Abstract
Diffuse intrinsic pontine glioma (DIPG) has proven to be one of the most challenging of all pediatric cancers. Owing to a historical reticence to obtain tumor tissue for study, and based on an erroneous assumption that the biology of DIPG would mirror that of supratentorial high-grade astrocytomas, innumerable studies have been undertaken—all of which have had a negligible impact on the natural history of this disease. More recently, improvements in neurosurgical techniques have allowed for the safe upfront biopsy of DIPG, which, together with a wider use of autopsy tissue, has led to an evolving understanding of the biology of this tumor. The discovery of a recurrent somatic gain-of-function mutation leading to lysine 27 to methionine (p.Lys27Met, K27M) substitution in histone 3 variants characterizes more than 85% of DIPG, suggesting for the first time the role of the epigenome and histones in the pathogenesis of this disease, and more unified diagnostic criteria. Along with further molecular insights into the pathogenesis of DIPG, rational targets are being identified and studied in the hopes of improving the otherwise dismal outcome for children with DIPG.
Keywords: DIPG, epigenetics, H3K27M, oncohistone
The diagnosis of a diffuse intrinsic pontine glioma (DIPG) in a child remains one of the most devastating diagnoses within pediatric cancers. While a myriad of treatments have been studied in hundreds of clinical trials, the outcome for these children remains abysmal. Almost all will die of their disease between 6 months and 2 years from the time of their initial diagnosis. Against the backdrop of this grim prognosis, there has been a growing understanding that if improvements are to be realized for these children, then historic assumptions need to be challenged. Notably, the long-standing assumption that adult-type infiltrating high-grade astrocytomas in the supratentorial compartment recapitulate the biology of DIPG was called into question. This has driven efforts to obtain tumor tissue either from autopsy specimens or more recently at the time of diagnosis. These efforts are now allowing investigators to understand much more about the unique biology of these tumors and to provide the first therapeutic hints to management.
Clinical Considerations
Clinical Presentation
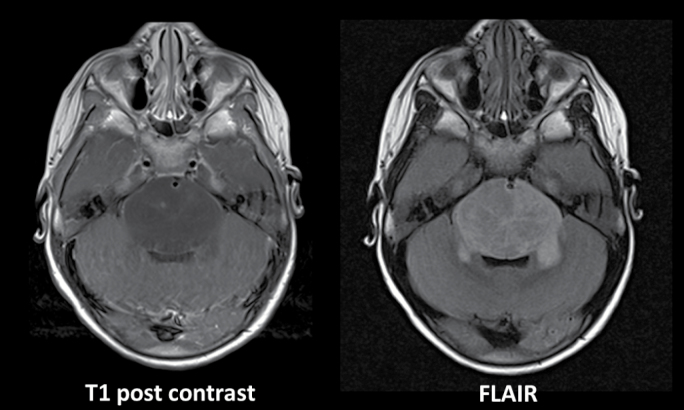
At initial diagnosis of a DIPG, 3 symptoms predominate: cranial neuropathies, long tract signs, and ataxia. In a subset of patients, hydrocephalus with symptoms of raised intracranial pressure will present. Similar symptoms may be noted with non-DIPG brainstem tumors such that clinical presentation alone is not pathognomonic of a DIPG. In most of the DIPG cases, the delay between first signs and diagnosis is shorter: less than 3 months. Classic features noted on MRI include a mass centered in the pons, encompassing more than 50% of the pons and causing enlargement of the pons. In most cases at initial diagnosis, little or no enhancement is noted after the administration of gadolinium. In some cases, areas of necrosis with surrounding serpiginous contrast enhancement are present. The basilar artery is often enveloped by the engorged pons (Fig. 1). Imaging features supportive of a DIPG are considered sufficient for the presumptive treatment in the absence of a biopsy, although as discussed below, there is a growing tendency to consider a biopsy at diagnosis. A survey on DIPG diagnosis by neurosurgeons based on imaging solely has shown striking discrepancies between observers.1 While prognosis remains poor for all children, features such as age ≥3 years, shorter duration of symptoms, and presence of ring enhancement on MRI at the time of diagnosis portend an even shorter survival.2
Fig. 1.
Classic imaging appearance of DIPG at diagnosis. MRI panels of these axial images demonstrate an enlarged pons with minimal contrast enhancement after the administration of gadolinium. Fluid attenuated inversion recovery imaging further elucidates the extent of involvement and demonstrates the envelopment of the basilar artery.
Standard Treatment of DIPG
The mainstay of treatment of children with newly diagnosed DIPG remains focal radiation therapy to the pons. In most centers this consists of 3D conformal photon-based radiotherapy to a range of 54‒59.4 Gy given in 30–33 fractions of 1.8 Gy daily. Recently, some centers have employed hypofractionation (eg, 39 Gy given in 13 fractions of 3.0 Gy daily) in an effort to reduce treatment burden. Small non-inferiority trials of the latter approach have been reported demonstrating clinically similar levels of disease control,3–6 although the ideal fractionation to be used has yet to be defined. To date, no chemotherapeutic strategy has been shown to improve overall survival in children with DIPG, including neoadjuvant chemotherapy, chemoradiotherapy, adjuvant chemotherapy, chemotherapy initiated at the time of clinical or radiologic progression post radiation, or high-dose therapy with stem cell rescue. The reader is directed to a number of contemporary reviews delineating individual trials and outcomes.7–9
The Role of Biopsy at Diagnosis
Until recently, typical clinical and imaging features were considered sufficient to make a presumptive diagnosis of DIPG. World Health Organization (WHO) grading was not prognostic, with outcomes for grade II tumors being as poor as those seen in grade III and IV tumors. Biopsy of the brainstem tended to be reserved for cases in which the imaging features were atypical (eg, focal exophytic, strongly contrast enhancing, well circumscribed lesions) recognizing that the approach to management and prognosis varies dramatically should a WHO grade I tumor be diagnosed. Beginning with the published report of Roujeau et al10 demonstrating that biopsy, performed by an experienced pediatric neurosurgeon, could be safely undertaken—there has been a growing tendency to perform biopsies at diagnosis. The largest single-institution series of 130 biopsies reported by Puget et al demonstrated a 3.9% incidence of transient worsening of clinical symptoms. Hospital stays were less than one week in greater than 90% of cases. Sufficient diagnostic material was almost always obtained and in greater than 80% of cases there was sufficient material for comprehensive molecular profiling.11 A small number of cases have been reported in which the diagnosis was other than DIPG, despite clinical and imaging features suggestive of that diagnosis. Conversely, some lesions with atypical presentations could also be identified as DIPG based on typical molecular and biological features. With the exception of a WHO grade I tumor, it remains unclear whether such non-DIPG diagnoses portend a more favorable prognosis. Whether a biopsy at the time of diagnosis should be obtained in the absence of a clinical trial remains contentious. The presumptive diagnosis of DIPG based on clinical and imaging findings is generally confirmed. Increasingly, however, material from upfront biopsies are being analyzed by advanced oncogenomic techniques that may provide important insights into prognosis and management.
Preclinical Biology
A Disease of the Developing Brain
The advent of biopsies has, for the first time, provided sufficient tumor tissue for studies using next-generation sequencing technologies and methylation arrays to interrogate the genome and epigenome of DIPG. These studies revealed a previously unsuspected role of the epigenome in the genesis, progression, and perhaps resistance to therapy of DIPG unseen in any other human disease to date. Although mutations were found in canonical signaling pathway genes, recurrent somatic gain-of-function mutation leading to lysine 27 to methionine (p.Lys27Met, K27M) substitution in histone 3 (H3) variants characterize more than 85% of DIPG.12–14 These studies were the first to show mutations in a histone gene in human disease, leading to a paradigm shift and potentially a “new hallmark” in cancer: the oncohistone. The groundbreaking discoveries of oncohistones implicate a direct effect of epigenetic dysregulation in tumorigenesis.
K27MH3 Mutations Are Location Specific and Characterize DIPG
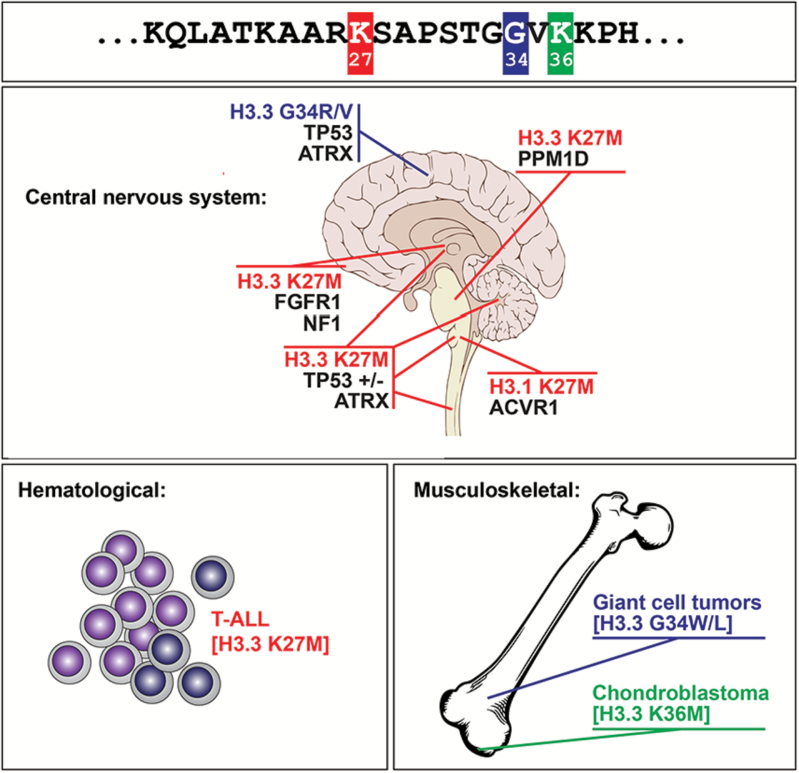
High-frequency recurrent, gain-of-function, somatic mutations at specific residues in H3 variants occur in high-grade astrocytomas (HGA) affecting children and young adults.12–14 H3 mutant oncohistones were later identified by another group in 2 bone cancers affecting children and young adults15 and in very rare leukemia samples16 (Fig. 2). The 2 mutations found in HGA lead to amino acid changes in key residues of H3 variants, K27M in one of the genes H3F3A/H3.3, HIST1H3B or HIST1H3C/H3.1 and in one case in HIST2H3C/H3.2, and G34V or G34R in H3F3A/H3.3. These oncohistones are the pediatric counterpart of the recurrent mutations in isocitrate dehydrogenase (IDH) enzymes identified in young adult gliomas,17,18 which we now know indirectly affect these histone marks. H3.3G34R/V occur in 36% of HGA in the cerebral hemispheres, while the K27M substitution in H3 variants (H3K27M) occurs in close to 85% of DIPG. This consistent finding has led to a revision in the WHO classification of CNS tumors such that the neuropathologic diagnosis of a tumor with imaging features of a DIPG is now reported as “diffuse midline glioma, H3 K27M-mutant.” This nomenclature eliminates a specific reference to diffuse astrocytoma grade, reflecting the uniform behavior of such tumors regardless of historic grade. K27M and G34R/V H3 mutations are tightly correlated with a distinct global DNA methylation pattern19,20 and have neuroanatomical specificity.12,13,19–22 Indeed, H3K27M mutations occur in 80% of brain midline HGA (pons, thalamus, cerebellum, spine), while alterations directly or indirectly affecting the K36 mark in H3 variants (SETD2, H3.3G34R/V) occur in ~40% of HGA of the cerebral hemispheres.
Fig. 2.
Known oncohistone mutations in human disease. Three specific residues in the histone 3 tail schematized at the amino acid level in the top panel are affected in cancer. Oncohistones are associated with cancers of ectomesenchyme origin in children and young adults, namely high-grade astrocytomas (HGA), chondroblastomas, giant cell tumors of the bone, and rarely acute T-cell lymphoblastic leukemias. K27M mutations target H3.3 and H3.1 variants and specify HGA of the brain midline. G34X exclusively targets noncanonical H3.3: H3F3A/G34R/V specifies HGA of the cortex while H3F3B/G34W specifies giant cell tumors of bone. H3.3 K36M mutations are identified in chondroblastoma. Note that oncohistones in HGA show specific associated mutations and brain location.
Obligate High-Fidelity Partnership with Specific Genetic Alterations Based on Age and H3 Variant in Oncohistone Mutagenesis
Each oncohistone variant seems to have selective age and spatial clustering of associated molecular alterations which are seemingly “obligate partners” in tumorigenesis. Indeed, despite a low mutational rate relative to other cancers, H3K27M mutants are in the vast majority if not in all cases associated with other mutations which mainly include (Fig. 3):
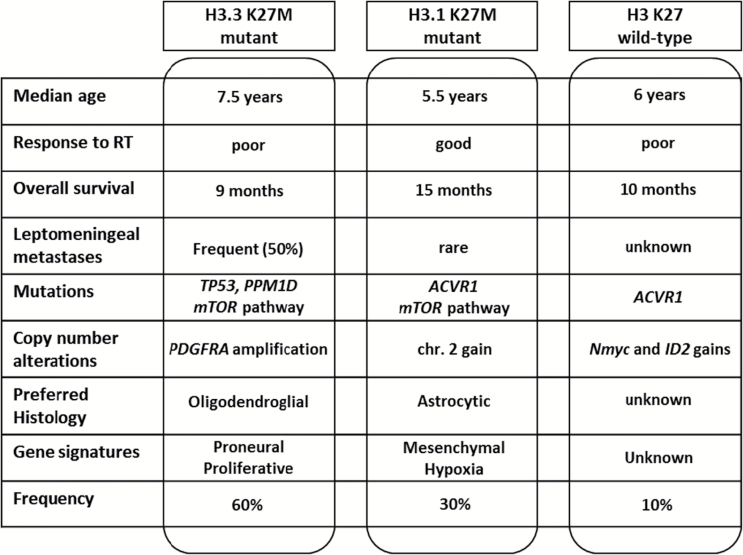
Fig. 3.
Identified subtypes of DIPG. Response to RT (Response to Radiotherapy); leptomeningeal metastases (at any time during the course of the disease).
Loss-of-function mutations in TP53 or related pathway members PPM1D and CHEK2.12,13,19,23–25
Loss-of-function mutations in ATRX (alpha thalassemia/mental retardation syndrome X-linked)13 and rarely in DAXX (death domain associated protein), 2 subunits of a chromatin remodeling complex required for H3.3 incorporation at pericentric heterochromatin and telomeres26,27 and implicated in chromatin remodeling. The presence of this alteration is frequent at autopsy but rare at diagnosis,24 suggesting that it may be a secondary event in this type of oncogenesis.
Mutually exclusive gain-of-function mutation or genetic amplification of growth factor receptors involved in brain development, namely ACVR1 (also known as anaplastic lymphoma kinase 2 [ALK2]) (hindbrain, left-right patterning), FGFR1 (diencephalon), PI3KR1 and PDGFRA (hindbrain, diencephalon, telencephalon).19,23–25,28
Analysis of published genomic DIPG datasets (N = 130) shows the presence of H3K27M in 116/130 (89%) patients (29 H3.1K27M, 1 H3.2K27M, 86 H3.3K27M).29 H3K27M mutants are in the vast majority of cases (96%) associated with alterations affecting the TP53 pathway exclusively (61%), growth factor pathways exclusively (24%), or a combination of both (15%). Mutations in TP53 or PPM1D, or other genes controlling this cell-cycle checkpoint,21 were preferentially associated with noncanonical H3.3K27M. Platelet derived growth factor receptor alpha (PDGFRA) mutation and amplification are preferentially associated with H3.3K27M mutation.30 Recurrent activating ACVR1 somatic mutations favor association with the canonical H3.1 or H3.2K27M histones. In rare cases H3K27M associates with loss-of-function mutations in the regulatory subunit of phosphatidylinositol-3 kinase (PI3K), PIK3R1 (3/111).29
Strikingly, the H3 variant to be affected by K27M varies with age and tumor location, H3.1K27M mutations being more frequent in younger children and almost exclusive to the pons, while H3.3K27M mutations are more frequent in older people and distributed all over the midline structures of the brain and spine.25,30 In younger children with DIPG (mean age 3 y), the H3.1 or H3.2K27M alteration is mainly associated with ACVR1 somatic gain-of-function mutations. ACVR1/ALK2 is a type I receptor of the mammalian transforming growth factor beta signaling family with critical developmental roles in mouse embryo31 and in early left-right patterning.32 The ACVR1 somatic mutations we and others observed have previously been reported as germline mutations causing fibrodysplasia ossificans progressiva,33 an inherited musculoskeletal disease. The specific amino acid substitutions identified in DIPG were shown previously to be gain-of-function (p.R206H, p.G328E, p.G356D)34,35 and to result in hyperactivation of bone morphogenetic protein (BMP) signaling, and interestingly, ventralization of zebrafish embryos.34–36 In older children (mean age 5–7 y), H3.3K27M mutants predominate in the pons and mainly associates with tumor protein (TP)53 pathway alterations (TP53/protein phosphatase, Mg2+/Mn2+ dependent 1D [PPM1D]/checkpoint kinase 2 [CHEK2]). Interestingly, older children and young adults will have HGA in other midline locations within the brain (thalamus, spine, cerebellum), and in these locations H3.3K27M is the main oncohistone. It mainly associates with TP53 pathway alterations except for rare cases in the thalamus where it associates with gain-of-function somatic mutations in fibroblast growth factor receptor 1 (FGFR1). Notably, activin A receptor type 1 (ACVR1) mutations are the same as those identified in the germline of patients with a debilitating musculoskeletal disease. FGFR1 mutations are the same as those identified in children with thalamic or cerebellar low-grade gliomas. In these low-grade tumors, FGFR1 mutants are isolated and samples do not harbor H3.3K27M mutants or alterations in the TP53 pathways.37,38 In addition, several cases of low-grade and high-grade midline gangliogliomas have been reported with dual BRAF-V600E and H3.3K27M mutations.39,40 More studies are needed on these rare cases to identify the relative and cumulative prognostic impact of these concurring mutations. Although pathognomonic to DIPG (and similar midline gliomas), the H3-K27M mutations may not always be associated with the same dismal prognosis in all other midline gliomas.
Remarkably, DIPGs show a previously unsuspected homogeneity for the main driver mutations in DIPG across the course of the disease and in tumor spread. Indeed, it appears that H3K27M is likely the first mutation and remains linked to specific partners throughout the course of the disease.29 Analysis of tumors, often at the time of autopsy, note new subclones often with PI3K pathway alterations. Notably, studies have shown early tumor spread outside the brainstem, including the cerebrum.41 The preservation of these mutations throughout the disease course suggests that the small amount of biopsy material obtained at diagnosis should be sufficient to identify relevant oncohistone therapeutic targets. The needle biopsies recommended to orient care appear representative of the main drivers in DIPG even if the regional heterogeneity of other secondary targetable alterations, such as mutations of PIK3CA (phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha), may not be fully captured. Based on evidence of early tumor spread to several parts of the brain, future efforts to cure DIPG may require a component of systemic tumor control as opposed to regimens focused exclusively on the pons.29
Disease Modeling in DIPG
In order to exploit this wealth of novel and very specific findings to the benefit of patients, additional next steps include the identification and validation of suitable diagnostic, prognostic, and predictive biomarkers, the generation of faithful genetic mouse models, as well as the establishment and molecular characterization of a repertoire of patient derived xenografts (PDX) in animal models. These are essential tools for preclinical drug screening and in vivo drug testing and for the identification of resistance mechanisms to inform rational combination strategies (reverse translation). Ideal preclinical models faithfully recapitulate human disease, as assessed by their molecular features, including genetic and epigenetic landscape and expression signatures, and by their in vivo growth properties. Furthermore, these models must be amenable to experimentation, requiring robust in vitro and in vivo growth, and the ability to experimentally manipulate them. In addition, the blood–brain barrier especially in the pons is another challenge that will need to be addressed in any PDX or genetically engineered animal model.
There are no reliable in vitro and in vivo models recapitulating oncohistone pathogenesis. In addition, overexpression of tagged mutant histones addresses specific questions that need validation in endogenous models of oncohistone, as there is exquisite dosage of histone genes and chromatin modifiers and remodelers in cells that also vary with the cell of origin. We and others have found that pediatric high-grade gliomas with histone H3 K27M mutation grow well in culture transiently using neural stem cell culture conditions, but exhibit progressively slowing growth in vitro with increasing passage.42 While the mutation can be lost after several passages, some culture conditions with enriched medium used for amniotic cell culture maintain the histone H3-K27M in culture for longer passages (>30).42 In contrast, these mutations are maintained over multiple passages of PDX models implanted orthotopically in the brain. No model is perfect, and a combination of patient tumor-derived xenografts and/or neurosphere cell lines along with genetically engineered mouse models generally will offer complementary advantages and are being tackled in multiple laboratories working on oncohistones.
Translational Aspects in DIPG
Biomarkers
Diagnostic biomarkers
The diagnosis of DIPG has long relied on imaging only. However, these criteria are not necessarily homogeneously applied and recent studies have shown the limitation of this approach. In addition, several diseases can mimic DIPG and some atypical radiological aspects of brainstem tumors may still correspond to true DIPG in terms of biology and clinical behavior.43,44 Although in the past, biopsies of these infiltrative neoplasms brought limited information as grading was not correlated with survival, mutations in the regulatory tail of the histone H3 variants at the position of the Lys 27 may become diagnostic criteria for the vast majority of DIPG. Indeed, such mutations are present in 80%–90% on average of the radiologically defined DIPG.12,23,30,45 There is a subset of DIPG without H3.1K27M or H3.2K27M mutations23 that deserve further characterization; they comprise ill-defined tumors either with N-myc amplification or alternative mutations on the histone H3 variant such as K27I, as they are predicted to disrupt the Polycomb repressive complex 2 similarly30,46 or alternative K27M mutations on the histone H3.2 variant.30 The loss of an H3K27 trimethylation mark on immunohistochemistry (IHC) as a consequence of Polycomb repressive complex 2 inactivation is, however, present in most DIPGs,47,48 except the N-myc amplified ones; this alteration is well correlated with the presence of the mutation by sequencing, which in turn is also almost always correlated with the identification of the K27M mutation by IHC.45 As the mutation is detected in 100% of the tumor cells by IHC,45 the abnormal cells can be detected even when the degree of infiltration is limited.30 The diagnosis of a DIPG may be difficult on a stereotactic biopsy, especially if tumor infiltration is limited. The loss of H3K27me3 nuclear staining may be difficult to identify, as well as the presence of the mutation; if only a limited number of tumor cells are present, deep sequencing may be necessary with its own limitations. While IHC is the most versatile technique and permits the diagnosis with the minimum of tissue, sequencing is still necessary to ascertain the type of histone H3 variant mutated, since the available antibody recognizes both H3.3 and H3.1-K27M mutations, although with a weaker binding on H3.1-K27M.
In the presence of a brainstem glioma, the loss of H3K27me3 and the presence of the K27M mutation confirm the diagnosis of DIPG. These tests should therefore be performed in all cases of a biopsied brainstem tumor independent of the radiologic features. There is presently no definitive consensus for the biologic diagnosis of DIPG, but a unified pathologic diagnosis has been defined in the new update of the WHO classification.49 This new entity is now described as “diffuse midline glioma, H3 K27M mutant.” This strict definition will leave some cases in a gray zone, such as classical DIPG without histone H3 mutation. Conversely, one will not be able to confer on these gliomas a definitive malignant behavior, since H3 K27M mutations were also found in low-grade neoplasms.37,50
Prognostic biomarkers
In cases of a brainstem glioma, the presence of an H3.3K27M mutation is associated with a worse prognosis and a close to 100% death rate, while the tumors without this mutation had a better survival and long-term survival around 30%.12 These tumors may not all have been DIPG, since one would have expected a close to 100% death rate. If the survival analyses are restricted to the H3K27M-mutated DIPG, it appears in at least 2 case series that the tumors with H3.3K27M mutation are associated with an even shorter survival than those with H3.1K27M mutation.25,30 The substratum for these 2 different phenotypes and outcome has still to be elucidated.
The recently described ACVR1 mutations appear to be largely predominant in the H3.1K27M-mutated tumors and are also associated with a better outcome.23–25 This mutation is, however, probably secondary to the H3.1K27M mutation, since most H3.1K27M DIPGs have an ACVR1 mutation at autopsy,23 while not all of them have an ACVR1 mutation at diagnosis.24 In a recent study, the prognostic impact of the H3.1K27M mutation was shown to be stronger than that of the ACVR1 mutations.30 Differences in survival length do not exceed 6 months, but H3.3K27M-mutated tumors seem to show more metastatic relapses and poorer response to radiotherapy.25,30 This later finding could have some importance in treatment planning.
Conversely, alterations in the p53 pathway including PPMD1 have been strongly associated with H3.3K27M mutation,24,38 as well as with a worse outcome.51 If confirmed in prospective trials, these biomarkers will need to be taken into account when interpreting the results of a given trial and could eventually be used to stratify therapy if the behavior of these 2 major subgroups are confirmed to be different.
Theranostic Biomarkers to Be Used to Stratify Treatment
Targeted therapies against epidermal growth factor receptor, PDGFR, or vascular endothelial growth factor pathways have been explored already in DIPG without taking into account the specific biology of the tumor. Rare long-term survival exceeding 2 years has been observed in these studies. The absence of a pretreatment biopsy has not made it possible to distinguish why occasional patients have a more sustained response to treatment and whether this in any way correlates with specific alterations in tumor biology. Clearly, next-generation trials will need to be more informative about the biology of the tumors.
It is unlikely that this tumor will be curable with one single drug. Some of the drugs may have to target the epigenetic drivers of DIPG such as histone deacetylase inhibitors52 or histone demethylase inhibitors,53 which may correspond to most of the DIPG, but some others may have to target a more specific pathway in given tumors, such as the sonic hedgehog pathway,54 the PDGFRA pathway,51 or the BMP pathway.24 It would therefore be of paramount importance to know in advance for each tumor which pathways are involved in order to tailor therapy or enrich the early phase trials with patients more likely to respond to a given treatment. Such trials are under way.
MRI as Surrogate to Predict and Judge Treatment Efficacy
MRI remains the mainstay for the diagnosis and follow-up of DIPG despite some of the limitations described above.55 None of the structural characteristics of MRI can be linked to prognosis independently,56 although the presence of a ring enhancement as part of a multiparametric prognostic score has been published.2 Two studies have suggested that the diffusion values may be used to separate entities with different prognoses.30,57
Interobserver variability has been reported when measuring the tumor volume in 3D and the size of the pons in 1D or 2D. Fluid attenuated inversion recovery has been proposed to measure the effect of treatment with less variability apparently.58 Optimal timing to measure the effect of radiation therapy in terms of tumor volume could be set anywhere between the 2 months post irradiation, since little changes have been observed between 2-week and 6- to 8-week scans, responses being already measurable early after radiation therapy.59 While the risk of metastases is low at diagnosis, their presence is not unusual at progression.30,60
Decreased tumor size and decreased diffusion values have been associated with better survival.55 However, some of the DIPG subtypes present with an important edema which may confound the correlation between cell density and apparent diffusion coefficient values. Diffusion tensor imaging could describe the tumor infiltration around the white matter tracts. Mean diffusivity (MD) histograms of pediatric DIPG show significant interpatient and intratumoral differences and quantifiable changes in tumor structure over time. Corticosteroids greatly affected MD and must be considered a confounding factor when interpreting MD results in the context of treatment response.61
Magnetic resonance spectroscopy showing a high ratio of choline to N-acetylaspartate after radiation therapy has also been associated with worse survival.62,63 Single-voxel spectroscopy or maximum values of multivoxel spectroscopy give equivalent information albeit the latter will also give useful information on tumor heterogeneity.64 Several groups have reported that citrate is present in high concentration in DIPG tumors while it cannot be detected in normal brain tissue after the age of 6 months.65,66 This metabolite decreases after treatment and could be a marker of treatment efficacy.66
Some studies have shown an association of the appearance of contrast enhancement with shorter survival.55,62 Contrast enhancement can be “occult” in some cases, and only visible after subtraction.67 The interpretation of tumor changes after radiation therapy can be problematic, since pseudoprogression is not unusual, especially when radiosensitizers are employed.68 The evolution of clinical signs and multimodal MRI sequences may be of some help to separate pseudoprogression and pseudoresponses.69 Perfusion evaluated with dynamic susceptibility contrast MRI has been explored prospectively by several independent groups,62,67,70,71 with higher values at diagnosis being associated with worse survival. Two studies have linked early (immediately after radiotherapy) post-irradiation increased relative cerebral blood volume with improved survival,71 consistent with similar data acquired in adult glioblastoma; the pathophysiological effect of this phenomenon is, however, not elucidated. If confirmed in prospective trials, these type of sequences may be very useful to determine as early as possible the effect of a given therapy including irradiation.
Other Surrogates for Diagnosis and Monitoring
PET imaging has been recently introduced in the evaluation of brainstem glioma. Values of the normal brainstem are remarkably stable across ages and sex.72 Combining multimodal MRI and PET may help to identify higher-grade tumors.70 Considering the possibility to label drugs with PET tracers, PET imaging could be used to study drug penetration in the tumor.73
There is definitively tumor circulating DNA in gliomas, and IDH1 point mutation, for example, could be detected in some cases when the blood–brain barrier was disrupted.74,75 Since the histone mutation is present in all DIPG tumor cells, detection of the mutation in the blood or in the CSF could be used as a diagnostic marker.
CSF protein profiling of DIPG has been performed in a preliminary study revealing specific upregulation of cyclophilin A and dimethylarginase compared with controls.76 While its use for diagnosis may be less effective than the detection of the histone mutation, changes in protein content could be followed to assess treatment response.
Evolving Therapeutic Strategies
Given the frequent absence or minimal contrast enhancement of the tumor at the time of diagnosis, some investigators have speculated that the failure of chemotherapy to impact survival has been related to the inability to deliver biologically meaningful doses of these agents to the pons, presumably across an intact blood–brain barrier. A number of strategies, including blood–brain barrier disruption, convection enhanced delivery, and intra-arterial delivery, are being tested to determine whether delivery could be improved and, if so, whether this impacts overall survival. Osmotic blood–brain barrier disruption has been reported with evidence that osmotic disruption leads to increased delivery of therapeutics to the pons. Limited reports have shown some activity.77 An alternate strategy designed to inhibit P-glycoprotein efflux of chemotherapeutics by the use of cyclosporine was excessively toxic and the trial was terminated early due to excessive toxicity.78 Direct delivery of therapeutics can be accomplished by placing catheters in the pons and infusing chemotherapy via convection enhanced delivery. Small case series and reports79,80 have demonstrated feasibility and safety, but the experience to date is too limited to determine whether overall survival will be impacted. An alternate strategy designed to deliver high concentrations of chemotherapeutics to the pons is intra-arterial administration. The advent of microcatheters now allows therapeutics to be infused at the level of the midbasilar artery. Trials are ongoing testing this approach.
Immunotherapeutic approaches are also being tested in the management of children with DIPG. Strategies have included peptide-based vaccine therapy81 and the initial testing of checkpoint inhibition utilizing anti–programmed cell death protein (PD) 1 and anti–PD-ligand 1 antibodies.
The finding of specific histone mutations, and associated mutations such as TP53, ATRX, ACVR1/ALK2 and PI3KR1, and PDGFRA, has provided new targets that were unrecognized prior to the advent of diagnostic biopsies and rapid autopsies.52 Numerous clinical trials are under way testing histone deacetylase inhibitors, (eg, panobinostat), Cdk4/6 inhibitors, tyrosine kinase inhibitors, and other agents that more specifically target these mutations in vitro and in xenograft models. Whether these agents will impact survival in children with DIPG is currently unknown.
Conclusions
While the prognosis for DIPG remains poor, a growing understanding of the biology of the tumor, notably the apparent homogeneity of the disease from a biologic perspective, has generated some tempered optimism that targeted therapies may yield therapeutic advances. None of these gains in the understanding of the biology could have been accomplished without the commitment of patients and their families who agreed to upfront biopsies and rapid autopsies at the time of death. From the first preclinical successes to the implementation of new therapies in patients, there must be continued focus on biologically driven hypotheses, proper delivery of agents, and precise measurement of treatment efficacy. Importantly, the pediatric neuro-oncology community will need to achieve consensus on how best to define eligible populations for future studies. Will clinical findings and characteristic imaging be sufficient for clinical trials enrollment, or will confirmation of a specific biology be necessary to ensure that trials are appropriately enriched for specified targets? Many would argue the latter in the hopes that such strategies will finally lead to appreciable improvements in survival.
Funding
J.G. is supported by Institut National du Cancer grant PL-BIO 14-253 STROMAD, Etoile de Martin and Fondation Lemos.
Conflict of interest statement. None.
References
- 1. Hankinson TC, Campagna EJ, Foreman NK, Handler MH. Interpretation of magnetic resonance images in diffuse intrinsic pontine glioma: a survey of pediatric neurosurgeons. J Neurosurg Pediatr. 2011;8(1):97–102. [DOI] [PubMed] [Google Scholar]
- 2. Jansen MH, Veldhuijzen van Zanten SE, Sanchez Aliaga E, et al. Survival prediction model of children with diffuse intrinsic pontine glioma based on clinical and radiological criteria. Neuro Oncol. 2015;17(1):160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Janssens GO, Gidding CE, Van Lindert EJ, et al. The role of hypofractionation radiotherapy for diffuse intrinsic brainstem glioma in children: a pilot study. Int J Radiat Oncol Biol Phys. 2009;73(3):722–726. [DOI] [PubMed] [Google Scholar]
- 4. Janssens GO, Jansen MH, Lauwers SJ, et al. Hypofractionation vs conventional radiation therapy for newly diagnosed diffuse intrinsic pontine glioma: a matched-cohort analysis. Int J Radiat Oncol Biol Phys. 2013;85(2):315–320. [DOI] [PubMed] [Google Scholar]
- 5. Negretti L, Bouchireb K, Levy-Piedbois C, et al. Hypofractionated radiotherapy in the treatment of diffuse intrinsic pontine glioma in children: a single institution’s experience. J Neurooncol. 2011;104(3):773–777. [DOI] [PubMed] [Google Scholar]
- 6. Zaghloul MS, Eldebawy E, Ahmed S, et al. Hypofractionated conformal radiotherapy for pediatric diffuse intrinsic pontine glioma (DIPG): a randomized controlled trial. Radiother Oncol. 2014;111(1):35–40. [DOI] [PubMed] [Google Scholar]
- 7. Bredlau AL, Korones DN. Diffuse intrinsic pontine gliomas: treatments and controversies. Adv Cancer Res. 2014;121:235–259. [DOI] [PubMed] [Google Scholar]
- 8. Frazier JL, Lee J, Thomale UW, Noggle JC, Cohen KJ, Jallo GI. Treatment of diffuse intrinsic brainstem gliomas: failed approaches and future strategies. J Neurosurg Pediatr. 2009;3(4):259–269. [DOI] [PubMed] [Google Scholar]
- 9. Khatua S, Moore KR, Vats TS, Kestle JR. Diffuse intrinsic pontine glioma-current status and future strategies. Childs Nerv Syst. 2011;27(9):1391–1397. [DOI] [PubMed] [Google Scholar]
- 10. Roujeau T, Machado G, Garnett MR, et al. Stereotactic biopsy of diffuse pontine lesions in children. J Neurosurg. 2007;107(1 Suppl):1–4. [DOI] [PubMed] [Google Scholar]
- 11. Puget S, Beccaria K, Blauwblomme T, et al. Biopsy in a series of 130 pediatric diffuse intrinsic pontine gliomas. Childs Nerv Syst. 2015;31(10):1773–1780. [DOI] [PubMed] [Google Scholar]
- 12. Khuong-Quang DA, Buczkowicz P, Rakopoulos P, et al. K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol. 2012;124(3):439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schwartzentruber J, Korshunov A, Liu XY, et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482(7384):226–231. [DOI] [PubMed] [Google Scholar]
- 14. Wu G, Broniscer A, McEachron TA, et al. ; St. Jude Children’s Research Hospital–Washington University Pediatric Cancer Genome Project. Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet. 2012;44(3):251–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Behjati S, Tarpey PS, Presneau N, et al. Distinct H3F3A and H3F3B driver mutations define chondroblastoma and giant cell tumor of bone. Nat Genet. 2013;45(12):1479–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Atak ZK, Gianfelici V, Hulselmans G, et al. Comprehensive analysis of transcriptome variation uncovers known and novel driver events in T-cell acute lymphoblastic leukemia. PLoS Genet. 2013;9(12):e1003997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fontebasso AM, Papillon-Cavanagh S, Schwartzentruber J, et al. Recurrent somatic mutations in ACVR1 in pediatric midline high-grade astrocytoma. Nat Genet. 2014;46(5):462–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sturm D, Witt H, Hovestadt V, et al. Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell. 2012;22(4):425–437. [DOI] [PubMed] [Google Scholar]
- 21. Fontebasso AM, Gayden T, Nikbakht H, et al. Epigenetic dysregulation: a novel pathway of oncogenesis in pediatric brain tumors. Acta Neuropathol. 2014;128(5):615–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fontebasso AM, Schwartzentruber J, Khuong-Quang DA, et al. Mutations in SETD2 and genes affecting histone H3K36 methylation target hemispheric high-grade gliomas. Acta Neuropathol. 2013;125(5):659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Buczkowicz P, Hoeman C, Rakopoulos P, et al. Genomic analysis of diffuse intrinsic pontine gliomas identifies three molecular subgroups and recurrent activating ACVR1 mutations. Nat Genet. 2014;46(5):451–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Taylor KR, Mackay A, Truffaux N, et al. Recurrent activating ACVR1 mutations in diffuse intrinsic pontine glioma. Nat Genet. 2014;46(5):457–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu G, Diaz AK, Paugh BS, et al. ; St. Jude Children’s Research Hospital–Washington University Pediatric Cancer Genome Project. The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat Genet. 2014;46(5):444–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dhayalan A, Tamas R, Bock I, et al. The ATRX-ADD domain binds to H3 tail peptides and reads the combined methylation state of K4 and K9. Hum Mol Genet. 2011;20(11):2195–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lewis PW, Elsaesser SJ, Noh KM, Stadler SC, Allis CD. Daxx is an H3.3-specific histone chaperone and cooperates with ATRX in replication-independent chromatin assembly at telomeres. Proc Natl Acad Sci U S A. 2010;107(32):14075–14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Buczkowicz P, Bartels U, Bouffet E, Becher O, Hawkins C. Histopathological spectrum of paediatric diffuse intrinsic pontine glioma: diagnostic and therapeutic implications. Acta Neuropathol. 2014;128(4):573–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nikbakht H, Panditharatna E, Mikael LG, et al. Spatial and temporal homogeneity of driver mutations in diffuse intrinsic pontine glioma. Nat Commun. 2016;7:11185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Castel D, Philippe C, Calmon R, et al. Histone H3F3A and HIST1H3B K27M mutations define two subgroups of diffuse intrinsic pontine gliomas with different prognosis and phenotypes. Acta Neuropathol. 2015;130(6):815–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mishina Y, Crombie R, Bradley A, Behringer RR. Multiple roles for activin-like kinase-2 signaling during mouse embryogenesis. Dev Biol. 1999;213(2):314–326. [DOI] [PubMed] [Google Scholar]
- 32. Kishigami S, Yoshikawa S, Castranio T, Okazaki K, Furuta Y, Mishina Y. BMP signaling through ACVRI is required for left-right patterning in the early mouse embryo. Dev Biol. 2004;276(1):185–193. [DOI] [PubMed] [Google Scholar]
- 33. Shore EM, Xu M, Feldman GJ, et al. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet. 2006;38(5):525–527. [DOI] [PubMed] [Google Scholar]
- 34. Chaikuad A, Alfano I, Kerr G, et al. Structure of the bone morphogenetic protein receptor ALK2 and implications for fibrodysplasia ossificans progressiva. J Biol Chem. 2012;287(44):36990–36998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fukuda T, Kanomata K, Nojima J, et al. A unique mutation of ALK2, G356D, found in a patient with fibrodysplasia ossificans progressiva is a moderately activated BMP type I receptor. Biochem Biophys Res Commun. 2008;377(3):905–909. [DOI] [PubMed] [Google Scholar]
- 36. Shen Q, Little SC, Xu M, et al. The fibrodysplasia ossificans progressiva R206H ACVR1 mutation activates BMP-independent chondrogenesis and zebrafish embryo ventralization. J Clin Invest. 2009;119(11):3462–3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jones DT, Hutter B, Jäger N, et al. ; International Cancer Genome Consortium PedBrain Tumor Project. Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma. Nat Genet. 2013;45(8):927–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang J, Wu G, Miller CP, et al. ; St. Jude Children’s Research Hospital–Washington University Pediatric Cancer Genome Project. Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet. 2013;45(6):602–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Joyon N, Tauziede-Espariat A, Alentorn A, et al. K27M mutation in H3F3A in ganglioglioma grade I with spontaneous malignant transformation extends the histopathological spectrum of the histone H3 oncogenic pathway. Neuropathol Appl Neurobiol. 2016; doi:10.1111/nan.12329. [DOI] [PubMed] [Google Scholar]
- 40. Ryall S, Krishnatry R, Arnoldo A, et al. Targeted detection of genetic alterations reveal the prognostic impact of H3K27M and MAPK pathway aberrations in paediatric thalamic glioma. Acta Neuropathol Commun. 2016;4(1):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Caretti V, Bugiani M, Freret M, et al. Subventricular spread of diffuse intrinsic pontine glioma. Acta Neuropathol. 2014;128(4):605–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Truffaux N, Philippe C, Paulsson J, et al. Preclinical evaluation of dasatinib alone and in combination with cabozantinib for the treatment of diffuse intrinsic pontine glioma. Neuro Oncol. 2015;17(7):953–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schumacher M, Schulte-Mönting J, Stoeter P, Warmuth-Metz M, Solymosi L. Magnetic resonance imaging compared with biopsy in the diagnosis of brainstem diseases of childhood: a multicenter review. J Neurosurg. 2007;106(2 Suppl):111–119. [DOI] [PubMed] [Google Scholar]
- 44. Sufit A, Donson AM, Birks DK, et al. Diffuse intrinsic pontine tumors: a study of primitive neuroectodermal tumors versus the more common diffuse intrinsic pontine gliomas. J Neurosurg Pediatr. 2012;10(2):81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bechet D, Gielen GG, Korshunov A, et al. Specific detection of methionine 27 mutation in histone 3 variants (H3K27M) in fixed tissue from high-grade astrocytomas. Acta Neuropathol. 2014;128(5):733–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lewis PW, Müller MM, Koletsky MS, et al. Inhibition of PRC2 activity by a gain-of-function H3 mutation found in pediatric glioblastoma. Science. 2013;340(6134):857–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bender S, Tang Y, Lindroth AM, et al. Reduced H3K27me3 and DNA hypomethylation are major drivers of gene expression in K27M mutant pediatric high-grade gliomas. Cancer Cell. 2013;24(5):660–672. [DOI] [PubMed] [Google Scholar]
- 48. Venneti S, Garimella MT, Sullivan LM, et al. Evaluation of histone 3 lysine 27 trimethylation (H3K27me3) and enhancer of Zest 2 (EZH2) in pediatric glial and glioneuronal tumors shows decreased H3K27me3 in H3F3A K27M mutant glioblastomas. Brain Pathol. 2013;23(5):558–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 50. Hochart A, Escande F, Rocourt N, et al. Long survival in a child with a mutated K27M-H3.3 pilocytic astrocytoma. Ann Clin Transl Neurol. 2015;2(4):439–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Puget S, Philippe C, Bax DA, et al. Mesenchymal transition and PDGFRA amplification/mutation are key distinct oncogenic events in pediatric diffuse intrinsic pontine gliomas. PLoS One. 2012;7(2):e30313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Grasso CS, Tang Y, Truffaux N, et al. Functionally defined therapeutic targets in diffuse intrinsic pontine glioma. Nat Med. 2015;21(6):555–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hashizume R, Andor N, Ihara Y, et al. Pharmacologic inhibition of histone demethylation as a therapy for pediatric brainstem glioma. Nat Med. 2014;20(12):1394–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Monje M, Mitra SS, Freret ME, et al. Hedgehog-responsive candidate cell of origin for diffuse intrinsic pontine glioma. Proc Natl Acad Sci U S A. 2011;108(11):4453–4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Poussaint TY, Kocak M, Vajapeyam S, et al. MRI as a central component of clinical trials analysis in brainstem glioma: a report from the Pediatric Brain Tumor Consortium (PBTC). Neuro Oncol. 2011;13(4):417–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hargrave D, Chuang N, Bouffet E. Conventional MRI cannot predict survival in childhood diffuse intrinsic pontine glioma. J Neurooncol. 2008;86(3):313–319. [DOI] [PubMed] [Google Scholar]
- 57. Lober RM, Cho YJ, Tang Y, et al. Diffusion-weighted MRI derived apparent diffusion coefficient identifies prognostically distinct subgroups of pediatric diffuse intrinsic pontine glioma. J Neurooncol. 2014;117(1):175–182. [DOI] [PubMed] [Google Scholar]
- 58. Steffen-Smith EA, Baker EH, Venzon D, Shandilya S, Bent RS, Warren KE. Measurements of the pons as a biomarker of progression for pediatric DIPG. J Neurooncol. 2014;116(1):127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ko C, Kaushal A, Hammoud DA, et al. Role of early postradiation magnetic resonance imaging scans in children with diffuse intrinsic pontine glioma. Int J Radiat Oncol Biol Phys. 2012;83(4):1252–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wagner S, Benesch M, Berthold F, et al. Secondary dissemination in children with high-grade malignant gliomas and diffuse intrinsic pontine gliomas. Br J Cancer. 2006;95(8):991–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Steffen-Smith EA, Sarlls JE, Pierpaoli C, et al. Diffusion tensor histogram analysis of pediatric diffuse intrinsic pontine glioma. Biomed Res Int. 2014;2014:647356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hipp SJ, Steffen-Smith E, Hammoud D, Shih JH, Bent R, Warren KE. Predicting outcome of children with diffuse intrinsic pontine gliomas using multiparametric imaging. Neuro Oncol. 2011;13(8):904–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Steffen-Smith EA, Shih JH, Hipp SJ, Bent R, Warren KE. Proton magnetic resonance spectroscopy predicts survival in children with diffuse intrinsic pontine glioma. J Neurooncol. 2011;105(2):365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Steffen-Smith EA, Venzon DJ, Bent RS, Hipp SJ, Warren KE. Single- and multivoxel proton spectroscopy in pediatric patients with diffuse intrinsic pontine glioma. Int J Radiat Oncol Biol Phys. 2012;84(3):774–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Seymour ZA, Panigrahy A, Finlay JL, Nelson MD, Jr, Blüml S. Citrate in pediatric CNS tumors? AJNR Am J Neuroradiol. 2008;29(5):1006–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yeom KW, Lober RM, Nelson MD, Jr, Panigrahy A, Blüml S. Citrate concentrations increase with hypoperfusion in pediatric diffuse intrinsic pontine glioma. J Neurooncol. 2015;122(2):383–389. [DOI] [PubMed] [Google Scholar]
- 67. Conway AE, Reddick WE, Li Y, et al. “Occult” post-contrast signal enhancement in pediatric diffuse intrinsic pontine glioma is the MRI marker of angiogenesis? Neuroradiology. 2014;56(5):405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chassot A, Canale S, Varlet P, et al. Radiotherapy with concurrent and adjuvant temozolomide in children with newly diagnosed diffuse intrinsic pontine glioma. J Neurooncol. 2012;106(2):399–407. [DOI] [PubMed] [Google Scholar]
- 69. Dhermain FG, Hau P, Lanfermann H, Jacobs AH, van den Bent MJ. Advanced MRI and PET imaging for assessment of treatment response in patients with gliomas. Lancet Neurol. 2010;9(9):906–920. [DOI] [PubMed] [Google Scholar]
- 70. Goda JS, Dutta D, Raut N, et al. Can multiparametric MRI and FDG-PET predict outcome in diffuse brainstem glioma? A report from a prospective phase-II study. Pediatr Neurosurg. 2013;49(5):274–281. [DOI] [PubMed] [Google Scholar]
- 71. Sedlacik J, Winchell A, Kocak M, Loeffler RB, Broniscer A, Hillenbrand CM. MR imaging assessment of tumor perfusion and 3D segmented volume at baseline, during treatment, and at tumor progression in children with newly diagnosed diffuse intrinsic pontine glioma. AJNR Am J Neuroradiol. 2013;34(7):1450–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jansen MH, Kloet RW, van Vuurden DG, et al. 18F-FDG PET standard uptake values of the normal pons in children: establishing a reference value for diffuse intrinsic pontine glioma. EJNMMI Res. 2014;4(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. van Dongen GA, Poot AJ, Vugts DJ. PET imaging with radiolabeled antibodies and tyrosine kinase inhibitors: immuno-PET and TKI-PET. Tumour Biol. 2012;33(3):607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Catteau A, Girardi H, Monville F, et al. A new sensitive PCR assay for one-step detection of 12 IDH1/2 mutations in glioma. Acta Neuropathol Commun. 2014;2:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chen WW, Balaj L, Liau LM, et al. BEAMing and droplet digital PCR analysis of mutant IDH1 mRNA in glioma patient serum and cerebrospinal fluid extracellular vesicles. Mol Ther Nucleic Acids. 2013;2:e109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Saratsis AM, Kambhampati M, Snyder K, et al. Comparative multidimensional molecular analyses of pediatric diffuse intrinsic pontine glioma reveals distinct molecular subtypes. Acta Neuropathol. 2014;127(6):881–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hall WA, Doolittle ND, Daman M, et al. Osmotic blood-brain barrier disruption chemotherapy for diffuse pontine gliomas. J Neurooncol. 2006;77(3):279–284. [DOI] [PubMed] [Google Scholar]
- 78. Greenberg ML, Fisher PG, Freeman C, et al. Etoposide, vincristine, and cyclosporin A with standard-dose radiation therapy in newly diagnosed diffuse intrinsic brainstem gliomas: a pediatric oncology group phase I study. Pediatr Blood Cancer. 2005;45(5):644–648. [DOI] [PubMed] [Google Scholar]
- 79. Anderson RC, Kennedy B, Yanes CL, et al. Convection-enhanced delivery of topotecan into diffuse intrinsic brainstem tumors in children. J Neurosurg Pediatr. 2013;11(3):289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Barua NU, Lowis SP, Woolley M, O’Sullivan S, Harrison R, Gill SS. Robot-guided convection-enhanced delivery of carboplatin for advanced brainstem glioma. Acta Neurochir (Wien). 2013;155(8):1459–1465. [DOI] [PubMed] [Google Scholar]
- 81. Pollack IF, Jakacki RI, Butterfield LH, et al. Antigen-specific immune responses and clinical outcome after vaccination with glioma-associated antigen peptides and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in children with newly diagnosed malignant brainstem and nonbrainstem gliomas. J Clin Oncol. 2014;32(19):2050–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]