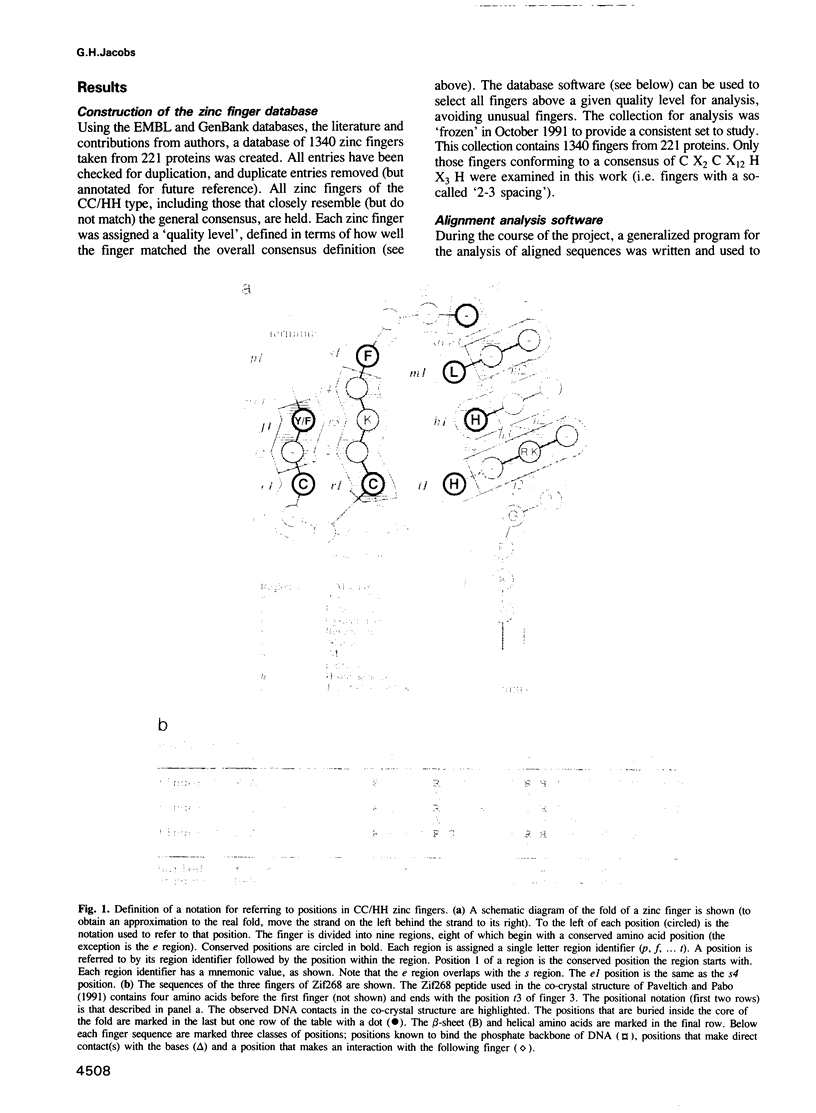
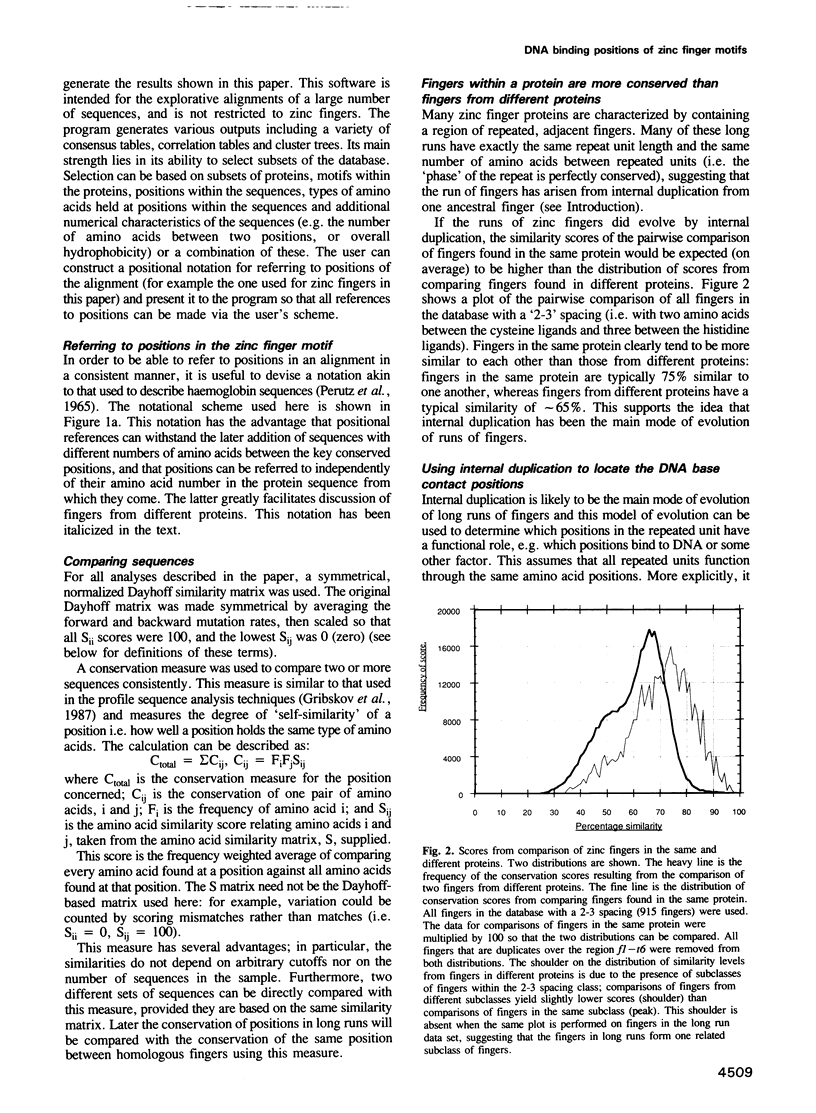
Abstract
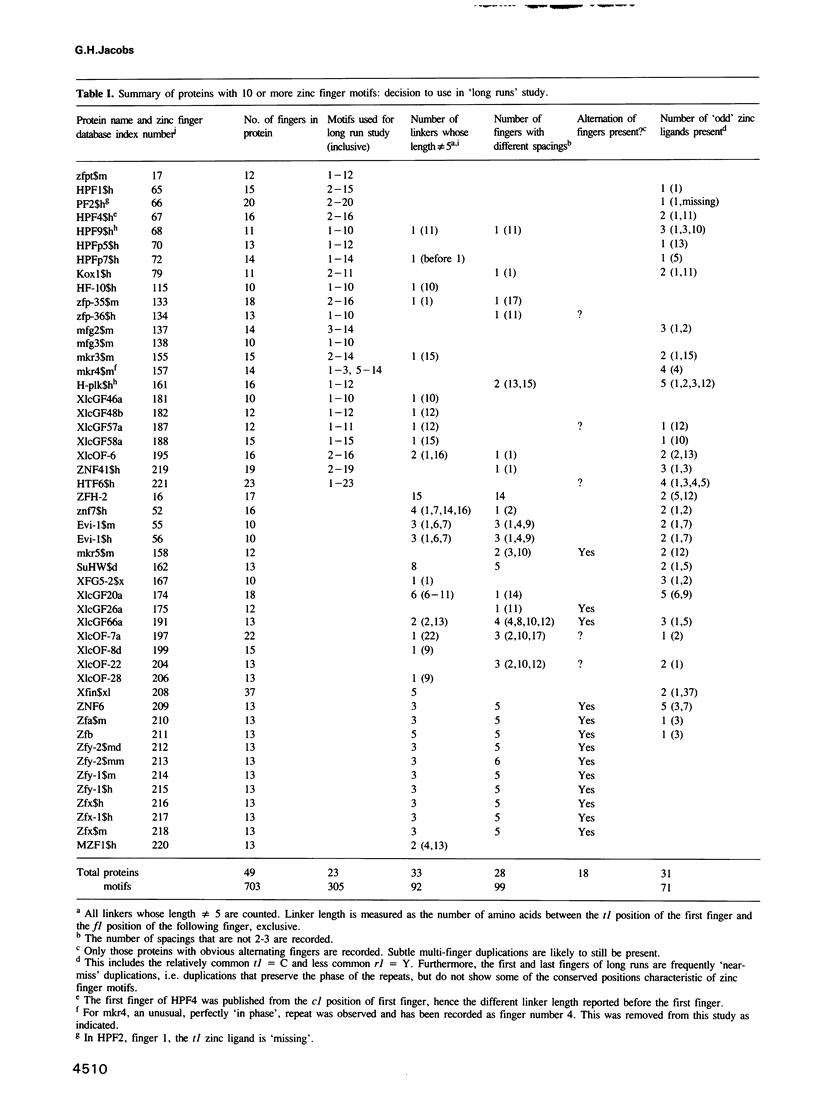
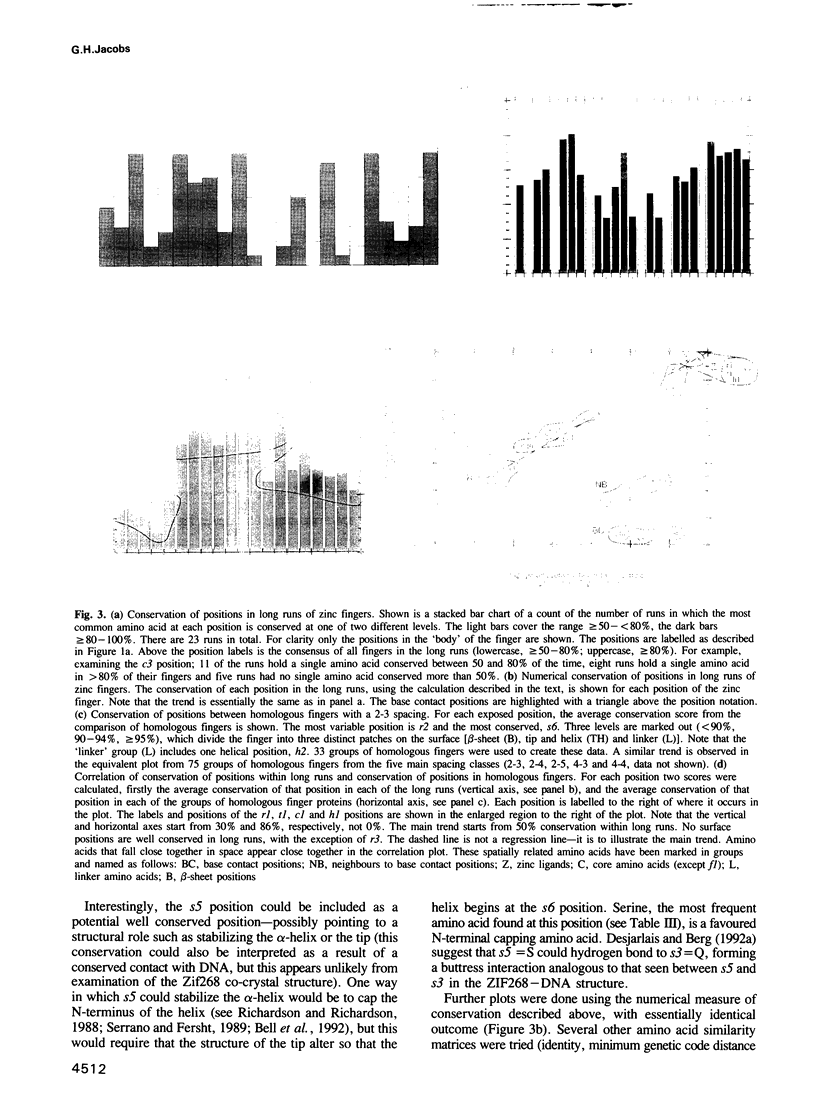
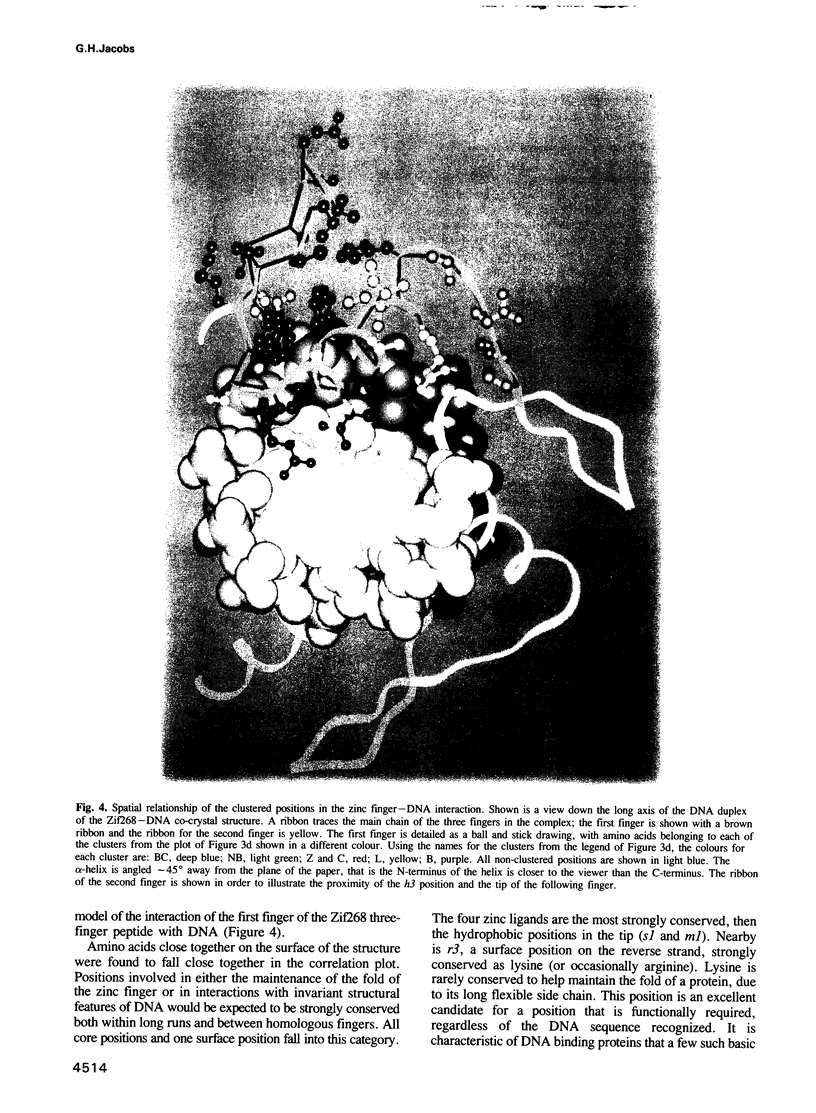
The CC/HH zinc finger is a small independently folded DNA recognition motif found in many eukaryotic proteins, which ligates zinc through two cysteine and two histidine ligands. A database of 1340 zinc fingers from 221 proteins has been constructed and a program for analysis of aligned sequences written. This paper describes sequence analysis aimed at determining the amino acid positions that recognize the DNA bases, by comparing two types of sequence variation. Using the idea that long runs of adjacent zinc fingers have arisen from internal gene duplication, the conservation of each position of the finger within the runs was calculated. The conservation of each position of the finger between homologous proteins from different species was also noted. A correlation of the two types of conservation showed clusters of related amino acids. One cluster of three positions was found to be especially variable within long runs, but highly conserved between corresponding fingers of homologous proteins; these positions are predicted to be the base contact positions. They match the amino acid positions that contact the bases in the co-crystal structure determined by Pavletich and Pabo [Science, 240, 809-817 (1991)]. An adjacent cluster of four positions on the plot may also be associated with DNA binding. This analysis shows that the base recognition positions can be identified even in the absence of a known structure for a zinc finger. These results are applicable to zinc fingers where the structure of the complex is unknown, in particular suggesting that the individual finger--DNA interaction seen in the Zif268--DNA structure has been conserved in many zinc finger--DNA interactions.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell J. A., Becktel W. J., Sauer U., Baase W. A., Matthews B. W. Dissection of helix capping in T4 lysozyme by structural and thermodynamic analysis of six amino acid substitutions at Thr 59. Biochemistry. 1992 Apr 14;31(14):3590–3596. doi: 10.1021/bi00129a006. [DOI] [PubMed] [Google Scholar]
- Bellefroid E. J., Lecocq P. J., Benhida A., Poncelet D. A., Belayew A., Martial J. A. The human genome contains hundreds of genes coding for finger proteins of the Krüppel type. DNA. 1989 Jul-Aug;8(6):377–387. doi: 10.1089/dna.1.1989.8.377. [DOI] [PubMed] [Google Scholar]
- Berg J. M. Proposed structure for the zinc-binding domains from transcription factor IIIA and related proteins. Proc Natl Acad Sci U S A. 1988 Jan;85(1):99–102. doi: 10.1073/pnas.85.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. S., Sander C., Argos P. The primary structure of transcription factor TFIIIA has 12 consecutive repeats. FEBS Lett. 1985 Jul 8;186(2):271–274. doi: 10.1016/0014-5793(85)80723-7. [DOI] [PubMed] [Google Scholar]
- Churchill M. E., Tullius T. D., Klug A. Mode of interaction of the zinc finger protein TFIIIA with a 5S RNA gene of Xenopus. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5528–5532. doi: 10.1073/pnas.87.14.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley P. H., Little P. F. A cluster of related zinc finger protein genes is deleted in the mouse embryonic lethal mutation tw18. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):7923–7927. doi: 10.1073/pnas.88.18.7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjarlais, Berg J. M. Redesigning the DNA-binding specificity of a zinc finger protein: a data base-guided approach. Proteins. 1992 Jul;13(3):272–272. doi: 10.1002/prot.340130309. [DOI] [PubMed] [Google Scholar]
- Desjarlais J. R., Berg J. M. Redesigning the DNA-binding specificity of a zinc finger protein: a data base-guided approach. Proteins. 1992 Feb;12(2):101–104. doi: 10.1002/prot.340120202. [DOI] [PubMed] [Google Scholar]
- Fairall L., Rhodes D., Klug A. Mapping of the sites of protection on a 5 S RNA gene by the Xenopus transcription factor IIIA. A model for the interaction. J Mol Biol. 1986 Dec 5;192(3):577–591. doi: 10.1016/0022-2836(86)90278-0. [DOI] [PubMed] [Google Scholar]
- Gibson T. J., Postma J. P., Brown R. S., Argos P. A model for the tertiary structure of the 28 residue DNA-binding motif ('zinc finger') common to many eukaryotic transcriptional regulatory proteins. Protein Eng. 1988 Sep;2(3):209–218. doi: 10.1093/protein/2.3.209. [DOI] [PubMed] [Google Scholar]
- Ginsberg A. M., King B. O., Roeder R. G. Xenopus 5S gene transcription factor, TFIIIA: characterization of a cDNA clone and measurement of RNA levels throughout development. Cell. 1984 Dec;39(3 Pt 2):479–489. doi: 10.1016/0092-8674(84)90455-0. [DOI] [PubMed] [Google Scholar]
- Gribskov M., McLachlan A. D., Eisenberg D. Profile analysis: detection of distantly related proteins. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4355–4358. doi: 10.1073/pnas.84.13.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs G., Michaels G. Zinc finger gene database. New Biol. 1990 Jun;2(6):583–583. [PubMed] [Google Scholar]
- Klevit R. E., Herriott J. R., Horvath S. J. Solution structure of a zinc finger domain of yeast ADR1. Proteins. 1990;7(3):215–226. doi: 10.1002/prot.340070303. [DOI] [PubMed] [Google Scholar]
- Kochoyan M., Havel T. F., Nguyen D. T., Dahl C. E., Keutmann H. T., Weiss M. A. Alternating zinc fingers in the human male associated protein ZFY: 2D NMR structure of an even finger and implications for "jumping-linker" DNA recognition. Biochemistry. 1991 Apr 9;30(14):3371–3386. doi: 10.1021/bi00228a004. [DOI] [PubMed] [Google Scholar]
- Lee M. S., Gippert G. P., Soman K. V., Case D. A., Wright P. E. Three-dimensional solution structure of a single zinc finger DNA-binding domain. Science. 1989 Aug 11;245(4918):635–637. doi: 10.1126/science.2503871. [DOI] [PubMed] [Google Scholar]
- Lee M. S., Gottesfeld J. M., Wright P. E. Zinc is required for folding and binding of a single zinc finger to DNA. FEBS Lett. 1991 Feb 25;279(2):289–294. doi: 10.1016/0014-5793(91)80170-8. [DOI] [PubMed] [Google Scholar]
- Miller J., McLachlan A. D., Klug A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J. 1985 Jun;4(6):1609–1614. doi: 10.1002/j.1460-2075.1985.tb03825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardelli J., Gibson T. J., Vesque C., Charnay P. Base sequence discrimination by zinc-finger DNA-binding domains. Nature. 1991 Jan 10;349(6305):175–178. doi: 10.1038/349175a0. [DOI] [PubMed] [Google Scholar]
- Neuhaus D., Nakaseko Y., Nagai K., Klug A. Sequence-specific [1H]NMR resonance assignments and secondary structure identification for 1- and 2-zinc finger constructs from SW15. A hydrophobic core involving four invariant residues. FEBS Lett. 1990 Mar 26;262(2):179–184. doi: 10.1016/0014-5793(90)80184-k. [DOI] [PubMed] [Google Scholar]
- Neuhaus D., Rhodes D. Putting the finger on DNA. Curr Biol. 1991 Aug;1(4):268–270. doi: 10.1016/0960-9822(91)90080-g. [DOI] [PubMed] [Google Scholar]
- Nietfeld W., el-Baradi T., Mentzel H., Pieler T., Köster M., Pöting A., Knöchel W. Second-order repeats in Xenopus laevis finger proteins. J Mol Biol. 1989 Aug 20;208(4):639–659. doi: 10.1016/0022-2836(89)90155-1. [DOI] [PubMed] [Google Scholar]
- Omichinski J. G., Clore G. M., Appella E., Sakaguchi K., Gronenborn A. M. High-resolution three-dimensional structure of a single zinc finger from a human enhancer binding protein in solution. Biochemistry. 1990 Oct 9;29(40):9324–9334. doi: 10.1021/bi00492a004. [DOI] [PubMed] [Google Scholar]
- Omichinski J. G., Clore G. M., Robien M., Sakaguchi K., Appella E., Gronenborn A. M. High-resolution solution structure of the double Cys2His2 zinc finger from the human enhancer binding protein MBP-1. Biochemistry. 1992 Apr 28;31(16):3907–3917. doi: 10.1021/bi00131a004. [DOI] [PubMed] [Google Scholar]
- Pavletich N. P., Pabo C. O. Zinc finger-DNA recognition: crystal structure of a Zif268-DNA complex at 2.1 A. Science. 1991 May 10;252(5007):809–817. doi: 10.1126/science.2028256. [DOI] [PubMed] [Google Scholar]
- Párraga G., Horvath S. J., Eisen A., Taylor W. E., Hood L., Young E. T., Klevit R. E. Zinc-dependent structure of a single-finger domain of yeast ADR1. Science. 1988 Sep 16;241(4872):1489–1492. doi: 10.1126/science.3047872. [DOI] [PubMed] [Google Scholar]
- Rhodes D., Klug A. An underlying repeat in some transcriptional control sequences corresponding to half a double helical turn of DNA. Cell. 1986 Jul 4;46(1):123–132. doi: 10.1016/0092-8674(86)90866-4. [DOI] [PubMed] [Google Scholar]
- Richardson J. S., Richardson D. C. Amino acid preferences for specific locations at the ends of alpha helices. Science. 1988 Jun 17;240(4859):1648–1652. doi: 10.1126/science.3381086. [DOI] [PubMed] [Google Scholar]
- Serrano L., Fersht A. R. Capping and alpha-helix stability. Nature. 1989 Nov 16;342(6247):296–299. doi: 10.1038/342296a0. [DOI] [PubMed] [Google Scholar]
- Tso J. Y., Van Den Berg D. J., Korn L. J. Structure of the gene for Xenopus transcription factor TFIIIA. Nucleic Acids Res. 1986 Mar 11;14(5):2187–2200. doi: 10.1093/nar/14.5.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrana K. E., Churchill M. E., Tullius T. D., Brown D. D. Mapping functional regions of transcription factor TFIIIA. Mol Cell Biol. 1988 Apr;8(4):1684–1696. doi: 10.1128/mcb.8.4.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Baradi T., Pieler T. Zinc finger proteins: what we know and what we would like to know. Mech Dev. 1991 Nov;35(3):155–169. doi: 10.1016/0925-4773(91)90015-x. [DOI] [PubMed] [Google Scholar]