Abstract
Trisomy 21 and the consequent extra copy of the amyloid precursor protein (APP) gene and increased beta-amyloid (Aβ) peptide production underlie the universal development of Alzheimer’s disease (AD) pathology and high risk of AD dementia in people with Down syndrome (DS). Trisomy 21 and other forms of aneuploidy also arise among neurons and peripheral cells in both sporadic and familial AD and in mouse and cell models thereof, reinforcing the conclusion that AD and DS are two sides of the same coin. The demonstration that 90% of the neurodegeneration in AD can be attributed to the selective loss of aneuploid neurons generated over the course of the disease indicates that aneuploidy is an essential feature of the pathogenic pathway leading to the depletion of neuronal cell populations. Trisomy 21 mosaicism also occurs in neurons and other cells from patients with Niemann-Pick C1 disease and from patients with familial or sporadic frontotemporal lobar degeneration (FTLD), as well as in their corresponding mouse and cell models. Biochemical studies have shown that Aβ induces mitotic spindle defects, chromosome mis-segregation, and aneuploidy in cultured cells by inhibiting specific microtubule motors required for mitosis. These data indicate that neuronal trisomy 21 and other types of aneuploidy characterize and likely contribute to multiple neurodegenerative diseases and are a valid target for therapeutic intervention. For example, reducing extracellular calcium or treating cells with lithium chloride (LiCl) blocks the induction of trisomy 21 by Aβ. The latter finding is relevant in light of recent reports of a lowered risk of dementia in bipolar patients treated with LiCl and in the stabilization of cognition in AD patients treated with LiCl.
Keywords: Alzheimer’s disease, down syndrome, mitosis, aneuploidy, trisomy 21, neurogenesis
INTRODUCTION
People with Down syndrome (DS) often have special medical challenges, but they are also relatively protected from other medical conditions. For example, people with DS are at much higher risk of developing autism, obesity, childhood leukemia, autoimmune disorders, including diabetes, and Alzheimer’s disease (AD), but they almost never develop solid tumors or cardiovascular disease [1–3]. Research into these special characteristics of people with DS may open the door to new opportunities for understanding and developing approaches to diagnose and treat some of the most prevalent and debilitating human disorders. In this review, we will focus our attention on AD, because the cause of AD in people with DS is well understood and because the most specific genetic feature of DS—trisomy 21—is increasingly recognized as also being associated with AD in the typical population.
The brain lesions that define AD—neuritic and amyloid plaques, neurofibrillary tangles, neuroinflammation, and neurodegeneration—begin to arise in people with DS during their late teens and become fully developed AD-like neuropathology by the age of 30–40 years [1, 4, 5]. By the age of 50–60 years, the majority of people with DS exhibit AD-like dementia. Because the biochemistry and pathology of AD in people with DS and in the typical population are essentially identical, the current ‘amyloid cascade hypothesis’ is believed to apply to both populations [6].
Since 1984, when Glenner and Wong [7] and then Masters, Beyreuther, and colleagues [8] reported that the major component of the amyloid deposits found in both AD and DS is a small peptide now termed beta-amyloid (Aβ3, most models of the pathogenic pathway to AD have included Aβ as a central molecule. Justification for this focus was made stronger by subsequent discoveries from a number of laboratories that have made it increasingly likely that Aβ is itself a pathogenic molecule, although the toxic structure (i.e., oligomer, protofibril, or filament) and mechanism remain unclear. For example, the following discoveries have solidified the importance of Aβ and have led to the development of the ‘amyloid cascade hypothesis’ [1, 5, 9–15]:
All people with DS develop AD neuropathology by the age of 35 years.
The amyloid precursor protein (APP) gene, which encodes Aβ, is located on chromosome 21.
AD-causing mutations have been identified in the APP gene and in the presenilin 1 and 2 (PS1 and PS2) genes, which encode components of the γ-secretase enzyme responsible for one of the Aβ-generating cleavages of APP.
Additional insights into the amyloid cascade hypothesis came when two amyloid-associated proteins—apolipoprotein E (ApoE) and antichymotrypsin (ACT)—were shown to bind directly to Aβ and promote its macromolecular assembly in vitro and in transgenic mouse models of AD [16–23]. Indeed, the inheritance of one and especially two copies of the ε4 allele of the ApoE gene is the strongest risk factor for developing AD, besides age itself [24], and ApoE4 is the strongest catalyst of Aβ polymerization [16,23]. The ApoE-catalyzed polymerization of monomeric Aβ in the brain results in large non-physiological structures that cannot be as easily cleared, particularly in the case of ApoE4, and likely underlies the apparent clearance differences associated with the ApoE isoforms [22, 23, 25].
Although mouse models with familial AD-associated mutations in the APP, PS1, and/or PS2 genes accumulate amyloid deposits, exhibit cognitive impairment, and serve as the primary animal models of AD, they lack the tau pathology characteristic of AD. However, tau pathology can be induced in transgenic mice that express a mutant version of the human microtubule-associated protein tau (MAPT) that causes the pure tauopathy FTLD [26]. A transgenic rat model of AD, whose endogenous MAPT gene is more similar to the human gene, was recently developed that co-expresses the familial AD-associated “Swedish” allele of the APP gene (APPswe) and the exon 9-deleted variant of PS1 gene (PS1ΔE9). The APPswe/PS1ΔE9 rat first develops human amyloid deposits, then develops tangles composed of endogenous rat tau protein, and, ultimately, exhibits neurodegeneration, providing evidence that AD-associated tau pathology and neuronal loss in humans is a natural consequence of the amyloid cascade [27]. Key unanswered questions include how does amyloid formation arise in the majority of AD patients (> 95%) who do not inherit autosomal dominant mutations in AD-associated genes, and what initiates the neuronal cell death that accompanies AD?
ALZHEIMER’S DISEASE AND DOWN SYNDROME: TWO SIDES OF THE SAME COIN
Most people with DS harbor three complete copies of chromosome 21 in all of their cells starting at conception. Consequently, it has remained difficult to determine whether three copies of the chromosome 21-residing APP gene alone is sufficient to cause AD, or whether additional genes, either on chromosome 21 or whose expression is modified due to trisomy 21, are also required for AD development [2, 28]. This uncertainty was clarified recently by the identification of families with familial AD and only one observable genetic alteration in affected individuals: a duplication of the APP-encoding region of one of their copies of chromosome 21, resulting in a third copy of the APP gene [29,30]. This finding provided the first direct evidence that three copies of the APP gene alone are sufficient to yield early-onset, fully penetrant familial AD, although it is noteworthy that some people with trisomy 21 DS appear to be protected from developing AD dementia, despite having AD neuropathology [3]. Early on, Miriam Schweber [31] predicted that typical AD was due to the duplication of a “Down syndrome critical region” on chromosome 21. Although this was quickly proven not to be the case for all types of AD patients studied at the time, the finding decades later that a few families carry three copies of the APP gene and develop early-onset AD validates the original prediction that there might be a gene copy number-based mechanistic link between AD and DS. Furthermore, a few individuals with DS and trisomy 21 have been reported who only carry two copies of the APP gene due to a deletion of that region on one of their three copies of chromosome 21 [32] and I. Lott personal communication]. These individuals with DS but only two copies of the APP gene do not develop AD dementia, even by the age of 60 years, reinforcing the conclusion that an alteration in the APP gene alone, either via increased gene dosage or mutation, is sufficient to yield AD.
IS ALZHEIMER’S DISEASE A MOSAIC FORM OF DOWN SYNDROME?
Almost 25 years ago, we proposed that typical age-related AD, both familial and sporadic, might be caused, or at least promoted, not through the acquisition of a third copy of the APP gene, but through the development of a mosaic population of cells with complete trisomy 21 that arises by stochastic chromosome mis-segregation during the lifetime of an individual [33]. That is, if complete trisomy 21 could cause AD in individuals with DS, then mosaicism for trisomy 21 that arises over time due to chromosome segregation defects could cause age-related AD.
Our trisomy 21 mosaicism model for AD explains many seemingly unrelated facts about AD and can be applied to both familial and sporadic forms of the disease, depending upon whether the chromosome segregation defect is due to a genetic mutation or an environmental insult [30]. The trisomy 21 mosaicism model for AD makes the following three testable predictions:
AD patients should develop a small population of trisomy 21 cells in their somatic tissues produced over time by unequal chromosome segregation during mitosis. Ultimately, individuals with mosaic populations of trisomy 21 cells could acquire AD through the same mechanism by which DS patients acquire AD (i.e., the presence of three copies of the APP gene), but at a later age due to the modulating effect of the majority population of normal diploid cells.
There should be an inherited risk of both DS and AD in certain families who carry mutations in genes associated with chromosome segregation.
Mutations that cause AD should occur in genes encoding proteins directly or indirectly involved in mitosis and chromosome segregation.
Each of the three predictions for the trisomy 21 mosaicism model of AD has been tested and validated, either by re-examination of the literature or by new experimentation. That is, we and others have found that chromosome mis-segregation and mosaic aneuploidy are the result of a general cell cycle defect in AD that affects all chromosomes in all tissues, both peripheral and central, and may be responsible for neurodegeneration in the brain.
MOSAIC ANEUPLOIDY IN DOWN SYNDROME AND ALZHEIMER’S DISEASE
Although the vast majority of people with DS have complete trisomy 21, around 1% of people with DS are mosaic for trisomy 21. Individuals with trisomy 21 mosaicism exhibit varying levels of DS-associated phenotypes, but they are still at increased risk of developing early-onset AD [33,34]. For example, trisomy 21 mosaicism was discovered in two women who were not characterized as having DS or as being mentally disabled, but developed AD-like dementia by age 40 [35, 36]. One of the two women with trisomy 21 mosaicism who developed early-onset AD also had a child with DS. These and other case studies of patients with trisomy 21 mosaicism who did not exhibit intellectual impairments associated with DS, but who developed AD at a young age, have demonstrated that a low level of chromosomal instability is sufficient to result in early-onset AD [37, 38]. The often late-onset dementia associated with classic AD—both sporadic and familial—could thus result from an even smaller population of trisomy 21 cells that may go undetected by standard karyotype analysis of peripheral blood lymphocytes.
It is clearly important to determine directly whether AD patients are mosaic for trisomy 21, as our model predicts. However, this apparently simple experiment is not straightforward because it requires the use of techniques capable of accurately detecting low levels of mosaicism. Cytogenetic analyses of lymphocytes from AD patients have been carried out in a number of laboratories, with mixed results. Some investigators reported small increases in aneuploidy or other chromosomal abnormalities, as measured by direct karyotyping of mitotically active cells, whereas other groups have failed to confirm this finding, especially if they analyzed a small number of families or analyzed families without many members [39–46].
In contrast to metaphase karyotyping, fluorescence in situ hybridization (FISH) allows the number of copies of a particular chromosome to be determined regardless of the stage of the cell cycle (mitotic or interphase). The ability to examine interphase nuclei also greatly increases the number of cells that can be analyzed and, in principle, should facilitate the detection of very low levels of aneuploidy, including in neuronal cells. Furthermore, lymphocytes are under great selective pressure, which may lead to the selective loss of (abnormal) aneuploid cells in the individual or in culture. Therefore, we used FISH to determine the extent of aneuploidy, particularly trisomy 21, in primary fibroblasts from AD patients and age-matched normal control individuals [47–49]. Our analysis of thousands of cells from 27 AD and 13 control individuals showed that fibroblasts from AD patients were more than twice as likely to exhibit trisomy 21 compared to fibroblasts from control individuals. The increased frequency of trisomy 21 cells in fibroblasts from AD patients was significant (p = 0.007) and independent of age. Furthermore, we found that chromosome mis-segregation was associated with all types of AD, including sporadic and familial AD (i.e., those carrying a mutation in either PS1 or PS2). Finally, we also detected chromosome 18 aneuploidy, indicating that the cell cycle defect leading to chromosome mis-segregation was not specific to chromosome 21, but seemed to be associated with chromosome 21 most often, possibly due to trisomy 21 cells being less prone to die in vivo than other types of aneuploid cells [49]. Chromosome mis-segregation in patients with sporadic AD, particularly involving, but not restricted to, chromosome 21, has been confirmed in blood lymphocytes [50, 51], in buccal cells [52], and in brain neurons [43–57].
INCREASED GENETIC RISK OF BOTH ALZHEIMER’S DISEASE AND DOWN SYNDROME
An early clue that DS may be genetically related to AD came from epidemiological studies showing that women from some families with a history of autosomal dominant AD give birth to a significantly higher than normal percentage of children with DS [58–60]. Other studies failed to confirm the increased incidence of DS in families with inherited autosomal dominant AD, but the authors acknowledged that the number of relatives analyzed was not sufficient to yield statistically significant results [61–63]. Although the data from the largest studies were strongly suggestive of a potential link between inherited AD and an increased frequency of DS in the same family, additional work will be required to confirm this connection. Also, since these early reports, the APP, PS1, and PS2 genes, which harbor mutations that cause almost all inherited forms of AD, have been identified, making it easier to carry out this type of analysis.
In a more rigorous retrospective study, Schupf et al. showed that younger mothers (age < 35 years) of a child with DS have a five-fold greater risk of developing AD later in life, when compared to older mothers of a child with DS, to all fathers of a child with DS, or to the general population, as though they suffer from a novel form of “accelerated aging” [64, 65]. We interpret this result, instead, as indicating that young mothers of a child with DS are most likely mosaic for trisomy 21, indicating that they may have a predisposition to chromosome mis-segregation, and that this mosaicism is reflected in their trisomy 21 offspring and in their own increased risk for developing AD [49]. Indeed, later work by Migliore et al. [66–68] confirmed the susceptibility to aneuploidy and trisomy 21 nondisjunction in young mothers of children with DS, providing support for our hypothesis that AD is associated with trisomy 21 mosaicism.
DEFECTS IN MITOSIS ASSOCIATED WITH ALZHEIMER’S DISEASE
Several independent lines of investigation have provided evidence that defects in mitosis and/or in mitosis-specific proteins occur in AD patients—supporting the third prediction of the trisomy 21 mosaicism model for AD. These defects could potentially lead to chromosome mis-segregation, which could in turn result in the trisomy 21 mosaicism and other types of aneuploidy observed in AD cells and individuals. Defects in mitosis may also lead to other features of AD, such as apoptosis and altered APP processing.
Studies showing abnormal mitotic spindles in dividing cells from AD patients have provided the most direct evidence for mitotic defects in AD. For example, we briefly treated lymphocyte cell lines from patients with familial AD who carried a PS1 mutation and from control individuals with the microtubule-disrupting agent colchicine, and analyzed their karyotypes 40 hours later. This treatment causes many of the cells to exhibit separated chromatids or premature centromere division (PCD) in metaphase spreads, which is a known risk factor for chromosome mis-segregation that increases with age [69]. Karyotype analyses of colchicine-treated cells showed that those from AD patients exhibited significantly more PCD than those from normal age-matched controls [47]. This type of AD-specific increase in PCD induced by microtubule-disrupting agents has been confirmed by other labs [50, 68, 70, 71]. Interestingly, a similar study of cultured lymphocytes and fibroblasts from a young mother who had three trisomy 21 conceptuses and one normal child showed that her cells exhibited trisomy 21, 18, and X, suggesting a predisposition to PCD and a susceptibility of these three chromosomes to mis-segregation [69]. A related defect in mitosis—an increased frequency of chromosomes displaced from the mitotic spindle after colchicine treatment—was reported in cells derived from mothers of children with DS [72]. In addition, the rate of hyperdiploidy for every chromosome was found to be elevated in cells from mothers with a DS child [73].
Together, these data indicate that young mothers of a child with DS may be generally prone to chromosome mis-segregation, which can lead to gonadal trisomy 21 mosaicism, meiotic non-disjunction, or both, resulting in their trisomy 21 children. Based on the work of Schupf and Migliore and their colleagues discussed above, young mothers of a child with DS have an increased risk of developing AD, which again implicates chromosome mis-segregation in this disease process.
MITOTIC FUNCTION OF THE PRESENILIN AND APP PROTEINS
Many of the AD patients whose fibroblasts were found to exhibit trisomy 21 mosaicism belonged to families that were subsequently shown to carry familial mutations in the genes encoding the presenilin proteins, PS1 and PS2. This finding provided the first indication that familial AD genes predispose to chromosome mis-segregation, and that their encoded proteins are likely involved in the process of mitosis—providing additional support for the third prediction of the trisomy 21 mosaicism model for AD.
The two familial AD genes encoding the presenilin proteins were identified on chromosome 14 (PS1) by St. George-Hyslop and colleagues based on previous genetic mapping [74–76], and on chromosome 1 (PS2) by Schellenberg, Tanzi, and colleagues [76–78], and independently by us [79]. Nearly 200 point mutations that cause early-onset AD have been identified throughout the PS1 gene, and, much more rarely, in the PS2 gene. The fact that the vast majority of familial AD mutations reside within the coding regions of the PS1 and PS2 genes suggests that a dominant gain- or loss-of-function mutation that results in altered presenilin function, localization, or structure, rather than in altered expression, is likely to underlie the involvement of these two genes in AD (see, for example, [80]).
Because presenilin proteins are typically expressed at very low levels in the cell, and overexpressed proteins can easily become mislocalized, we generated highly sensitive, affinity-purified presenilin antibodies and used them to label the endogenous proteins in cultured cells by immunoelectron microscopy [81]. Both endogenous PS1 and PS2 localized to the nuclear membrane, kinetochores, and centrosomes, indicating that they are likely involved in mitosis. Several other laboratories have confirmed that presenilin and APP proteins localize to the nuclear envelope and/or centrosome and that APP exhibits cell cycle-dependent phosphorylation [82–87]. Furthermore, the specific localization of presenilin proteins to centrosomes and kinetochore microtubules in early mouse embryos is induced by halting the cells in mitosis [88]. These results indicate that the presenilins and APP may be involved in mitosis and that familial AD mutations in these genes may alter the cell cycle as part of their causal effect on AD development.
FAMILIAL ALZHEIMER’S DISEASE MUTATIONS IN PS1 AND APP INDUCE CHROMOSOME MIS-SEGREGATION AND ANEUPLOIDY
To investigate the function of presenilins, APP, or their product, the Aβ peptide, in mitosis, we asked whether the chromosome mis-segregation and trisomy 21 mosaicism observed in human fibroblasts from AD patients with PS1 or APP mutations could be mimicked in peripheral and brain tissues of familial AD-transgenic mice. Whole brains from transgenic mice harboring familial AD-associated mutant PS1 (M146L or M146V) or APP (V717F) and their non-transgenic littermates were processed to yield primary cell cultures that were hybridized with a mouse chromosome 16 bacterial artificial chromosome (BAC) probe [89], followed by Neu-N immunocytochemistry to identify neurons. The mouse chromosome 16 BAC was selected because mouse chromosome 16 has a large region that is syntenic with human chromosome 21. Most of the neurons from normal littermates were disomic, with two copies of chromosome 16, whereas up to 4% of neurons from the PS1 transgenic mice exhibited trisomy 16 [90], and 6.5% of neurons from the APP transgenic mice exhibited trisomy 16 [91].
To determine whether the aneuploidy observed in the familial AD mouse models was caused directly by the mutated genes and not by some other factors, parallel cultures of the hTERT-HME1 immortalized primary mammary epithelial cell line (Clontech), which has a stable karyotype, were transiently transfected with a vector for the expression of wild-type PS1, a single mutant PS1 (M146L), a single mutant APP (V717I), or a double mutant APP (K595N/M596L plus V642I), or with an control empty vector. FISH was used to assess aneuploidy levels for chromosomes 21 and 12. Overexpression of familial AD-associated genes induced between 2–3% of trisomy 21 and/or trisomy 12, and up to 30% of the cells either gained or lost chromosomes and were aneuploid within 48 hours [90, 91]. Furthermore, immunocytochemistry of PS1-transfected cells revealed several mitotic spindle abnormalities, including disarrayed microtubules, multiple centrosomes, and lagging chromosomes as the most prominent spindle malformations [90]. These robust results indicated that the aneugenic effect of expressing familial AD mutations likely affects all chromosomes, with random gains and losses, and that chromosome mis-segregation may not be restricted to the cells expressing mutated genes, but may also extended to nearby, non-transfected cells due to increased Aβ secretion. We hypothesized that Aβ peptide, which is elevated in both familial and sporadic AD, is the key effector molecule responsible for interfering with mitosis and chromosome segregation [90, 92].
To more directly assess the role of Aβ peptide with regard to genetic instability, we treated hTERT-HME1 cells with either 1 μM Aβ40 or Aβ42 or with control peptides and found that the Aβ-treated cells develop more than 20% aneuploid metaphases with 2% of the cells trisomic for chromosome 21 or 12 within 48 hours of exposure, compared to 6% aneuploid metaphases with less than 1% of the cells trisomic for chromosome 21 or 12 in negative controls [91]. These results indicated that AD might be a self-propagating disorder in which the Aβ peptide—the product of both familial AD mutations and trisomy 21—induces further chromosome mis-segregation and the generation of trisomy 21 and other types of aneuploidy. That is, the trisomy 21 cells that accumulate will over-express APP and Aβ, and this imbalance would promote neurodegeneration as it does in people with DS, in people with mosaic trisomy 21, or in people carrying three copies of the APP gene who are born asymptomatic but later develop Aβ and tau pathology and dementia [29, 30, 33].
To investigate the potential mechanisms by which Aβ exerts its aneugenic effect on dividing cells, we analyzed the peptide’s other toxic activities with a focus on those related to microtubule function. Several lines of investigation have indicated that Aβ induces and requires downstream changes and/or defects in microtubules to exert its neurodegenerative activity. For example, in vitro and in vivo studies have shown that Aβ induces the phosphorylation of tau (e.g., [93]), and that Aβ toxicity depends on the presence of tau [94]. Therefore, we investigated the role of tau in Aβ-induced aneuploidy. Splenocytes prepared from Tau+/+, Tau+/−, and Tau −/− mice were treated with Aβ peptide and analyzed for aneuploidy 48 hours later. Prior to Aβ treatment, Tau+/− and Tau −/− cells exhibited up to 5% trisomy 16. However, the aneugenic effect of Aβ was greatly attenuated in Tau −/− cells, but not in normal cells, indicating that Aβ-induced chromosome mis-segregation requires tau and is likely to disrupt the normal microtubule stabilizing function of tau. The mechanism by which Aβ induces chromosome mis-segregation and aneuploidy was revealed when Aβ42 peptide was added to Xenopus egg extracts and found to impair the structure and stability of mitotic spindles by inhibiting three motor kinesins, including Eg5, KIF4A, and MCAK, which are required for normal mitotic spindle function and proper chromosome segregation [95].
CELL CYCLE DEFECTS IN OTHER NEURODE-GENERATIVE DISEASES
Because neurogenesis occurs throughout life, especially following neuronal loss, we and others investigated the possibility that aneuploidy might be associated with other neurodegenerative diseases, in addition to AD. For example, brains from patients with ataxia-telangiectasia (AT) exhibit mosaic aneuploidy in both neurons and glia, including trisomy 21, and hippocampi from patients with Lewy body diseases exhibit higher levels of mosaic aneuploidy in neurons than glia [55, 96, 97]. Furthermore, Rossi et al. discovered aneuploidy in peripheral tissues from patients with sporadic FTLD, and we found trisomy 21 and other aneuploidy in tau knockout and mutant MAPT transgenic mice [91, 98–100]. Recently, we have identified trisomy 21 and other aneuploidy in brain neurons and glia from patients with sporadic FTLD and with all forms of familial FTLD (MAPT, PRGN, and C9ORF72), in brain neurons and glia from mutant MAPT transgenic mice, and in cells transfected with a mutant form of MAPT (submitted). These trisomy 21 and other aneuploidy cells would not be fully functional and would also be particularly prone to apoptosis/degeneration, as has been shown in many experimental systems [56, 101–106]. Taken together with the finding of Arendt et al. [56] that the specific loss of aneuploid cells in AD accounts for 90% of the neurodegeneration in AD, these findings support the view that aneuploidy is an essential component of the pathogenic pathway in both AD and FTLD.
ROLE OF CHOLESTEROL AND LIPOPROTEINS IN THE CELL CYCLE
High levels of dietary cholesterol and plasma LDL are common risk factors for atherosclerosis, cardiovascular disease, and AD, and intracellular cholesterol levels are increased in patients with the inherited disease Niemann-Pick C1 [107–112]. In AD patients, the severity of atherosclerotic lesions in the brain correlates with the extent of AD pathology [113, 114]. Furthermore, a direct association between disrupted intracellular cholesterol trafficking and consequent neuronal loss, gliosis, and neurofibrillary tangle formation in people with Niemann-Pick C1 disease who carry a mutation in the NPC1 or NPC2 gene has been established. However, the mechanisms by which increased cholesterol causes disease are incompletely understood. We sought to determine whether there is a common pathogenic pathway by which cholesterol and LDL promote the development of AD, Niemann-Pick C1, and cardiovascular disease, and whether chromosome mis-segregation is involved. The latter hypothesis was reinforced by reports that each atherosclerotic plaque in a person with cardiovascular disease harbors a monoclonal population of aneuploid smooth muscle cells [115–121].
To test these hypotheses, we examined tissues from Niemann-Pick C1 patients and from Niemann-Pick C1 mouse models and found that both brain and peripheral tissues exhibited trisomy 21 mosaicism and trisomy 16 mosaicism, respectively [122]. Furthermore both LDL (but not HDL) and cyclodextrin-solubilized cholesterol induce mitotic spindle abnormalities and aneuploidy in cultured cells. Cell cycle abnormalities are associated with AD, Niemann-Pick C1, and cardiovascular disease, and excess lipoproteins, particularly LDL, could potentially cause or contribute to the chromosome mis-segregation and aneuploidy observed in these diseases. Interestingly, although amyloidogenic fragments of APP, including Aβ42, have been found in neurons from Niemann-Pick C1 brains [123], LDL induces chromosome mis-segregation even in the absence of APP [118]. Furthermore, ethanol treatment of cells blocks the aneugenic effect of cholesterol, just as it reduces atherosclerosis [118,124].
DOES NEURONAL ANEUPLOIDY ARISE VIA NEUROGENESIS AND/OR CELL CYCLE RE-ENTRY?
Abnormal mitotic spindle function is the clear underlying mechanism by which aneuploidy arises in neurodegenerative diseases, and this defect would be expected to affect all dividing cells. Indeed, glia and peripheral dividing cells, such as fibroblasts, buccal cells, and lymphocytes, from people with these disorders exhibit mosaic aneuploidy. However, it is less clear how large numbers of neurons (up to 30%) can become aneuploid in people with neurodegenerative diseases, as many investigators have found [56, 59, 90, 91, 122, 124–126].
Two potential mechanisms could explain the formation of aneuploid neurons in the brains of individuals with AD or other neurodegenerative diseases: 1) cell cycle re-entry of mature neurons, and 2) neurogenesis. Data provide support for both mechanisms. For example, neurons in the AD brain express phosphoproteins that are usually only found during mitosis, such as cyclin B1, cyclin D1, cdc2, and Ki67 [53, 126–132]. Some of these changes can also be detected in peripheral cells, suggesting the presence of a systemic cell cycle alteration [133]. How these “post-mitotic” cells acquire the ability to express mitosis-specific proteins remains unknown, but it is interesting that low concentrations of Aβ can induce the expression of mitotic proteins and cell cycle reentry in mature neurons in culture, as can the MiR-26b micro-RNA that is upregulated during AD [134–136]. Similar findings have been reported in mouse models of AD, in which the loss of pre-existing neurons, rather than amyloid or tangle pathology, appears to induce neuronal re-entry into the cell cycle [135,137]. Neurons re-entering an aberrant cell cycle in the AD brain could gain or lose chromosomes, exhibit changes in mitosis-specific gene expression, or undergo apoptosis in response to an untenable physiological state—any or all of which could stimulate the development of AD pathology [81, 126].
Neurogenesis is increasingly recognized as occurring in the normal adult brain, not only in the olfactory epithelium and bulb, but also in the dentate gyrus and the striatum [138–147]. Indeed, the rate of new neuron formation in the dentate gyrus of the hippocampus is reported to be on the order of 1,000–2,000 cells per day—more than enough to cause significant numbers of trisomy 21 cells or other types of aneuploid cells to accumulate over the course of 50 years under the influence of, for instance, an APP or presenilin mutation. Although neurogenesis has not been measured directly during the human neurodegenerative disease process, neuronal cell loss has been shown to induce neurogenesis in mice (see, for example, [148–150]). Thus, it would not be surprising if neuronal cell death during neurodegeneration induces neurogenesis and the production of aneuploid neurons in the presence of an aneugen, such as, for example, Aβ, mutant or abnormally phosphorylated Tau, or excess cholesterol. Furthermore, apoptotic neurons generate and release more Aβ than healthy neurons [151, 152], potentially initiating a positive feedback loop. Finally, individual cells with increased copies of the APP gene have been found in the AD brain, which would essentially have the same effect [153]. It is also possible for aneuploidy in glia to contribute to the neurodegenerative process. For example, trisomy 21 microglia express excess IL-1β, which can initiate an inflammatory cascade that leads to increased production of the amyloid catalysts ApoE and ACT [16,154,155].
CONCLUSION: ANEUPLOIDY UNDERLIES THE NEURODEGENERATIVE DISEASE PROCESS
Together, the findings reviewed herein support the conclusion that widespread aneuploidy occurs in patients with sporadic AD, familial AD, FTLD, and Niemann-Pick C1, as well as in mouse and cell models thereof. Thus, although the mosaic aneuploidy associated with each of these neurodegenerative diseases may arise through different mechanisms (for example, Aβ-mediated inhibition of microtubule motors in AD, MAPT-associated effects on a microtubule-binding protein in one form of FTLD, and cholesterol-induced changes in membrane fluidity in Niemann-Pick C1), the different pathogenic pathways all result in the production of aneuploid cells. Because the mosaic aneuploidy associated with these diseases affects both peripheral and brain cells, including neurons and glia, and the underlying mutations all cause chromosome mis-segregation and aneuploidy in mouse and cell models, it seems most likely that the mosaic brain aneuploidy shared by all of these diseases contributes to their associated neurodegeneration. Indeed, aneuploidy has been shown to underlie the neurodegeneration associated with AD.
IMPLICATIONS FOR TREATMENT
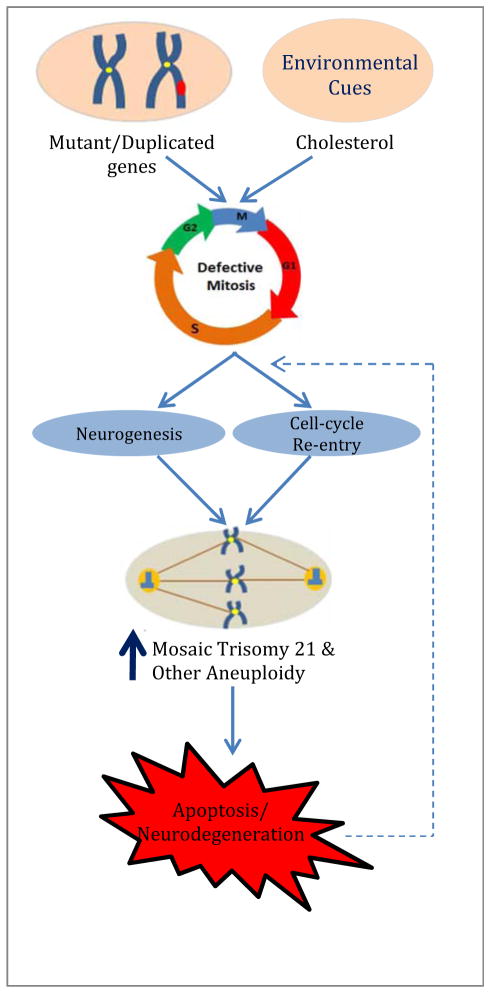
The mechanistic implication of the findings discussed above is that an early step in the pathogenic pathway to multiple neurodegenerative diseases involves a defect in chromosome segregation that leads to trisomy 21 and other types of mosaic aneuploidy and ultimately to apoptosis and neurodegeneration (Fig. 1). This conclusion can be exploited in the search for more effective diagnoses and treatments for AD and related disorders. For example, a very straightforward potential diagnostic test based on our finding of trisomy 21 cells among AD patient fibroblasts would be to use chromosome analysis to directly assess the level of trisomy 21 mosaicism in an individual. Although we have found that cell lines from AD patients as a group have increased trisomy 21 mosaicism, there is some overlap in the data between the AD patient group data and the control group data, possibly because the cell lines have undergone many mitoses in culture. Clearly, this type of test must be made more sensitive to be useful. Another approach would be to use buccal cells or fibroblasts directly from the patients [52].
Fig. 1. Generation of Aneuploidy and Neurodegeneration in Neurons from AD Patients.
Both neurogenesis and abnormal cell cycle re-entry have been shown to occur in many regions of the brain, and both conditions lead to cell proliferation (possibly to compensate for cell loss). As cell proliferation progresses, gene duplications, mutations, and/or environmental cues (i.e., cholesterol) affect cell cycle molecules, thereby causing mitotic defects, such as multiple/defective centrosomes, disorganized mitotic spindles, disrupted microtubule motors, etc. These defects may, in turn, lead to chromosome non-disjunction, chromosome mis-segregation, and elevated levels of trisomy 21 and other forms of aneuploidy. As a consequence of this genetic instability, the cells become more prone to undergoing apoptosis, thereby promoting neurodegeneration.
The implication from the data showing that trisomy 21 and other forms of mosaic aneuploidy may be one of the very first steps in the AD pathogenic pathway also suggests new approaches to prevention and therapy. For example, reducing exposure to molecules that induce chromosome mis-segregation, of which there are many, including cholesterol, may reduce the risk of developing AD [33,156,157]. Furthermore, molecules that reverse the inhibitory activity of Aβ on microtubule motors could be identified. Indeed, we have already found that reducing extracellular calcium levels with BAPTA or treating cells with LiCl blocks the aneugenic effect of Aβ [91]. Interestingly, LiCl treatment is becoming increasingly appreciated as a potentially effective AD therapy. For example, LiCl treatment reduces the incidence of dementia in people being treated for bipolar disorder [158–160]. Furthermore, LiCl stabilizes cognitive decline and reduces Aβ and amyloid production in cell and mouse models of AD [161,162]. Finally, recent studies have indicated that, although short-term (10 week) LiCl treatment was ineffective, longer term (15 month, low dose) LiCl treatment prevented cognitive decline in AD patients [163, 164]. How LiCl reduces the aneugenic effect of Aβ or prevents the cognitive deficits associated with AD remains unclear, and these two effects may or may not be related. One possible mechanism is based on the finding that LiCl inhibits GSK-3β, a kinase known to regulate the phosphorylation of tau, which is also implicated in Aβ-induced chromosome mis-segregation [91,165–167]. However, LiCl may not be as effective in vivo as it is in vitro with regard to GSK-3β inhibition [166], suggesting that LiCl may impact Aβ toxicity and cognition in AD in more than one way.
Molecules that block the ability of Aβ to inhibit microtubule motors may have an additional benefit: they would also block the ability of Aβ to reduce microtubule motor-dependent cell surface presentation of receptors essential for neuronal function, including the LDL receptor, the p75 NGF receptor, and the NMDA receptor [168, 169]. The target of Aβ in these studies—the kinesin-like microtubule motor Eg5/kinesin-5—has been shown to control neurite outgrowth, growth cone turning, and process size [170–172]. Thus, the restoration of kinesin-5 activity and consequent improvements in neuronal growth cone function and receptor localization and function could benefit people with AD, DS, or both. Alternatively, if trisomy 21 cells themselves are a particular problem, then it may be possible to target them for removal from the body by exploiting distinctive features of their cell biology.
In summary, many, and possibly all, patients with AD and other neurodegenerative diseases are mosaic for trisomy 21 and other types of aneuploidy. The mechanism(s) by which these abnormal cells arise and whether or how they contribute to the pathogenesis of the disease are areas of active investigation. Further exploration of these novel findings has the potential to contribute to the development of diagnoses and therapies for AD and other neurodegenerative diseases and to increase our understanding of basic cellular processes.
Acknowledgments
The work from our laboratory was supported by grants from the NIH (AG09665, AG025711, AG037942, and NS076291), the Alzheimer’s Association, and start-up funds from University of Colorado and the Linda Crnic Institute for Down Syndrome. We thank Bruce Lamb for the BAC 16 probe and Dennis Dickson and the Mayo Clinic for brain samples. We are also grateful to the Banner Sun Health Research Institute Brain and Body Donation Program of Sun City, AZ, for the provision of human brain tissue. The Brain and Body Donation Program is supported by the National Institute of Neurological Disorders and Stroke (U24 NS072026 National Brain and Tissue Resource for Parkinson’s Disease and Related Disorders), the National Institute on Aging (P30 AG19610 Arizona Alzheimer’s Disease Core Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901 and 1001 to the Arizona Parkinson’s Disease Consortium), and the Michael J. Fox Foundation for Parkinson’s Research.
Footnotes
Send Orders for Reprints to reprints@benthamscience.ae
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
References
- 1.Wisniewski HM, Rabe A, Wisniewski KE. Neuropathology and dementia in people with Down’s syndrome. In: Davies P, Finch C, editors. Mol Neuropathol Aging. 1988. pp. 399–413. [Google Scholar]
- 2.Epstein CJ. The consequences of chromosome imbalance. Am J Med Genet: Suppl. 1990;7:31–37. doi: 10.1002/ajmg.1320370706. [DOI] [PubMed] [Google Scholar]
- 3.Lott IT. Neurological phenotypes for Down syndrome across the life span. Prog Brain Res. 2012;197:101–121. doi: 10.1016/B978-0-444-54299-1.00006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lemere CA, Blusztajn JK, Yamaguchi H, Wisniewski T, Saido TC, Selkoe DJ. Sequence of deposition of heterogeneous amyloid beta-peptides and APO E in Down syndrome: implications for initial events in amyloid plaque formation. Neurobiol Dis. 1996;3(1):16–32. doi: 10.1006/nbdi.1996.0003. [DOI] [PubMed] [Google Scholar]
- 5.Olson MI, Shaw CM. Presenile dementia and Alzheimer’s disease in mongolism. Brain. 1969;92(1):147–156. doi: 10.1093/brain/92.1.147. [DOI] [PubMed] [Google Scholar]
- 6.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 297(5580):353–356. doi: 10.1126/science.1072994. Review Erratum in: Science 297(5590), 2209, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Glenner G, Wong CW. Alzheimer disease and Down’s syndrome: Sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984;122:1131–1135. doi: 10.1016/0006-291x(84)91209-9. [DOI] [PubMed] [Google Scholar]
- 8.Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci USA. 1985;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldgaber D, Lerman MJ, McBride OW, Saffiotti U, Gajdusek DC. Characterization and chromosomal localization of cDNA encoding brain amyloid of Alzheimer’s disease. Science. 1987;235(4791):877–880. doi: 10.1126/science.3810169. [DOI] [PubMed] [Google Scholar]
- 10.Neve RL, Finch EA, Dawes LR. Expression of the Alzheimer amyloid precursor gene transcripts in the human brain. Neuron. 1988;1(8):669–677. doi: 10.1016/0896-6273(88)90166-3. [DOI] [PubMed] [Google Scholar]
- 11.Petterson D, Gardiner K, Kao FT, Tanzi R, Watkins P, Gusella JF. Mapping of the gene encoding the β-amyloid precursor protein and its relationship to the Down syndrome region of chromosome 21. Proc Nat Acad Sci USA. 1988;85(21):8266–8270. doi: 10.1073/pnas.85.21.8266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanzi RE, Gusella JF, Watkins PC, Bruns GA, St George-Hyslop P, Van Keuren ML, et al. Amyloid β-protein gene cDNA, mRNA distributions, and genetic linkage near the Alzheimer locus. Science. 1987;235(4791):880–884. doi: 10.1126/science.2949367. [DOI] [PubMed] [Google Scholar]
- 13.Blennow K, de Leon M, Zetterberg H. Alzheimer’s disease. Lancet. 2006;368(9533):387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 14.Lee VM, Trojanowski JQ. Progress from Alzheimer’s tangles to pathological tau points towards more effective therapies now. J Alzheimers Dis. 2006;9:257–262. doi: 10.3233/jad-2006-9s328. [DOI] [PubMed] [Google Scholar]
- 15.Hardy J. Alzheimer’s disease: the amyloid cascade hypothesis: an update and reappraisal. Alzheimers Dis. 2006;9(3):151–153. doi: 10.3233/jad-2006-9s317. [DOI] [PubMed] [Google Scholar]
- 16.Ma J, Yee A, Brewer HB, Das S, Potter H. The Alzheimer amyloid-associated proteins a1-antichymotrypsin and apolipoprotein E promote the assembly of the Alzheimer β-protein into filaments. Nature. 1994;372:92–94. doi: 10.1038/372092a0. (see also “News and Views,” same issue) [DOI] [PubMed] [Google Scholar]
- 17.Wisniewski T, Castaño EM, Golabek A, Vogel T, Frangione B. Acceleration of Alzheimer’s fibril formation by apolipoprotein E in vitro. Am J Pathol. 1994;145:1030–1035. [PMC free article] [PubMed] [Google Scholar]
- 18.Bales KR, Verina T, Dodel RC, Du Y, Altstiel L, Bender M, et al. Lack of apolipoprotein E dramatically reduces amyloid β-peptide deposition. Nat Genet. 1997;17:263–264. doi: 10.1038/ng1197-263. [DOI] [PubMed] [Google Scholar]
- 19.Holzman DM, Bales KR, Tenkova T, Fagan AM, Parsadanian M, Sartorius L, et al. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2000;97:2892–2897. doi: 10.1073/pnas.050004797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mucke L, Yu G-Q, McConlogue L, Rockenstein EM, Abraham CR, Masliah E. Astroglial expression of human α1-antichymotrypsin enhances Alzheimer-like pathology in amyloid protein precursor transgenic mice. Am J Pathol. 2000;157:2003–2010. doi: 10.1016/s0002-9440(10)64839-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nilsson LNG, Bales KR, DiCarlo D, Gordon MN, Morgan D, Paul SM, et al. α-1-antichymotrypsin promotes beta-sheet amyloid plaque deposition in transgenic mouse model of Alzheimer’s disease. J Neurosci. 2001;21:1444–1451. doi: 10.1523/JNEUROSCI.21-05-01444.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nilsson LNG, Gografe S, Costa DA, Hughes T, Dressler D, Potter H. Use of fused circulations to investigate the role of apolipoprotein E as amyloid catalyst and peripheral sink in Alzheimer’s disease. Technol Innov. 2012;14:199–208. doi: 10.3727/194982412X13462021398010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Potter H, Wisniewski T. Apolipoprotein E: Essential Catalyst of the Alzheimer Amyloid Cascade. Intern Alz Dis. 2012;2012:489428. doi: 10.1155/2012/489428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roses AD. Apolipoprotein E alleles as risk factor in Alzheimer’s disease. Annu Rev Med. 1996;47:387–400. doi: 10.1146/annurev.med.47.1.387. [DOI] [PubMed] [Google Scholar]
- 25.Castellano JM, Kim J, Stewart FR, Jiang H, DeMattos RB, Patterson BW, et al. Human apoE isoforms differentially regulate brain amyloid-β peptide clearance. Sci Transl Med. 2011;3(89):89ra57. doi: 10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberson ED. Mouse models of frontotemporal dementia. Ann Neurol. 2012;72(6):837–849. doi: 10.1002/ana.23722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen R, Rezai-Zadeh K, Weitz TM, Rentsendorj A, Gate D, Spivak I, et al. A transgenic Alzheimer rat with plaques, tau pathology, behavioral impairment, oligomeric aβ, and frank neuronal loss. J Neurosci. 2013;33(15):6245–6256. doi: 10.1523/JNEUROSCI.3672-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hartley D, Blumenthal T, Carrillo M, DiPaolo G, Esralew L, Gardiner K, et al. Down syndrome and Alzheimer’s disease: Common pathways, common goals. Alzheimers Dement. 2015;11(6):700–709. doi: 10.1016/j.jalz.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rovelet-Lecrux A, Hannequin D, Raux G, Le Meur N, Laquerrière A, Vital A, et al. APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nat Genet. 2006;38(1):11–12. doi: 10.1038/ng1718. [DOI] [PubMed] [Google Scholar]
- 30.Sleegers K, Brouwers N, Gijselinck I, Theuns J, Goossens D, Wauters J, et al. APP duplication is sufficient to cause early onset Alzheimer’s dementia with cerebral amyloid angiopathy. Brain. 2006;129(11):2977–2983. doi: 10.1093/brain/awl203. [DOI] [PubMed] [Google Scholar]
- 31.Schweber M. A possible unitary genetic hypothesis for Alzheimer’s disease and Down syndrome. Ann NY Acad Sci. 1985;450:223–238. doi: 10.1111/j.1749-6632.1985.tb21495.x. [DOI] [PubMed] [Google Scholar]
- 32.Prasher VP, Farrer JM, Kessling AM, Fisher EM, West RJ, Barber PC, et al. Molecular mapping of Alzheimer-type dementia in Down’s syndrome. Ann Neurol. 1998;43:380–383. doi: 10.1002/ana.410430316. [DOI] [PubMed] [Google Scholar]
- 33.Potter H. Review and hypothesis: Alzheimer disease and Down syndrome--chromosome 21 nondisjunction may underlie both disorders. Am J Hum Genet. 1991;48(6):1192–1200. [PMC free article] [PubMed] [Google Scholar]
- 34.Hultén MA, Jonasson J, Nordgren A, Iwarsson E. Germinal somatic trisomy 21 mosaicism: how common is it, what are the implications for individual carriers and how does it come about? Curr Genom. 2010;11(6):1389–2029. doi: 10.2174/138920210793176056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schapiro MB, Kumar A, White B, Grandy CL, Friedland RP, Rappaport SI. Alzheimer’s disease (AD) in mosaic/translocation Down’s syndrome (Ds) without mental retardation. Neurology. 1989;39:169. [Google Scholar]
- 36.Rowe IF, Ridler MAC, Gibberd FB. Presenile dementia associated with mosaic trisomy 21 in a patient with a Down syndrome child. Lancet. 1989;2(8656):229. doi: 10.1016/s0140-6736(89)90421-2. [DOI] [PubMed] [Google Scholar]
- 37.Hardy J, Goate A, Owen M, Rossor M. Presenile dementia associated with mosaic trisomy 21 in a patient with a Down syndrome child. Lancet. 1989;2(8665):743. doi: 10.1016/s0140-6736(89)90805-2. [DOI] [PubMed] [Google Scholar]
- 38.Ringman JM, Rao PN, Lu PH, Cederbaum S. Mosaicism for trisomy 21 in a patient with young-onset dementia: a case report and brief literature review. Arch Neurol. 2008;65(3):412–415. doi: 10.1001/archneur.65.3.412. [DOI] [PubMed] [Google Scholar]
- 39.Jarvik LF, Yen F-S, Goldstein F. Chromosomes and mental status. A study of women residing in institutions for the elderly. Arch Gen Psychiatry. 1974;30:186–190. doi: 10.1001/archpsyc.1974.01760080046007. [DOI] [PubMed] [Google Scholar]
- 40.Ward BE, Cook RH, Robinson A, Austin JH. Increased aneuploidy in Alzheimer disease. Am J Med Genet. 1979;3:137–144. doi: 10.1002/ajmg.1320030204. [DOI] [PubMed] [Google Scholar]
- 41.Nordenson I, Adolfsson R, Beckman G, Bucht G, Winblad B. Chromosomal abnormality in dementia of Alzheimer type. Lancet. 1980;1:481–482. doi: 10.1016/s0140-6736(80)91020-x. [DOI] [PubMed] [Google Scholar]
- 42.White BJ, Crandall C, Goudsmit J, Morrow CH, Alling DW, Gajdusek DC, et al. Cytogenetic studies of familial and sporadic Alzheimer disease. Am J Med Genet. 1981;10:77–89. doi: 10.1002/ajmg.1320100110. [DOI] [PubMed] [Google Scholar]
- 43.Buckton KE, Whalley LJ, Lee M. Chromosome changes in Alzheimer’s presenile dementia. J Med Genet. 1983;20:46–51. doi: 10.1136/jmg.20.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moorhead PS, Heyman A. Chromosome studies of patients with Alzheimer’s disease. Am J Med Genet. 1983;14:545–556. doi: 10.1002/ajmg.1320140319. [DOI] [PubMed] [Google Scholar]
- 45.Kormann-Bortolotto MH, Smith MAC, Neto JT. Alzheimer’s disease and ageing: a chromosomal approach. Gerontology. 1993;39:1–6. doi: 10.1159/000213508. [DOI] [PubMed] [Google Scholar]
- 46.Lao JI, Beyer K, Fernadez-Novoa L. Cytogenetic signs of genomic instability in Alzheimer’s disease. Neurobiol Aging. 1996;17:S62. [Google Scholar]
- 47.Potter H, Ma J, Das S, Geller LN, Benjamin M, Kayyali US, et al. Beyond β-protein: new steps in the pathogenic pathway to Alzheimer’s disease. In: Iqbal K, Mortimer JA, Winblad B, Wisnicuski HM, editors. Research Advances in Alzheimer’s Disease and Related Disorders. New York: John Wiley and Sons; 1995. pp. 643–654. [Google Scholar]
- 48.Potter H, Geller LN. Alzheimer disease, Down’s syndrome, and chromosome segregation. Lancet. 1996;348:66. doi: 10.1016/s0140-6736(05)64399-1. [DOI] [PubMed] [Google Scholar]
- 49.Geller LN, Potter H. Chromosome missegregation and trisomy 21 mosaicism in Alzheimer’s disease. Neurobiol Dis. 1999;6(3):167–179. doi: 10.1006/nbdi.1999.0236. [DOI] [PubMed] [Google Scholar]
- 50.Migliore L, Testa A, Scarpato R, Pavese N, Petrozzi L, Bonuccelli U. Spontaneous and induced aneuploidy in peripheral blood lymphocytes of patients with Alzheimer’s disease. Hum Genet. 1997;101(3):299–305. doi: 10.1007/s004390050632. [DOI] [PubMed] [Google Scholar]
- 51.Migliore L, Botto N, Scarpato R, Petrozzi L, Cipriani G, Bonuccelli U. Preferential occurrence of chromosome 21 malsegregation in peripheral blood lymphocytes of Alzheimer disease patients. Cytogenet Cell Genet. 1999;87(1–2):41–46. doi: 10.1159/000015389. [DOI] [PubMed] [Google Scholar]
- 52.Thomas P, Fenech M. Chromosome 17 and 21 aneuploidy in buccal cells is increased with ageing and in Alzheimer’s disease. Mutagenesis. 2008;23(1):57–65. doi: 10.1093/mutage/gem044. [DOI] [PubMed] [Google Scholar]
- 53.Kingsbury MA, Yung YC, Peterson SE, Westra JW, Chun J. Aneuploidy in the normal and diseased brain. Cell Mol Life Sci. 2006;63:2626–2641. doi: 10.1007/s00018-006-6169-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mosch B, Morawski M, Mittag A, Lenz D, Tarnok A, Arendt T. Aneuploidy and DNA replication in the normal human brain and Alzheimer’s disease. J Neurosci. 2007;27(26):6859–6867. doi: 10.1523/JNEUROSCI.0379-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iourov IY, Vorsanova SG, Liehr T, Yurov YB. Aneuploidy in the normal, Alzheimer’s disease and ataxia-telangiectasia brain: differential expression and pathological meaning. Neurobiol Dis. 2009a;34(2):212–220. doi: 10.1016/j.nbd.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 56.Arendt T, Brückner MK, Mosch B, Lösche A. Selective cell death of hyperploid neurons in Alzheimer’s disease. Am J Pathol. 2010;177(1):15–20. doi: 10.2353/ajpath.2010.090955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arendt T. Cell cycle activation and aneuploid neurons in Alzheimer’s disease. Mol Neurobiol. 2012;46:125–135. doi: 10.1007/s12035-012-8262-0. [DOI] [PubMed] [Google Scholar]
- 58.Heston LL, Mastri AR. The genetics of Alzheimer’s disease. Arch Gen Psychiatry. 1977;34:976–981. doi: 10.1001/archpsyc.1977.01770200114017. [DOI] [PubMed] [Google Scholar]
- 59.Heston LL, Mastri AR, Anderson VE, White J. Dementia of the Alzheimer type. Arch Gen Psychiatry. 1981;38:1084–1090. doi: 10.1001/archpsyc.1981.01780350019001. [DOI] [PubMed] [Google Scholar]
- 60.Heyman A, Wilkinson W, Hurwitz B, Schmechel D, Sigmon AH, Weinberg T, et al. Alzheimer’s disease: Genetic aspects and associated clinical disorders. Ann Neurol. 1983;14:507–515. doi: 10.1002/ana.410140503. [DOI] [PubMed] [Google Scholar]
- 61.Whalley LJ, Carothers AD, Collyer S, De Mey R, Frackiewicz A. A study of familial factors in Alzheimer’s disease. Br J Psychiatry. 1982;140:249–256. doi: 10.1192/bjp.140.3.249. [DOI] [PubMed] [Google Scholar]
- 62.Amaducci LA, Fratiglioni L, Rocca WA, Fieschi C, Livrea P, Pedone D, et al. Risk factors for clinically diagnosed Alzheimer’s disease: A case-control study of an Italian population. Neurology. 1986;36:922–931. doi: 10.1212/wnl.36.7.922. [DOI] [PubMed] [Google Scholar]
- 63.Chandra V, Philipose V, Bell PA, Lazaroff A, Schoenberg BS. Case-control study of late onset “probable Alzheimer’s disease. Neurology. 1987;37:1295–1300. doi: 10.1212/wnl.37.8.1295. [DOI] [PubMed] [Google Scholar]
- 64.Schupf N, Kapell D, Lee JH, Ottman R, Mayeux R. Increased risk of Alzheimer’s disease in mothers of adults with Down’s syndrome. Lancet. 1994;344(8919):353–356. doi: 10.1016/s0140-6736(94)91398-6. [DOI] [PubMed] [Google Scholar]
- 65.Schupf N, Kapell D, Naghtingale B, Lee JH, Mohlenhoff J, Bewley S, et al. Specificity of the fivefold increase in AD in mothers of adults with Down syndrome. Neurology. 2001;57(6):979–984. doi: 10.1212/wnl.57.6.979. [DOI] [PubMed] [Google Scholar]
- 66.Migliore L, Boni G, Bernardini F, Trippi F, Colognato R, Fontana I, et al. Susceptibility to chromosome malsegregation in lymphocytes of women who had a Down syndrome child in young age. Neurobiol Aging. 2006;27(5):710–716. doi: 10.1016/j.neurobiolaging.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 67.Migliore L, Migheli F, Coppedé F. Susceptibility to aneuploidy in young mothers of Down syndrome children. Scientific World J. 2009;9:1052–1060. doi: 10.1100/tsw.2009.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Migliore L, Coppede F, Fenech M, Thomas P. Association of micronucleus frequency with neurodegenerative diseases. Mutagenesis. 2011;26(1):85–92. doi: 10.1093/mutage/geq067. [DOI] [PubMed] [Google Scholar]
- 69.Fitzgerald PH, Archer SA, Morris CM. Evidence for the repeated primary non-disjunction of chromosome 21 as a result of premature centromere division (PCD) Hum Genet. 1986;72(1):58–62. doi: 10.1007/BF00278818. [DOI] [PubMed] [Google Scholar]
- 70.Spremo-Potparević B, Živković L, Djelić N, Plećas-Solarović B, Smith MA, Bajić V. Analysis of premature centromere division (PCD) of the chromosome 18 in peripheral blood lymphocytes in Alzheimer disease patients. Mech Ageing Develop. 2006;127:892–896. doi: 10.1016/j.mad.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 71.Spremo-Potparević B, Živković L, Djelić N, Plećas-Solarović B, Smith MA, Bajić V. Premature centomere division of the X chromosome in neurons in Alzheimer’s disease. J Neurochem. 2008;106(5):2218–2223. doi: 10.1111/j.1471-4159.2008.05555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ford JH. Spindle microtubular dysfunction in mothers of Down syndrome children. Hum Genet. 1984;68:295–298. doi: 10.1007/BF00292587. [DOI] [PubMed] [Google Scholar]
- 73.Staessen C, Maes AM, Kirsch-Volders M, Susanne C. Is there a predisposition for meiotic non-disjunction that may be detected by mitotic hyperploidy? Clin Genet. 1983;24:184–190. [PubMed] [Google Scholar]
- 74.Schellenberg GD, Bird TD, Wijsman EM, Orr HT, Anderson L, Nemens E, et al. Genetic linkage evidence for a familial Alzheimer’s disease locus on chromosome 14. Science. 1993;258:668–671. doi: 10.1126/science.1411576. [DOI] [PubMed] [Google Scholar]
- 75.Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, Ikeda M, et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature. 1995;375:754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- 76.Levy-Lahad E, Wijsman EM, Nemens E, Anderson L, Goddard KA, Weber JL, et al. A familial Alzheimer’s disease locus on chromosome. Science. 1995a;269:970–973. doi: 10.1126/science.7638621. [DOI] [PubMed] [Google Scholar]
- 77.Levy-Lahad E, Wasco W, Poorkaj P, Ramano Dm, Oshima J, Pettingell WH, et al. Candidate gene for the chromosome 1 familial Alzheimer’s disease locus. Science. 1995b;269:973–977. doi: 10.1126/science.7638622. [DOI] [PubMed] [Google Scholar]
- 78.Rogaev EI, Sherrington R, Rogaeva EA, Levesque G, Ikeda M, Liang Y, et al. Familial Alzheimer’s disease in kindreds with mis-sense mutations in a gene on chromosome 1 related to the Alzheimer’s disease type 3 gene. Nature. 1995;376:775–778. doi: 10.1038/376775a0. [DOI] [PubMed] [Google Scholar]
- 79.Li J, Ma J, Potter H. Identification and expression analysis of a potential familial Alzheimer’s disease gene on chromosome 1 related to AD3. Proc Natl Acad Sci USA. 1995;92:12180–12184. doi: 10.1073/pnas.92.26.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xia D, Watanabe H, Wu B, Lee SH, Li Y, Tsvetkov E, et al. Presenilin-1 knockin mice reveal loss-of-function mechanism for familial Alzheimer’s disease. Neuron. 2015;85:967–981. doi: 10.1016/j.neuron.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li J, Xu M, Zhou H, Ma J, Potter H. Alzheimer presenilins in the nuclear membrane, interphase kinetochores, and centrosomes suggest a role in chromosome segregation. Cell. 1997;90:917–927. doi: 10.1016/s0092-8674(00)80356-6. [DOI] [PubMed] [Google Scholar]
- 82.Annaert WG, Levesque L, Craessaerts K, Dierinck I, Snellings G, Westaway D, et al. Presenilin 1 controls gamma-secretase processing of amyloid precursor protein in pregolgi compartments of hippocampal neurons. J Cell Biol. 1999;147:277–294. doi: 10.1083/jcb.147.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Honda T, Nihonmatsu N, Yasutake K, Ohtake A, Sato K, Tanaka S, et al. Familial Alzheimer’s disease-associated mutations block translocation of full-length presenilin 1 to the nuclear envelope. Neurosci Res. 2000;37:101–111. doi: 10.1016/s0168-0102(00)00106-1. [DOI] [PubMed] [Google Scholar]
- 84.Kimura N, Nakamura SI, Honda T, Takashima A, Nakayama H, Ono F, et al. Age-related changes in the localization of presenilin-1 in cynomolgus monkey brain. Brain Res. 2001;922:30–41. doi: 10.1016/s0006-8993(01)03146-8. [DOI] [PubMed] [Google Scholar]
- 85.Nizzari M, Venezia V, Bianchini P, Caorsi V, Diaspro A, Repetto E, et al. Amyloid precursor protein and presenilin 1 interaction studied by FRET in human H4 cells. Ann NY Acad Sci. 2007;1096:249–257. doi: 10.1196/annals.1397.091. [DOI] [PubMed] [Google Scholar]
- 86.Young-Pearse TL, Suth S, Luth ES, Sawa A, Selkoe DJ. Biochemical and functional interaction of disrupted-in-schizophrenia 1 and amyloid precursor protein regulates neuronal migration during mammalian cortical development. J Neurosci. 2010;30(31):10431–10440. doi: 10.1523/JNEUROSCI.1445-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Judge M, Hornbeck L, Potter H, Padmanabhan J. Mitosis-specific phosphorylation of amyloid precursor protein at threonine 668 leads to its altered processing and association with centrosomes. Mol Neurodegen. 2011;6:80. doi: 10.1186/1750-1326-6-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jeong SJ, Kim HS, Chang KA, Geum DH, Park CH, Seo JH, et al. Subcellular localization of presenilins during mouse preimplantation development. FASEB. 2000;14:2171–2176. doi: 10.1096/fj.99-1068com. [DOI] [PubMed] [Google Scholar]
- 89.Kulnane LS, Lehman EJ, Hock BJ, Tsuchiya KD, Lamb BT. Rapid and efficient detection of transgene homozygosity by FISH of mouse fibroblasts. Mamm Genom. 2002;13(4):223–226. doi: 10.1007/s00335-001-2128-5. [DOI] [PubMed] [Google Scholar]
- 90.Boeras DI, Granic A, Crespo NC, Rojiani AM, Potter H. Alzheimer’s presenilin 1 causes chromosome missegregation and aneuploidy. Neurobiol Aging. 2008;29(3):319–328. doi: 10.1016/j.neurobiolaging.2006.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Granic A, Padmanabhan J, Norden M, Potter H. Alzheimer Aβ peptide induces chromosome mis-segregation and aneuploidy, including trisomy 21: requirement for tau and APP. Mol Biol Cell. 2010;21(4):511–520. doi: 10.1091/mbc.E09-10-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Potter H. Down syndrome and Alzheimer’s disease: two sides of the same coin. Future Neurol. 2008;(1):29–37. 1479–6708. [Google Scholar]
- 93.Small SA, Duff K. Linking Abeta and tau in late-onset Alzheimer’s disease: a dual pathway hypothesis. Neuron. 2008;60(4):534–542. doi: 10.1016/j.neuron.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rapoport M, Dawson HN, Binder LI, Vitek MP, Ferreira A. Tau is essential to beta-amyloid-induced neurotoxicity. Proc Natl Acad Sci USA. 2002;99(9):6364–6369. doi: 10.1073/pnas.092136199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Borysov SI, Granic A, Padmanabhan J, Potter H. Alzheimer Aβ disrupts the mitotic spindle by inhibiting mitotic motors. Cell Cycle. 2011;10(9):1397–1410. doi: 10.4161/cc.10.9.15478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Iourov IY, Vorsanova SG, Liehr T, Kolotii AD, Yurov YB. Increased chromosome instability dramatically disrupts neural genome integrity and mediates cerebellar degeneration in the ataxia-telangiectasia brain. Hum Mol Genet. 2009b;18:2656–2669. doi: 10.1093/hmg/ddp207. [DOI] [PubMed] [Google Scholar]
- 97.Yang Y, Shepherd D, Halliday G. Aneuploidy in Lewy body diseases. Neurobiol Aging. 2015;36(3):1253–1260. doi: 10.1016/j.neurobiolaging.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 98.Rossi G, Dalprà L, Crosti F, Lissoni S, Sciacca FL, Catania M, et al. A new function of microtubule-associated protein tau: involvement in chromosome stability. Cell Cycle. 2008;15:1788–1794. doi: 10.4161/cc.7.12.6012. [DOI] [PubMed] [Google Scholar]
- 99.Rossi G, Conconi D, Panzeri E, Redaelli S, Piccoli E, Paoletta L. Mutations in MAPT gene cause chromosome instability and introduce copy number variations widely in the genome. J Alzheimers Dis. 2013;33:969–982. doi: 10.3233/JAD-2012-121633. [DOI] [PubMed] [Google Scholar]
- 100.Rossi G, Conconi D, Panzeri E, Paoletta L, Piccoli E, Ferretti MG, et al. Mutations in MAPT give rise to aneuploidy in mouse models of tauopathy. Neurogenetics. 2014;15:31–40. doi: 10.1007/s10048-013-0380-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Busciglio J, Yankner BA. Apoptosis and increased generation of reactive oxygen species in Down’s syndrome neurons in vitro. Nature. 1997;378:776–779. doi: 10.1038/378776a0. [DOI] [PubMed] [Google Scholar]
- 102.McConnell MJ, Kaushal D, Yang AH, Kingsbury MA, Rehen SK, et al. Failed clearance of aneuploid embryonic neural progenitor cells leads to excess aneuploidy in the Atm-deficient but not the Trp53-deficient adult cerebral cortex. J Neurosci. 2004;241(37):8090–8096. doi: 10.1523/JNEUROSCI.2263-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rajendran RS, Wellbrock UM, Zupanc GK. Apoptotic cell death, long-term persistence, and neuronal differentiation of aneuploid cells generated in the adult brain of teleost fish. Dev Neurobiol. 2008;68:1257–1268. doi: 10.1002/dneu.20656. [DOI] [PubMed] [Google Scholar]
- 104.Kai Y, Wang CC, Kishigami S, Kazuki Y, Abe S, Takiguchi M, Treunes K, et al. Enhanced apoptosis during early neuronal differentiation in mouse ES cells with autosomal imbalance. Cell Res. 2009;19:247–258. doi: 10.1038/cr.2008.305. [DOI] [PubMed] [Google Scholar]
- 105.Peterson S, Yang AH, Bushman DM, Westra JW, Yung YC, Baral S, et al. Aneuploid cells are differentially susceptible to caspase-mediated death during embryonic cerebral cortical development. J Neurosci. 2012;32(46):16213–16222. doi: 10.1523/JNEUROSCI.3706-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Oromendia AB, Amon A. Aneuploidy: implications for protein homeostasis and disease. Dis Model Mech. 2014;7:15–20. doi: 10.1242/dmm.013391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sparks DL. Coronary artery disease, hypertension, ApoE, and cholesterol: a link to Alzheimer’s disease. Ann NY Acad Sci. 1997;826:128–146. doi: 10.1111/j.1749-6632.1997.tb48466.x. [DOI] [PubMed] [Google Scholar]
- 108.Burns M, Duff K. Cholesterol in Alzheimer’s disease and tauopathy. Ann NY Acad Sci. 2002;977:367–375. doi: 10.1111/j.1749-6632.2002.tb04839.x. [DOI] [PubMed] [Google Scholar]
- 109.Wolozin B. Cholesterol and the biology of Alzheimer’s disease. Neuron. 2004;41:7–10. doi: 10.1016/s0896-6273(03)00840-7. [DOI] [PubMed] [Google Scholar]
- 110.Anstey KJ, Lipnicki DM, Low LF. Cholesterol as a risk factor for dementia and cognitive decline: A systematic review of prospective studies with meta-analysis. Am J Geriatr Psychiatry. 2008;16:343–354. doi: 10.1097/JGP.0b013e31816b72d4. [DOI] [PubMed] [Google Scholar]
- 111.Dolan H, Crain B, Troncoso J, Resnik SM, Zonderman AB, O’Brien R. Atherosclerosis, dementia, and Alzheimer’s disease in the Baltimore Longitudinal Study of Aging Cohort. Ann Neurol. 2010;68:231–240. doi: 10.1002/ana.22055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vance J. Dysregulation of cholesterol balance in the brain: contribution to neurodegenerative disease. Dis Model Mech. 2012;5:746–755. doi: 10.1242/dmm.010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sparks DL, Hunsaker JC, Scheff SW, Kryscio RJ, Henderson JL, Markersbery WR. Cortical senile plaques in coronary artery disease, aging and Alzheimer’s disease. Neurobiol Aging. 1990;11:601–607. doi: 10.1016/0197-4580(90)90024-t. [DOI] [PubMed] [Google Scholar]
- 114.Beach TG, Wilson JR, Sue LI, Newell A, Poston M, Cisneros R. Circle of Willis atherosclerosis: association with Alzheimer’s disease, neuritic plaques and neurofibrillary tangles. Acta Neuropathol. 2007;113:13–21. doi: 10.1007/s00401-006-0136-y. [DOI] [PubMed] [Google Scholar]
- 115.Casalone R, Granata P, Minelli E, Portentoso P, Giudici A, Righi R, et al. Cytogenetic analysis reveals clonal proliferation of smooth muscle cells in atherosclerotic plaques. Hum Genet. 1991;87:139–143. doi: 10.1007/BF00204169. [DOI] [PubMed] [Google Scholar]
- 116.Vanni R, Licheri S. Clonal cytogenetic changes in atherosclerotic plaques including trisomy 20. Dis Markers. 1991;9:81–85. [PubMed] [Google Scholar]
- 117.Matturri L, Cazzullo A, Turconi P, Lavezzi AM. Cytogenetic aspects of cell proliferation in atherosclerotic plaques. Cardiologia. 1997;42:833–836. [PubMed] [Google Scholar]
- 118.Matturri L, Cazzullo A, Turconi P, Lavezzi AM, Vandone PR, Gabrielli L, et al. Chromosomal alterations in atherosclerotic plaques. Atherosclerosis. 2001;154:755–761. doi: 10.1016/s0021-9150(00)00488-3. [DOI] [PubMed] [Google Scholar]
- 119.Murry CE, Gipaya CT, Bartosek T, Benditt EP, Schwartz SM. Monoclonality of smooth muscle cells in human atherosclerosis. Am J Pathol. 1997;151:697–705. [PMC free article] [PubMed] [Google Scholar]
- 120.Andreassi MG, Botto N, Colombo MG, Biagini A, Clerico A. Genetic instability and atherosclerosis: can somatic mutations account for the development of cardiovascular diseases? Environ Mol Mutagen. 2000;35:265–269. doi: 10.1002/1098-2280(2000)35:4<265::aid-em1>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 121.Miniati P, Sourvinos G, Michalodimitrakis M. Spandidos DA of heterozygosity on chromosomes 1, 2, 8, 9 and 17 in cerebral atherosclerotic plaques. Int J Biol Markers. 2001;16:167–171. doi: 10.1177/172460080101600302. [DOI] [PubMed] [Google Scholar]
- 122.Granic A, Potter H. Cholesterol induces mitotic spindle disruption and chromosome mis-segregation—implications for Niemann Pick, cardiovascular, and Alzheimer’s disease. PLoS One. 2013;8(4):e60718. doi: 10.1371/journal.pone.0060718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jin LW, Maezawa I, Vincent I, Bird T. Intracellular accumulation of amyloidogenic fragments of amyloid-b precursor protein in neurons with niemann-pick type c defects is associated with endosomal abnormalities. Am J Pathol. 2004;164(3):975–985. doi: 10.1016/s0002-9440(10)63185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Granic A, Potter H. Down syndrome model of Alzheimer’s disease: beyond trisomy 21 nondisjunction. In: Dey S, editor. Genetics and etiology of Down syndrome. Rijeka: In Tech; 2011. pp. 159–176. [Google Scholar]
- 125.Rehen SK, McConnell MJ, Kaushal D, Kingsbury MA, Yang AH, Chun J. Chromosomal variation in neurons of the developing and adult mammalian nervous system. Proc Natl Acad Sci USA. 2001;98:13361–13366. doi: 10.1073/pnas.231487398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yang Y, Geldmacher DS, Herrup K. DNA replication precedes neuronal cell death in Alzheimer’s disease. J Neurosci. 2001;21:2661–2668. doi: 10.1523/JNEUROSCI.21-08-02661.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Arendt T, Holzer M, Grossmann A, Zedlick D, Brückner MK. Increased expression and subcellular translocation of the mitogen activated protein kinase and mitogen-activated protein kinase in Alzheimer’s disease. Neuroscience. 1995;68:5–18. doi: 10.1016/0306-4522(95)00146-a. [DOI] [PubMed] [Google Scholar]
- 128.Arendt T, Rodel L, Gartner U, Holzer M. Expression of the cyclin-dependent kinase inhibitor p16 in Alzheimer’s disease. Neuro Report. 1996;7:3047–3049. doi: 10.1097/00001756-199611250-00050. [DOI] [PubMed] [Google Scholar]
- 129.Smith TW, Lippa CF. TI Ki-67 immunoreactivity in Alzheimer’s disease and other neurodegenerative disorders. J Neuropathol Exp Neurology. 1995;54:297–303. doi: 10.1097/00005072-199505000-00002. [DOI] [PubMed] [Google Scholar]
- 130.Vincent I, Rosado M, Davies P. Mitotic mechanisms in Alzheimer’s disease? J Cell Biol. 1996;132:413–425. doi: 10.1083/jcb.132.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vincent I, Jicha G, Rosado M, Dickson DW. Aberrant expression of mitotic Cdc2/cyclin B1 kinase in degenerating neurons of Alzheimer’s disease brain. J Neurosci. 1997;17:3588–3598. doi: 10.1523/JNEUROSCI.17-10-03588.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.McShea A, Harris PLR, Webster KR, Wahl AF, Smith MA. Abnormal expression of the cell cycle regulators P16 and CDK4 in Alzheimer’s disease. Am J Pathol. 1997;150:1933–1939. [PMC free article] [PubMed] [Google Scholar]
- 133.Song J, Wang S, Tan M, Jia J. G1/S checkpoint proteins in peripheral blood lymphocytes are potentially diagnostic biomarkers for Alzheimer’s disease. Neurosci Lett. 2012;526(2):144–1449. doi: 10.1016/j.neulet.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 134.Majd S, Zarifkar A, Rastegar K, Takhshid MA. Different fibrillar Abeta 1-42 concentrations induce adult hippocampal neurons to reenter various phases of the cell cycle. Brain Res. 2008;1218:224–229. doi: 10.1016/j.brainres.2008.04.050. [DOI] [PubMed] [Google Scholar]
- 135.Seward ME, Swanson E, Norambuena A, Reimann A, Cochran JN, Li R, et al. Amyloid-beta signals through tau to drive ectopic neuronal cell cycle re-entry in Alzheimer’s disease. J Cell Sci. 2013;126(Pt 5):1278–1286. doi: 10.1242/jcs.1125880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Absalon S, Kochanek DM, Raghavan V, Krichevsky AM. MiR-26b, upregulated in Alzheimer’s disease, activates cell cycle entry, tau-phosphorylation, and apoptosis in postmitotic neurons. J Neurosci. 2013;33(37):14645–14659. doi: 10.1523/JNEUROSCI.1327-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lopes JP, Blurton-Jones M, Yamasaki TR, Agostinho P, LaFerla FM. Activation of cell cycle proteins in transgenic mice in response to neuronal loss but not amyloid-beta and tau pathology. J Alzheimers Dis. 2009;16:541–549. doi: 10.3233/JAD-2009-0993. [DOI] [PubMed] [Google Scholar]
- 138.Altman J, Das GD. Autoradiographic and histologic evidence of postnatal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 139.Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977;197:1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- 140.Bayer SA, Yackel JW, Puri PS. Neurons in the rat dentate gyrus granular layer substantially increase during juvenile and adult life. Science. 1982;216:890–892. doi: 10.1126/science.7079742. [DOI] [PubMed] [Google Scholar]
- 141.Cameron HA, Woolley CS, McEwen BS, Gould E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993;56:337–344. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- 142.Kaplan MS, Bell DH. Mitotic neuroblasts in the 9-day-old and 11-month-old rodent hippocampus. J Neurosci. 1984;4:1429–1441. doi: 10.1523/JNEUROSCI.04-06-01429.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kuhn GH, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16(6):2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gould E, Reeves AJ, Graziano MS, Gross CG. Neurogenesis in the neocortex of adult primates. Science. 1999a;286:548–52. doi: 10.1126/science.286.5439.548. [DOI] [PubMed] [Google Scholar]
- 145.Gould E, Reeves AJ, Fallah M, Tanapat P, Gross CG, Fuchs E. Hippocampal neurogenesis in adult Old World primates. Proc Natl Acad Sci USA. 1999b;96:5263–7. doi: 10.1073/pnas.96.9.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mu Y, Gage FH. Adult hippocampal neurogenesis and its role in Alzheimer’s disease. Mol Neurodegen. 2011;6:85. doi: 10.1186/1750-1326-6-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ernst A, Alkass K, Bernard S, Salehpour M, Perl S, Tisdale J, et al. Neurogenesis in the striatum of the adult human brain. Cell. 2014;156(5):1072–1083. doi: 10.1016/j.cell.2014.01.044. [DOI] [PubMed] [Google Scholar]
- 148.Zhou L, Del Villar K, Dong Z, Miller CA. Neurogenesis response to hypoxia-induced cell death: map kinase signal transduction mechanisms. Brain Res. 2004;1021(1):8–19. doi: 10.1016/j.brainres.2004.05.115. [DOI] [PubMed] [Google Scholar]
- 149.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 150.Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, et al. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153:1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.LeBlank A. Increased production of 4KDa amyloid beta peptide in serum-deprived human primary neuron cultures: possible involvement of apoptosis. J Neurosci. 1995;15:7837–7846. doi: 10.1523/JNEUROSCI.15-12-07837.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Galli C, Piccini A, Ciotti MT, Castellani L, Calissano P, Zaccheo D, et al. Increased amyloidogenic secretion in cerebellar granule cells undergoing apoptosis. Proc Natl Acad Sci USA. 1998;95:1247–1252. doi: 10.1073/pnas.95.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bushman DM, Kaeser GE, Siddoway B, Westra JW, Rivera RR, Rehen SK, et al. Genomic mosaicism with increased amyloid precursor protein (APP) gene copy number in single neurons from sporadic Alzheimer’s disease brains. Elife. 2015;4 doi: 10.7554/eLife.05116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Griffin WST, Stanley LC, Ling C, White L, MacLeod V, Perrot LJ, et al. Brain interleukin-1 and S100 immunoreactivity elevated in Down’s syndrome and Alzheimer’s disease. Proc Natl Acad Sci USA. 1989;86:7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Das S, Potter H. Expression of the Alzheimer amyloid-promoting factor antichymotrypsin is induced in human astrocytes by IL-1. Neuron. 1995;14:447–456. doi: 10.1016/0896-6273(95)90300-3. [DOI] [PubMed] [Google Scholar]
- 156.Parry EM, Parry JM, Corso C, Doherty A, Haddad F, Hermine TF, et al. Detection and characterization of mechanisms of action of aneugenic chemicals. Mutagenesis. 2002;17(6):509–521. doi: 10.1093/mutage/17.6.509. [DOI] [PubMed] [Google Scholar]
- 157.Hernández LG, van Benthem J, Johnson GE. A mode-of-action approach for the identification of genotoxic carcinogens. PLoS One. 2013;8(5):e64532. doi: 10.1371/journal.pone.0064532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nunes PV, Forlenza OV, Gattaz WF. Lithium and risk for Alzheimer’s disease in elderly patients with bipolar disorder. Br J Psychiatry. 2007;190:359–360. doi: 10.1192/bjp.bp.106.029868. [DOI] [PubMed] [Google Scholar]
- 159.Kessing LV, Søndergård L, Forman JL, Andersen PK. Lithium treatment and risk of dementia. Arch Gen Psychiatry. 2008;65(11):1331–1335. doi: 10.1001/archpsyc.65.11.1331. [DOI] [PubMed] [Google Scholar]
- 160.Forlenza OV, Diniz BS, Radanovic M, Santos FS, Talib LL, Gattaz WF. Disease-modifying properties of long-term lithium treatment for amnestic mild cognitive impairment: randomised controlled trial. Br J Psychiatry. 2011;198(5):351–356. doi: 10.1192/bjp.bp.110.080044. [DOI] [PubMed] [Google Scholar]
- 161.Alvarez G, Munoz-Montano JR, Satrustegui J, Avila J, Bogonez E, Diaz-Nido J. Regulation of tau phosphorylation and protection against beta-amyloid-induced neurodegeneration by lithium. Possible implications for Alzheimer’s disease. Bipolar Disord. 2002;4(3):153–165. doi: 10.1034/j.1399-5618.2002.01150.x. [DOI] [PubMed] [Google Scholar]
- 162.Zhang X, Heng X, Li T, Li L, Yang D, Zhang X, et al. Long-term treatment with lithium alleviates memory deficits and reduces amyloid-β production in an aged Alzheimer’s disease transgenic mouse model. J Alzheimers Dis. 2011;24(4):739–749. doi: 10.3233/JAD-2011-101875. [DOI] [PubMed] [Google Scholar]
- 163.Hampel H, Ewers M, Burger K, Annas P, Mortberg A, Bogstedt A, et al. Lithium trial in Alzheimer’s disease: a randomized, single-blind, placebo-controlled, multicenter 10-week study. J Clin Psychiatry. 2009;70(6):922–931. [PubMed] [Google Scholar]
- 164.Nunes MA, Viel TA, Buck HS. Microdose lithium treatment stabilized cognitive impairment in patients with Alzheimer’s disease. Curr Alzheimer Res. 2013;10(1):104–107. doi: 10.2174/1567205011310010014. [DOI] [PubMed] [Google Scholar]
- 165.Takashima A. GSK-3 is essential in the pathogenesis of Alzheimer’s disease. J Alzheimer’s Dis. 2006;9:309–317. doi: 10.3233/jad-2006-9s335. [DOI] [PubMed] [Google Scholar]
- 166.Kremer A, Louis JV, Jaworski T, Van Leuven F. GSK3 and Alzheimer’s disease: facts and fiction. Front Mol Neurosci. 2011;4:1–10. doi: 10.3389/fnmol.2011.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.King MK, Pardo M, Cheng Y, Downey K, Jope RS, Beurel E. Glycogen synthase kinase-3 inhibitors: rescuers of cognitive impairments. Pharmacol Ther. 2014;141(1):1–12. doi: 10.1016/j.pharmthera.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Abisambra JF, Fiorelli T, Padmanabhan J, Neame P, Wefes I, Potter H. LDLR expression and localization are altered in and in vivo and in vitro models of Alzheimer’s disease. PLoS One. 2010;5:e8556. doi: 10.1371/journal.pone.0008556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ari A, Borysov SI, Wu J, Padmanabhan J, Potter H. Alzheimer Ab inhibition of Eg5/kinesin5 disrupts neurotrophin and neurotransmitter receptor localization and function. Neurobiol Aging. 2014;35:1839–1849. doi: 10.1016/j.neurobiolaging.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Nadar VC, Ketschek A, Myers KA, Gallo G, Baas PW. Kinesin-5 is essential for growth-cone turning. Curr Biol. 2008;18:1972–1977. doi: 10.1016/j.cub.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Falnikar A, Tole S, Baas PW. Kinesin-5, a mitotic microtubule-associated motor protein, modulates neuronal migration. Mol Biol Cell. 2011;2:1561–1574. doi: 10.1091/mbc.E10-11-0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kahn OI, Sharma V, González-Billault C, Baas PW. Effects of kinesin-5 inhibition on dendritic architecture and microtubule organization. Mol Biol Cell. 2015;26(1):66–77. doi: 10.1091/mbc.E14-08-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]