Abstract
Background
Extracorporeal membrane oxygenation (ECMO) provides a rescue for children with severe cardiac failure. It has previously been shown that triiodothyronine (T3) improves cardiac function by modulating pyruvate oxidation during weaning. This study focused on fatty acid (FA) metabolism modulated by T3 for weaning from ECMO after cardiac injury.
Methods and Results
Nineteen immature piglets (9.1–15.3 kg) were separated into 3 groups with ECMO (6.5 h) and wean: normal circulation (Group-C); transient coronary occlusion (10 min) for ischemia-reperfusion (IR) followed by ECMO (Group-IR); and IR with T3 supplementation (Group-IR-T3). 13-Carbon (13C)-labeled lactate, medium-chain and long-chain FAs, was infused as oxidative substrates. Substrate fractional contribution (FC) to the citric acid cycle was analyzed by 13C-nuclear magnetic resonance. ECMO depressed circulating T3 levels to 40% of the baseline at 4 h and were restored in Group-IR-T3. Group-IR decreased cardiac power, which was not fully restorable and 2 pigs were lost because of weaning failure. Group-IR also depressed FC-lactate, while the excellent contractile function and energy efficiency in Group-IR-T3 occurred along with a marked FC-lactate increase and [adenosine triphosphate]/[adenosine diphosphate] without either decreasing FC-FAs or elevating myocardial oxygen consumption over Group-C or -IR.
Conclusions
T3 releases inhibition of lactate oxidation following IR injury without impairing FA oxidation. These findings indicate that T3 depression during ECMO is maladaptive, and that restoring levels improves metabolic flux and enhances contractile function during weaning.
Keywords: ECMO, Myocardial metabolism, Pediatric
Extracorporeal membrane oxygenation (ECMO) is commonly used in children with postoperative cardiopulmonary failure and offers a route to recovery.1,2 Veno-arterial ECMO for cardiac support unloads the ventricles and reduces oxygen consumption requirements, while maintaining systemic pressure and oxygen delivery. Theoretically, readjustment of the balance between energy production and utilization allows the heart to rest and recover. However, rates for weaning from the ECMO circuit for infants with cardiac failure still remain low, and overall mortality exceeds 40%.3,4 Despite the positive effects, ventricular unloading through ECMO promotes an inflammatory response, which has a deleterious impact on the failing heart.5,6 The inflammatory cytokine surge associated with ECMO produces a disruption of thyroid hormone homeostasis production, which results in low circulating levels of triiodothyronine (T3).7 Moreover, T3 decreases in children undergoing cardiac surgery for congenital heart disease.8 A deficit in myocardial stimulation by T3 is known to impair oxidative metabolism and adenosine triphosphate (ATP) synthesis, and affects arterial stiffening and cardiac contractility.9,10
Accordingly, we have pursued a hypothesis that these metabolic disturbances impair ATP production and limit the ability of the heart on ECMO to re-establish contractile function. We further postulated that manipulation of substrate supply or hormonal readjustment such as T3 replacement would improve contractile function. We have followed a systematic experimental plan in a porcine model to carefully dissect out the important factors determining substrate utilization both before and after weaning from the ECMO circuit. Initially, we found that ventricular unloading by ECMO preserved metabolic flexibility and the myocardium’s ability to respond to a high carbohydrate loading by increasing pyruvate oxidation.6 Under ECMO conditions using supraphysiological concentrations of pyruvate in the coronary artery supply,6 T3 supplementation promoted pyruvate flux through the citric acid cycle (CAC).11 With more physiological substrate supply consistent with clinical management, ECMO shifted substrate preference towards free fatty acids (FAs).12 Furthermore, successful weaning from ECMO required the flexibility to access both medium-chain and long-chain FAs (MCFAs and LCFAs) for oxidation and ATP production.13
ECMO frequently provides support after ischemia-reperfusion (IR) injury, which is associated with cardiac surgery or arrest. We also demonstrated that both pyruvate loading and T3 supplementation promoted pyruvate flux through the CAC and improved cardiac contractile function after ischemia, which is associated with cardiopulmonary bypass.9,14 Finally, thyroid hormone re-established the ability of the heart to increase pyruvate oxidative flux during weaning from ECMO support for IR injury.15 Enhanced pyruvate flux occurred in conjunction with improved myocardial oxidative capacity and contractile function. The porcine studies were performed with the experiments using a high supraphysiological pyruvate concentration within the coronary arteries. Results from those studies also suggest that the heart relies heavily on an alternate undefined substrate during weaning. Free FAs, both MCFAs and LCFAs, are used clinically for nutritional support during ECMO,16 and at least theoretically provide efficient carbon substrate sources for ATP production. Therefore, we tested the hypothesis that thyroid hormone modulates pyruvate and FA oxidation under relatively physiological substrate conditions during weaning from ECMO.
Methods
Animal Models
Nineteen male Yorkshire piglets (body weight 9.1–15.3 kg, age 30–57 days) were used for the experiments. All experimental procedures were approved by Seattle Children’s Institutional Animal Use Committee. The piglets were premedicated with an intramuscular injection of ketamine (33 mg/kg) and xylazine (2 mg/kg). After intubation through surgical tracheostomy, the piglets were mechanically ventilated with FiO2 40–60%, volume control 15 ml/kg, PEEP 3 cmH2O, respiratory rate 14–18/min and isoflurane 1–2% to maintain general anesthesia.
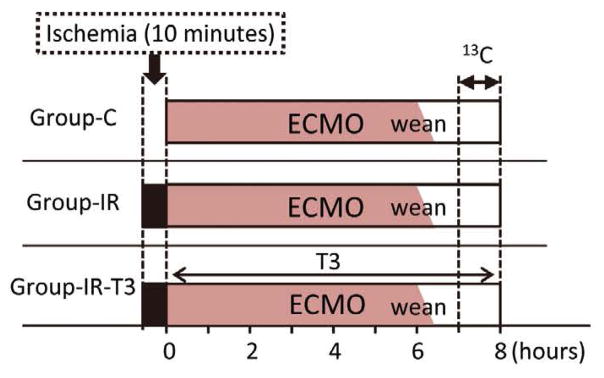
Pigs were randomly divided into 3 study groups that underwent ECMO and weaning (Figure 1). Pigs in Group-IR and -IR-T3 were exposed to IR injury prior to ECMO. T3 (Sigma, St. Louis, MO, USA) was administrated at a bolus of 0.6 μg/kg and then continuous infusion was administered at a rate of 0.2 μg·kg−1·h−1 in Group-IR-T3. As control group, pigs did not receive either IR injury or T3 supplementation (Group-C). The ECMO duration was for 6.5 h, and the study endpoints of each group were at 1.5 h after weaning from ECMO. Plasma T3 concentrations were measured using commercial kits (Endocrine Technology, Newark, CA, USA).
Figure 1.
Diagram of the experimental protocols. Three protocols were designed. The duration of 13-Carbon (13C)-labeled substrate infusion was 60 min. Hemodynamic measurements at the endpoint were performed near labeled substrate infusion completion. Samples of left ventricular tissue were collected at the end of the infusion.
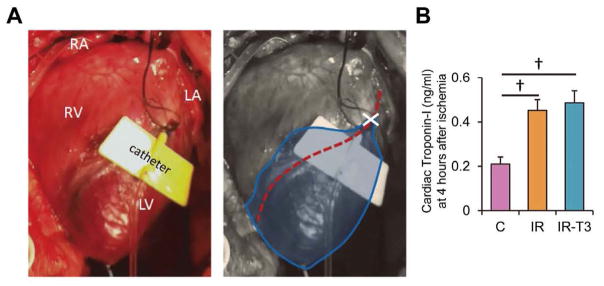
After the performance of a median sternotomy, aortic flow and coronary flow probes (TS420; Transonic Systems Inc, Ithaca, NY, USA) and a left ventricular (LV) pressure catheter (Millar Instruments, Houston, TX, USA) were organized and then a 24-gauge BD Saf-T-Infusion catheter (Becton Dickinson, Sandy, UT, USA) was advanced just distal to the origin of the first branch from the left anterior descending coronary artery (LAD), as previously described.6,9,12 Coronary arterial occlusion was created by LAD ligation with a 4-0 monofilament sutures for 10 min, and then the suture was removed. Occlusion was visually confirmed by the change of color of the myocardium. We performed these experiments in immature piglets exposed to 10 min of ischemia created by LAD occlusion (Figure 2A). Our prior work showed that although coronary flow is fully re-established, this duration of ischemia causes high mortality due to cardiac contractile failure and marked contractile dysfunction in the survivors.15
Figure 2.
Ischemic injury area. (A) Piglets were exposed to 10 min of coronary occlusion by left anterior descending coronary artery (LAD) ligation. Ischemic injury area is surrounded by a blue line. Red dot line, LAD; X mark, ligation point of LAD. A catheter with yellow wings was inserted into the LAD for the infusion of 13-Carbon (13C)-labeled substrates. RA, right atrium; LA, left atrium; RV, right ventricle; LV, left ventricle. (B) Plasma cardiac troponin-I concentration at 4 h after starting ECMO. Values are presented as mean ± SE; n=5–6 per group. †P<0.01 vs. Group-C. Group-C, control group.
ECMO consisted of a roller peristaltic pump console (Sarns 8000; Terumo, Tokyo, Japan) and a hollow fiber membrane oxygenator (CX-RX05RW; Terumo, Tokyo, Japan). The circuit was primed with dextran 40 in 0.9% sodium chloride, 5% dextrose and 2,000 units of heparin. The total prime volume was approximately 80 ml. A veno-arterial ECMO circuit was established by central cannulation into the ascending aorta and right atrium. Management during ECMO kept the pump flow rates at 80–100ml·kg−1·min−1. We maintained a pH of 7.35–7.45, an arterial pCO2 of 35–45 mmHg, an arterial pO2 of >120 mmHg, and a rectal temperature of 36–37.5°C. The ECMO duration time was 6.5 h. Perfusion flow of ECMO was decreased gradually for 30 min and then ECMO was weaned. The animals did not receive any blood transfusions or inotropic or vasoactive drugs.
Myocardial Oxygen Consumption and Cardiac Parameters
Cardiac output (CO) was measured by aortic flow meter directly. Cardiac power [Watt] was calculated as the mean arterial pressure ×CO/451, where mean arterial pressure=(systolic pressure+2×diastolic pressure)/3. Myocardial oxygen consumption (MV̇O2) [μmol·min−1·g−1] was calculated from the coronary flow and the difference in oxygen content of systemic artery and coronary venous. Cardiac energy efficiency [%] was calculated as the cardiac power/MV̇O2. For this formula, MV̇O2 was converted to Joules [J/min] using the conversion of 1 μmol O2=0.4478 J, as described by Suga.17
Evaluation of Myocardial Injury Levels
As an index of ischemic myocardial injury, the plasma cardiac troponin-I concentration was measured at 4 h after starting ECMO, using a commercially available ELISA kit (Life Diagnostics, West Chester, PA, USA).
Metabolic Analysis
We examined substrate utilization to the CAC using 13-Carbon (13C)-labeled substrates and nuclear magnetic resonance (NMR) analysis, as previously described.12 We analyzed the central portion of the myocardial ischemic injured zone in strict concordance with the perfused area of 13C-substrates for these metabolic studies. [2-13C]lactate and [2,4,6,8-13C]octanoate, MCFA (Sigma St. Louis, MO, USA) and [U-13C] LCFAs (Cambridge Isotope Laboratories, Andover, MA, USA) were used as the metabolic markers. [U-13C]LCFAs consist of palmitic acid (45–55%), palmitoleic acid (10–15%), oleic acid (20–30%) and linoleic acid (10–15%). Each 13C-labeled substrate labels 13C-labeled acetyl-CoA differently, and the pattern is reflected by the pattern of 13C-glutamate in the NMR spectrum.
Infusion of labeled Substrates
The 13C-labeled substrates were infused directly into the LAD for the final 60 min of the protocol. Based on coronary artery flow, intracoronary concentrations of infusing labeled substrates were adjusted to 1.2mmol/L [2-13C]lactate, 0.1 mmol/L [2,4,6,8-13C]octanoate and 0.1 mmol/L [U-13C]LCFAs to closely match the physiological concentration. Immediately upon completion of the 13C-labeled substrates infusion, portions of LV myocardial tissue perfused by the LAD were quickly freeze-clamped and stored at −80°C for later extraction.
NMR
13C-NMR was performed on the myocardium for the determination of specific carbon glutamate labeling.18 We used methanol extraction for the LV tissue, and NMR spectra were acquired on a Varian Direct Drive (VNMRS) 600 MHz spectrometer (Varian Inc, Palo Alto, CA, USA), as previously described.6,9,12 The labeled carbon resonances (C3–C5) of glutamate were integrated using commercial software (NUTS; Acorn NMR, Livermore, CA, USA). The individual integral values were used as starting parameters for the CAC analysis-fitting algorithm, tcaCALC, kindly provided by Drs C. R. Malloy and F. M. Jeffrey.19 This algorithm provided the fractional enrichment for each substrate to the acetyl-CoA pool entering the CAC. Energy metabolites, lactate and pyruvate, were also measured by 1H-NMR spectra from LV tissues prepared by using methanol, as previously described.12 Collected spectra were analyzed using Chenomx 7.6 software (Edmonton, Alberta, Canada), with quantifications based on spectral intensities relative to 0.5 mmol/L 2,2-dimethyl-2-silapentane-5-sulfonate, which was added as a spike to each sample.
Western Blotting
Immunoblot analyses were used to evaluate the expression of key proteins regulating substrate oxidation. Fifty micrograms of total protein extract from the heart tissue were electrophoresed through 4.5% stacking and 10% running SDS-polyacrylamide gels, and electroblotted onto PDVF-plus membranes. Membranes were blocked for 1 h at room temperature with 5% non-fat milk in Tris-buffered saline plus Tween-20 (TBST: 10 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, and 0.05% Tween-20). Equal protein loading of samples was determined by a protein assay (BioRad, Hercules, CA, USA) and confirmed by using a reversible protein stain kit for PVDF membranes (Thermo Scientific, Rockford, IL, USA). The primary antibodies used in this study were 5′ adenosine monophosphate-activated protein kinase α (AMPKα), phospho-AMPKα-Thr172, acetyl-CoA carboxylase (ACC), phospho-ACC-Ser79 and pyruvate dehydrogenase (PDH), obtained from Cell Signaling Technology (Danvers, MA, USA), and phospho-PDH-Ser293, which was obtained from Millipore (Billerica, MA, USA). Blots were incubated at room temperature for 1 h with the appropriate secondary antibody conjugated to horseradish peroxidase. The blots were visualized with enhanced chemiluminescence after exposure to Kodak Biomax light ML-2 film. The densitometric intensities were determined using Image J analysis software (National Institutes of Health, Bethesda, MD, USA). Western blots were repeated in triplicate to confirm the findings.
Statistical Analyses
Reported values are mean ± standard error (SE) in figures, text, and tables. All data were evaluated by one-way ANOVA with Tukey’s post-hoc test. The criterion for significance was P<0.05 for all comparisons.
Results
Survivors and Validation of Ischemic Injury
We directly observed color change as demarcated in Figure 2A during coronary constriction. Survival was determined by the ability to wean from the ECMO and maintain circulation without support for 1 h. All animals survived except for 2 in the IR group, leaving Control 5/5, IR 6/8 and IR-T3 6/6 for further analyses. Further validation of ischemic injury was provided by plasma troponin-I concentrations, which increased significantly in our ischemic groups. Plasma cardiac troponin-I concentrations in Group-IR and Group-IR-T3 were 0.45± 0.05 and 0.49±0.05 ng/ml, respectively, at 4 h after starting ECMO, and were significantly higher than that in Group-C (0.21±0.03ng/ml, P<0.01; Figure 2B).
Plasma T3 Levels
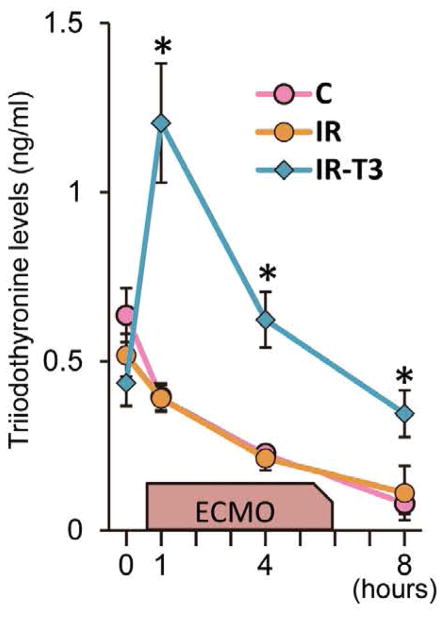
We measured plasma T3 levels before ischemic injury and ECMO as a baseline, at 1 and 4 h after starting ECMO, and just before completion of the labeled infusion as an endpoint. The T3 levels in Group-C and Group-IR gradually dropped off after starting ECMO, whereas they were maintained at baseline levels until the end of the protocol for Group-IR-T3 (Figure 3).
Figure 3.
Plasma triiodothyronine (T3) levels. In Group-C and Group-IR, plasma T3 levels were decreased gradually after starting extracorporeal membrane oxygenation (ECMO). T3 supplementation significantly prevented the reduction of total T3 levels. Values are presented as mean ± SE; n=5–6 per group. *P<0.05 vs. Group-C and Group-IR. Group-C, control group; Group-IR, Ischemia-reperfusion injury group.
Cardiac Function
Baseline hemodynamics were not significantly different among the 3 groups (Table 1). During ECMO, the LV end-diastolic pressure significantly dropped below 4 mmHg in all animals. Table 2 showed parameters of cardiac function relative to baseline at the endpoint of each protocol. Hemoglobin, heart rate and mean systemic blood pressure at the endpoint of the protocol were not significantly different among the 3 groups. As noted in previous publications, hemoglobin dropped slightly due to the lack of blood pump prime, and ranged it between 6.7 and 8.8 g/dl in all animals.12,13 IR produced low CO (P=0.06 vs. Group-C), whereas Group-IR-T3 surpassed Group-C in CO and cardiac power. MV̇O2 was similar among all groups. The cardiac energy efficiency in Group-IR-T3 was also higher than that in Group-IR (P=0.03).
Table 1.
Parameters of Baseline Cardiac Function Just After Cannulation for ECMO
| Group-C (n=5) | Group-IR (n=6) | Group-IR-T3 (n=6) | |
|---|---|---|---|
| Hemoglobin (g/dl) | 9.3±0.9 | 9.1±0.3 | 9.5±0.5 |
| HR (beats/min) | 117±4 | 105±5 | 111±8 |
| Mean SBP (mmHg) | 57±3 | 63±3 | 61±3 |
| LVEDP (mmHg) | 7±1 | 7±1 | 6±1 |
| CO (L/min) | 1.14±0.08 | 1.05±0.13 | 1.04±0.04 |
| Power (Watt) | 0.20±0.01 | 0.21±0.04 | 0.20±0.01 |
| MV̇O2 (μmol·min−1·g−1) | 3.79±0.28 | 4.36±0.43 | 3.61±0.43 |
| Efficiency (%) | 24.9±3.2 | 21.2±4.3 | 21.6±1.5 |
There were no significant differences among groups. Values are presented as mean ± SE.
CO, cardiac output; ECMO, extracorporeal membrane oxygenation; Group-C, control group; Group-IR, Ischemia-reperfusion group; Group-IR-T3, T3 treatment group; HR, heart rate; LVEDP, left ventricular end-diastolic pressure; MV̇O2, myocardial oxygen consumption; SBP, systemic blood pressure.
Table 2.
Parameters of Cardiac Function at the End of the Protocol
| Group-C (n=5) | Group-IR (n=6) | Group-IR-T3 (n=6) | |
|---|---|---|---|
| Survival | 5/5 | 6/8 | 6/6 |
| Hemoglobin (g/dl) | 7.4±0.8 | 7.0±0.4 | 7.5±0.5 |
| HR (beats/min) | 129±10 | 133±7 | 133±8 |
| Mean SBP (mmHg) | 49±3 | 51±5 | 58±6 |
| LVEDP (mmHg) | 7±1 | 9±1 | 6±1† |
| CO (% of baseline) | 89.7±7.9 | 85.3±7.7 | 114.8±9.2† |
| Power (% of baseline) | 81.9±10.3 | 71.9±12.4 | 117.9±9.5*,† |
| MV̇O2 (μmol·min−1·g−1) | 3.76±0.43 | 2.72±0.41 | 2.93±0.70 |
| Efficiency (%) | 23.3±5.9 | 18.9±3.0 | 29.3±2.7† |
Values are presented as mean ± SE.
P<0.05 vs. Group-C,
P<0.05 vs. Group-IR.
Abbreviations as in Table 1.
High Energy Phosphate Metabolism
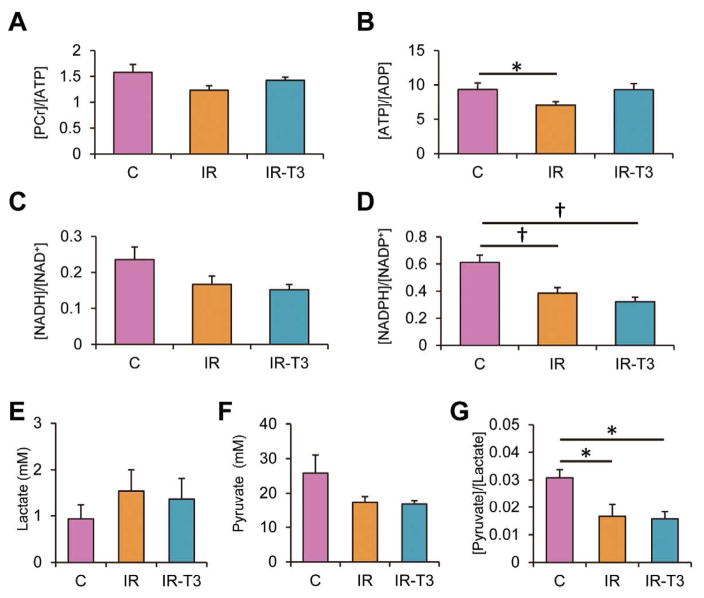
We used 1H-NMR to determine myocardial energy metabolite ratios following weaning and identified modest though not significant decreases in [Phosphocreatine; PCr]/[ATP] in survivors (Figure 4A). These changes were partially reversed with T3 supplementation. This ratio can be increased by depletion of the ATP pool. We therefore also analyzed [ATP]/[adenosine diphosphate: ADP]; another index for phosphorylation potential (Figure 4B). IR injury significantly decreased this index, with reversal by T3 supplementation. Therefore, the data, which included only survivors, suggests that T3 at least partially reverses abnormalities in high energy phosphate metabolism induced by IR injury. The [reduced nicotinamide adenine dinucleotide: NADH]/[oxidized nicotinamide adenine dinucleotide: NAD] and [reduced nicotinamide adenine dinucleotide phosphate: NADPH]/[oxidized nicotinamide adenine dinucleotide phosphate: NADP] data are indicators of a cellular redox state (Figures 4C, D). Ischemia-reperfusion decreases these ratios, though only reaching significance for [NADPH]/[NADP]. Ischemic injury tended to increased lactate concentration of LV tissues compared with non-ischemia, and T3 did not influence in lactate and pyruvate accumulations (Figures 4E, F). The [Pyruvate]/[Lactate] ratio (Figure 4G), another surrogate for redox state, decreases with ischemia, with no reversal by T3.
Figure 4.
Energy metabolites by 1H-NMR. Myocardial [PCr]/[ATP] and [ATP]/[ADP], and [NADH]/[NAD+] and [NADPH]/[NADP+] changed among the 3 groups, respectively (A–D). Group-IR demonstrated significantly lower [ATP]/[ADP] and [NADPH]/[NADP+] than Group-C, and T3 improved [ATP]/[ADP]. Ischemic injury increased the lactate concentration of left ventricular tissues compared with non-ischemia, but this did not reach statistical significance (E). The pyruvate concentration of left ventricular tissue was not statistically different among the 3 groups (F). The ratio of [Pyruvate]/[Lactate] in Group-IR and Group-IR-T3 was significantly decreased compared with Group-C (G). Values are presented as mean ± SE; n=5–6 per group. *P<0.05, †P<0.01 vs. Group-C. ATP, adenosine triphosphate; ADP, adenosine diphosphate; NADH, reduced nicotinamide adenine dinucleotide; NAD, oxidized nicotinamide adenine dinucleotide; NADPH, reduced nicotinamide adenine dinucleotide phosphate; NADP+, oxidized nicotinamide adenine dinucleotide phosphate; Group-C, control group; Group-IR, Ischemia-reperfusion group; Group-IR-T3, T3 treatment group; PCr, Phosphocreatine; T3, triiodothyronine.
Substrate Metabolism
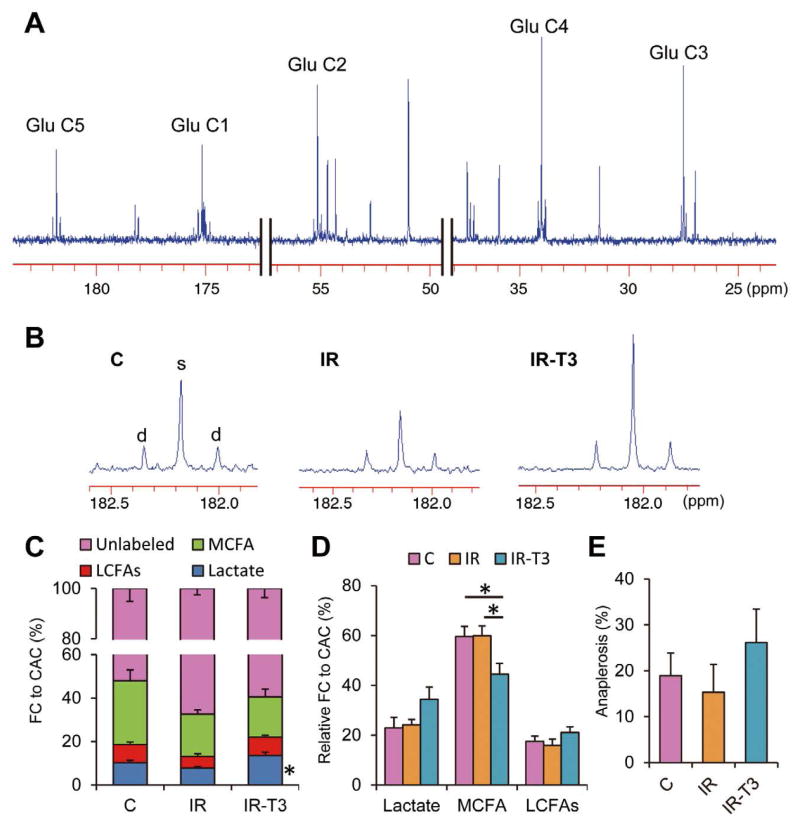
The 13C-NMR analyses provide fractional contributions (FCs) of acetyl-CoA to the CAC from 3 labeled substrates and a fourth-but-undefined unlabeled component. Typical and representative 13C-spectra identifying glutamate peaks are shown in Figures 5A and B. In all studies, 13C-enrichment was modest, and the unlabeled fraction represented the majority of oxidized substrate. Ischemia decreased FC from 13C-labeled substrates (32.6±2.5%) compared to the control (48.0±5.1%, P<0.05), and this was reversed by T3 supplementation (Figure 5C). The FC-lactate in Group-IR (10.3±1.0%) was lower than that in Group-C (7.7±0.7%); however, that did not reach statistical difference (P=0.08). Furthermore, FC-lactate was significantly higher in the T3-supplemented group (near 70% compared to Group-IR, P<0.05). The FC for 13C-labeled substrates can be influenced by their uptake as well as the size of the unlabeled pool in the myocardium. Absolute lactate levels and pyruvate detected by 1H-NMR were not altered by the protocol, suggesting that the substrate pool size was not responsible for these alterations in FC from labeled substrate (Figures 4E, F). Of note, our prior studies showed that T3 decreased total lactate and pyruvate concentration. However, the discrepancy might be related to higher pyruvate loading with supraphysiological concentrations in those experiments.15 We also analyzed the relative contribution among the 13C-labeled substrates (Figure 5D). Lactate contribution was higher in Group-IR-T3 than in the other groups, but it did not reach significant difference (P=0.08). The MCFA, octanoate, provided the majority of the contribution from 13C-labeled substrate through all 3 protocols. Interestingly, T3 significantly decreased FC-MCFA and tended to increase FC-LCFAs; however, contribution of total FAs (sum of MCFA and LCFAs) was not significantly different among the 3 groups.
Figure 5.
Typical 13C-NMR full spectrum (A), representative spectra for glutamates carbon 5 (B) and analyzed data (C–E). Chemical shifts in parts per million (ppm) were as follows: C3, 27.5; C4, 34.2; C2, 55.2; C1, 175.0; C5 of glutamate, 182.1. Marked differences occur in C5-glutamate peak complexes among the 3 groups. C5 in Group-IR-T3 shows prominently increased singlet peak area (s) relative to doublet spike areas (d), indicating increased lactate contribution compared to Group-IR. Substrate fractional contribution (C) and relative contribution except unlabeled substrates (D) to acetyl-CoA and anaplerotic component (E) were analyzed by 13C-NMR. Ischemic injury decreased lactate oxidation compared with the control at the end of the protocol (P=0.07). T3 restored lactate oxidation that was decreased by ischemia. On relative contribution among the 13C-labeled substrates, T3 significantly decreased MCFAs contribution and tended to increase LCFAs contribution. Values are presented as mean ± SE; n=5–6 per group. *P<0.05 vs. Group-IR. Glu, glutamate; FA, fatty acid; NMR, nuclear magnetic resonance; T3, triiodothyronine; MCFAs, medium-chain fatty acids; LCFAs, long-chain fatty acids; Group-IR, Ischemia-reperfusion group; Group-IR-T3, T3 treatment group.
Anaplerosis can replenish the CAC intermediates. However, these pathways produce futile cycling as they bypass substrate oxidation for entry into the CAC. We previously studied a major route of anaplerosis, pyruvate carboxylation, and found that its contribution, relative to pyruvate carboxylation, was not altered by IR or short-term T3 supplementation.9 In the current study, the labeling strategy did not permit specific analyses for pyruvate carboxylation. However, the tcaCALC algorithm yields data for total anaplerotic carbon contribution relative to the citric synthetase flux. Although we identified a considerable contribution to the CAC in each group, there were no significant differences (Figure 5E).
Immunoblot Analyses
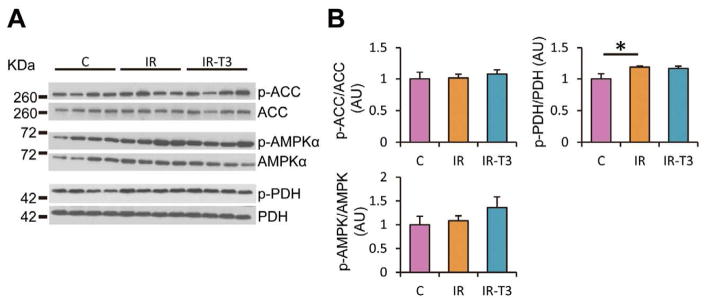
We measured protein expression levels of phospho-ACC (inactivate form of ACC), which inhibits the β-oxidation of FAs in the mitochondria; phospho-AMPKα, which activates the cellular uptake of glucose through glucose transporter 4 and the β-oxidation of FAs through the phosphorylation of ACC, and phospho-PDH (inactivate form of PDH), which inhibits pyruvate decarboxylation as myocardial metabolism-related proteins (Figure 6). These protein levels were similar among the 3 groups, except for the increase of the phospho-PDH relative to total PDH caused by ischemic injury. Inactivation of PDH in Group-IR corresponded to the results of 13C-NMR, showing lower FC-lactate in Group-IR than that in Group-C.
Figure 6.
Representative immunoblot data (A) and pooled data (B). Phospholylated AMPKα, ACC and PDH were at Thr172, Ser79 and Ser293, respectively. Group-IR demonstrated higher expression levels of phosphorylated PDH relative to total PDH compared with Group-C. Values are presented as mean ± SE; n=5–6 per group. *P<0.05 vs. Group-C. AMPKα, 5′ adenosine monophosphate-activated protein kinase; ACC, acetyl-CoA carboxylase; PDH, pyruvate dehydrogenase; Group-IR, Ischemia-reperfusion group; Group-C, control group; AU, arbitrary unit.
Discussion
Our study shows that thyroid hormone repletion during ECMO modifies cardiac substrate oxidation by promoting the relative contribution of lactate to the CAC and thereby providing a potential mechanism for elevating contractile function during weaning. We cannot define in these experiments whether increased contractile function stems from the ischemic, border or non-ischemic zones. However, prior studies have in other experimental models shown that T3 provided acutely in similar doses promotes contractile function in IR but not in uninjured myocardium.20 In addition, our previous study assessed the effectiveness of T3 in the uninjured heart during ECMO.11 This data demonstrated that T3 markedly increased lactate oxidation in the uninjured heart. Unfortunately, cardiac performances were not clearly expressed under supported by ECMO. We cannot exclude the potential effects that uninjured myocardium contributes to the improvement of cardiac work compared with injured myocardium. To summarize, our previous and present results suggest that T3 improves cardiac work, and that activation of carbohydrate oxidation in both ischemic injured and uninjured myocardium is one potential mechanism of this effect.
Results from our current study support data from our previous study that related to thyroid hormone regulation of pyruvate decarboxylation.9,15 In those studies, we used supraphysiological doses of pyruvate without providing FAs in the coronary artery in order to maximize flux through the pyruvate decarboxylation pathway. In the current study, we confirmed the effect on pyruvate decarboxylation using more physiological concentrations for substrates, which are typically oxidized by the immature heart. Although FAs provide superior ATP production per carbon compared to carbohydrates, the latter are more oxygen efficient for ATP synthesis. Improved efficiency for ATP synthesis is not really relevant under conditions with ample O2 supply. In this model, T3 improves efficiency and [ATP]/[ADP], suggesting that a limitation exists in oxygen delivery, which can be accommodated by shifting substrate preference towards a carbohydrate such as lactate. Contractile function improved in conjunction with myocardial cellular energetic, suggesting that impaired weaning in piglets not receiving T3 has a metabolic basis.
Prior studies that used isolated perfused rat hearts showed that T3 after IR promoted glucose oxidation without any significant change in palmitate oxidation.20 This response occurred in conjunction with an increase in activity for PDH, which is responsible for the metabolism of pyruvate to acetyl-CoA. Unlike the rat, porcine myocardium uses minimal glucose for oxidation under IR conditions, but shows preference for lactate or pyruvate, which then enters the CAC via PDH.21 Although the relative metabolic flux response to T3 after IR in our porcine myocardium resembles that found in isolated rat myocardium, we could not document similar changes in activation through the phosphorylation of PDH. Furthermore, we also found no change in the phosphorylation of ACC, a primary regulator of free FA flux. Thus, the mechanisms driving these relative flux changes still require clarification in vivo.
We supplied both MCFAs and LCFAs in these experiments in order to emulate the clinical scenario. The immature porcine heart rapidly transitions the primary oxidative substrates from carbohydrates to FAs after birth.22 These studies confirm heavy reliance on these FAs, as they accounted for the majority of labeled substrate entering the CAC as acetyl-CoA. As noted in other experiments performed in multiple models, IR appears to impair overall FA oxidation.23,24 Our prior studies in isolated perfused rat heart showed that T3 infusion abruptly increases FC-LCFAs.25 However, this affect was reversed by epinephrine, which also enhanced T3 promotion of lactate oxidation. In the current study, we did not use adrenergic or any inotropic stimulation, although inotropic agents are frequently used clinically. Conceivably ambient levels of circulating catecholamines following IR are enough to emulate the experiments performed in the isolated rat heart and modify the T3 affect on FA metabolism. In this study, lactate provided the principal source of acetyl-CoA produced by pyruvate decarboxylation. Lactate, in addition to FAs, generally supplies the overwhelming majority of oxidative substrate to the heart, while glucose provides a minor contribution.26 These substrates are also typically provided as nutritional support to infants during ECMO.16 Our data show that T3 increases FC from 13C-lactate by nearly 70%. The NMR resolution in our studies and our ability to discriminate differences in FCs among groups depends, in part, on the level of glutamate enrichment. As these studies are performed in vivo, we could not achieve more than modest enrichment of the glutamate pool while using physiological concentrations of 13C-labeled substrate. The majority of acetyl-CoA contribution in this model is therefore derived from unlabeled sources and may reflect oxidation of endogenous substrates within the heart such as triglycerides in addition to circulating substrates. Despite the low glutamate enrichment, we were able to identify differences in substrate utilization among the 3 groups. Our previous studies compared the high- and low-dose 13C-labeled substrate within the coronary artery and showed that proportional changes in the FCs of these substrates are not altered by dose within the coronary artery.12 However, the cellular redox state appeared highly sensitive to FA supply and FC to the CAC. With the substrate concentrations used in this study, we noted that ischemic injury reduces cellular redox state despite T3 supplementation. Our indices for redox state reflect total cellular values and do not address compartmentation between mitochondria and cytosol. The inflammatory response during mechanical circulatory support has been directly linked to the disruption of thyroid hormone homeostasis in human infants, as well as our immature pig model.7 Surges in cytokines, including interleukin-6, inhibit stimulation of the thyroid hormone secretion along many points in the control axis, extending from the hypothalamus to the thyroid itself.27 Cytokines also block conversion of thyroxine (T4) to T3 and reduce end-organ response.28 Marked elevation in these pro-inflammatory cytokines can play a role in the reduction in insulin sensitivity noted during ECMO, as well as frequent hyperglycemia observed in patients following mechanical circulation.29 Our data suggest that cytokine-induced abnormalities can be circumvented by T3 supplementation.
There are some limitations in this study. Ten minutes of cardiac ischemia is arguably considered a modest insult in many models. Thus, the degree of cardiac injury might be questioned in our porcine experiments. However, our preliminary experiments showed that few pigs survived to weaning after longer periods of coronary occlusion. We documented ischemia through direct observation, and noted marked increases in troponin leak, thereby validating ischemic injury. Accordingly, our observed changes in cardiac function and metabolism caused by T3 appear modest, although some were statistically significant. Our study design did introduce some bias by the exclusion of non-survivors. Obviously, we could not measure function or metabolism in the two piglets, which could not maintain circulation and wean. We chose not to assume a contractile functional status for these piglets, but recognize that any incorporation of their data would enhance the functional differences between Group-IR and Group-IR-T3. Second, MV̇O2 was calculated using total coronary flow reflected by blood return to coronary sinus as a simple technique, because direct measurement of coronary flow by using flow pad or probe potentially might change the flow in a subtle way in a small piglet heart. As noted previously, inotropic agents are used in the majority of patients supported by ECMO. The basis for their utilization is not well substantiated and remains mainly theoretical. A recent clinical trial in infants undergoing cardiopulmonary bypass showed that T3 supplementation, similar to that used in our experiments, reduced postoperative inotropic use.30 Accordingly, we did not feel it was necessary to add an inotropic agent to our protocol.
In summary, we have noted that ischemia-reperfusion in this immature pig model alters substrate metabolism, causing impairment in high energy phosphate status. T3 supplementation reverses some of these impairments, in part, by increasing relative flux through PDH without substantial disturbance of FA metabolism, and facilitates weaning from ECMO following cardiac injury in an immature swine model. These data support the notion that metabolic manipulation by thyroid hormone can improve weaning from ECMO. Although these experiments evaluated responses in immature myocardium, adults also undergo ECMO support.31 The relevance of these studies for adults undergoing ECMO can be a subject for future studies.
Acknowledgments
This work was supported by the National Institutes of Health [R01HL60666 to M.A.P.]. A portion of the research was performed using the Environmental Molecular Sciences Laboratory, a national scientific user facility sponsored by the Department of Energy’s Office of Biological and Environmental Research and is located at Pacific Northwest National Laboratory.
Footnotes
Disclosures
None.
References
- 1.Undar A, Wang S. Current devices for pediatric extracorporeal life support and mechanical circulatory support systems in the United States. Biomed Mater Eng. 2013;23:57–62. doi: 10.3233/BME-120732. [DOI] [PubMed] [Google Scholar]
- 2.Friedland-Little JM, Aiyagari R, Yu S, Donohue JE, Hirsch-Romano JC. Survival through staged palliation: Fate of infants supported by extracorporeal membrane oxygenation after the Norwood operation. Ann Thorac Surg. 2014;97:659–665. doi: 10.1016/j.athoracsur.2013.10.066. [DOI] [PubMed] [Google Scholar]
- 3.Mascio CE, Austin EH, 3rd, Jacobs JP, Jacobs ML, Wallace AS, He X, et al. Perioperative mechanical circulatory support in children: An analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database. J Thorac Cardiovasc Surg. 2014;147:658–664. doi: 10.1016/j.jtcvs.2013.09.075. discussion 664–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shuhaiber J, Thiagarajan RR, Laussen PC, Fynn-Thompson F, del Nido P, Pigula F. Survival of children requiring repeat extracorporeal membrane oxygenation after congenital heart surgery. Ann Thorac Surg. 2011;91:1949–1955. doi: 10.1016/j.athoracsur.2011.01.078. [DOI] [PubMed] [Google Scholar]
- 5.Stewart DL, Ssemakula N, MacMillan DR, Goldsmith LJ, Cook LN. Thyroid function in neonates with severe respiratory failure on extracorporeal membrane oxygenation. Perfusion. 2001;16:469–475. doi: 10.1177/026765910101600606. [DOI] [PubMed] [Google Scholar]
- 6.Priddy CM, Kajimoto M, Ledee DR, Bouchard B, Isern N, Olson AK, et al. Myocardial oxidative metabolism and protein synthesis during mechanical circulatory support by extracorporeal membrane oxygenation. Am J Physiol Heart Circ Physiol. 2013;304:H406–H414. doi: 10.1152/ajpheart.00672.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Priest JR, Slee A, Olson AK, Ledee D, Morrish F, Portman MA. Triiodothyronine supplementation and cytokines during cardiopulmonary bypass in infants and children. J Thorac Cardiovasc Surg. 2012;144:938–943. e932. doi: 10.1016/j.jtcvs.2012.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cantinotti M, Lorenzoni V, Storti S, Moschetti R, Murzi B, Marotta M, et al. Thyroid and brain natriuretic peptide response in children undergoing cardiac surgery for congenital heart disease- age-related variations and prognostic value. Circ J. 2013;77:188–197. doi: 10.1253/circj.cj-12-0834. [DOI] [PubMed] [Google Scholar]
- 9.Olson AK, Bouchard B, Ning XH, Isern N, Rosiers CD, Portman MA. Triiodothyronine increases myocardial function and pyruvate entry into the citric acid cycle after reperfusion in a model of infant cardiopulmonary bypass. Am J Physiol Heart Circ Physiol. 2012;302:H1086–H1093. doi: 10.1152/ajpheart.00959.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masaki M, Komamura K, Goda A, Hirotani S, Otsuka M, Nakabo A, et al. Elevated arterial stiffness and diastolic dysfunction in subclinical hypothyroidism. Circ J. 2014;78:1494–1500. doi: 10.1253/circj.cj-13-1556. [DOI] [PubMed] [Google Scholar]
- 11.Kajimoto M, O’Kelly Priddy CM, Ledee DR, Xu C, Isern N, Olson AK, et al. Effects of continuous triiodothyronine infusion on the tricarboxylic acid cycle in the normal immature swine heart under extracorporeal membrane oxygenation in vivo. Am J Physiol Heart Circ Physiol. 2014;306:H1164–H1170. doi: 10.1152/ajpheart.00964.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kajimoto M, O’Kelly Priddy CM, Ledee DR, Xu C, Isern N, Olson AK, et al. Extracorporeal membrane oxygenation promotes long chain fatty acid oxidation in the immature swine heart in vivo. J Mol Cell Cardiol. 2013;62:144–152. doi: 10.1016/j.yjmcc.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kajimoto M, O’Kelly Priddy CM, Ledee DR, Xu C, Isern N, Olson AK, et al. Myocardial reloading after extracorporeal membrane oxygenation alters substrate metabolism while promoting protein synthesis. J Am Heart Assoc. 2013;2:e000106. doi: 10.1161/JAHA.113.000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olson AK, Hyyti OM, Cohen GA, Ning XH, Sadilek M, Isern N, et al. Superior cardiac function via anaplerotic pyruvate in the immature swine heart after cardiopulmonary bypass and reperfusion. Am J Physiol Heart Circ Physiol. 2008;295:H2315–H2320. doi: 10.1152/ajpheart.00739.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Files MD, Kajimoto M, O’Kelly Priddy CM, Ledee DR, Xu C, Des Rosiers C, et al. Triiodothyronine facilitates weaning from extra-corporeal membrane oxygenation by improved mitochondrial substrate utilization. J Am Heart Assoc. 2014;3:e000680. doi: 10.1161/JAHA.113.000680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaksic T, Hull MA, Modi BP, Ching YA, George D, Compher C. A.S.P.E.N Clinical guidelines: Nutrition support of neonates supported with extracorporeal membrane oxygenation. JPEN J Parenter Enteral Nutr. 2010;34:247–253. doi: 10.1177/0148607110369225. [DOI] [PubMed] [Google Scholar]
- 17.Suga H. Ventricular energetics. Physiol Rev. 1990;70:247–277. doi: 10.1152/physrev.1990.70.2.247. [DOI] [PubMed] [Google Scholar]
- 18.Des Rosiers C, Lloyd S, Comte B, Chatham JC. A critical perspective of the use of (13)C-isotopomer analysis by GCMS and NMR as applied to cardiac metabolism. Metab Eng. 2004;6:44–58. doi: 10.1016/j.ymben.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Malloy CR, Sherry AD, Jeffrey FM. Analysis of tricarboxylic acid cycle of the heart using 13C isotope isomers. Am J Physiol. 1990;259:H987–H995. doi: 10.1152/ajpheart.1990.259.3.H987. [DOI] [PubMed] [Google Scholar]
- 20.Liu Q, Clanachan AS, Lopaschuk GD. Acute effects of triiodothyronine on glucose and fatty acid metabolism during reperfusion of ischemic rat hearts. Am J Physiol. 1998;275:E392–E399. doi: 10.1152/ajpendo.1998.275.3.E392. [DOI] [PubMed] [Google Scholar]
- 21.Kudej RK, White LT, Kudej AB, Vatner SF, Lewandowski ED. Brief increase in carbohydrate oxidation after reperfusion reverses myocardial stunning in conscious pigs. Circulation. 2002;106:2836–2841. doi: 10.1161/01.cir.0000039326.87475.98. [DOI] [PubMed] [Google Scholar]
- 22.Werner JC, Sicard RE, Schuler HG. Palmitate oxidation by isolated working fetal and newborn pig hearts. Am J Physiol. 1989;256:E315–E321. doi: 10.1152/ajpendo.1989.256.2.E315. [DOI] [PubMed] [Google Scholar]
- 23.Lou PH, Zhang L, Lucchinetti E, Heck M, Affolter A, Gandhi M, et al. Infarct-remodelled hearts with limited oxidative capacity boost fatty acid oxidation after conditioning against ischaemia/reperfusion injury. Cardiovasc Res. 2013;97:251–261. doi: 10.1093/cvr/cvs323. [DOI] [PubMed] [Google Scholar]
- 24.Liedtke AJ, Nellis SH. Effects of carnitine in ischemic and fatty acid supplemented swine hearts. J Clin Invest. 1979;64:440–447. doi: 10.1172/JCI109481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krueger JJ, Ning XH, Argo BM, Hyyti O, Portman MA. Triidothyronine and epinephrine rapidly modify myocardial substrate selection: A (13)C isotopomer analysis. Am J Physiol Endocrinol Metab. 2001;281:E983–E990. doi: 10.1152/ajpendo.2001.281.5.E983. [DOI] [PubMed] [Google Scholar]
- 26.Sherry AD, Nunnally RL, Peshock RM. Metabolic studies of pyruvate- and lactate-perfused guinea pig hearts by 13C NMR: Determination of substrate preference by glutamate isotopomer distribution. J Biol Chem. 1985;260:9272–9279. [PubMed] [Google Scholar]
- 27.Bartalena L, Bogazzi F, Brogioni S, Grasso L, Martino E. Role of cytokines in the pathogenesis of the euthyroid sick syndrome. Eur J Endocrinol. 1998;138:603–614. doi: 10.1530/eje.0.1380603. [DOI] [PubMed] [Google Scholar]
- 28.Boelen A, Maas MA, Lowik CW, Platvoet MC, Wiersinga WM. Induced illness in interleukin-6 (IL-6) knock-out mice: A causal role of IL-6 in the development of the low 3,5,3′-triiodothyronine syndrome. Endocrinology. 1996;137:5250–5254. doi: 10.1210/endo.137.12.8940342. [DOI] [PubMed] [Google Scholar]
- 29.Grimble RF. Inflammatory status and insulin resistance. Curr Opin Clin Nutr Metab Care. 2002;5:551–559. doi: 10.1097/00075197-200209000-00015. [DOI] [PubMed] [Google Scholar]
- 30.Portman MA, Slee A, Olson AK, Cohen G, Karl T, Tong E, et al. Triiodothyronine Supplementation in Infants and Children Undergoing Cardiopulmonary Bypass (TRICC): A multicenter placebo-controlled randomized trial: Age analysis. Circulation. 2010;122:S224–S233. doi: 10.1161/CIRCULATIONAHA.109.926394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chung SY, Sheu JJ, Lin YJ, Sun CK, Chang LT, Chen YL, et al. Outcome of patients with profound cardiogenic shock after cardio-pulmonary resuscitation and prompt extracorporeal membrane oxygenation support: A single-center observational study. Circ J. 2012;76:1385–1392. doi: 10.1253/circj.cj-11-1015. [DOI] [PubMed] [Google Scholar]