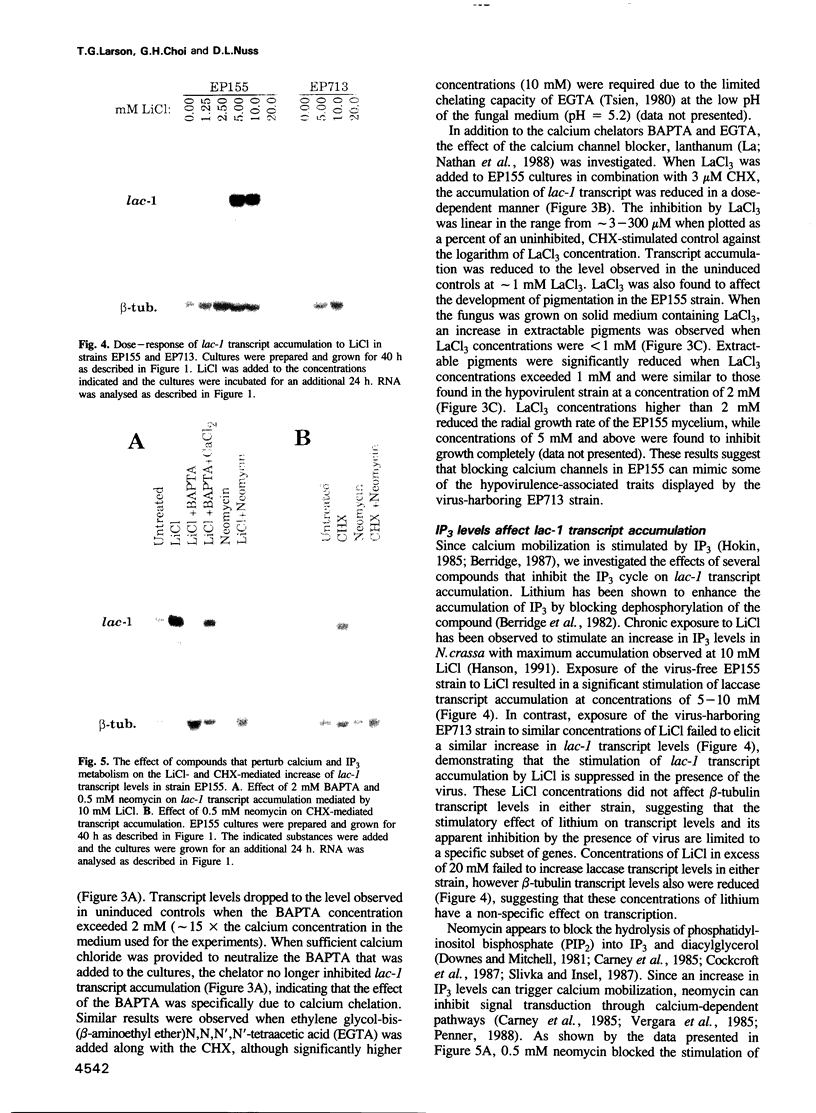
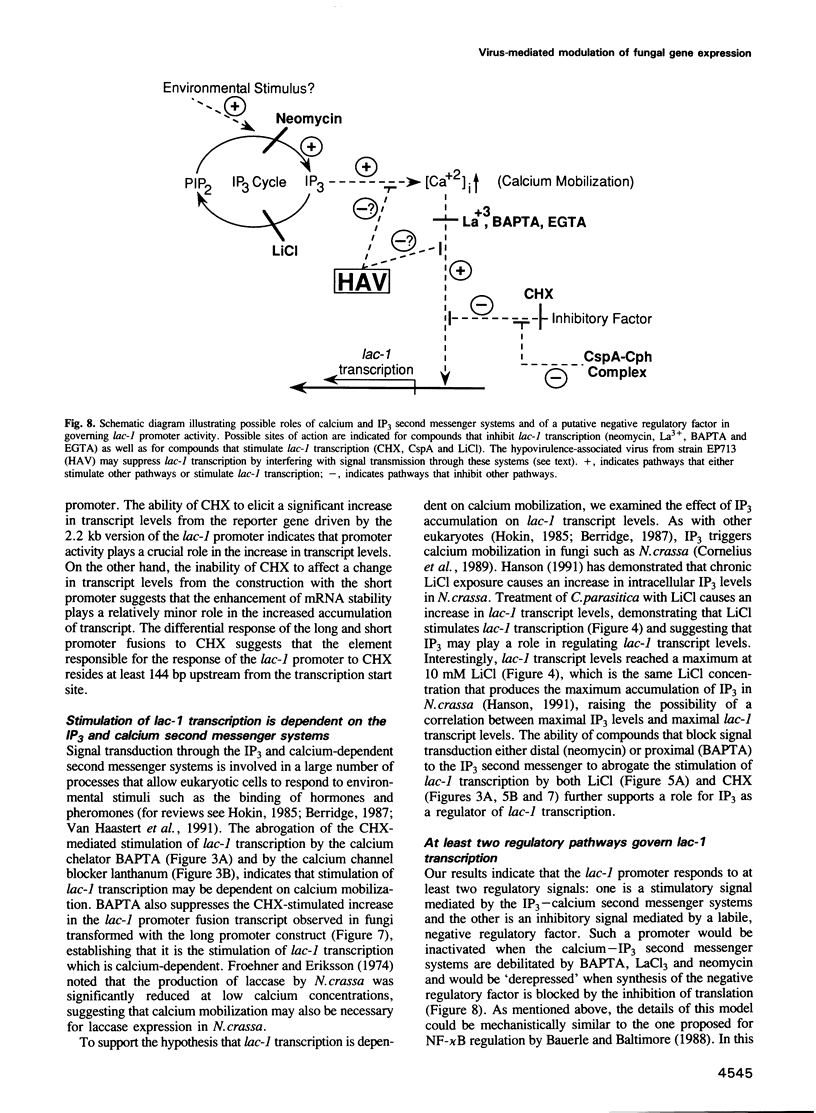
Abstract
A viral double-stranded RNA associated with virulence attenuation (hypovirulence) in the chestnut blight fungus (Cryphonectria parasitica) has been shown by DNA-mediated transformation to be responsible for transmissible hypovirulence. In addition to reduced virulence, the fungal strain harboring this virus exhibits a diverse array of characteristics, termed hypovirulence-associated traits, which distinguish it from an isogenic virus-free strain. We have investigated one of these traits, suppressed lac-1 (laccase) transcript accumulation. Two different and opposing regulatory pathways appear to govern lac-1 transcript levels in the virus-free strain: a stimulatory pathway was found to be dependent on the inositol trisphosphate (IP3) and calcium second messenger systems. A second pathway limiting transcript accumulation was shown to require ongoing protein synthesis. Additionally, changes in the level of lac-1 transcript accumulation were found to be related to modulation of promoter activity and this activity was shown to be suppressed in the virus-containing strain. We conclude that this hypovirulence-associated virus interferes with transduction of an IP3-calcium-dependent signal that is required for stimulation of lac-1 transcription. The perturbation of such signal transduction pathways by hypovirulence-associated viruses may account for the manifold symptoms associated with transmissible hypovirulence.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostakis S. L. Biological control of chestnut blight. Science. 1982 Jan 29;215(4532):466–471. doi: 10.1126/science.215.4532.466. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A., Baltimore D. I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science. 1988 Oct 28;242(4878):540–546. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- Baldari C. T., Macchia G., Heguy A., Melli M., Telford J. L. Cyclosporin A blocks calcium-dependent pathways of gene activation. J Biol Chem. 1991 Oct 5;266(28):19103–19108. [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982 Sep 15;206(3):587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carney D. H., Scott D. L., Gordon E. A., LaBelle E. F. Phosphoinositides in mitogenesis: neomycin inhibits thrombin-stimulated phosphoinositide turnover and initiation of cell proliferation. Cell. 1985 Sep;42(2):479–488. doi: 10.1016/0092-8674(85)90105-9. [DOI] [PubMed] [Google Scholar]
- Choi G. H., Larson T. G., Nuss D. L. Molecular analysis of the laccase gene from the chestnut blight fungus and selective suppression of its expression in an isogenic hypovirulent strain. Mol Plant Microbe Interact. 1992 Mar-Apr;5(2):119–128. doi: 10.1094/mpmi-5-119. [DOI] [PubMed] [Google Scholar]
- Choi G. H., Nuss D. L. A viral gene confers hypovirulence-associated traits to the chestnut blight fungus. EMBO J. 1992 Feb;11(2):473–477. doi: 10.1002/j.1460-2075.1992.tb05077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi G. H., Nuss D. L. Hypovirulence of chestnut blight fungus conferred by an infectious viral cDNA. Science. 1992 Aug 7;257(5071):800–803. doi: 10.1126/science.1496400. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Howell T. W., Gomperts B. D. Two G-proteins act in series to control stimulus-secretion coupling in mast cells: use of neomycin to distinguish between G-proteins controlling polyphosphoinositide phosphodiesterase and exocytosis. J Cell Biol. 1987 Dec;105(6 Pt 1):2745–2750. doi: 10.1083/jcb.105.6.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius G., Gebauer G., Techel D. Inositol trisphosphate induces calcium release from Neurospora crassa vacuoles. Biochem Biophys Res Commun. 1989 Jul 31;162(2):852–856. doi: 10.1016/0006-291x(89)92388-7. [DOI] [PubMed] [Google Scholar]
- Cullen D., Leong S. A., Wilson L. J., Henner D. J. Transformation of Aspergillus nidulans with the hygromycin-resistance gene, hph. Gene. 1987;57(1):21–26. doi: 10.1016/0378-1119(87)90172-7. [DOI] [PubMed] [Google Scholar]
- Downes C. P., Michell R. H. The polyphosphoinositide phosphodiesterase of erythrocyte membranes. Biochem J. 1981 Jul 15;198(1):133–140. doi: 10.1042/bj1980133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont F. J., Staruch M. J., Koprak S. L., Melino M. R., Sigal N. H. Distinct mechanisms of suppression of murine T cell activation by the related macrolides FK-506 and rapamycin. J Immunol. 1990 Jan 1;144(1):251–258. [PubMed] [Google Scholar]
- Edwards D. R., Mahadevan L. C. Protein synthesis inhibitors differentially superinduce c-fos and c-jun by three distinct mechanisms: lack of evidence for labile repressors. EMBO J. 1992 Jul;11(7):2415–2424. doi: 10.1002/j.1460-2075.1992.tb05306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmel E. A., Verweij C. L., Durand D. B., Higgins K. M., Lacy E., Crabtree G. R. Cyclosporin A specifically inhibits function of nuclear proteins involved in T cell activation. Science. 1989 Dec 22;246(4937):1617–1620. doi: 10.1126/science.2595372. [DOI] [PubMed] [Google Scholar]
- Feldman J. F., Thayer J. P. Cyclic AMP-induced tyrosinase synthesis in Neurospora crassa. Biochem Biophys Res Commun. 1974 Dec 11;61(3):977–982. doi: 10.1016/0006-291x(74)90251-4. [DOI] [PubMed] [Google Scholar]
- Flanagan W. M., Corthésy B., Bram R. J., Crabtree G. R. Nuclear association of a T-cell transcription factor blocked by FK-506 and cyclosporin A. Nature. 1991 Aug 29;352(6338):803–807. doi: 10.1038/352803a0. [DOI] [PubMed] [Google Scholar]
- Froehner S. C., Eriksson K. E. Induction of Neurospora crassa laccase with protein synthesis inhibitors. J Bacteriol. 1974 Oct;120(1):450–457. doi: 10.1128/jb.120.1.450-457.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartshorn K. L., Collamer M., Auerbach M., Myers J. B., Pavlotsky N., Tauber A. I. Effects of influenza A virus on human neutrophil calcium metabolism. J Immunol. 1988 Aug 15;141(4):1295–1301. [PubMed] [Google Scholar]
- Hermann T. E., Kurtz M. B., Champe S. P. Laccase localized in hulle cells and cleistothecial primordia of Aspergillus nidulans. J Bacteriol. 1983 May;154(2):955–964. doi: 10.1128/jb.154.2.955-964.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokin L. E. Receptors and phosphoinositide-generated second messengers. Annu Rev Biochem. 1985;54:205–235. doi: 10.1146/annurev.bi.54.070185.001225. [DOI] [PubMed] [Google Scholar]
- Horowitz N. H., Feldman H. M., Pall M. L. Derepression of tyrosinase synthesis in Neurospora by cycloheximide, actinomycin D, and puromycin. J Biol Chem. 1970 Jun 10;245(11):2784–2788. [PubMed] [Google Scholar]
- Linden R. M., Schilling B. C., Germann U. A., Lerch K. Regulation of laccase synthesis in induced Neurospora crassa cultures. Curr Genet. 1991 May;19(5):375–381. doi: 10.1007/BF00309598. [DOI] [PubMed] [Google Scholar]
- Mahadevan L. C., Edwards D. R. Signalling and superinduction. Nature. 1991 Feb 28;349(6312):747–748. doi: 10.1038/349747c0. [DOI] [PubMed] [Google Scholar]
- Mattila P. S., Ullman K. S., Fiering S., Emmel E. A., McCutcheon M., Crabtree G. R., Herzenberg L. A. The actions of cyclosporin A and FK506 suggest a novel step in the activation of T lymphocytes. EMBO J. 1990 Dec;9(13):4425–4433. doi: 10.1002/j.1460-2075.1990.tb07893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister R. K., Hulman S. E., Johnson L. F. Rapid changes in poly (A)(+) mRNA content in growth stimulated fibroblasts following perturbations in protein synthesis. J Cell Physiol. 1979 Sep;100(3):531–538. doi: 10.1002/jcp.1041000315. [DOI] [PubMed] [Google Scholar]
- Nathan R. D., Kanai K., Clark R. B., Giles W. Selective block of calcium current by lanthanum in single bullfrog atrial cells. J Gen Physiol. 1988 Apr;91(4):549–572. doi: 10.1085/jgp.91.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhäuser A., Klingmüller W., Kaudewitz F. Selektion Actidion-resistenter Mutanten bei Neurospora crassa sowie ihre genetische und biochemische Analyse. Mol Gen Genet. 1970;106(2):180–194. doi: 10.1007/BF00323837. [DOI] [PubMed] [Google Scholar]
- Penner R. Multiple signaling pathways control stimulus-secretion coupling in rat peritoneal mast cells. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9856–9860. doi: 10.1073/pnas.85.24.9856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez V. L., Rowe T., Justement J. S., Butera S. T., June C. H., Folks T. M. An HIV-1-infected T cell clone defective in IL-2 production and Ca2+ mobilization after CD3 stimulation. J Immunol. 1991 Nov 1;147(9):3145–3148. [PubMed] [Google Scholar]
- Perlman J., Feldman J. F. Cycloheximide and heat shock induce new polypeptide synthesis in Neurospora crassa. Mol Cell Biol. 1982 Oct;2(10):1167–1173. doi: 10.1128/mcb.2.10.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell W. A., Van Alfen N. K. Differential accumulation of poly(A)+ RNA between virulent and double-stranded RNA-induced hypovirulent strains of Cryphonectria (Endothia) parasitica. Mol Cell Biol. 1987 Oct;7(10):3688–3693. doi: 10.1128/mcb.7.10.3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigling D., Van Alfen N. K. Regulation of laccase biosynthesis in the plant-pathogenic fungus Cryphonectria parasitica by double-stranded RNA. J Bacteriol. 1991 Dec;173(24):8000–8003. doi: 10.1128/jb.173.24.8000-8003.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Slivka S. R., Insel P. A. Alpha 1-adrenergic receptor-mediated phosphoinositide hydrolysis and prostaglandin E2 formation in Madin-Darby canine kidney cells. Possible parallel activation of phospholipase C and phospholipase A2. J Biol Chem. 1987 Mar 25;262(9):4200–4207. [PubMed] [Google Scholar]
- Tsien R. Y. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980 May 27;19(11):2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- Van Haastert P. J., Janssens P. M., Erneux C. Sensory transduction in eukaryotes. A comparison between Dictyostelium and vertebrate cells. Eur J Biochem. 1991 Jan 30;195(2):289–303. doi: 10.1111/j.1432-1033.1991.tb15706.x. [DOI] [PubMed] [Google Scholar]
- Vergara J., Tsien R. Y., Delay M. Inositol 1,4,5-trisphosphate: a possible chemical link in excitation-contraction coupling in muscle. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6352–6356. doi: 10.1073/pnas.82.18.6352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright K. A., Dezzutti C. S., Lewis M. G., Olsen R. G. FeLV-induced immunosuppression through alterations in signal transduction: changes in intracellular free calcium levels. Vet Immunol Immunopathol. 1989 May;21(1):47–53. doi: 10.1016/0165-2427(89)90129-3. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]