A number of retrospective and prospective series have assessed local control outcomes of brain metastases based on primary histology.1–4 These studies have revealed mixed results, with some studies showing poorer local control with histologies typically classified as radioresistant, including melanoma and renal cell cancers. The current determinants of stereotactic radiation dose are lesion size and receipt of previous radiation treatment.5 Histology does not routinely play a primary role in the determination of radiation dose.
We have previously developed a multigene expression model of tumor radiosensitivity.6 This model has been validated in multiple independent clinical cohorts, including breast, rectal, esophageal, head and neck, glioblastoma, pancreas, and prostate malignancies.6 This model predicts a radiosensitivity index (RSI) that is directly proportional to tumor radioresistance (RSI, high index = radioresistance). A recent report from our group demonstrated significant differences in RSI based on the anatomical location of colon metastases7 and in liver metastases based on primary histology.8 The purpose of this analysis was to assess the RSI of brain metastases based on primary histology.
Patients were identified from the institutional review board–approved Total Cancer Care (TCC) prospective observational protocol at Moffitt Cancer Center. Deidentified data from a total of 277 metastatic brain lesions were obtained from the TCC meta-data pool. RNA preparation and gene expression profiling methods have been described.7 RSI was calculated using the previously published ranked based algorithm on metastatic brain samples.6 When comparing RSI values between groups, the Kruskal‒Wallis test was used. For pairwise comparisons, Bonferroni adjusted P values were calculated when considering multiple comparisons.
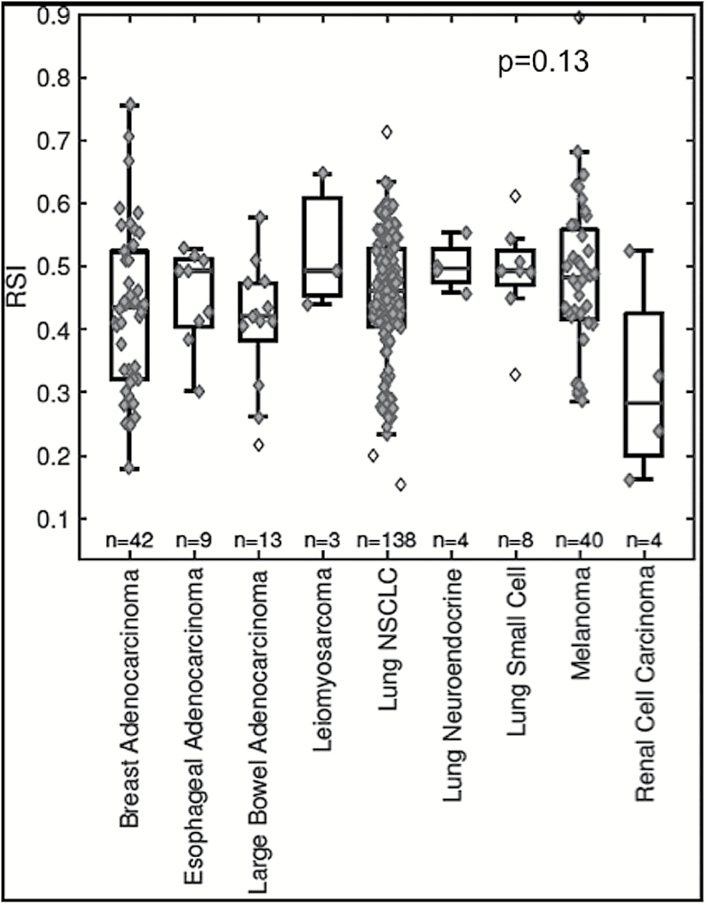
The analysis was restricted to primary histologies with at least 3 tumor samples, leaving 261 samples for analysis. The median RSI for all brain metastases was 0.46 (quartile [Q]1, 0.40; Q3, 0.52). The most common primary histologies for brain metastases were non–small cell lung cancer (n = 138; 53%), breast adenocarcinoma (n = 42; 16%), melanoma (n = 40; 15%), and colorectal adenocarcinoma (n = 13; 5%).
Across the group, no significant differences in radiosensitivity were noted. The median RSIs for brain metastases in descending order of radioresistance were lung neuroendocrine (0.50), esophageal adenocarcinoma (0.49), leiomyosarcoma (0.49), small cell lung cancer (0.49), melanoma (0.48), non–small cell lung cancer (0.46), breast adenocarcinoma (0.44), colorectal adenocarcinoma (0.42), and renal cell carcinoma (0.28), P = 0.13; Fig. 1. No significant differences were noted in pairwise comparisons between the histologies (all Bonferroni adjusted P > 0.05).
Fig. 1.
Box plot of RSI values based on primary histology. Unfilled diamonds represent outliers using the standard 1.5 interquartile range rule.
A total of 9 patients had multiple samples available for analysis (n = 19). The mean standard deviation for tumor samples from the same patient with the same histology was 0.01 (range: 0–0.06). We assessed the degree of consistency among repeated RSI measurements within a patient by calculating the intraclass coefficient. The intraclass coefficient of samples from the same patient was 0.78 (95% CI: 0.49–0.94, P < 0.001), suggesting an excellent agreement based on Cicchetti’s guidelines.
There was a wide distribution that was noted across histologies revealing that radiosensitivity can vary between metastases. However, when comparing the median RSI of histologies, the majority were found to be radioresistant. Across the 14000 samples in the TCC database, we identified a cutpoint of 0.375, with samples ≥0.375 deemed to be more radioresistant and those samples with an RSI of <0.375 deemed to be more radiosensitive.7 Thus, the majority of metastatic brain histologies in this study had a median RSI that was radioresistant.
Previous studies have revealed mixed results in the management of brain metastases based upon primary histology. Studies have found worse local control outcomes with treatment of histologies traditionally thought of as being radioresistant (melanoma, sarcoma, and renal cell).1,2 However, this finding has not been confirmed by a multi-institutional report from Flickinger et al and a study by Rades et al.3,4 Given these mixed results based on primary histology, it is clear to see why primary histology is not a primary determinant of radiotherapy dosing.
Our results indicate that radioresistant and radiosensitive brain metastases exist across all histologies, and varying RT doses may not be appropriate based on primary histology of brain metastases. Moreover, since the median RSIs of the majority of brain metastases based on histology were deemed radioresistant, ablative stereotactic radiosurgery doses may be most appropriate to achieve local control. When assessing lesions from the same patient, similarity was noted in RSI indicating similarity in the radiosensitivity of lesions from the same patient. Our findings have implications for future studies adjusting dose to brain metastases based on primary histology, suggesting that such a strategy may not improve local control outcomes.
Funding
This work was supported by National Institutes of Health Grants [R21CA101355/R21CA135620]; US Army Medical Research and Materiel Command [W81XWH-08-2-0101, Subaward 12-15479-01-07]; National Functional Genomics Center [Award 170220051]; Bankhead-Coley Foundation [Award 09BB-22]; the Debartolo Family Personalized Medicine Institute; and also supported in part under the Merck-Moffitt Cancer Center Research Collaboration.
Conflict of interest statement. S.A.E. and J.F.T-R. report stock and leadership in Cvergenx Inc. and royalty and patents on RSI.
References
- 1. Sia J, Paul E, Dally M, Ruben J. Stereotactic radiosurgery for 318 brain metastases in a single Australian centre: the impact of histology and other factors. J Clin Neurosci. 2015;22(2):303–307. [DOI] [PubMed] [Google Scholar]
- 2. Oermann EK, Kress MA, Todd JV, et al. The impact of radiosurgery fractionation and tumor radiobiology on the local control of brain metastases. J Neurosurg. 2013;119(5):1131–1138. [DOI] [PubMed] [Google Scholar]
- 3. Rades D, Hornung D, Blanck O, et al. Stereotactic radiosurgery for newly diagnosed brain metastases: comparison of three dose levels. Strahlenther Onkol. 2014;190(9):786–791. [DOI] [PubMed] [Google Scholar]
- 4. Flickinger JC, Kondziolka D, Lunsford LD, et al. A multi-institutional experience with stereotactic radiosurgery for solitary brain metastasis. Int J Radiat Oncol Biol Phys. 1994;28(4):797–802. [DOI] [PubMed] [Google Scholar]
- 5. Shaw E, Scott C, Souhami L, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000;47(2):291–298. [DOI] [PubMed] [Google Scholar]
- 6. Eschrich SA, Pramana J, Zhang H, et al. A gene expression model of intrinsic tumor radiosensitivity: prediction of response and prognosis after chemoradiation. Int J Radiat Oncol Biol Phys. 2009;75(2):489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ahmed KA, Fulp WJ, Berglund AE, et al. Differences between colon cancer primaries and metastases using a molecular assay for tumor radiation sensitivity suggest implications for potential oligometastatic SBRT patient selection. Int J Radiat Oncol Biol Phys. 2015;92(4):837–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ahmed KA, Caudell JJ, El-Haddad G, et al. Radiosensitivity differences between liver metastases based on primary histology suggest implications for clinical outcomes after stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2016;95(5):1399–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]