Abstract
Aim
Rare variants of genes encoding the cardiac sodium channel and associated compounds have been linked with atrial fibrillation (AF). Nevertheless, current expert consensus does not support genetic testing in AF, which is in part based on the fact that “there is no therapeutic impact derived from AF genetic test results”. However, there are no studies available supporting this recommendation. Consequently, this study analyzed the impact of rare variants affecting the cardiac sodium channel on rhythm outcome of AF catheter ablation.
Methods and results
In 137 consecutive patients with lone AF enrolled in the Leipzig Heart Center AF ablation registry, screening for mutations in SCN5A, SCN1B – 4B, CAV3, GPD1L, SNTA1 and MOG1 was performed. We identified 3 rare non-synonymous variants in SCN5A, 5 in SCN1B, 1 in SCN4B, 1 in CAV3, 6 in GPD1L, 3 in SNTA1 and 3 in MOG1 (16%). Variant carriers were otherwise comparable with non-variant carriers. Analysis of AF recurrence rates after radiofrequency AF catheter ablation by serial 7-day Holter ECG monitoring between 3 and 12 months revealed no difference between groups, i.e. 45% in variant carriers vs. 49% in non-variant carriers.
Conclusions
Rare variants in genes encoding the cardiac sodium channel and associated compounds are frequently found in lone AF but were not found to impact the outcome of AF catheter ablation. This finding supports current recommendations not to screen for rare variants for the ablation outcome in AF. Nevertheless, it may at least be helpful for understanding AF mechanisms and larger studies are needed to further explore the possible association between genotype and response to AF therapies.
Introduction
Rare variants of genes encoding the cardiac sodium channel have been linked with atrial fibrillation (AF). For instance, mutations in the SCN5A gene that encodes the alpha-subunit of the sodium current (Nav1.5) have been found in 9% of an unselected AF population and in 6% of patients with lone AF.[1] Mutations of the SCN1B –SCN4B genes that encode the modifying beta-subunits of the Navß1-ß4 have been detected in an additional 2%.[2, 3] Other genes play an important role for the generation of a healthy Nav1.5 current by coding for accessory channel anchoring or trafficking proteins. Mutations in four of these genes, i.e. GPD1L, MOG1, SNTA1, CAV3 have been linked with AF [4] and long QT syndrome (LQTS),[5, 6] Brugada syndrome (BrS) [7] or sudden infant death syndrome (SIDS) [8].
However, current expert consensus does not support genetic testing in AF. This recommendation is in part based on the fact that “there is no prognostic or therapeutic impact derived from AF genetic test result”.[9] Since there are no studies available supporting this recommendation we sought to investigate the frequency and potential impact of rare sodium channel or associated compound variants on rhythm outcome of catheter ablation of AF.
Methods
Study population
The study included 137 consecutive patients of Caucasian ancestry with drug refractory paroxysmal or persistent lone AF enrolled in the Leipzig Heart Center AF Ablation and Genetics Registry, who underwent AF radiofrequency ablation. The patient characteristics are summarized in Table 1 and S1 Table.
Table 1. Rare variants in AF.
| Gene | n | Locus of mutation | NCBI Reference Sequence |
|---|---|---|---|
|
SCN5A |
1 1 1 |
SCN5A/Exon2/c.73G>A/p.E25K SCN5A/Exon9/c.1036G>A/p.E346K SCN5A/Exon17/c.3190G>A/p. E1064K |
NM_000335.4 |
|
SCN1B |
3 2 |
SCN1B/Exon3_ext/c.448+193G>A/p.R214Q | NM_199037.3 |
| SCN1B/Exon3_ext/c.448+321G>A/p.G257R | |||
| SCN4B | 1 | SCN4B/Exon6/c.632C>G/p.T211R | NM_174934.3 |
|
GPD1L |
4 1 1 |
GPD1L/Exon4/c.370A>G/p.I124V GPD1L/Exon3/c.267C>A/p.D89E GPD1L/Exon4/c.391C>A/p.L131M |
NM_015141.3 |
| MOG1 | 3 | MOG1, Exon 1_2/c.181G>T/p.E61X | NM_016492.4 |
|
SNTA1 |
1 2 |
SNTA1/Exon4/c.787G>T/p.A263S SNTA1/Exon3/c.556C>T/p.S189L |
NM_003098.2 |
| CAV3 | 1 | CAV3/Exon2/c.433G>A/p.V145M | NM_033337.2 |
Paroxysmal AF was defined as self-terminating episodes of AF within 7 days after onset documented by ECG or an ambulatory ECG monitor. Persistent AF was defined as an AF episode either lasting longer than 7 days or requiring drug or direct current cardioversion for termination.
In all patients, transthoracic and transesophageal echocardiography was performed prior to catheter ablation. Left atrial diameter and left ventricular ejection fraction were determined using standard measurements and a left atrial thrombus was excluded. All class I or III antiarrhythmic medications with the exception of amiodarone were discontinued at least 5 half-lives before the procedure.
The study was approved by the institutional review board (Medical Faculty, Leipzig University) and performed in agreement with the declaration of Helsinki. All patients provided written informed consent for study participation.
Genotyping
Whole blood was collected for genomic DNA extraction and analysis from all subjects. We directly sequenced the coding regions of high priority candidate ion channel genes SCN5A (sodium channel, voltage-gated, type V, alpha subunit), SCN1B (sodium channel, voltage-gated, type I, beta subunit), SCN2B (sodium channel, voltage-gated, type II, beta subunit), SCN3B (sodium channel, voltage-gated, type III, beta subunit), and SCN4B (sodium channel, voltage-gated, type IV, beta subunit) as well as non-ion channel candidate genes SNTA1 (syntrophin, alpha 1), CAV3 (caveolin 3), MOG1 (RAN guanine nucleotide release factor) and GPD1L (glycerol-3-phosphate dehydrogenase 1-like protein).
Screening for variants was performed by PCR amplification of coding regions and flanking intronic sequences followed by direct sequencing of amplicons on an ABI prism 3730 DNA Sequence Detection System. All variants were validated by re-sequencing an independent PCR-generated amplicon from the subject.
Variant selection and functional analysis
All non-synonymous variants (missense, nonsense, frame-shift, and splice-site) were screened against dbSNP build 137, National Heart, Lung, Blood Institute (NHLBI) Exome Sequencing Project (ESP) and the Exome Aggregation Consortium (ExAC, http://exac.broadinstitute.org). Variants were defined as being rare if a minor allele frequency < 1% was reported in all of the interrogated databases and variants absent from these were defined as being novel. Single base conservation scores and predicted functional effects were evaluated by PhastCons, Genomic Evolutionary Rate Profiling (GERP), Grantham scores, and PolyPhen2 using the SeattleSeq Genomic Variation Server and are presented in Table 2 and S2 Table.
Table 2. Patient characteristics.
| Total | Variant carriers (N = 22) |
Variant non-carriers (N = 115) |
P-value | |
|---|---|---|---|---|
| Age, yrs | 51±11 | 52±14 | 51±11 | 0.595 |
| Male (%) | 93 | 96 | 93 | 0.676 |
| Persistent AF (%) | 34 | 41 | 32 | 0.427 |
| AF history, months | 73±66 | 74±74 | 72±65 | 0.868 |
| BB† / CCB‡ use (%) | 80 | 86 | 78 | 0.388 |
| Digitalis use (%) | 10 | 18 | 9 | 0.241 |
| AAD§ use (%) | 43 | 45 | 43 | 0.805 |
| LAD¶, mm | 42±6 | 43±5 | 42±6 | 0.604 |
| LVEF# (%) | 61±11 | 63±11 | 61±11 | 0.455 |
†BB—beta blockers
‡CCB—calcium channel blockers
§AAD—antiarrthythmic drugs
¶LAD—left atrial diameter
#LVEF—left ventricular ejection fraction.
Catheter ablation
Left atrial catheter ablation was performed using a previously described approach.[10] In brief, patients were studied under deep propofol sedation with continuous invasive monitoring of arterial blood pressure and oxygen saturation. Non-fluoroscopic 3D catheter orientation, CT image integration, and tagging of the ablation sites were performed using Ensite NavX, Ensite Velocity (St. Jude Medical, St. Paul, MN, USA) or CARTO 3 (Biosense Webster, Diamond Bar, CA, USA). Trans-septal access and catheter navigation were performed with a steerable sheath (Agilis, St. Jude Medical, St. Paul, MN, USA). Patients presenting with AF at the beginning of the procedure were electrically cardioverted and ablation was performed during sinus rhythm (i.e. AF termination with ablation was not attempted). In all patients circumferential left atrial ablation lines were placed around the antrum of the ipsilateral pulmonary veins (irrigated tip catheter, pre-selected tip temperature of 48°C, and maximum power of 30–50 W). In patients with persistent AF, additional linear lesions were added at the left atrial roof, the basal posterior wall and the left atrial isthmus. Ablation of complex fractionated electrograms was not performed.
After circumferential line placement, voltage and pace mapping along the ablation line were used to identify and close gaps. The isolation of all pulmonary veins with bidirectional block was verified with a multipolar circular mapping catheter and was defined as the procedural endpoint.
Follow-up
After ablation, class I and III antiarrhythmic drugs were not reinitiated. Oral anticoagulation was prescribed for 6 months, and proton pump inhibitors were added for 4 weeks. All patients were followed in the outpatient clinic for 12 months after the ablation. During this follow-up period, 7-day Holter ECG recordings were performed 3, 6 and 12 months after the ablation. Additional ECGs and Holter ECG recordings were obtained when patients’ symptoms were suggestive of AF. AF recurrence was defined as a documented AF episode lasting longer than 30 seconds between 3 and 12 months after the ablation (thus, including a 3-month “blanking period”). All patients with sustained early recurring AF underwent direct cardioversion. Additional drug administration was left to the discretion of the treating physician.
Statistical analysis
Continuous variables are presented as mean values ± one standard deviation and categorical variables as frequencies. Comparison of continuous variables was performed using the unpaired Student’s t-test and of categorical variables using the Pearson chi-square test. A two-sided p-value < .05 was considered as statistically significant.
Considering a case–control ratio of 1:5 and assuming large variant effects, a difference of 57% versus 25% AF recurrence rates could have been detected with power of 80%.
Results
Sodium channel and associated compound rare variants in AF
In 22 patients (16%), we identified 3 rare non-synonymous variants in SCN5A, 2 in SCN1B, 1 in SCN4B, 1 in CAV3, 3 in GPD1L, 2 in SNTA1 and 1 in MOG1 (Table 1). Variant details and in-silico prediction is summarized in S2 Table.
There were no significant differences in clinical characteristics between variant carriers and non-variant carriers (Table 2). The individual clinical and ECG characteristics of the variant carriers are summarized in S1 Table. None of the patients exhibited a typical BrS pattern on 12-lead ECG or showed a prolonged QT interval duration suggestive of LQTS. Patients’ history and family history was not suspicious with respect to syncope, aborted sudden cardiac death or SIDS. Three patients each had a brother with AF who either had died or did not consent for genetic testing.
Outcome of AF catheter ablation
Complete pulmonary vein isolation as a procedural end point was achieved in all cases. Eleven patients did not have a complete follow-up and were therefore excluded from the analysis. AF recurrence within the first week (ERAF) and between 3 and 12 months (LRAF) were observed in 49% and 48%, respectively.
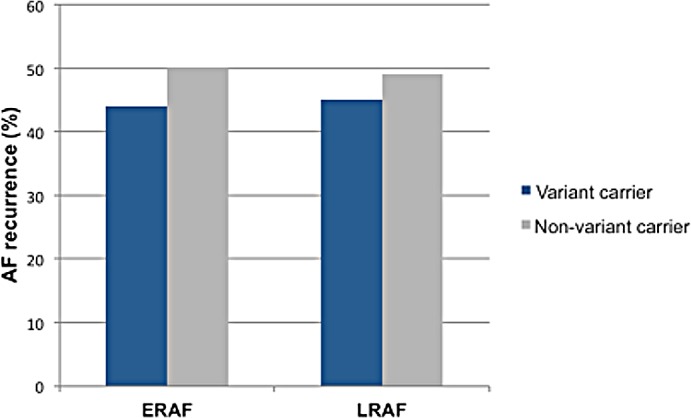
Patients with AF recurrence are compared to patients without AF recurrence in Table 3. There were no differences in clinical characteristics between patients with and without ERAF and LRAF. Patients’ variant carrier status had also no impact on the recurrence of AF (Fig 1). In patients with LRAF, reablation rates were comparable between both groups (78% in variant carriers versus 68% in variant non-carriers, p = .709). All of those patients had reconnected pulmonary veins.
Table 3. Comparison of patients with and without early (ERAF) and late recurring AF (LRAF).
| ERAF+ n = 62 |
ERAF- n = 65 |
P value |
LRAF+ n = 62 |
LRAF- n = 67 |
P value |
|
|---|---|---|---|---|---|---|
| Male % | 97 | 94 | 0.680 | 94 | 94 | >0.999 |
| Persistent AF % | 32 | 28 | 0.613 | 34 | 30 | 0.624 |
| AF history (months) | 74 ± 63 | 77 ± 72 | 0.958 | 82± 73 | 66 ± 60 | 0.251 |
| Age (years) | 52 ± 11 | 50 ± 12 | 0.380 | 53 ± 11 | 50 ± 11 | 0.131 |
| BB†/CCB‡ use % | 86 | 78 | 0.285 | 86 | 78 | 0.251 |
| Digitalis use % | 11 | 9 | 0.724 | 13 | 9 | 0.471 |
| Previous AAD§ use % | 44 | 45 | 0.842 | 48 | 40 | 0.355 |
| LAD (mm) | 43 ± 6 | 41 ± 5 | 0.323 | 42 ± 6 | 42 ± 6 | 0.945 |
| LVEF (%) | 61 ± 9 | 61 ± 13.0 | 0.480 | 62 ± 8 | 61 ± 13 | 0.933 |
| IVSd¶ (mm) | 12 ± 2 | 12 ± 2 | 0.816 | 12 ± 2 | 12 ± 2 | 0.667 |
| LVED# (mm) | 50 ± 6 | 48 ± 9 | 0.219 | 49 ± 9 | 49 ± 6 | 0.396 |
| Additional linear lesions (%) | 53 | 44 | 0.286 | 56 | 47 | 0.323 |
| Ablation time (s) | 3177 ± 1433 | 3158 ± 1117 | 0.950 | 3472 ± 1501 | 3005 ± 1174 | 0.158 |
| Variant carriers % | 13 | 16 | 0.662 | 15 | 16 | 0.766 |
†BB—beta blockers
‡CCB—calcium channel blockers
§AAD—antiarrthythmic drugs
¶IVSd—interventricular septal enddiastolic dimension
#LVED—left ventricular end diastolic diameter.
Fig 1. AF recurrence stratified by variant carrier status.
Discussion
Main findings
To the best of our knowledge, this is the first study to analyze the impact of rare variants in cardiac sodium channel and associated compound genes on outcome after catheter ablation for AF. Analysis of 137 patients with lone AF revealed a prevalence of sodium channel and associated compound gene rare variants of 16%, but no large impact on outcome of ablation.
Rare variants in genes responsible for the sodium current in AF
The voltage-gated cardiac sodium channel is a multiprotein complex in which accessory proteins interact with the alpha-subunit to regulate its gating, cellular localization, intracellular transport, and degradation. It conducts the inward sodium current (INa) that initiates action potential depolarization and is essential for action potential propagation in the heart but also influences repolarization and refractoriness.[11]
Several genetic variants in SCN5A, causing both loss and gain of INa, have been associated with lone AF,[1, 12] thereby expanding the number of phenotypes associated with rare variants in the SCN5A gene. Although the fundamental mechanisms of each of these SCN5A associated disorders may differ considerably, phenotypic overlap exists, including individuals with both the LQTS and BrS [13] and those with both BrS and conduction system disease.[14] Also, up to 3% of patients with LQTS and 22% with BrS suffer from AF.[15, 16]
Selection of other candidate genes (SCN1B-4B, GPD1L, MOG1, SNTA1, CAV3) was based on previously described associations between variants in these genes and AF, LQTS or BrS. The encoded accessory proteins play an important role in the generation of healthy cardiac sodium current. The protein encoded by the GPD1L gene is found in the cytoplasm, associated with the plasma membrane, where it binds the sodium channel, voltage-gated, type V, alpha subunit. Rare variants in this gene are a cause of BrS type 2 [7] as well as SIDS [8] by decreasing INa through a reduction in SCN5A cell surface expression.[7]
The MOG1 gene plays an important role in sodium channel trafficking by increasing plasma membrane expression of Na(v)1.5 and sodium current (I(Na)) density. A disturbed function due to rare variants and resulting loss-of/reduced sodium current has been shown to cause a BrS type 11, sick-sinus syndrome and lone AF.[4, 17, 18]
The N-terminal domain of the syntrophin protein encoded by the SNTA1 gene interacts with the C-terminus of the pore-forming alpha subunit of the cardiac sodium channel Nav1.5. This gene is a susceptibility locus for LQTS type 12 and SIDS due to an increase in late sodium current.[19, 20]
Caveolin-3, encoded by CAV3 is identified as an important negative regulator for cardiac late INa via nNOS dependent direct S-nitrosylation of SCN5A, however CAV3 mutations increase late INa and cause LQTS type 9.[21, 22]
In our cohort, 16% patients were carriers of rare variants. However, aside from lone AF they did not express any particular ECG phenotype, i.e. PR prolongation, LQTS or BrS patterns. In order to predict functionality we reported the results of recognized in-silico tools. However, the accuracy of these tools is limited, especially for the sodium channel.[23] There were contradictory results between tools and some variants that had previously been reported in association with BrS [7] and SIDS [8] were reported as “benign”. In that context, it also needs to be pointed out that a comprehensive mutational analysis of 829 unrelated subjects estimated that about 5% of healthy individuals harbor a rare non-synonymous variant in SCN5A.[24] Thus, it remains elusive whether or not those rare variants were disease causing.
Associations between genotype and AF ablation outcomes
Over the last 5 years, several studies have analyzed possible associations between common genetic variants and AF ablation outcomes. Our group was the first to report an association between common AF-susceptibility alleles on chromosome 4q25 and rhythm outcomes of catheter ablation.[25] Meanwhile, this finding has been replicated by another single-center study [26] and very recently by a meta-analysis including 991 patients from 3 centers.[27]
Several other, smaller studies have used a candidate gene approach focusing on different AF pathophysiological pathways. So far, polymorphisms in the soluble epoxide hydrolase gene (EPHX2),[28] the heme oxygenase-1 gene (HO-1),[29] the angiotensin-converting enzyme gene (ACE),[30] the interleukin-6 receptor gene (IL6R),[31] and the angiotensinogen gene (AGT) [32] have been found to associate with recurring atrial arrhythmias but replication studies are lacking.
All these studies point to the potential role of genotypes for the individual stratification of therapeutic interventions in AF patients with the goal of improving rhythm outcome and increasing patient safety for instance through better patient selection, tailored ablation and post ablation management.
However, unselected screening for rare variants was not helpful for this purpose in our cohort consequently supporting current recommendations [9] and clinical practice. Ablation outcomes in our cohort that represents the largest study with known rare variant carrier status were in agreement with two previous case series in 9 and 5 BrS patients in whom mutations were, however, not reported.[33, 34]
Limitations
Although we included patients with lone AF, left atrial diameter was somewhat enlarged with low prevalence of diastolic dysfunction with no differences among both groups. Overall we found no large impact of rare variants on outcome of de-novo catheter ablation. Moreover, although reablation rates were comparable with all patients showing reconnected pulmonary veins, the sample size of this subgroup is rather small, therefore, no meaningful comparison of impact on subsequent outcome or AF mechanisms can be made. However, this does not rule out that private mutations may play a role in the outcome of therapeutic interventions for AF. While rare variants are more likely to be associated with large effect sizes, we do acknowledge that functional studies or co-segregation data are required to support disease-causality. Importantly, co-segregation could not be assessed (e.g. 3 brothers were not screened). Moreover, additional functional analysis on any of the detected rare non-synonymous mutations that have not been described previously was not performed. Hence, we are unable to determine whether the identified variants are pathogenic or benign or have smaller effects that could not be detected with this sample size. Although we did not include a non-AF sample, the majority of variants are very rare in reference samples. However, there is no phenotype data available and consequently, controls may in fact contain AF cases or other heart disease.
While we re-sequenced several high priority candidate AF genes, we did not perform whole exome/next generation sequencing. Therefore, we cannot exclude the possibility of our findings being influenced by variants residing in genes that were not screened. It is also possible that by evaluating coding regions only we may have missed potentially important variants in non-coding regions. Lastly, by focusing on rare variants only, we are unable to assess the effects of common variants including combinations of variants, with small or intermediate effects. Furthermore it should be noted that this study applies only to males, since they represent >90% of the study population.
Conclusions
Rare variants of genes encoding the cardiac sodium channel and associated compounds are frequently found in lone AF but seem not to impact on outcome of AF catheter ablation. This finding is in agreement with current recommendations not to screen for rare variants for the ablation outcome in AF. Nevertheless, it may at least be helpful for understanding AF mechanisms and larger studies are needed to further explore the possible association between genotype and response to AF therapies.
Supporting information
(DOCX)
(DOCX)
Abbreviations
- AF
atrial fibrillation
- LQTS
long QT syndrome
- BrS
Brugada syndrome
- SIDS
sudden infant death syndrome
- SCN5A
sodium channel, voltage-gated, type V, alpha subunit
- SCN1B
sodium channel, voltage-gated, type I, beta subunit
- SCN2B
sodium channel, voltage-gated, type II, beta subunit
- SCN3B
sodium channel, voltage-gated, type III, beta subunit
- SCN4B
sodium channel, voltage-gated, type IV, beta subunit
- SNTA1
syntrophin, alpha 1
- CAV3
caveolin 3
- MOG1
RAN guanine nucleotide release factor
- GPD1L
glycerol-3-phosphate dehydrogenase 1-like protein
- ERAF
early recurrence of atrial fibrillation
- LRAF
late recurrence of atrial fibrillation
- INa
inward sodium current
- EPHX2
soluble epoxide hydrolase gene
- HO-1
heme oxygenase-1 gene
- ACE
angiotensin-converting enzyme gene
- IL6R
interleukin-6 receptor gene
- AGT
angiotensinogen gene
- BB
beta blockers
- CCB
calcium channel blockers
- AAD
antiarrthythmic drugs
- LAD
left atrial diameter
- LVEF
left ventricular ejection fraction
- IVSd
interventricular septal enddiastolic dimension
- LVED
left ventricular end diastolic diameter
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
DH was supported by the Volkswagen Foundation Germany (84901), LU was supported by the German Cardiac Society (Otto Hess Stipend). MBS was supported by grants from the National Institutes of Health (NIH) (1K23HL127704) and American Heart Association (AHA) (11CRP7420009). DR was supported by grants from the NIH (U19HL65962 and UL1RR024975). DD was supported by grants from the NIH (R01HL092217) and AHA (EIA094116N).
References
- 1.Darbar D, Kannankeril PJ, Donahue BS, Kucera G, Stubblefield T, Haines JL, et al. Cardiac sodium channel (SCN5A) variants associated with atrial fibrillation. Circulation 2008;117(15):1927–1935. doi: 10.1161/CIRCULATIONAHA.107.757955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olesen MS, Holst AG, Svendsen JH, Haunso S, Tfelt-Hansen J. SCN1Bb R214Q found in 3 patients: 1 with Brugada syndrome and 2 with lone atrial fibrillation. Heart Rhythm 2012;9(5):770–773. doi: 10.1016/j.hrthm.2011.12.005 [DOI] [PubMed] [Google Scholar]
- 3.Watanabe H, Darbar D, Kaiser DW, Jiramongkolchai K, Chopra S, Donahue BS, et al. Mutations in sodium channel beta1- and beta2-subunits associated with atrial fibrillation. Circ Arrhythm Electrophysiol 2009;2(3):268–275. doi: 10.1161/CIRCEP.108.779181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olesen MS, Jensen NF, Holst AG, Nielsen JB, Tfelt-Hansen J, Jespersen T, et al. A novel nonsense variant in Nav1.5 cofactor MOG1 eliminates its sodium current increasing effect and may increase the risk of arrhythmias. Can J Cardiol 2011;27(4):523.e17-23. [DOI] [PubMed] [Google Scholar]
- 5.Vatta M, Ackerman MJ, Ye B, Makielski JC, Ughanze EE, Taylor EW, et al. Mutant caveolin-3 induces persistent late sodium current and is associated with long-QT syndrome. Circulation 2006;114(20):2104–2112. doi: 10.1161/CIRCULATIONAHA.106.635268 [DOI] [PubMed] [Google Scholar]
- 6.Hu RM, Tan BH, Orland KM, Valdivia CR, Peterson A, Pu J, et al. Digenic inheritance novel mutations in SCN5a and SNTA1 increase late I(Na) contributing to LQT syndrome. Am J Physiol Heart Circ Physiol 2013;304(7):H994–H1001. doi: 10.1152/ajpheart.00705.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.London B, Michalec M, Mehdi H, Zhu X, Kerchner L, Sanyal S, et al. Mutation in glycerol-3-phosphate dehydrogenase 1 like gene (GPD1-L) decreases cardiac Na+ current and causes inherited arrhythmias. Circulation 2007;116(20):2260–2268. doi: 10.1161/CIRCULATIONAHA.107.703330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Norstrand DW, Valdivia CR, Tester DJ, Ueda K, London B, Makielski JC, et al. Molecular and functional characterization of novel glycerol-3-phosphate dehydrogenase 1 like gene (GPD1-L) mutations in sudden infant death syndrome. Circulation 2007;116(20):2253–2259. doi: 10.1161/CIRCULATIONAHA.107.704627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Priori SG, Wilde AA, Horie M, Cho Y, Behr ER, Berul C, et al. Executive summary: HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Europace 2013;15(10):1389–1406. doi: 10.1093/europace/eut272 [DOI] [PubMed] [Google Scholar]
- 10.Eitel C, Hindricks G, Sommer P, Gaspar T, Kircher S, Wetzel U, et al. Circumferential pulmonary vein isolation and linear left atrial ablation as a single-catheter technique to achieve bidirectional conduction block: the pace-and-ablate approach. Heart Rhythm 2010;7(2):157–164. doi: 10.1016/j.hrthm.2009.10.003 [DOI] [PubMed] [Google Scholar]
- 11.Wilde AA, Brugada R. Phenotypical manifestations of mutations in the genes encoding subunits of the cardiac sodium channel. Circ Res 2011;108(7):884–897. doi: 10.1161/CIRCRESAHA.110.238469 [DOI] [PubMed] [Google Scholar]
- 12.Ellinor PT, Nam EG, Shea MA, Milan DJ, Ruskin JN, MacRae CA. Cardiac sodium channel mutation in atrial fibrillation. Heart Rhythm 2008;5(1):99–105. doi: 10.1016/j.hrthm.2007.09.015 [DOI] [PubMed] [Google Scholar]
- 13.Bezzina C, Veldkamp MW, van Den Berg MP, Postma AV, Rook MB, Viersma JW, et al. A single Na(+) channel mutation causing both long-QT and Brugada syndromes. Circ Res 1999;85(12):1206–1213. [DOI] [PubMed] [Google Scholar]
- 14.Shirai N, Makita N, Sasaki K, Yokoi H, Sakuma I, Sakurada H, et al. A mutant cardiac sodium channel with multiple biophysical defects associated with overlapping clinical features of Brugada syndrome and cardiac conduction disease. Cardiovasc Res 2002;53(2):348–354. [DOI] [PubMed] [Google Scholar]
- 15.Johnson JN, Tester DJ, Perry J, Salisbury BA, Reed CR, Ackerman MJ. Prevalence of early-onset atrial fibrillation in congenital long QT syndrome. Heart Rhythm 2008;5(5):704–709. doi: 10.1016/j.hrthm.2008.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kusano KF, Taniyama M, Nakamura K, Miura D, Banba K, Nagase S, et al. Atrial fibrillation in patients with Brugada syndrome relationships of gene mutation, electrophysiology, and clinical backgrounds. J Am Coll Cardiol 2008;51(12):1169–1175. doi: 10.1016/j.jacc.2007.10.060 [DOI] [PubMed] [Google Scholar]
- 17.Chakrabarti S, Wu X, Yang Z, Wu L, Yong SL, Zhang C, et al. MOG1 rescues defective trafficking of Na(v)1.5 mutations in Brugada syndrome and sick sinus syndrome. Circ Arrhythm Electrophysiol 2013;6(2):392–401. doi: 10.1161/CIRCEP.111.000206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kattygnarath D, Maugenre S, Neyroud N, Balse E, Ichai C, Denjoy I, et al. MOG1: a new susceptibility gene for Brugada syndrome. Circ Cardiovasc Genet 2011;4(3):261–268. doi: 10.1161/CIRCGENETICS.110.959130 [DOI] [PubMed] [Google Scholar]
- 19.Cheng J, Van Norstrand DW, Medeiros-Domingo A, Valdivia C, Tan BH, Ye B, et al. Alpha1-syntrophin mutations identified in sudden infant death syndrome cause an increase in late cardiac sodium current. Circ Arrhythm Electrophysiol 2009;2(6):667–676. doi: 10.1161/CIRCEP.109.891440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu G, Ai T, Kim JJ, Mohapatra B, Xi Y, Li Z, et al. Alpha-1-syntrophin mutation and the long-QT syndrome: a disease of sodium channel disruption. Circ Arrhythm Electrophysiol 2008;1(3):193–201. doi: 10.1161/CIRCEP.108.769224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng J, Valdivia CR, Vaidyanathan R, Balijepalli RC, Ackerman MJ, Makielski JC. Caveolin-3 suppresses late sodium current by inhibiting nNOS-dependent S-nitrosylation of SCN5A. J Mol Cell Cardiol 2013;61:102–110. doi: 10.1016/j.yjmcc.2013.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaidyanathan R, Vega AL, Song C, Zhou Q, Tan B, Berger S, et al. The interaction of caveolin 3 protein with the potassium inward rectifier channel Kir2.1: physiology and pathology related to long qt syndrome 9 (LQT9). J Biol Chem 2013;288(24):17472–17480. doi: 10.1074/jbc.M112.435370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leong IU, Stuckey A, Lai D, Skinner JR, Love DR. Assessment of the predictive accuracy of five in silico prediction tools, alone or in combination, and two metaservers to classify long QT syndrome gene mutations. BMC Med Genet 2015;16:34 doi: 10.1186/s12881-015-0176-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ackerman MJ, Splawski I, Makielski JC, Tester DJ, Will ML, Timothy KW, et al. Spectrum and prevalence of cardiac sodium channel variants among black, white, Asian, and Hispanic individuals: implications for arrhythmogenic susceptibility and Brugada/long QT syndrome genetic testing. Heart Rhythm 2004;1(5):600–607. doi: 10.1016/j.hrthm.2004.07.013 [DOI] [PubMed] [Google Scholar]
- 25.Husser D, Adams V, Piorkowski C, Hindricks G, Bollmann A. Chromosome 4q25 variants and atrial fibrillation recurrence after catheter ablation. J Am Coll Cardiol 2010;55(8):747–753. doi: 10.1016/j.jacc.2009.11.041 [DOI] [PubMed] [Google Scholar]
- 26.Shoemaker MB, Muhammad R, Parvez B, White BW, Streur M, Song Y, et al. Common atrial fibrillation risk alleles at 4q25 predict recurrence after catheter-based atrial fibrillation ablation. Heart Rhythm 2013;10(3):394–400. doi: 10.1016/j.hrthm.2012.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shoemaker MB, Bollmann A, Lubitz SA, Ueberham L, Saini H, Montgomery J, et al. Common genetic variants and response to atrial fibrillation ablation. Circ Arrhythm Electrophysiol 2015;8(2):296–302. doi: 10.1161/CIRCEP.114.001909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wutzler A, Kestler C, Perrot A, Loehr L, Huemer M, Parwani AS, et al. Variations in the human soluble epoxide hydrolase gene and recurrence of atrial fibrillation after catheter ablation. Int J Card 2013;168(4):3647–3651. [DOI] [PubMed] [Google Scholar]
- 29.Hu YF, Lee KT, Wang HH, Ueng KC, Yeh HI, Chao TF, et al. The association between heme oxygenase-1 gene promoter polymorphism and the outcomes of catheter ablation of atrial fibrillation. PLoS One 2013;8(2):e56440 doi: 10.1371/journal.pone.0056440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ueberham L, Bollmann A, Shoemaker MB, Arya A, Adams V, Hindricks G, et al. Genetic ACE I/D polymorphism and recurrence of atrial fibrillation after catheter ablation. Circ Arrhythm Electrophysiol 2013;6(4):732–737. doi: 10.1161/CIRCEP.113.000253 [DOI] [PubMed] [Google Scholar]
- 31.Wu G, Cheng M, Huang H, Yang B, Jiang H, Huang C. A variant of IL6R is associated with the recurrence of atrial fibrillation after catheter ablation in a Chinese Han population. PLoS One 2014;9(6):e99623 doi: 10.1371/journal.pone.0099623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Q, Hu X, Li S, Wang X, Wang J, Zhang R, et al. Association of the angiotensinogen M235T polymorphism with recurrence after catheter ablation of acquired atrial fibrillation. J Renin Angiotensin Aldosterone Syst 2015;16(4):888–897. doi: 10.1177/1470320315594315 [DOI] [PubMed] [Google Scholar]
- 33.Conte G, Chierchia GB, Wauters K, De Asmundis C, Sarkozy A, Levinstein M, et al. Pulmonary vein isolation in patients with Brugada syndrome and atrial fibrillation: a 2-year follow-up. Europace 2014;16(4):528–532. doi: 10.1093/europace/eut309 [DOI] [PubMed] [Google Scholar]
- 34.Sairaku A, Yoshida Y, Nakano Y, Kihara Y. Ablation of atrial fibrillation in Brugada syndrome patients with an implantable cardioverter defibrillator to prevent inappropriate shocks resulting from rapid atrial fibrillation. Int J Cardiol 2013;168(6):5273–5276. doi: 10.1016/j.ijcard.2013.08.016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.


