on Visual Analog Scale score for olfactory function +5.7 ± 2.2).38 Patients who had undergone surgery before radiotherapy (OP+RT) had significantly better mental health (SF-36) compared with patients who only received radiotherapy (RT): before RT (OP+RT 43, RT 32, P = .04), at the end of radiotherapy (OP+RT 42, RT 29, P = .014), and at 6/12/18/24 months follow-up (6 mo: OP+RT 45, RT 36; 12 mo: OP+RT 43, RT 33; 18 mo: OP+RT 44, RT 31; 24 mo: OP+RT 42, RT 34, all P = .004).29 Moreover, the addition of RT to surgery resulted in worse scores on the following domains: physical functioning (OP+RT 55 ± 55, RT 73 ± 33, P = .05), role limitations caused by physical functioning (OP+RT 34 ± 39, RT 61 ± 43, P = .03), and the physical component score (OP+RT 33 ± 11, RT 52 ± 12, P = .007).31 However, these differences could be explained by the longer disease length for patients treated with OP+RT compared with those treated with OP only (7.6 vs 3.0 y after diagnoses, respectively).31
Factors negatively influencing HRQoL in meningioma patients
A larger tumor size (P = .037), higher histological grade (P = .011), and tumor recurrence (P = .018) were all associated with lower overall HRQoL.21 In addition, larger tumor size was associated with more physical mobility impairment.21,26 The presence of a meningioma was associated with emotional well-being in a univariable analysis (r = −0.14, P = .048); however, this association was not confirmed in the multivariable analysis.37 Waagemans et al found that meningioma patients who used anti-epileptic drugs had lower scores on physical health (P < .01), social functioning (P < .05), mental health (P < .05), vitality (P < .01), and overall health status (P < .05) compared with healthy controls.23 They also found significant associations between impaired HRQoL and problems in neurocognitive functioning (executive functioning, information processing, verbal memory, psychomotor speed).23 Furthermore, shorter time since diagnosis (P = .013), more posttraumatic stress (P = .005), confusion (P = .000), and tumor location in the left hemisphere (P = .009) were negatively associated with HRQoL in meningioma patients.30
Factors positively influencing HRQoL in meningioma patients
A longer follow-up was associated with better HRQoL outcomes (SF-36); meningioma patients scoring on more than 4 subscales below the 25th percentile of normative data of healthy controls had a mean follow-up period of 2.9 years, whereas patients scoring less than 4 subscales below the 25th percentile had a mean follow-up period of 5.4 years (P < .05).34 Furthermore, less emotional impairment was associated with longer follow-up time after surgery (Innsbruck Health Dimensions Questionnaire for Neurosurgical Patients [IHD-NS]).26
Assessment of Reporting Level of PRO Data in the Included Articles
Reporting level of PRO data in the included studies is depicted in Table 2. Median reporting level score was 8 points (range: 6–14 points) and in 5 articles PRO data could be classified as sufficiently reported (≥11 points).22,23,30–32 All articles described the PRO in the title or abstract and included or cited the used questionnaire. However, most articles did not report the PRO methods (none), used statistical methods (for missing data, 5%),22 or explained how the results should be interpreted (eg, presenting the number of patients with a minimal important change or describing the cutoff for normal scores for the used scale, 5%).22 On all other criteria, 26% to 84% of the articles scored the highest possible score.
Table 2.
Assessment of PRO reporting level of included studies
| Author (year) |
Title and Abstract
(1 pnt) |
Introduction Background and Objectives (1 pnt) | Methods (6 pnt) | Results (3 pnt) | Discussion (4 pnt) | Protocol / Copy of Instrument (1 Pnt) |
Total Points
(Ma x 16 Points) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcomes (4 Pnt) | Statistical Methods (2 Pnt) | Participant Flow / Missing Data (1 Pnt) | Baseline Data (1 Pnt) | Outcomes and Estimation (1 Pnt) | Limitations (1 Pnt) | Generalizability (1 Pnt) | Interpretation (2 Pnt) | |||||
| Miao (2010)21 | 1 | 1 | 2 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 8 |
| Jakola (2012)*22 | 1 | 0 | 3 | 2 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 14 |
| Waagemans (2010)*23 | 1 | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 13 |
| Mathiesen (2007)34 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 6 |
| Neil-Dwyer (2000)24 | 1 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 6 |
| Neil-Dwyer (2001)40 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 6 |
| Lang (1999)39 | 1 | 0 | 3 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 8 |
| Konglund (2012)25 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 7 |
| Shin (2013)33 | 1 | 1 | 2 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 9 |
| Mohsenipour (2001)26 | 1 | 0 | 2 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 7 |
| Kalkanis (2000)27 | 1 | 0 | 3 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 8 |
| Salo (2002)28 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 6 |
| Henzel (2013)29 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 8 |
| Kangas (2012)*30 | 1 | 1 | 3 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 11 |
| Van Nieuwenhuizen31 (2007)* | 1 | 1 | 3 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 12 |
| Van Nieuwenhuizen (2013)*32 | 1 | 0 | 3 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 11 |
| Bunevicius (2014)37 | 1 | 0 | 2 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 8 |
| Krupp (2009)36 | 1 | 0 | 3 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 10 |
| Curey (2012)33 | 1 | 0 | 2 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 7 |
| Percentage of studies scoring maximum score per criterion | 100% | 32% | 0% | 5% | 53% | 42% | 84% | 26% | 58% | 5% | 100% | Mean 8 points |
* Articles with sufficient reporting level (predefined cutoff ≥11 points).
Quality Assessment of Used Questionnaires
Of the 13 used questionnaires, 3 were validated in meningioma patients, the FACT-G/FACT-BR14 and an SSQ.41 In addition, 5 questionnaires were (partially) validated for other types of acquired brain injury or brain cancer: EQ-5D,42,43 SF-36,44 EORTC QLQ-C30 and QLQ-BN20,15,45 and the IHD(NS).46 Validity and reliability varied among all questionnaires and none of the questionnaires met all requirements as specified in the COSMIN criteria. Data are presented in Supplementary Table 3.
Discussion
Although HRQoL is an important outcome for meningioma patients, this systematic literature review showed that only a few studies are published describing HRQoL in this patient group. Of those published, unfortunately, the level of PRO reporting of most articles was of low quality; only 3 HRQoL questionnaires have been validated in meningioma patients, and only one study reported minimal important changes of the PRO results, all hampering interpretation of HRQoL results. Nevertheless, based on the available results, we can conclude that in general, meningioma patients had a clinically relevant worse HRQoL than healthy controls. Tumor resection improved HRQoL, but long-term follow-up still showed reduced HRQoL compared with healthy controls. In addition, meningioma patients seemed to have a better HRQoL than (brain) cancer patients after surgery, although this difference was not clinically relevant. These results suggest an impaired HRQoL in some meningioma patients even years after tumor resection.
In general, meningioma patients reported worse HRQoL than healthy controls both before and after surgery. However, because of the few available studies, the use of different questionnaires, and low PRO reporting level, PRO results could not be pooled and results could not be compared for patients with different tumor locations (eg, convexity vs skull base). When comparing results of the EORTC QLQ-C30 of postoperative meningioma patients25,33 with normative data of healthy controls,47 meningioma patients had a clinically relevant lower score on the following domains: physical functioning, role functioning, emotional functioning, cognitive functioning, social functioning, and insomnia. In different studies, meningioma patients scored both better and worse on overall health status and fatigue. Likewise, when comparing preoperative results of the SF-36 for meningioma patients with matched controls (age, sex, and education) in a small study, meningioma patients had clinically significantly more role limitations caused by physical and emotional problems, worse general health, and less vitality.32 However, these clinically relevant differences between meningioma patients and healthy controls disappeared after surgery,23,31 except for the role limitations caused by physical problems.23 These seemingly confounding findings may be the result of psychological mechanisms of coping with surgery and illness, which may lead to a positive mental change, also called posttraumatic growth, a known phenomenon generally found in long-term follow-up of patients with different types of cancer or acquired brain injury.48–50 In addition, a mental change often causes a “response shift,” that is, a change in patients’ internal standards, values, and consequently perception of HRQoL.51
Results of the included studies further showed that compared with glioma patients, meningioma patients generally had a statistically significantly better HRQoL. One study, however, showed that meningioma patients had more insomnia than glioma patients.33 Comparing scores of newly diagnosed glioblastoma patients with scores of meningioma patients on the EORTC QLQ-C30 and QLQ-BN20 questionnaires,25,33 these scores were surprisingly similar between both patient groups.52 Although differences in 11 HRQoL domains were statistically significant, these results were not clinically relevant. Moreover, meningioma patients experienced more pain and visual problems than other brain cancer patients.52 Compared with a meta-analysis on SF-36 data in rheumatoid arthritis patients, the study by Waagemans et al showed that meningioma patients scored similar on the mental and physical component score 5 years after tumor removal.23 This implies that 5 years after tumor removal, HRQoL scores of meningioma patients are similar to those of a chronic disease and substantially lower than HRQoL scores of healthy controls.53
Results on the impact of different therapies on both survival and HRQoL/cognition may be used to determine the net clinical benefit of specific therapies.6 This information is important for clinical decision making and patient-tailored therapy. Although 2 studies showed a statistically significant improvement in HRQoL after surgery, this improvement was not clinically relevant in one study and not interpretable in the other study, as characteristics of the used questionnaire were not presented.21,22 Patients who underwent radiotherapy perceived a clinically relevant reduction in role limitations caused by physical problems immediately after radiotherapy and a clinically relevant reduction in role limitations caused by emotional problems 6 months after radiotherapy. However, both of these differences disappeared after 2 years of follow-up,29 suggesting that HRQoL returns to pre-radiotherapy levels in the long term. However, studies in low-grade glioma patients give strong evidence that radiotherapy causes long-term (after 6 y) cognitive problems and a decline in HRQoL.54–56 These results, while not in all respects comparable with those from meningioma patients due to different radiation fields and/or techniques, suggest that meningioma patients who receive radiotherapy might also experience a decline in HRQoL and cognitive performance in the long term. As the results of the impact of surgery and radiotherapy are not conclusive and potentially suffer from confounding by indication, prospective studies are needed to investigate the impact of treatment on both HRQoL and cognition in the long term.
PRO reporting of the included articles was on average of low quality. While the used study design, data acquisition methods, and analysis of the results may be correctly performed, it was not adequately described by the authors. As patient and tumor characteristics (eg, WHO grade, tumor location) were often not fully reported, and HRQoL data not stratified for these characteristics, generalizability of the results is hampered.
Studies comparing HRQoL results after radiotherapy and surgery may suffer from confounding by indication, as patients who are only treated with radiotherapy may have a worse prognosis due to unfavourable tumor location (close to critical structures) and/or higher WHO grade (WHO grades II and III). Moreover, most studies did not report whether they included consecutive patients in a predefined time period and did not describe characteristics of nonresponders. Since reasons for patients not to participate in a study are frequently poor health status and age,57 this could result in an overestimation of HRQoL of meningioma patients in the included studies. Another major limitation of the included studies is that no article clearly reported the PRO data registration and intended collection schedule, while both can influence results.58 Self-report tools suffer more from patients’ cognitive deficits than interviews, while both may be hampered by aphasia.6 Interpretation of HRQoL results depends on the intended moment of measurement, short-term or long-term, which may lead to different outcomes and interpretations. Indeed, Jakola and colleagues22 showed that compared with preoperative HRQoL, the mean improvement of patients’ HRQoL was not significantly improved 6 weeks after surgery, while it was improved 10–58 months after surgery.
There is great variety of available HRQoL questionnaires and a lack of argumentation for choosing a particular questionnaire, prohibiting comparison of results between studies. The most commonly used questionnaires were the SF-36,9,10 the FACT-G and FACT-BR,14 and the EORTC QLQ-C3012 and QLQ-BN20.15 Of these questionnaires, the FACT-G and FACT-BR were also validated in meningioma patients. In addition, the minimal important change is determined for the SF-36, FACT, and EORTC questionnaires, which is necessary for critical appraisal of found differences. Currently, the SF-36, FACT, and EORTC questionnaires seem most suitable for measuring HRQoL in meningioma patients.
In conclusion, this systematic review describes 19 studies reporting on HRQoL in meningioma patients. Most questionnaires that were used to assess HRQoL were not validated in meningioma patients and the reporting quality of the PRO data in the included studies was on average of low quality, both hampering interpretation of the results. In contrast to the current impression of patients and physicians, data are still insufficient and not conclusive on the effect of interventions on HRQoL in meningioma patients. To improve clinical decision making, more high-quality evidence is needed on the effect of meningioma and its different treatment modalities on HRQoL. Therefore, new prospective studies, validated meningioma-specific instruments to assess HRQoL in meningioma patients, and improved level of reporting seem warranted. Current data suggest that even though tumor removal through surgery may be beneficial, some meningioma patients have long-term clinically significantly impaired HRQoL.
Supplementary Material
Supplementary material is available online at Neuro-Oncology (http://neuro-oncology.oxfordjournals.org/).
Funding
None.
Conflicts of interest statement. None of the authors declares a conflict of interest.
Acknowledgments
J. W. Schoones helped with the systematic literature search.
References
- 1. Wiemels J, Wrensch M, Claus EB. Epidemiology and etiology of meningioma. J Neurooncol. 2010;99(3):307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ostrom QT, Gittleman H, Fulop J, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 2015;14(suppl. 5):1–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Whittle IR, Smith C, Navoo P, et al. Meningiomas. Lancet. 2004;363(9420):1535–1543. [DOI] [PubMed] [Google Scholar]
- 4. van Alkemade H, de Leau M, Dieleman EM, et al. Impaired survival and long-term neurological problems in benign meningioma. Neuro Oncol. 2012;14(5):658–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dirven L, Armstrong TS, Taphoorn MJ. Health-related quality of life and other clinical outcome assessments in brain tumor patients: challenges in the design, conduct and interpretation of clinical trials. Neurooncol Pract. 2015;2(1):2–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dirven L, Reijneveld JC, Taphoorn MJ. Health-related quality of life or quantity of life: a difficult trade-off in primary brain tumors? Semin Oncol. 2014;41(4):541–552. [DOI] [PubMed] [Google Scholar]
- 7. Guyatt GH, Feeny DH, Patrick DL. Measuring health-related quality of life. Ann Intern Med. 1993;118(8):622–629. [DOI] [PubMed] [Google Scholar]
- 8. Slevin ML, Plant H, Lynch D, et al. Who should measure quality of life, the doctor or the patient? Br J Cancer. 1988;57(1):109–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 10. McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-item short-form health survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31(3):247–263. [DOI] [PubMed] [Google Scholar]
- 11. Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337–343. [DOI] [PubMed] [Google Scholar]
- 12. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. [DOI] [PubMed] [Google Scholar]
- 13. Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000;89(7):1634–1646. [DOI] [PubMed] [Google Scholar]
- 14. Weitzner MA, Meyers CA, Gelke CK, et al. The Functional Assessment of Cancer Therapy (FACT) scale. Development of a brain subscale and revalidation of the general version (FACT-G) in patients with primary brain tumors. Cancer. 1995;75(5):1151–1161. [DOI] [PubMed] [Google Scholar]
- 15. Taphoorn MJ, Claassens L, Aaronson NK, et al. EORTC Quality of Life Group, and Brain Cancer, NCIC and Radiotherapy Groups. An international validation study of the EORTC brain cancer module (EORTC QLQ-BN20) for assessing health-related quality of life and symptoms in brain cancer patients. Eur J Cancer. 2010;46(6):1033–1040. [DOI] [PubMed] [Google Scholar]
- 16. Armstrong TS, Mendoza T, Gning I, et al. Validation of the M.D. Anderson Symptom Inventory Brain Tumor Module (MDASI-BT). J Neurooncol. 2006;80(1):27–35. [DOI] [PubMed] [Google Scholar]
- 17. Moher D, Liberati A, Tetzlaff J, et al. PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9, W64. [DOI] [PubMed] [Google Scholar]
- 18. Brundage M, Blazeby J, Revicki D, et al. Patient-reported outcomes in randomized clinical trials: development of ISOQOL reporting standards. Qual Life Res. 2013;22(6):1161–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dirven L, Taphoorn MJ, Reijneveld JC, et al. EORTC Quality of Life Group (Patient Reported Outcome Measurements Over Time In ONcology-PROMOTION Registry). The level of patient-reported outcome reporting in randomised controlled trials of brain tumour patients: a systematic review. Eur J Cancer. 2014;50(14):2432–2448. [DOI] [PubMed] [Google Scholar]
- 20. Terwee CB, Bot SD, de Boer MR, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007;60(1):34–42. [DOI] [PubMed] [Google Scholar]
- 21. Miao Y, Lu X, Qiu Y, et al. A multivariate analysis of prognostic factors for health-related quality of life in patients with surgically managed meningioma. J Clin Neurosci. 2010;17(4):446–449. [DOI] [PubMed] [Google Scholar]
- 22. Jakola AS, Gulati M, Gulati S, et al. The influence of surgery on quality of life in patients with intracranial meningiomas: a prospective study. J Neurooncol. 2012;110(1):137–144. [DOI] [PubMed] [Google Scholar]
- 23. Waagemans ML, van Nieuwenhuizen D, Dijkstra M, et al. Long-term impact of cognitive deficits and epilepsy on quality of life in patients with low-grade meningiomas. Neurosurgery. 2011;69(1):72–78; discussion 78. [DOI] [PubMed] [Google Scholar]
- 24. Neil-Dwyer G, Lang DA, Davis A. Outcome from complex neurosurgery: an evidence based approach. Acta Neurochir (Wien). 2000;142(4):367–371. [DOI] [PubMed] [Google Scholar]
- 25. Konglund A, Rogne SG, Lund-Johansen M, et al. Outcome following surgery for intracranial meningiomas in the aging. Acta Neurol Scand. 2013;127(3):161–169. [DOI] [PubMed] [Google Scholar]
- 26. Mohsenipour I, Deusch E, Gabl M, et al. Quality of life in patients after meningioma resection. Acta Neurochir (Wien). 2001;143(6):547–553. [DOI] [PubMed] [Google Scholar]
- 27. Kalkanis SN, Quiñones-Hinojosa A, Buzney E, et al. Quality of life following surgery for intracranial meningiomas at Brigham and Women’s Hospital: a study of 164 patients using a modification of the functional assessment of cancer therapy-brain questionnaire. J Neurooncol. 2000;48(3):233–241. [DOI] [PubMed] [Google Scholar]
- 28. Salo J, Niemelä A, Joukamaa M, et al. Effect of brain tumour laterality on patients’ perceived quality of life. J Neurol Neurosurg Psychiatry. 2002;72(3):373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Henzel M, Fokas E, Sitter H, et al. Quality of life after stereotactic radiotherapy for meningioma: a prospective non-randomized study. J Neurooncol. 2013;113(1):135–141. [DOI] [PubMed] [Google Scholar]
- 30. Kangas M, Williams JR, Smee RI. The association between post-traumatic stress and health-related quality of life in adults treated for a benign meningioma. Appl Res Qual Life. 2012;7(2):163–182. [Google Scholar]
- 31. van Nieuwenhuizen D, Klein M, Stalpers LJ, et al. Differential effect of surgery and radiotherapy on neurocognitive functioning and health-related quality of life in WHO grade I meningioma patients. J Neurooncol. 2007;84(3):271–278. [DOI] [PubMed] [Google Scholar]
- 32. van Nieuwenhuizen D, Ambachtsheer N, Heimans JJ, et al. Neurocognitive functioning and health-related quality of life in patients with radiologically suspected meningiomas. J Neurooncol. 2013;113(3):433–440. [DOI] [PubMed] [Google Scholar]
- 33. Shin YS, Kim JH. Validation of the Korean version of the European Organization for Research and Treatment of Cancer brain cancer module (EORTC QLQ-BN20) in patients with brain tumors. Health Qual Life Outcomes. 2013;11:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mathiesen T, Gerlich Å, Kihlström L, et al. Effects of using combined transpetrosal surgical approaches to treat petroclival meningiomas. Neurosurgery. 2007;62(6):982–991. [DOI] [PubMed] [Google Scholar]
- 35. Frič R, Eide PK. The presigmoid approach for removal of tumours causing ventral compression of the brainstem. Surgical results and postoperative quality of life. Br J Neurosurg. 2011;25(1):86–93. [DOI] [PubMed] [Google Scholar]
- 36. Krupp W, Klein C, Koschny R, et al. Assessment of neuropsychological parameters and quality of life to evaluate outcome in patients with surgically treated supratentorial meningiomas. Neurosurgery. 2009;64(1):40–47; discussion 47. [DOI] [PubMed] [Google Scholar]
- 37. Bunevicius A, Tamasauskas S, Deltuva V, et al. Predictors of health-related quality of life in neurosurgical brain tumor patients: focus on patient-centered perspective. Acta Neurochir (Wien). 2014;156(2):367–374. [DOI] [PubMed] [Google Scholar]
- 38. Curey S, Derrey S, Hannequin P, et al. Validation of the superior interhemispheric approach for tuberculum sellae meningioma: clinical article. J Neurosurg. 2012;117(6):1013–1021. [DOI] [PubMed] [Google Scholar]
- 39. Lang DA, Neil-Dwyer G, Garfield J. Outcome after complex neurosurgery: the caregiver’s burden is forgotten. J Neurosurg. 1999;91(3):359–363. [DOI] [PubMed] [Google Scholar]
- 40. Neil-Dwyer G, Lang D, Garfield J. The realities of postoperative disability and the carer’s burden. Ann R Coll Surg Engl. 2001;83(3):215–218. [PMC free article] [PubMed] [Google Scholar]
- 41. Miao Y, Qiu Y, Lin Y, et al. Assessment of self-reported and health-related quality of life in patients with brain tumours using a modified questionnaire. J Int Med Res. 2008;36(6):1279–1286. [DOI] [PubMed] [Google Scholar]
- 42. Golicki D, Niewada M, Buczek J, et al. Validity of EQ-5D-5L in stroke. Qual Life Res. 2015;24(4):845–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sagberg LM, Jakola AS, Solheim O. Quality of life assessed with EQ-5D in patients undergoing glioma surgery: what is the responsiveness and minimal clinically important difference? Qual Life Res. 2014;23(5):1427–1434. [DOI] [PubMed] [Google Scholar]
- 44. Anderson C, Laubscher S, Burns R. Validation of the Short Form 36 (SF-36) health survey questionnaire among stroke patients. Stroke. 1996;27(10):1812–1816. [DOI] [PubMed] [Google Scholar]
- 45. Maringwa J, Quinten C, King M, et al. EORTC PROBE Project and Brain Cancer Group. Minimal clinically meaningful differences for the EORTC QLQ-C30 and EORTC QLQ-BN20 scales in brain cancer patients. Ann Oncol. 2011;22(9):2107–2112. [DOI] [PubMed] [Google Scholar]
- 46. Dos Santos CB, De Carvalho SCA, Da Silva MFG, et al. Cross-cultural adaptation of the Innsbruck health dimensions questionnaire for neurosurgical patients (IHD-NS). Arq Neuropsiquiatr. 2008;66(3B):698–701. [DOI] [PubMed] [Google Scholar]
- 47. Van de Poll-Franse LV, Mols F, Gundy CM et al. Normative data for the EORTC QLQ-C30 and EORTC-sexuality items in the general Dutch population. Eur J Cancer. 2011;47(5):667–675. [DOI] [PubMed] [Google Scholar]
- 48. Tedeschi RG, Calhoun LG Posttraumatic growth: conceptual foundations and empirical evidence. Psychol Inq. 2004;15(1):1–18. [Google Scholar]
- 49. Cordova MJ, Cunningham LL, Carlson CR, et al. Posttraumatic growth following breast cancer: a controlled comparison study. Health Psychol. 2001;20(3):176–185. [PubMed] [Google Scholar]
- 50. Powell T, Ekin-Wood A, Collin C. Post-traumatic growth after head injury: a long-term follow-up. Brain Inj. 2007;21(1):31–38. [DOI] [PubMed] [Google Scholar]
- 51. Sprangers MA, Schwartz CE. Integrating response shift into health-related quality of life research: a theoretical model. Soc Sci Med. 1999;48(11):1507–1515. [DOI] [PubMed] [Google Scholar]
- 52. Taphoorn MJ, Henriksson R, Bottomley A, et al. Health-related quality of life in a randomized phase III study of bevacizumab, temozolomide, and radiotherapy in newly diagnosed glioblastoma. J Clin Oncol. 2015;33(19):2166–2175. [DOI] [PubMed] [Google Scholar]
- 53. Matcham F, Scott IC, Rayner L, et al. The impact of rheumatoid arthritis on quality-of-life assessed using the SF-36: a systematic review and meta-analysis. Semin Arthritis Rheum. 2014;44(2):123–130. [DOI] [PubMed] [Google Scholar]
- 54. Douw L, Klein M, Fagel SS, et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol. 2009;8(9):810–818. [DOI] [PubMed] [Google Scholar]
- 55. Klein M, Heimans JJ, Aaronson NK, et al. Effect of radiotherapy and other treatment-related factors on mid-term to long-term cognitive sequelae in low-grade gliomas: a comparative study. Lancet. 2002;360(9343):1361–1368. [DOI] [PubMed] [Google Scholar]
- 56. Aaronson NK, Taphoorn MJ, Heimans JJ, et al. Compromised health-related quality of life in patients with low-grade glioma. J Clin Oncol. 2011;29(33):4430–4435. [DOI] [PubMed] [Google Scholar]
- 57. Walker M, Brown J, Brown K, et al. Practical problems with the collection and interpretation of serial quality of life assessments in patients with malignant glioma. J Neurooncol. 2003;63(2):179–186. [DOI] [PubMed] [Google Scholar]
- 58. Bowling A. Mode of questionnaire administration can have serious effects on data quality. J Public Health (Oxf). 2005;27(3):281–291. [DOI] [PubMed] [Google Scholar]