Abstract
Enzalutamide significantly improved radiographic progression-free survival (rPFS) and overall survival (OS) among men with chemotherapy-naïve metastatic castration-resistant prostate cancer at the prespecified interim analysis of PREVAIL, a phase 3, double-blind, randomized study. We evaluated the longer-term efficacy and safety of enzalutamide up to the prespecified number of deaths in the final analysis, which included an additional 20 mo of follow-up for investigator-assessed rPFS, 9 mo of follow-up for OS, and 4 mo of follow-up for safety. Enzalutamide reduced the risk of radiographic progression or death by 68% (hazard ratio [HR] 0.32, 95% confidence interval [CI] 0.28–0.37; p < 0.0001) and the risk of death by 23% (HR 0.77, 95% CI 0.67–0.88; p = 0.0002). Median investigator-assessed rPFS was 20.0 mo (95% CI 18.9–22.1) in the enzalutamide arm and 5.4 mo (95% CI 4.1–5.6) in the placebo arm. Median OS was 35.3 mo (95% CI 32.2–not yet reached) in the enzalutamide arm and 31.3 mo (95% CI 28.8–34.2) in the placebo arm. At the time of the OS analysis, 167 patients in the placebo arm had crossed over to receive enzalutamide. The most common adverse events in the enzalutamide arm were fatigue, back pain, constipation, and arthralgia. This final analysis of PREVAIL provides more complete assessment of the clinical benefit of enzalutamide.
Keywords: Enzalutamide, Metastatic castration-resistant prostate cancer, Androgen receptor signaling inhibitor, Overall survival, Radiographic progression-free survival
Identification of key drivers of cancer proliferation and spread has facilitated significant advances in the treatment of an increasing number of malignancies. For prostate cancer, the androgen receptor (AR) is the principal therapeutic target. A series of trials have demonstrated that potent suppression of androgen signaling via receptor blockade [1,2] or inhibition of ligand production [3,4] extends the survival of men with metastatic castration-resistant prostate cancer (mCRPC).
The PREVAIL study, which evaluated treatment with the AR signaling inhibitor enzalutamide compared to placebo before chemotherapy, was stopped at the interim analysis point for patient benefit. Thus, the primary analysis was limited in its reporting of longer-term outcomes, with median follow-up of approximately 20 mo. Median overall survival (OS) estimates were unstable (32.4 mo in the enzalutamide group and 30.2 mo in the placebo group), and median radiographic progression-free survival (rPFS) could not be estimated in the initial analysis. A recent analysis of an early phase 1–2 enzalutamide study demonstrated that approximately one-quarter of chemotherapy-naïve patients were still on enzalutamide treatment after several years [5]. These results indicate that a subset of patients experience long-term disease control on enzalutamide. Therefore, we undertook an analysis of efficacy and safety data for patients continuing treatment in the PREVAIL study.
The full methodology for PREVAIL, a multinational, randomized, double-blind, placebo-controlled phase 3 study (NCT01212991), has been published elsewhere [2] and is summarized in the Supplementary material.
Following a final analysis of centrally read rPFS (data cutoff May 6, 2012) and a planned interim analysis of OS (data cutoff September 16, 2013), which became the final OS analysis after enzalutamide demonstrated a significant benefit over placebo, patients receiving placebo were offered the opportunity to receive enzalutamide (crossover began January 1, 2014). The primary safety data were analyzed on September 16, 2013 [2].
The joint primary endpoints were rPFS assessed by central independent review and OS. In the post hoc extended analysis, only investigator-assessed rPFS (as previously defined [2]) was evaluated using Prostate Cancer Clinical Trials Working Group 2 [6] guidelines for bone disease and Response Evaluation Criteria in Solid Tumors version 1.1 for soft-tissue disease. The updated safety analysis was performed on January 15, 2014 as a 90-d safety update based on communication with the US Food and Drug Administration. The extended investigator-assessed rPFS analysis was carried out at the same time and included 929 events. The extended OS analysis was based on a June 1, 2014 data cutoff after 784 events, the original prespecified number of events for the final analysis.
A total of 1717 patients were enrolled in PREVAIL (enzalutamide, n = 872; placebo, n = 845); 1715 patients received at least one dose of study drug (Supplementary Fig. 1) [2]. Baseline characteristics were well balanced between the two treatment arms (Supplementary Table 1) [2]. At the extended OS analysis data cutoff, 787 patients had entered the open-label extension (Supplementary Fig. 1). Of these, 325 patients received enzalutamide (158 from the original enzalutamide arm and 167 from the placebo arm). An additional 68 patients in the enzalutamide arm who were not yet enrolled in the open-label extension continued to receive enzalutamide. Therefore, at the time of data cutoff for the OS extended analysis, 26% of all patients randomized to receive enzalutamide at study entry were still being treated with enzalutamide.
Subsequent antineoplastic therapies (with demonstrated effects on survival in phase 3 trials) were administered to 52% of patients in the enzalutamide arm and 81% in the placebo arm (Table 1). At least one AR-targeted therapy (enzalutamide or abiraterone acetate) was administered as a subsequent therapy to 265 patients (30%) originally randomized to enzalutamide and to 538 patients (64%) originally randomized to placebo.
Table 1.
Use of subsequent antineoplastic therapy
| Enzalutamide (n = 872) | Placebo (n = 845) | |
|---|---|---|
| Patients taking ≥1 subsequent therapy, n (%) | 457 (52.4) | 685 (81.1) |
| Docetaxel | 358 (41.1) | 504 (59.6) |
| Abiraterone acetatea | 256 (29.4) | 417 (49.3) |
| Enzalutamideb | 21 (2.4) | 249 (29.5) |
| Cabazitaxel | 79 (9.1) | 149 (17.6) |
| Radium-223 dichloride | 16 (1.8) | 22 (2.6) |
| Sipuleucel-T | 17 (1.9) | 11 (1.3) |
Concomitant abiraterone acetate use was allowed before study drug discontinuation in patients with confirmed radiographic progression or a skeletal-related event.
Placebo patients who received enzalutamide in the open-label extension period are included in the subsequent therapy of enzalutamide under the placebo column.
For the extended analysis of investigator-assessed rPFS, which included an additional 490 events during 20 mo of recording after the final rPFS analysis, the median follow-up was 22 mo for the enzalutamide arm and 11 mo for the placebo arm. Treatment with enzalutamide reduced the risk of radiographic progression or death by 68% compared to placebo (hazard ratio [HR] 0.32, 95% confidence interval [CI] 0.28–0.37; p < 0.0001; Fig. 1). Median rPFS was 20.0 mo (95% CI 18.9–22.1) in the enzalutamide arm and 5.4 mo (95% CI 4.0–5.6) in the placebo arm.
Fig. 1.
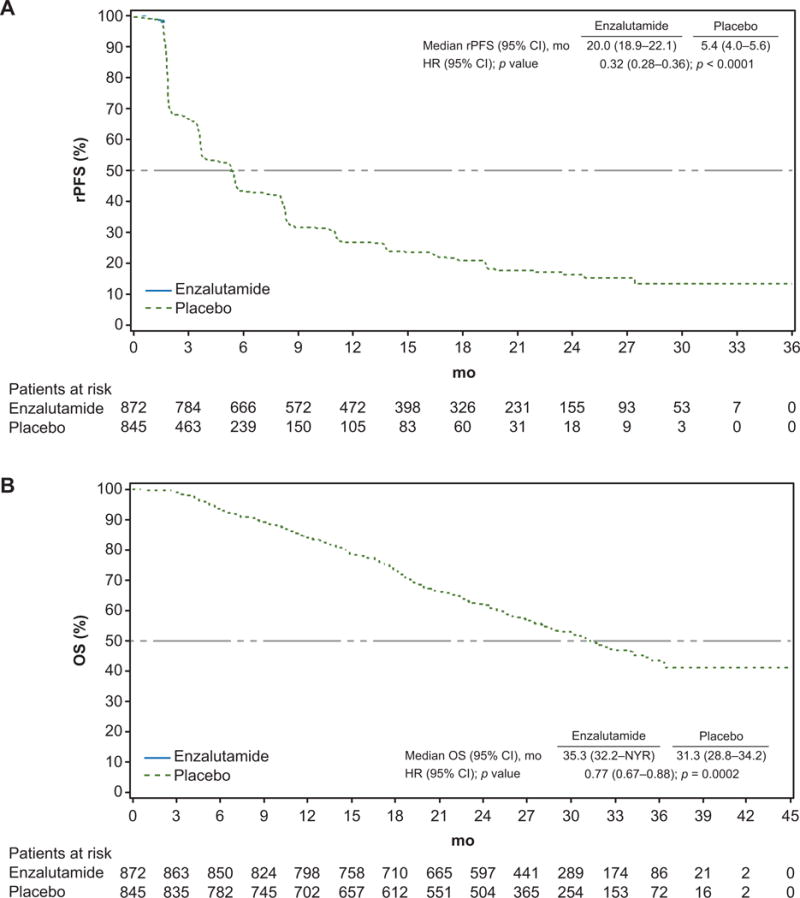
Kaplan-Meier estimates of radiographic progression-free survival (rPFS) and overall survival (OS) at the extended analysis. Dashed horizontal lines indicate median values. CI = confidence interval; HR = hazard ratio; NYR = not yet reached.
For the OS extended analysis, which included an additional 244 deaths during 9 mo of reporting, including 5 mo after patients in the open-label extension had crossed over from placebo to enzalutamide, the median follow-up was 31 mo. Treatment with enzalutamide reduced the risk of death by 23% compared to placebo (HR 0.77, 95% CI 0.67–0.88; p = 0.0002; Fig. 1). Median OS was 35.3 mo (95% CI 32.2–not yet reached) in the enzalutamide arm and 31.3 mo (95% CI 28.8–34.2) in the cohort originally randomized to placebo. The treatment effect on OS was consistent across all prespecified subgroups (Supplementary Fig. 2).
The updated safety analysis included an additional 4 mo of reporting after the primary analysis, with the majority of data collected from patients remaining on enzalutamide treatment (Supplementary Table 2). Safety data collected on enzalutamide crossover in the open-label extension or in the commercial setting were not analyzed. The median treatment duration was 18.2 mo for the enzalutamide arm and 5.4 mo for the placebo arm. Grade 3 or higher adverse events were reported for 46% of patients in the enzalutamide arm and 37% of patients in the placebo arm. Adverse events that led to treatment discontinuation were comparable between the enzalutamide and placebo arms (6% each). Adverse events that led to death occurred in 5% of patients in the enzalutamide arm and 4% of patients in the placebo arm.
The most common adverse events in the enzalutamide arm were fatigue, back pain, constipation, and arthralgia (Supplementary Table 2). The rate of fatigue when adjusted for duration of observation was 28 versus 42 events per 100 patient-years in the enzalutamide and placebo arms, respectively. The safety data reported here are similar to those reported in the primary analysis.
As PREVAIL was halted early because of patient benefit, these extended analyses provide an opportunity to include additional follow-up. We are now able to provide more stable estimates of the median values for both investigator-assessed rPFS and OS. Although OS was based on the prespecified number of events, both rPFS and safety were post hoc analyses. The demonstration of a large difference in rPFS and the confirmation of an OS benefit, even in the context of early use of extensive postprogression therapy in patients randomized to receive placebo (as part of crossover and in the course of standard patient care), affirms the PREVAIL results reported after the positive interim analysis.
Supplementary Material
Patient summary.
According to data from longer follow-up, enzalutamide continued to provide benefit over placebo in patients with metastatic castration-resistant prostate cancer.
Acknowledgments
Funding/Support and role of the sponsor: This study was supported by Medivation and Astellas Pharma, the developers of enzalutamide. The manuscript was reviewed and comments were provided to the authors by Medivation and Astellas Pharma. Writing and editorial support for the development of this manuscript was provided by Stephanie Vadasz and Shannon Davis from Infusion Communications and was funded by Medivation and Astellas Pharma.
Author contributions
Tomasz M. Beer had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Beer, Armstrong, Sternberg, Higano, Iversen, Tombal.
Acquisition of data: Beer, Armstrong, Rathkopf, Loriot, Sternberg, Higano, Iversen, Evans, Kim, Kimura, Miller, Saad, Bjartell, Borre, Mulders, Tammela, Tombal.
Analysis and interpretation of data: All authors.
Drafting of the manuscript: All authors.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Sari.
Obtaining funding: Study sponsors.
Administrative, technical, or material support: Study sponsors.
Supervision: Beer, Tombal.
Other: None.
Financial disclosures
Tomasz M. Beer certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Tomasz M. Beer has stock and other ownership interests in Salarius Pharmaceuticals; is an advisor for Astellas Pharma, AstraZeneca, Bayer, Churchill Pharmaceuticals, Dendreon, Janssen Japan, Novartis, and F. Hoffmann-La Roche; and has received institutional research funding from Astellas Pharma, Bristol-Myers Squibb, Dendreon, Janssen Research & Development, Medivation, OncoGenex, and Sotio. Andrew J. Armstrong is an advisor for Astellas, Bayer, Dendreon, Janssen, Medivation, and Sanofi Aventis; has received honoraria from Dendreon and Sanofi Aventis; has received speaker fees from Dendreon; has received institutional research funding from Active Biotech, Astellas, Bayer, Dendreon, Janssen, Medivation, Novartis, Pfizer, and Sanofi Aventis; has received travel expenses from Dendreon, Janssen, and Medivation; and has a patent pending involving his institution and Janssen for technology relating to oncology. Dana Rathkopf has received research funding from Celgene, Ferring, Janssen, Medivation, Millenium, and Novartis. Yohann Loriot is an advisor for Astellas, Bristol-Myers Squibb, Ipsen, Janssen, Roche, and Sanofi; has received institutional research funding from Sanofi; and has received travel expenses from Astellas, Bristol-Myers Squibb, and Sanofi. Cora N. Sternberg has received institutional research funding or honoraria from Astellas, Bayer, Cougar Biotechnology (now Janssen Oncology), Medivation, and Sanofi. Celestia S. Higano is an advisor for AbbVie, Bayer, BHR Pharma, Dendreon, Emergent BioSolutions, Ferring, Genentech, Medivation, Orion Corporation, Pfizer, and Sanofi; has received institutional research funding from Algeta/Bayer, Aragon Pharmaceuticals, AstraZeneca, Dendreon, Emergent BioSolutions, Exelixis, Genentech, Medivation, Millennium, Oncogenex, Sanofi, and Teva; and has received travel expenses from AbbVie, Amgen, Astellas Pharma, Bayer, Dendreon, Emergent BioSolutions, Ferring, Genentech, Johnson & Johnson, Medivation, Ockham, Orion Pharma, Pfizer, Sanofi, and Teva. Peter Iversen is an advisor for Astellas, Ferring, Janssen, and Medivation; and has received research funding from Astellas, Bavarian Nordic, and Medivation. Christopher P. Evans is an advisor and speaker for and has received research funding from in Medivation and is a shareholder in the company. Go Kimura has received honoraria and research funding from Astellas, Bayer, GlaxoSmithKline, Novartis, Ono Pharmaceutical, Pfizer, and Takeda. Kurt Miller is an advisor for Astellas, Amgen, Janssen-Cilag, Medivation, Novartis, and Roche; has received honoraria from Astellas and Medivation; and has received speaker fees from Janssen-Cilag, Novartis, and Pierre Fabre. Fred Saad has received honoraria and research funding from and is an advisor for Astellas, Janssen, and Medivation. Peter Mulders is an advisor for and has received honoraria from Astellas, AstraZeneca, GlaxoSmithKline, Janssen, and Johnson & Johnson; and has received institutional research support from Bayer. Teuvo L. Tammela is an advisor for Astellas, Ferring, Orion Pharma, Bayer, and Sanofi, and has received institutional research funding from Astellas, Ferring, Medivation, Orion, and Bayer. Teresa Parli and Suha Sari are employees of Medivation. Steve van Os and Ad Theeuwes are employees of Astellas. Bertrand Tombal has received research funding from Astellas, Ferring, and Sanofi, and honoraria from Astellas, Bayer, Ferring, Medivation, and Sanofi. Choung-Soo Kim, Anders S. Bjartell and Michael Borre have nothing to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
In this extended analysis of the PREVAIL study, enzalutamide continued to provide significant benefit over placebo even in the context of postprogression therapy.
PREVAIL is registered on ClinicalTrials.gov as NCT01212991.
References
- 1.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 2.Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–33. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–48. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Higano CS, Beer TM, Taplin ME, et al. Long-term safety and antitumor activity in the phase 1–2 study of enzalutamide in pre- and post-docetaxel castration-resistant prostate cancer. Eur Urol. 2015;68:795–801. doi: 10.1016/j.eururo.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: Recommendations of the prostate cancer clinical trials working group. J Clin Oncol. 2008;26:1148–59. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


