Abstract
Obesity remains a major public health concern and novel treatments are needed. Transcranial direct current stimulation (tDCS) is a neuromodulation technique shown to reduce food craving and consumption, especially when targeting the dorsolateral prefrontal cortex (DLPFC) with a right anode/left cathode electrode montage. Despite the implications to treat frank (non-bingeeating) obesity, no study has tested the right anode/left cathode montage in this population. Additionally, most tDCS appetite studies have not controlled for differences in traits under DLPFC control that may influence how well one responds to tDCS. Hence, N = 18 (10F/8M) adults with frank obesity completed the Dutch Eating Behavior Questionnaire-Restraint and Barratt Impulsiveness Scale, and received 20 min of 2 mA active tDCS and control tDCS session. Craving and eating was assessed at both sessions with a food photo “wanting” test and in-lab measures of total, preferred, and less-preferred kilocalories consumed of three highly palatable snack foods. While main effects of tDCS vs. control were not found, significant differences emerged when trait scores were controlled. tDCS reduced food craving in females with lower attention-type impulsiveness (p = 0.047), reduced preferred-food consumption in males with lower intent to restrict calories (p = 0.024), and reduced total food consumption in males with higher non-planning-type impulsiveness (p = 0.009) compared to control tDCS. This is the first study to find significant reductions in food craving and consumption in a sample with frank obesity using the most popular tDCS montage in appetite studies. The results also highlight the cognitive-based heterogeneity of individuals with obesity and the importance of considering these differences when evaluating the efficacy of DLPFC-targeted tDCS in future studies aimed at treating obesity.
Keywords: Neuromodulation, Dorsolateral prefrontal cortex, Sex differences, Impulsiveness, Dieting, Treatment, Cognitive control
1. Introduction
Obesity is a major public health concern as it affects over one-third of the United States population and results in life-threatening medical complications (Ogden, Carroll, Kit, & Flegal, 2014; Smith & Smith, 2016). Current weight loss strategies are moderately effective in producing initial weight loss but weight regain is common (Wing & Hill, 2001). Bariatric surgery is effective, but it is invasive and costly. Drug therapies are available but their side effects commonly lead to their discontinuation (Bray, Frübeck, Ryan, & Wilding, 2016). Therefore, a new obesity treatment or treatment adjunct is needed that is safe, economical, and long-lasting.
Transcranial direct current stimulation (tDCS) is an inexpensive, non-invasive neuromodulation technique that has been found to decrease food consumption and food craving, especially when using a right anode/left cathode montage over the dorsolateral prefrontal cortex (DLPFC; see Table 1). Anodal stimulation increases spontaneous neuronal excitation while cathodal stimulation inhibits it (Creutzfeldt, Fromm, & Kapp, 1962). The DLPFC is targeted because of its role in cognitive control (Gilbert & Burgess, 2008), a putative functional mechanism in the suppressing effects on food craving and consumption (Lapenta, Sierve, de Macedo, Fregni, & Boggio, 2014). However, despite the obvious implications of tDCS to potentially treat obesity, only one of the ten tDCS studies aiming to reduce craving and eating has used the popular right anode/left cathode montage in this population (Table 1).
Table 1.
Participant description and parameters of tDCS studies aimed at reducing food craving and intake.
| Reference | N (F/M) | Study Sample |
BMI Category |
Electrode Montage |
Current × sessionsa | Reduced Craving |
Reduced Intake |
Traits Controlledb |
|---|---|---|---|---|---|---|---|---|
| Anodal Stimulation to the Right DLPFC | ||||||||
| Fregni et al. (2008) | 21 (NR) | Adults w/FFC1 | NR | Bilateralc | 2 mA | Yes | Yes | No |
| Goldman et al. (2011) | 19 (13/6) | Adults w/FFC2 | Overweight | Bilateral | 2 mA | Yes | No | No |
| Lapenta et al. (2014) | 9 (9/0) | Adults w/FFC1 | Healthy | Bilateral | 2 mA | Yes | Yes | No |
| Kekic et al. (2014) | 17 (17/0) | Adults w/FFC3 | Healthy | Bilateral | 2 mA | Yes | No | Yes |
| Jauch-Chara et al. (2014) | 14 (0/14) | Adults | Healthy | Unilaterald | 1mA × 8 | Yes | Yes | No |
| Bravo et al. (2016) | 32 (NR) | Adults w/PWS | Overweighte | Unilateral | 2mA/30min × 5 | Yes | ___ | No |
| Burgess et al. (2016) | 30 (20/10) | Adults w/BED | Obese | Bilateral | 2mA | Yes | Yes | Yes |
| Ljubisavljevic, Maxood, Bjekic, Oommen, and Nagelkerke (2016) | 27(8/19) | Adults w/FFC4 | Overweight | Unilateralf | 2mA × 5 | Yes | ___ | No |
| Anodal Stimulation of the Left DLPFC | ||||||||
| Fregni et al. (2008) | 21 (NR) | Adults w/FFC1 | NR | Bilateral | 2 mA | No | Yes | No |
| Montenegro et al. (2012) | 9 (4/5) | Adults | Overweight | Unilateral | 2 mA | Yes | ___ | No |
| Gluck et al. (2016) | 9 (6/3) | Adults | Obese | Unilateral | 2mA/40min × 6active × 2control | ___ | Nog | No |
tDCS = transcranial direct current stimulation; BMI = mean body mass index; DLPFC = dorsolateral prefrontal cortex; NR = not reported; PWS = Prader-Willi Syndrome; BED = binge-eating disorder; __ = Not measured; FFC = frequent food cravings. FFC:
defined as having ≥3 cravings per day for the test foods used in-lab;
defined as having food cravings for sweets, fast food fats, high fats, or carbohydrates at least 3 times per week for 1 month determined by the Food Craving Inventory;
defined as self-reported food cravings at least once per day;
based on scores from two food craving questionnaires.
All studies used a single 20 min active and control session unless otherwise stated;
Traits were assessed to determine influence on main tDCS effects;
Bilateral-anode and cathode over DLPFC;
Unilateral-cathode over supraorbital area;
Used obese control group and found tDCS reduction only on Three-Factor Eating Questionnaire Scores vs. control;
Unilateral with cathode over left forehead;
3 anodal vs. 3 cathodal sessions within-subjects; anodal reduced fat intake, soda intake, and body weight vs. cathodal but not compared to a separate group receiving 2 control sessions.
The one study using the right anode/left cathode montage was conducted in our laboratory (Burgess et al., 2016) where we found reductions in food craving and food consumption. However, all of the participants with obesity were also diagnosed with binge-eating disorder (BED) or subthreshold BED, and their responses to tDCS cannot be generalized to frank obesity (defined here as non-BED obesity) since BED is a mental disorder with unique neural, behavioral, and psychopathological correlates from non-BED obesity (Balodis et al., 2016; Herbozo, Schaefer, & Thompson, 2015; Schag, Schönleber, Teufel, Zipfel, & Giel, 2013).
Therefore, the main aim of this study was to test the efficacy of the right anode/left cathode tDCS montage to reduce food craving and consumption in participants with frank obesity. The study also determined the degree to which tDCS suppression of craving and eating in frank obesity was influenced by individual baseline differences. Not everyone responds to the expected effects of tDCS. Individual differences in physiology or behavior may explain differential responding to tDCS in eating and other behaviors. For example, individual differences in certain gene variants (Wiegand, Nieratschker, & Plewnia, 2016), in brain current density following stimulation (Kim et al., 2016), and in baseline performance of targeted functions (Hsu, Juan, & Tseng, 2016), have been found to influence the magnitude of tDCS-induced improvement on the condition being tested. In tDCS appetite studies, only two studies, to date, have considered the influence of individual trait differences on tDCS efficacy (Table 1). Kekic et al. found that tDCS reduced food craving more effectively in those with lower vs. greater impulsiveness on a reward-choice task (Kekic et al., 2014), but these were healthy-weight participants. Our lab also found that greater vs. lower intent to restrict calories predicted tDCS suppression of food craving, but these were participants with BED (Burgess et al., 2016). However, both studies hint that greater baseline cognitive control may enhance the anti-obesity actions of tDCS. Hence, investigating the effect of individual differences in cognition-related traits on tDCS responses may elucidate the source for inconsistent results across participants and help predict who might benefit the most from tDCS-based treatments. Additionally, given the promise of a tDCS-based treatment for obesity, it is important to understand the effectiveness of tDCS in both males and females as obesity affects both groups (Ogden et al., 2014) and previous work in our lab found that males and females respond differently to tDCS (Burgess et al., 2016).
Given the results of this study in BED, the study by Kekic et al. and previous studies using the right anode/left cathode montage, it was hypothesized that a single session of right anode/left cathode tDCS of the DLPFC would suppress food craving and eating more than a session of control tDCS in a male and in a female sample of frank obesity. Furthermore, it was hypothesized that significant responses to tDCS would depend on baseline differences related to cognitive control, specifically, that those with greater intent to restrict calories and with lower trait impulsivity would exhibit a greater tDCS suppression of food craving and consumption.
2. Methods
2.1. Subjects
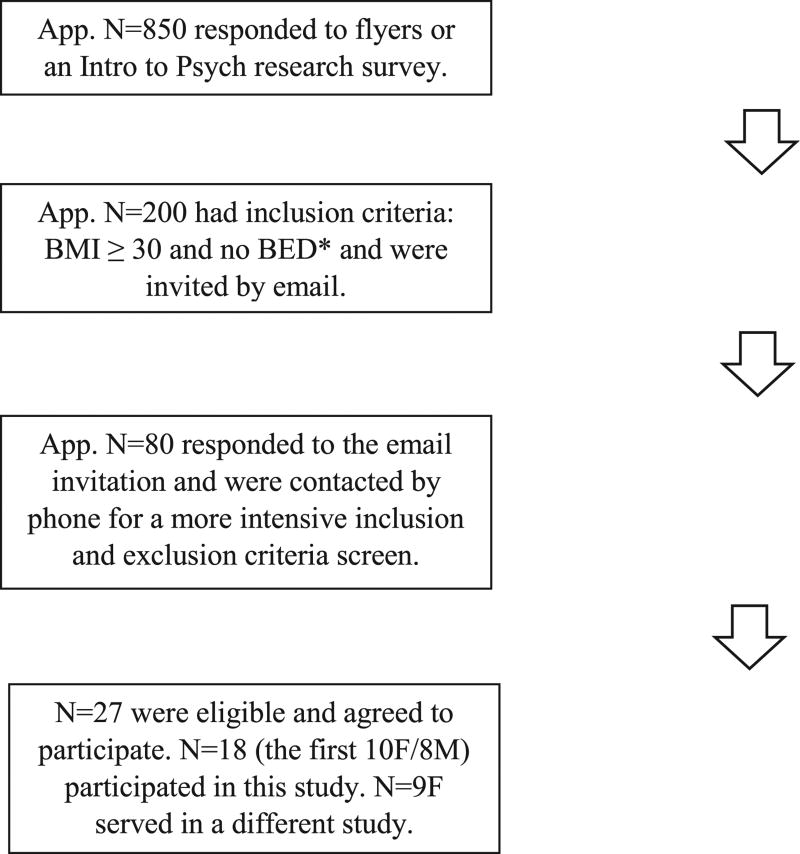
Participant recruitment and selection is outlined in Fig. 1. The most common reasons for exclusion were allergy to test-food ingredients, BED status, and disinterest in participating. Other exclusion criteria included pregnancy, breastfeeding, uncontrolled diabetes or hypertension, and standard tDCS study exclusions such as history of bipolar disorder and metal or electrical implants (Bikson et al., 2016). The study was approved by The University of Alabama at Birmingham (UAB) Institutional Review Board for Human Use.
Fig. 1.
Flow diagram of the participant selection process. *Self-reported height and weight and survey for DSM-5 BED criteria (American Psychiatric Association, 2013) (via research survey for Intro to Psych students and phone interview for flyer recruits).
2.2. Transcranial direct current stimulation (tDCS)
An active and control tDCS session was administered in counterbalanced order across the female and male subgroups. A 1ch stimulator from TCT Research Limited (Hong Kong, China) with 4 × 6 cm electrodes was used to deliver 2 mA (current density = 0.083 mA/cm2) of current for 20 min in the active tDCS condition. In the control condition, current was ramped up to 2 mA and back down in the first and last minute of the 20-minute session. Participants and researchers, other than the one delivering current, were blind to the stimulation condition. The cathode was placed over F3 (left DLPFC) and the anode over F4 (right DLPFC) based on the EEG 10–20 system. Participants completed physical sensation rating sheets to report any physical sensations including: some tingling, a lot of tingling, some itching, a lot of itching, cold or bright, warm, very warm, burning (like scalding water), burning (like sunburn), and hurts a lot. They were also asked to rate their discomfort level on a scale from 1 (no discomfort) to 10 (extreme discomfort) 5 min after the start of the sessions and at the end of the sessions. Both ratings were averaged for analyses.
2.3. Measures
2.3.1. Demographics, BMI, and hunger
Age, sex, and ethnicity were reported on a survey. Although all had self-reported height and weight during the screening process, height and weight were measured in the lab without shoes by an assistant and the formula k/m2 was used to calculate BMI. Current hunger was assessed with a 10-point scale from 0 = “I am not hungry at all” to 10 = “I have never been more hungry” (Flint, Raben, Blundell, & Astrup, 2000).
2.3.2. Psychological questionnaires. Barratt Impulsiveness Scale 11 (BIS-11)
The 30-item BIS uses three subscales to assess types of impulsiveness: “Attentional” which pertains to degree of concentration, degree of focus, and general cognitive instability; “Motor” which pertains to degree of acting without thinking and perseverance; and “Nonplanning” which pertains to degree of self-control and cognitive complexity in future planning (Patton, Stanford, & Barratt, 1995). Higher scores indicate greater impulsiveness.
2.3.3. Dutch Eating Behavior Questionnaire-Restraint (DEBQ-R)
The 10-item DEBQ-R measures constructs related to dieting with 2 subscales: “Intent” which assesses frequency of effort and preoccupation to restrict calories with the goal of losing weight; and “Behavior” which assesses frequency of successful caloric restriction. Higher scores indicate greater preoccupation with and restricting calories (Van Strien, Fritjers, Bergers, & Defares, 1986).
2.3.4. Food cravings test
Participants rated 24 food images on a computer screen for amount of “liking” and current “wanting” to eat each food (0 = “definitely not,” 1 = “not likely,” 2 = ”maybe,” 3 = “probably,” and 4 = “definitely”). The wanting questions served as a proxy for craving and were obtained before and immediately after the tDCS and control sessions for a pre-and post-stimulation craving rating of each food. For analyses, the food images were classified into 4 categories: sweets (e.g., cake, brownies), fatty proteins (e.g., bratwurst, ribs), carbohydrates (e.g., biscuits, fries), mixed foods (e.g., pizza, nachos), and an all-foods category (all 24 foods). For each individual participant, any food with a “liking” rating less than 2 during the first or second visit was removed from all analyses belonging to that particular individual. This avoided floor-effects since disliked foods were not expected to be craved. The number of foods removed are shown in Table 2. Pre-vs. post-craving scores for each stimulation condition were calculated by subtracting the post-stimulation from the pre-stimulation wanting ratings for each food. The control tDCS scores were subtracted from the active tDCS scores. Hence, positive values represent a greater craving reduction by active tDCS vs. control.
Table 2.
Number of individual food items removed for each food category from the food craving analyses. Data presented as group mean (range of number of food items removed).
| All (N = 18) | Female (N = 10) | Male (N = 8) | |
|---|---|---|---|
| Food Category | |||
| Sweets | 0.61 (0.00–3.00) | 0.80 (0.00–3.00) | 0.38 (0.00–3.00) |
| Fatty Proteins | 0.83 (0.00–3.00) | 1.10 (0.00–3.00) | 0.50 (0.00–2.00) |
| Carbs | 0.44 (0.00–2.00) | 0.50 (0.00–2.00) | 0.38 (0.00–2.00) |
| Mixed Foods | 0.28 (0.00–1.00) | 0.20 (0.00–1.00) | 0.38 (0.00–1.00) |
| All-foods | 2.17 (0.00–7.00) | 2.60 (0.00–7.00) | 1.63 (0.00–5.00) |
2.3.5. In-lab eating test
Participants were left alone in a room for 20 min with a generous amount of pre-measured Oreo Double-Stuff cookies, plain M&Ms, and Lay's potato chips. They were instructed to try at least some of each food so they could complete a palatability rating sheet which asked them to rate how much they liked the properties of each food (e.g., smell, taste). The rating scale was a ruse for the actual goal of measuring amount of food eaten. The participants were encouraged to eat as much food as they wanted and were instructed to discard any remaining food in a nearby closed trash bin to avoid any self-consciousness over amount eaten. All discarded food was weighed and converted to kilocalories (kcals) to determine amount of total food consumption. In addition, a measure of preferred- and less-preferred food intake was obtained. Participants were asked to rank their favorite of the three foods. Preferred-food consumed was the mean kcal intake of each participant's highest ranked food; less-preferred food consumed was the mean kcal intake of the two lower ranked foods averaged for each participant. The same script, foods, and time period were used in our previous study (Burgess et al., 2016). Eating difference scores were calculated by subtracting kcals eaten with active tDCS from kcals eaten with control tDCS. Hence, positive values represent a greater reduction in kcal consumption by active tDCS vs. control.
2.4. Procedures
Prior to each of the two lab visits, participants were instructed to “eat just enough food so you are not too hungry or too full when you come to the lab.” Research assistants verified this state of hunger prior to testing and rescheduled anyone that reported feeling overly hungry or full. The second lab visit was scheduled as close as possible to the same time as the first lab visit. Participants were then measured for a BMI and completed the hunger rating scale and battery of questionnaires, followed by the pre-stimulation food craving test. They were then administered active or control tDCS followed by completion of the post-stimulation craving test. They were then instructed to complete the palatability rating sheets during the eating test. Procedures for the second visit were the same as the first except that they did not complete the battery of questionnaires, they received the alternate stimulation condition, and they were asked to rank the three eating-test foods for preference. Participants were then debriefed and compensated.
2.5. Statistical analysis
Between-subject MANOVAs determined differences between males and females on descriptives and trait scores (Table 3). Within-subject MANOVAs were used to determine differences between active and control tDCS on the craving (Fig. 2) and eating measures (Figs. 3 and 4) for males and females separately. Despite small Ns, males (N = 8) and females (N = 10) were analyzed separately because of previous sex-divergent effects of tDCS on appetite variables (Burgess et al., 2016) and because to date, most tDCS studies on craving and eating have used samples composed of predominantly one sex over another (see Table 1). The separate analyses allowed for more comparable, albeit cursory, comparisons of results across studies. Age, hunger ratings, BMI, and time-of-day difference between visits were included as separate continuous covariates and ethnicity and order of stimulation condition were included as separate between-subject variables in the within-subject MANOVAs. Only covariates and between-subject variables with a significant effect on the active vs. control tDCS outcomes were retained when then testing for any moderating effects of psychological traits (BIS and DEBQ-R sub-scale scores on active vs. control tDCS outcomes). Finally, between-subject MANOVAs determined differences between males and females on craving ratings and kcals consumed (Table 4). Four male participants had a lab-measured BMI < 30, but in the upper overweight range: 28.0, 28.4, 29.4, and 29.5. They were included because of the difficulty in recruiting males with a BMI >30 and because analyses revealed no differences between these and the other males on the results reported. The data were tested for outliers and none were found. Data are reported as mean (M) and 95% confidence interval (CI).
Table 3.
Participant descriptives and psychological trait scores obtained at the onset of the study. Values reflect means (SD).
| All (N = 18) | Female (N = 10) | Male (N = 8) | |
|---|---|---|---|
| Body Mass Index | 37.4 (9.1) | 40.7 (9.9) | 33.3 (6.3) |
| Hunger Ratings | |||
| tDCS | 3.8 (2.2) | 3.5 (2.3) | 4.3 (2.1) |
| Control | 4.0 (1.4) | 3.6 (1.3) | 4.5 (1.4) |
| Age | 22.7 (7.9) | 24.4 (10.3) | 20.6 (2.7) |
| Ethnicity | |||
| White | 10 (56%) | 3 (30%) | 7 (88%) |
| Black | 6 (33%) | 6 (60%) | 0 (0%) |
| Asian or Hispanic | 2 (11%) | 1 (10%) | 1 (12%) |
| Barratt Impulsiveness Scale | |||
| Attention | 15.7 (3.8) | 16.6 (4.3) | 14.6 (2.9) |
| Motor | 20.3 (4.7) | 21.2 (4.1) | 19.1 (5.4) |
| Nonplanning | 21.6 (6.0) | 25.0 (4.9) | 17.4 (4.6)** |
| Dutch Eating Behavior Q-Restraint | |||
| Intent | 3.0 (0.8) | 3.0 (0.9) | 3.0 (0.7) |
| Behavior | 3.0 (0.9) | 3.2 (1.0) | 2.8 (0.7) |
p < 0.01 females vs. males.
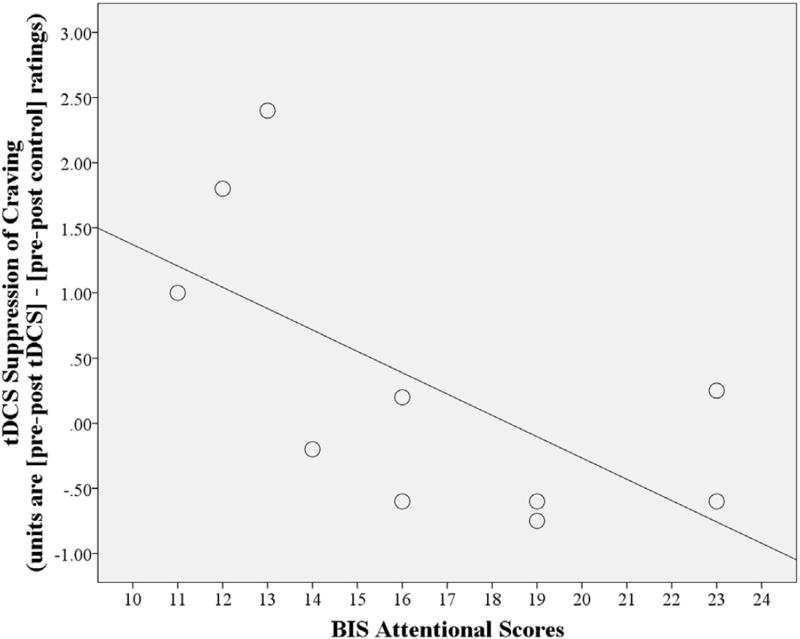
Fig. 2.
The effect of tDCS to suppress food craving for mixed foods is greater in females (N = 10) with lower Barratt Impulsiveness Scale (BIS) Attentional scores. Attentional scores × stimulation condition interaction, p = 0.047; R2 = 0.41.
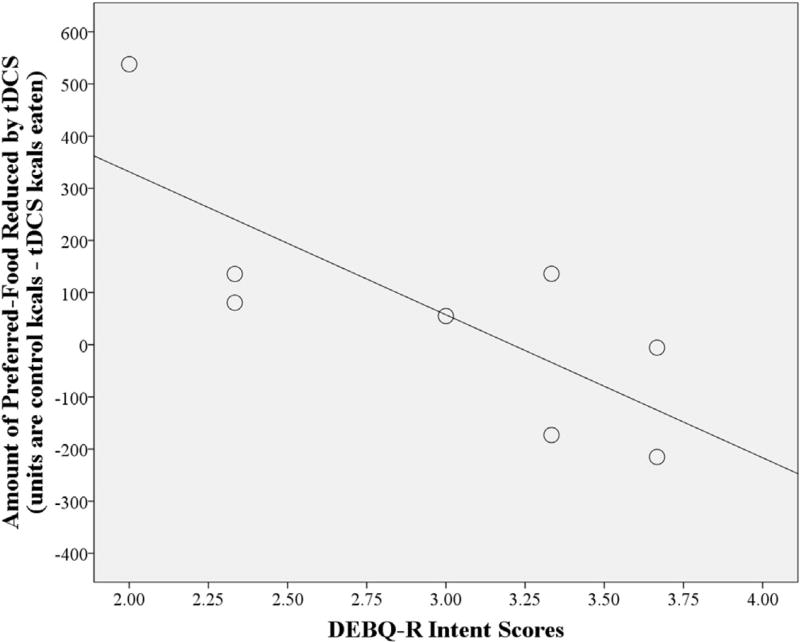
Fig. 3.
The effect of tDCS to suppress preferred food consumption is greater in males (N = 8) with lower Dutch Eating Behavior Questionnaire-Restraint (DEBQ-R) Intent scores. Intent scores × stimulation condition interaction, p = 0.024; R2 = 0.600.
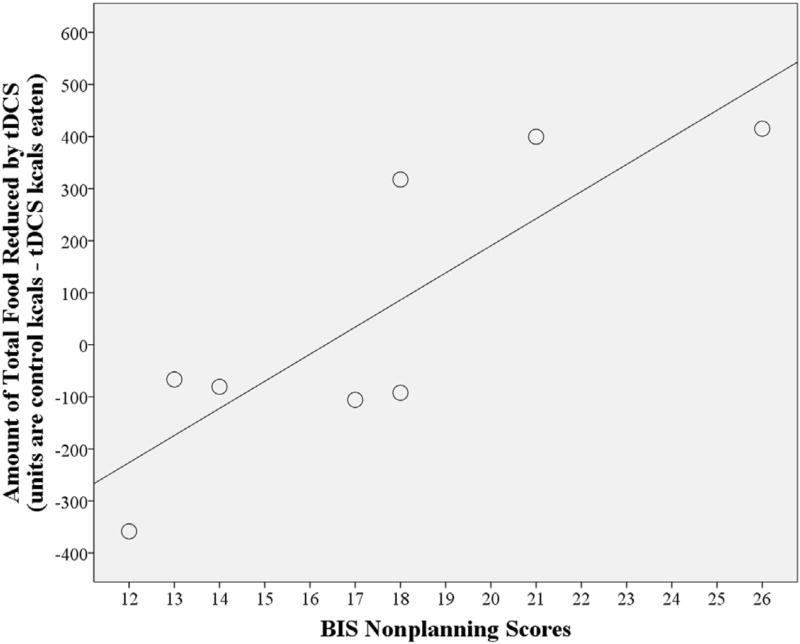
Fig. 4.
The effect of tDCS to suppress total food consumption is greater in males (N = 8) with higher Barratt Impulsiveness Scale (BIS) Nonplanning scores. Nonplanning scores × stimulation condition interaction, p = 0.009; R2 = 0.71.
Table 4.
Food craving scores for the all-foods category and kcals consumed.
| All (N = 18) | Female (N = 10) | Male (N = 8) | |
|---|---|---|---|
| Food Craving (rating scores) | |||
| Liking | |||
| Control | 2.9 (0.4) | 2.8 (0.4) | 3.0 (0.5) |
| tDCS | 2.9 (0.4) | 2.9 (0.4) | 2.9 (0.4) |
| Wanting | |||
| Pre-Control | 2.1 (0.8) | 2.2 (0.6) | 2.0 (1.1) |
| Post-Control | 2.1 (0.8) | 2.3 (0.7) | 1.9 (1.0) |
| Pre-tDCS | 2.4 (0.6) | 2.6 (0.5) | 2.1 (0.7) |
| Post-tDCS | 2.1 (0.9) | 2.3 (0.8) | 1.8 (1.0) |
| Food Consumption (kcals) | |||
| Preferred food | |||
| Control | 354.2 (244.5) | 223.1 (119.8) | 518.2 (267.3)** |
| tDCS | 344.1 (196.3) | 259.9 (114.8) | 449.3 (232.1)* |
| Less-preferred food | |||
| Control | 185.6 (166.6) | 130.7 (77.6) | 254.2 (223.6) |
| tDCS | 171.3 (143.3) | 98.8 (66.9) | 261.9 (165.1) * |
| Total food | |||
| Control | 719.9 (496.9) | 474.6 (227.7) | 1026.6 (582.6)* |
| tDCS | 686.6 (378.4) | 457.5 (164.3) | 973.1 (379.8)** |
p < 0.05,
p < 0.01 males vs. females. Rating scores could range from 0 to 4.
3. Results
3.1. Demographics, hunger, BMI, and psychological questionnaires
Table 3 lists the mean age, BMI, and hunger ratings for females, males, and the overall sample. These variables did not differ between females and males (all p > 0.05). Table 3 also lists mean BIS and DEBQ-R subscale scores. BIS Nonplanning scores were significantly higher in females compared to males in this sample [F(1,16) = 11.49, p = 0.004] but comparable to those obtained from healthy adult samples (Stanford et al., 2009) and samples with non-BED obesity (Nasser, Gluck, & Geliebter, 2004). The mean DEBQ-R scores for the Intent and Behavior subscales did not differ between females and males in the present sample and were are also comparable to other samples studied with obesity (Larsen, van Strien, Eisinga, Herman, & Engels, 2007).
In females, age, hunger ratings, BMI, time-of-day difference between visits, ethnicity, and order of stimulation condition did not influence the effect of tDCS on food craving or eating. In males, ethnicity and order of stimulation condition influenced the effect of tDCS on craving for mixed foods so were included as covariates. Some of the psychological trait scores influenced tDCS outcomes for females and for males as detailed below.
3.2. Effect of tDCS on food craving
For females, there was no main effect of stimulation condition (active vs. control tDCS). However, differences emerged when BIS Attentional scores were included as a covariate. Active tDCS reduced craving more than control. Specifically, active tDCS reduced craving for: sweets [F(1,8) = 5.4, p = 0.049, M = 0.2, CI = −0.4, 0.7], for fatty proteins [F(1,8) = 6.0, p = 0.04, M = 0.3, CI = −0.3, 0.8], for mixed foods [F(1,8) = 6.4, p = 0.036, M = 0.3, CI = −0.3, 0.9], and for the all-foods category [F(1,8) = 6.4, p = 0.035, M = 0.3, CI = −0.2, 0.7] compared to control tDCS: sweets (M = −0.2, CI = −0.6, 0.2), fatty proteins (M = −0.2, CI = −0.6, 0.3), mixed (M = 0.0, CI = −0.4, 0.4), and the all-foods category (M = −0.1, CI = −0.4, 0.2). There was also a significant BIS Attention × stimulation condition interaction on craving for mixed foods [F(1,8) = 5.5, p = 0.047] such that active vs. control tDCS exerted a stronger suppression of craving in females with lower BIS Attentional scores (Fig. 2).
For males, there was no main effect of stimulation condition (active vs. control tDCS) on any of the food craving categories with or without controlling for trait scores (all p > 0.05).
As shown in Table 4, liking ratings, pre-vs. post-control wanting ratings, or pre-vs. post-tDCS wanting ratings (all p > 0.05) did not differ between females and males.
3.3. Effect of tDCS on preferred, less-preferred, and total in-lab food consumption
For females, there was no main effect of stimulation condition (active vs. control tDCS) on preferred, non-preferred, or total food consumption with or without controlling for trait scores (all p > 0.05).
In males, there was no main effect of stimulation condition (active vs. control tDCS). However, differences emerged when DEBQ-R Intent scores were included as a covariate. Active tDCS reduced preferred-food kcals consumed compared to control (by 13.3% of control kcals) [F(1,6) = 10.2, p = 0.019; active tDCS M = 449.3, CI = 239.7, 658.9 vs. control tDCS M = 518.2, CI = 294.5, 741.8]. There was also a significant DEBQ-R Intent × stimulation condition interaction [F(1,6) = 9.0, p = 0.024] such that the reduction of preferred-food consumption by active tDCS was greater in males with lower DEBQ-R Intent scores (Fig. 3).
Additionally in males, when covarying for BIS Nonplanning scores, active tDCS significantly reduced total food consumption compared to control tDCS (by 5.2% of control kcals) [F(1,6) = 12.0, p = 0.014, active tDCS M = 973.1, CI = 692.1, 1254.0 vs. control tDCS M = 1026.6, CI = 706.6, 1346.7]. This reduction was primarily for preferred-vs. less-preferred food (reduction of 68.9 vs. an increase of 7.6 kcals, respectively). There was also a significant BIS Nonplanning × stimulation condition interaction [F(1,6) = 14.3, p = 0.009] such that tDCS reduced total food consumption more in males with higher BIS Nonplanning scores (Fig. 4). There was no effect of tDCS on less-preferred food consumption in males (p > 0.05).
As shown in Table 4, males ate significantly more kcals than females with active tDCS from preferred [F(1,16) = 5.1, p = 0.038, male M = 449.3, CI = 317.3, 581.2 vs. female M = 259.9, CI = 141.9, 377.9], less-preferred [F(1,16) = 8.1, p = 0.011, male M = 261.9, CI = 171.8, 352.0 vs. female M = 98.8, CI = 18.2, 179.4], and total food [F(1,16) = 15.1, p = 0.001, male M = 973.1, CI = 763.4, 1182.8 vs. female M = 457.5, CI = 269.9, 645.1]. As expected, males also ate significantly more kcals than females with control tDCS from preferred [F(1,16) = 9.8, p = 0.006, male M = 518.2, CI = 369.5, 666.8 vs. female M = 223.1, CI = 90.2, 356.0] and total food [F(1,16) = 7.6, p = 0.014, male M = 1026.7, CI = 710.7, 1342.6 vs. female M = 474.6, CI = 192.0, 757.1].
3.4. Stimulation tolerability
Participants reported comparable sensations for tDCS and control, mostly tingling, itching, and warmth under the electrodes. The mean discomfort ratings were not significantly different between stimulation conditions [active tDCS = 2.2 (1.9) vs. control tDCS = 1.8 (1.6); ns].
4. Discussion
The main aim of this study was to assess the effect of tDCS to suppress food craving and consumption in individuals with frank (non-bingeeating) obesity using the most efficacious electrode montage used in appetite studies. The study also assessed the moderating influence of cognitive-based functions of the DLPFC on the efficacy of tDCS to suppress craving and eating. Males and females were tested separately as previous data suggested that the sexes respond differently to tDCS and it is important to understand how each sex responds to tDCS given its promising treatment potential. The results of this single-session study provide favorable initial evidence for tDCS to serve as a treatment or adjunct treatment for frank obesity and highlight the importance of controlling for cognitive-based traits when targeting the DLPFC with tDCS to ameliorate obesogenic factors.
While there were no main effects of active vs. control tDCS, significant effects for active tDCS to suppress food craving and food consumption over control tDCS emerged when considering cognitive-based psychological traits. Specifically, intent to restrict calories, as measured by the DEBQ-R Intent subscale, and Non-planning impulsivity, as measured by the BIS subscales, influenced tDCS effects on eating in males; whereas Attentional impulsivity, as measured by the BIS, influenced tDCS effects on craving in females. Interestingly, individual differences on the Behavior subscale of the DEBQ-R and the Motor subscale of the BIS did not affect tDCS response. Together this pattern suggests that differences in traits involving cognitive vs. motor functions are more susceptible to anti-obesity effects of tDCS in frank obesity and support the right DLPFC as a rational target for stimulation given its critical role in cognitive control (Hare, Camerer, & Rangel, 2009).
We hypothesized that tDCS effects would be stronger in those with lower impulsivity because there might be less interference on tDCS to enhance cognitive control. In support of this hypothesis, tDCS was found to suppress craving more effectively in females with lower BIS Attentional scores, i.e. with greater ability to pay attention. Conversely, those with higher Attentional scores (lower ability to pay attention) have been found to, paradoxically, be hyper-attentive to rewarding stimuli including highly palatable food (Hou et al., 2011). Since the craving test used photos of highly palatable foods and because these foods play an integral role in obesity, more tDCS sessions may be required for this subgroup of individuals with obesity.
Greater attention to food also occurs in overweight and obese individuals that are dieting vs. not dieting (Bazzaz, Fadardi, & Parkinson, 2017). This may explain why tDCS was not as effective at reducing food consumption in those with higher intent to restrict as measured by the DEBQ-R, but significantly reduced eating in those with lower intent to restrict scores. This effect was significant for preferred-food and only in the male sample. The greater suppression of tDCS on preferred but not less-preferred food is important as overeating occurs more frequently with preferred foods (Epstein, Leddy, Temple, & Faith, 2007). The effect of tDCS to reduce preferred but not less-preferred food replicates findings from our previous BED obesity study (Burgess et al., 2016) and suggests that food-stimuli that normally arouses a greater need for cognitive control is more malleable to neuromodulation by tDCS.
The other significant effect of tDCS on food consumption was to suppress total consumption in males with higher Nonplanning impulsivity scores. This was contrary to our hypothesis that lower impulsivity scores would facilitate the effects of tDCS to suppress eating. Impulsiveness is complex and cannot be represented as an all-encompassing construct (Patton et al., 1995). However, it is interesting that others have found Attentional and Nonplanning scores to have opposite relationships on eating behavior. Specifically, higher Attentional scores are related to binge-eating (Meule & Platte, 2015) while higher Nonplanning scores are related to reduced food intake in laboratory settings (Nasser et al., 2004). If those with more difficulty in planning are already primed to eat less in controlled settings as suggested (Nasser et al., 2004), it may help explain why the males with higher Nonplanning scores (more difficulty planning) in this study were more sensitive to the consumption-suppressing effects of tDCS. Indeed, tDCS exerts its effects not by inducing action potentials but augmenting spontaneous or ‘primed’ neural activity (Creutzfeldt et al., 1962).
A last important finding revealed by the inclusion of a male and female sample in this study is that differences in baseline cognitive-related traits, alone, cannot account for differences in tDCS responding. For example, tDCS suppressed craving only in females with lower Attentional impulsivity scores but not in males, despite comparable Attentional scores in the males. tDCS also suppressed food intake only in males with lower Intent to Restrict scores and in males with higher Nonplanning impulsivity scores, despite that the females had comparable Intent to Restrict scores and higher Non-planning impulsivity scores. Clearly, both sex and baseline psychological differences play a role in tDCS responsiveness and should be considered in future tDCS studies aimed at treating obesity. Moreover, as a neuromodulator, tDCS can be used as a tool to elucidate the unique physiology underlying sex- and trait-based subgroups of obesity.
The study had some limitations. First, when comparing the effect of tDCS on craving and eating in this study with other tDCS studies, it should be kept in mind that the tests used to measure these constructs vary between studies and the food photo test has not been psychometrically validated. Second, the findings may not generalize to other populations since our sample was mainly college students. Third, ecological validity may have been compromised by asking the participants to “not be too hungry or too full” when coming to the lab. Lastly, this is an initial single-tDCS session study in individuals with non-BED, or “frank” obesity with small Ns representing the male and female subgroups. Larger studies administering multiple sessions of tDCS are needed with the right anode/left cathode montage in this important population. Future studies should also test tDCS effects in different obesity subgroups such as those seeking weight-loss and in different BMI-defined subgroups (i.e., Class I, II, and III). Other cognitive traits are also bound to vary among those with frank obesity and should likewise be considered when evaluating the promise of tDCS as a treatment or adjunct therapy.
Despite the study limitations, there are important strengths. Namely, the investigation tested individuals with frank obesity, a sample representing approximately 1/3rd of the U.S. population (Hudson, Hiripi, Pope, & Kessler, 2007; Ogden et al., 2014), with the most promising tDCS montage. Another strength of the study was the inclusion of males and females and their separate analysis because this elucidated trait × stimulation condition interactions that otherwise would not have been found if only one sex was tested.
5. Conclusion
The results may help to identify trait- and sex-based subgroups that respond best to tDCS as a treatment or adjunct therapy for obesity and to predict what subgroups will require a more intensive tDCS regimen. Finally, among the traits examined, the particular influence of cognitive-based baseline traits on tDCS responses provide additional support for the DLPFC as the ideal target in the neuromodulation treatment of obesity.
Acknowledgments
funding
The project described was supported by Award Number P30DK056336 from the National Institute Of Diabetes And Digestive And Kidney Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute Of Diabetes And Digestive And Kidney Diseases or the National Institutes of Health."
We also acknowledge the support of an NIH-T32 Pre-Doctoral training grant to MKR made possible by UAB's Nutrition Obesity Research Center and Dept. of Psychology Incentive Funds to MMB. We also acknowledge Dr. Edwin Cook's assistance with the Intro to Psych research survey.
Abbreviations
- BED
Binge-eating disorder
- BIS
Barratt Impulsiveness Scale
- DEBQ-R
Dutch Eating Behavior Questionnaire-Restraint.
Footnotes
Conflicts of interest
None.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Balodis IM, Molina ND, Kober H, Worhunsky PD, White MA, Sinha R, et al. Divergent neural substrates of inhibitory control in binge eating disorder relative to other manifestations of obesity. Obesity (Silver Spring) 2016;21:367–377. doi: 10.1002/oby.20068. http://dx.doi.org/10.1002/oby.20068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzaz MM, Fadardi JS, Parkinson J. Efficacy of the attention control training program on reducing attentional bias in obese and overweight dieters. Appetite. 2017;108:1–11. doi: 10.1016/j.appet.2016.08.114. http://dx.doi.org/10.1016/j.appet.2016.08.114. [DOI] [PubMed] [Google Scholar]
- Bikson M, Grossman P, Thomas C, Zannou AL, Jiang J, Adnan T, et al. Safety of transcranial direct current stimulation: Evidence based update 2016. Brain Stimulation. 2016;9:641–661. doi: 10.1016/j.brs.2016.06.004. http://dx.doi.org/10.1016/j.brs.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo GL, Poje AB, Perissinotti I, Marcondes BF, Villamar MF, Manzardo AM, et al. Transcranial direct current stimulation reduces food-craving and measures of hyperphagia behavior in participants with Prader-Willi syndrome. American Journal of Medical Genetics B Neuropsychiatric Genetics. 2016;171B:266–275. doi: 10.1002/ajmg.b.32401. http://dx.doi.org/10.1002/ajmg.b.32401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray GA, Frübeck G, Ryan DH, Wilding JP. Management of obesity. Lancet. 2016;7:1947–1956. doi: 10.1016/S0140-6736(16)00271-3. http://dx.doi.org/10.1016/S0140-6736(16)00271-3. [DOI] [PubMed] [Google Scholar]
- Burgess EE, Sylvester MD, Morse KE, Amthor FR, Mrug S, Lokken KL, et al. Effects of transcranial direct current stimulation (tDCS) on binge-eating disorder. International Journal of Eating Disorders. 2016;49:930–936. doi: 10.1002/eat.22554. http://dx.doi.org/10.1002/eat.22554. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt OD, Fromm GH, Kapp H. Influence of transcortical d-c currents on cortical neuronal activity. Experimental Neurology. 1962;5:436–452. doi: 10.1016/0014-4886(62)90056-0. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Leddy JJ, Temple JL, Faith MS. Food reinforcement and eating: A multilevel analysis. Psychological Bulletin. 2007;133:884–906. doi: 10.1037/0033-2909.133.5.884. http://dx.doi.org/10.1037/0033-2909.133.5.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. International Journal of Obesity. 2000;24:38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- Fregni F, Orsati F, Pedrosa W, Fecteau S, Tome FA, Nitsche MA, et al. Transcranial direct current stimulation of the prefrontal cortex modulates the desire for specific foods. Appetite. 2008;51:34–41. doi: 10.1016/j.appet.2007.09.016. http://dx.doi.org/10.1016/j.appet.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert SJ, Burgess PW. Executive function. Current Biology. 2008;18:R110–R114. doi: 10.1016/j.cub.2007.12.014. http://dx.doi.org/10.1016/j.cub.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Gluck ME, Alonso-Alonso M, Piaggi P, Weise CM, Jumpertz-von Schwartzenberg R, Reinhardt M, et al. Neuromodulation targeted to the prefrontal cortex induces changes in energy intake and weight loss in obesity. Obesity (Silver Spring) 2016;23:2149–2156. doi: 10.1002/oby.21313. http://dx.doi.org/10.1002/oby.21313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman RL, Borckardt JJ, Frohman HA, O'Neil PM, Madan A, Campbell LK, et al. Prefrontal cortex transcranial direct current stimulation (tDCS) temporarily reduces food cravings and increases the self-reported ability to resist food in adults with frequent food craving. Appetite. 2011;56:741–746. doi: 10.1016/j.appet.2011.02.013. http://dx.doi.org/10.1016/j.appet.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–648. doi: 10.1126/science.1168450. http://dx.doi.org/10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Herbozo S, Schaefer LM, Thompson JK. A comparison of eating disorder psychopathology, appearance satisfaction, and self-esteem in overweight and obese women with and without binge eating. Eating Behaviors. 2015;17:86–89. doi: 10.1016/j.eatbeh.2015.01.007. http://dx.doi.org/10.1016/j.eatbeh.2015.01.007. [DOI] [PubMed] [Google Scholar]
- Hou R, Mogg K, Bradley BP, Moss-Morris R, Peveler R, Roefs A. External eating, impulsivity and attentional bias to food cues. Appetite. 2011;56:424–427. doi: 10.1016/j.appet.2011.01.019. http://dx.doi.org/10.1016/j.appet.2011.01.019. [DOI] [PubMed] [Google Scholar]
- Hsu TY, Juan CH, Tseng P. Individual differences and state-dependent responses in transcranial direct current stimulation. Frontiers in Human Neuroscience. 2016;10:651. doi: 10.3389/fnhum.2016.00643. http://dx.doi.org/10.3389/fnhum.2016.00651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson JI, Hiripi E, Pope HG, Jr, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biological Psychiatry. 2007;61:348–358. doi: 10.1016/j.biopsych.2006.03.040. http://dx.doi.org/10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauch-Chara K, Kistenmacher A, Herzog N, Schwarz M, Schweiger U, Oltmanns KM. Repetitive electric brain stimulation reduces food intake in humans. American Journal of Clinical Nutrition. 2014;100:1003–1009. doi: 10.3945/ajcn.113.075481. http://dx.doi.org/10.3945/ajcn.113.075481. [DOI] [PubMed] [Google Scholar]
- Kekic M, McClelland J, Campbell I, Nestler S, Rubia K, David AS, et al. The effects of prefrontal cortex transcranial direct current stimulation (tDCS) on food craving and temporal discounting in women with frequent food cravings. Appetite. 2014;78:55–62. doi: 10.1016/j.appet.2014.03.010. http://dx.doi.org/10.1016/j.appet.2014.03.010. [DOI] [PubMed] [Google Scholar]
- Kim JH, Kim DW, Chang WH, Kim YH, Kim K, Im CH. Inconsistent outcomes of transcranial direct current stimulation may originate from anatomical differences among individuals: Electric field simulation using individual MRI data. Frontiers in Human Neuroscience. 2016;10:643. doi: 10.1016/j.neulet.2014.01.054. http://dx.doi.org/10.3389/fnhum.2016.00643. [DOI] [PubMed] [Google Scholar]
- Lapenta OM, Sierve KD, de Macedo EC, Fregni F, Boggio PS. Transcranial direct current stimulation modulates ERP-indexed inhibitory control and reduces food consumption. Appetite. 2014;83:42–48. doi: 10.1016/j.appet.2014.08.005. http://dx.doi.org/10.1016/j.appet.2014.08.005. [DOI] [PubMed] [Google Scholar]
- Larsen JK, van Strien T, Eisinga R, Herman CP, Engels RC. Dietary restraint: Intention versus behavior to restrict food intake. Appetite. 2007;49:100–108. doi: 10.1016/j.appet.2006.12.005. http://dx.doi.org/10.1016/j.appet.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Ljubisavljevic M, Maxood K, Bjekic J, Oommen J, Nagelkerke N. Long-term effects of repeated prefrontal cortex transcranial direct current stimulation (tDCS) on food craving in normal and overweight young adults. Brain Stimulation. 2016 doi: 10.1016/j.brs.2016.07.002. http://dx.doi.org/10.1016/j.brs.2016.07.002. Epub ahead of print. [DOI] [PubMed]
- Meule A, Platte P. Facets of impulsivity interactively predict body fat and binge eating in young women. Appetite. 2015;87:352–357. doi: 10.1016/j.appet.2015.01.003. http://dx.doi.org/10.1016/j.appet.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Montenegro RA, Okano AH, Cunha FA, Gurgel JL, Fontes EB, Farinatti PT. Prefrontal cortex transcranial direct current stimulation associated with aerobic exercise change aspects of appetite sensation in overweight adults. Appetite. 2012;58:333–338. doi: 10.1016/j.appet.2011.11.008. http://dx.doi.org/10.1016/j.appet.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Nasser JA, Gluck ME, Geliebter A. Impulsivity and test meal intake in obese binge eating women. Appetite. 2004;43:303–307. doi: 10.1016/j.appet.2004.04.006. http://dx.doi.org/10.1016/j.appet.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. Journal of the American Medical Association. 2014;311:806–814. doi: 10.1001/jama.2014.732. http://dx.doi.org/10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. Journal of Clinical Psychology. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Schag K, Schönleber J, Teufel M, Zipfel S, Giel KE. Food-related impulsivity in obesity and binge eating disorder–a systematic review. Obesity Reviews. 2013;14:477–495. doi: 10.1111/obr.12017. http://dx.doi.org/10.1111/obr.12017. [DOI] [PubMed] [Google Scholar]
- Smith KB, Smith MS. Obesity statistics. Primary Care. 2016;43:121–135. doi: 10.1016/j.pop.2015.10.001. http://dx.doi.org/10.1016/j.pop.2015.10.001. [DOI] [PubMed] [Google Scholar]
- Stanford MS, Mathias CW, Dougherty DM, Lake SL, Anderson NE, Patton JH. Fifty years of the Barratt impulsiveness Scale: An update and review. Personality and Individual Differences. 2009;47:385–395. http://dx.doi.org/10.1016/j.paid.2009.04.008. [Google Scholar]
- Van Strien T, Fritjers JER, Bergers GPA, Defares PB. Dutch Eating Behaviour Questionnaire for assessment of restrained, emotional and external eating behaviour. International Journal of Eating Disorders. 1986;20:295–315. http://dx.doi.org/10.1002/1098-108X(198602)5:2<295::AIDEAT2260050209>3.0.CO;2-T. [Google Scholar]
- Wiegand A, Nieratschker V, Plewnia C. Genetic modulation of transcranial direct current stimulation effects on cognition. Frontiers in Human Neuroscience. 2016;10:651. doi: 10.3389/fnhum.2016.00651. http://dx.doi.org/10.3389/fnhum.2016.00651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing RR, Hill JO. Successful weight loss maintenance. Annual Review of Nutrition. 2001;21:323–341. doi: 10.1146/annurev.nutr.21.1.323. http://dx.doi.org/10.1146/annurev.nutr.21.1.323. [DOI] [PubMed] [Google Scholar]