Abstract
Background
Abiraterone acetate (AA) is the prodrug of abiraterone, which inhibits CYP17A1 and testosterone synthesis and prolongs the survival of patients with metastatic castration-resistant prostate cancer (mCRPC). AA plus prednisone (AA + P) is approved for the treatment of patients with mCRPC.
Objective
To investigate whether long-term use of low-dose prednisone with or without AA leads to corticosteroid-associated adverse events (CA-AEs) in mCRPC patients.
Design, setting, and participants
The study included 2267 patients in COU-AA-301 and COU-AA-302. We used an inclusive Standardized MedDRA Queries–oriented approach to identify 112 preferred terms for known CA-AEs, and assessed the incidence of CA-AEs during 3-mo exposure intervals and across all prednisone exposure levels.
Intervention
All 2267 patients received 5 mg of prednisone twice daily, and 1333/2267 received AA (1 g) plus prednisone.
Results and limitations
The CA-AE incidence after any prednisone exposure was 25%, 26%, and 23% for any grade, and 5%, 5%, and 4% for grade ≥3 CA-AEs for all patients and the AA + P and P alone groups, respectively. The most common any-grade CA-AEs were hyperglycemia (7.4%, 7.8%, and 6.9% for all patients, AA + P, and P alone, respectively) and weight increase (4.3%, 3.9%, and 4.8%, respectively). When assessed by duration of exposure (3-mo intervals up to ≥30 mo), no discernable trend was observed for CA-AEs, including hyperglycemia and weight increase. The investigator-reported study discontinuation rate due to CA-AEs was 11/2267 (0.5%), and one patient had a CA-AE resulting in death.
Conclusions
Low-dose prednisone given with or without AA is associated with low overall incidence of CA-AEs. The frequency of CA-AEs remained low with increased duration of exposure to prednisone.
Patient summary
We assessed adverse events in patients with metastatic castration-resistant prostate cancer during long-term treatment with a low dose of a corticosteroid. We found that long-term treatment with this low-dose corticosteroid is safe and tolerable.
Keywords: Abiraterone acetate, Adverse events, Corticosteroids, Glucocorticoid, Long term, Metastatic castration-resistant prostate cancer, Tolerability
1. Introduction
Abiraterone acetate (AA) is the prodrug of abiraterone, which selectively blocks CYP17. CYP17 is required for androgen biosynthesis, which occurs in testicular, adrenal, and prostatic tumor tissue [1–3]. AA plus prednisone (AA + P) is approved for the treatment of patients with metastatic castration-resistant prostate cancer (mCRPC) on the basis of a demonstrated survival benefit and a favorable tolerability profile in both prechemotherapy and postchemotherapy settings [4–8]. In the final analysis of the phase 3 COU-AA-301 trial of postchemotherapy patients with mCRPC, AA + P significantly prolonged median overall survival (OS) by 4.6 mo (26% reduction in risk of death compared to placebo plus prednisone, hereafter denoted P alone) [4,5]. Compared with P alone, AA + P significantly prolonged OS by 4.4 mo (19% reduction in risk of death) in the final analysis of the phase 3 COU-AA-302 trial of prechemotherapy patients with mCRPC [6–8].
Corticosteroids may be used by patients with cancer, including those with mCRPC, to manage cancer-related pain and weight loss and the side effects of chemotherapy [9,10]. Long-term use of moderate or high corticosteroid doses (eg, ≥20 mg/d prednisone) has an established adverse event (AE) profile [11,12]. A list of common corticosteroid-associated AEs (CA-AEs) is presented in Section 2. There are some concerns about whether the frequency and severity of side effects would be similar for long-term coadministration of AA and low-dose P. To address this question, we used the COU-AA-301 and COU-AA-302 data sets to investigate whether long-term use of low-dose P with or without AA in patients with mCRPC leads to CA-AEs.
2. Patients and methods
The design, eligibility criteria, and efficacy results for the COU-AA-301 (NCT00638690) and COU-AA-302 (NCT00887198) phase 3 trials have been described previously [4–8]. In brief, COU-AA-301 and COU-AA-302 randomized post-docetaxel and chemotherapy-naïve mCRPC patients 2:1 and 1:1 to AA (1 g) plus P or to P alone (5 mg twice a day [BID]), respectively [4–8]. A total of 1195 patients were enrolled in COU-AA-301 (AA + P, n = 797; P alone, n = 398) [4,5] and 1088 patients in COU-AA-302 (AA + P, n = 546; P alone, n = 542) [6–8]. The combined total of 2267 patients in COU-AA-301 and COU-AA-302 were included in the safety population and received 5 mg BID prednisone. Of these, 1333 patients received AA + P.
We used an inclusive Standardized MedDRA Queries–oriented approach to identify 116 preferred terms for known CA-AEs according to the prednisone label to interrogate the COU-AA-301 and COU-AA-302 databases. Irrelevant preferred terms were excluded, and upcoding strategies were applied to convert and match with legacy MedDRA versions (version 17 used for these analyses). CA-AEs of interest were as follows:
Endocrine disorders: adrenal insufficiency, Cushing’s syndrome, Cushingoid state, pituitary-dependent Cushing’s syndrome
Eye disorders: cataract, cataract cortical, cataract subcapsular
Gastrointestinal disorders: chronic gastrointestinal bleeding, duodenal perforation, duodenal ulcer, duodenal ulcer hemorrhage, duodenal ulcer perforation, duodenal ulcer perforation (obstructive), duodenal ulcer (obstructive), duodenitis (hemorrhagic), erosive duodenitis, erosive esophagitis, feces discolored, gastric hemorrhage, gastric perforation, gastric ulcer, gastric ulcer hemorrhage, gastric ulcer hemorrhage (obstructive), gastric ulcer perforation, gastric ulcer perforation (obstructive), gastritis (erosive), gastritis (hemorrhagic), gastroduodenal hemorrhage, gastroduodenal ulcer, gastroduodenitis (hemorrhagic), gastrointestinal erosion, gastrointestinal hemorrhage, gastrointestinal perforation, gastrointestinal ulcer, gastrointestinal ulcer hemorrhage, gastrointestinal ulcer perforation, hematemesis, hematochezia, hemorrhagic erosive gastritis, jejunal ulcer, jejunal ulcer perforation, melena, esophageal hemorrhage, esophageal perforation, esophageal ulcer, esophageal ulcer hemorrhage, esophageal ulcer perforation, esophageal varices hemorrhage, esophagitis (hemorrhagic), esophagitis (ulcerative), peptic ulcer, peptic ulcer hemorrhage, peptic ulcer perforation, peptic ulcer perforation (obstructive), peptic ulcer (obstructive), proctitis (hemorrhagic), upper gastrointestinal hemorrhage
General disorders and administration site conditions: impaired healing, perforated ulcer
Infections and infestations: peptic ulcer Helicobacter
Injury, poisoning, and procedural complications: acetabulum fracture, atypical femur fracture, cervical vertebral fracture, femoral neck fracture, forearm fracture, hip fracture, lumbar vertebral fracture, rib fracture, spinal compression fracture, thoracic vertebral fracture, wrist fracture
Investigations: blood glucose abnormal, blood glucose fluctuation, blood glucose increased, body mass index increased, bone density decreased, gastric occult blood positive, glucose tolerance decreased, glucose tolerance test abnormal, glucose urine present, glycosylated hemoglobin increased, occult blood positive, weight increase
Metabolism and nutrition disorders: abnormal weight gain, central obesity, diabetes mellitus, diabetes mellitus inadequate control, glucose tolerance impaired, hyperglycemia, impaired fasting glucose, insulin-requiring type 2 diabetes mellitus, metabolic syndrome
Musculoskeletal and connective tissue disorders: bone formation decreased, bone loss, myopathy, myopathy toxic, osteopenia, osteoporosis, osteoporotic fracture, resorption bone increased, spinal deformity
Renal and urinary disorders: glycosuria
Skin and subcutaneous disorders: ecchymosis, hemorrhage (subcutaneous), hemorrhage (subepidermal), purpura, skin atrophy, skin fragility, skin hemorrhage, skin striae
Surgical and medical procedures: cataract operation, duodenal ulcer repair, gastrointestinal ulcer management, perforated peptic ulcer oversewing
The incidence of CA-AEs during 3-mo exposure intervals and across all exposure levels was assessed as the number of patients with an AE divided by the number of patients at risk. Data are presented for all patients (any P), AA + P, and P alone.
3. Results
Patient demographics were similar in COU-AA-301 and COU-AA-302. Patients enrolled in COU-AA-302 were asymptomatic or mildly symptomatic and had no visceral metastases, and therefore had a lower disease burden than patients in COU-AA-301, who had more advanced disease (Table 1).
Table 1.
Baseline characteristics of patients in COU-AA-301 and COU-AA-302
| Baseline characteristic | COU-AA-301 (N = 1195) |
COU-AA-302 (N = 1088) |
|---|---|---|
| Median age, yr (range) | 69 (39–95) | 70 (44–95) |
| Gleason score at initial diagnosis (n) | 1047 | 996 |
| ≤7, n (%) | 502 (48) | 479 (48) |
| ≥8, n (%) | 545 (52) | 517 (52) |
| Extent of disease (n) | 1190 | 1086 |
| Bone, n (%) | 1066 (90) | 884 (81) |
| Bone only, n (%) | 474 (40) | 541 (50) |
| Soft tissue or node, n (%) | 709 (60) | 538 (50) |
| Other, n (%) | 60 (5) | 11 (1) |
| Median time from initial diagnosis to first dose, yr (range) | 6.02 (0.17–25.01) | 5.3 (0–28) |
| Median weight, kg (range) | 82.6 (39.2–203.3) | 87.5 (45.3–168.3) |
| Median PSA, ng/ml (range) | 131.4 (0.4–10114) | 39.51 (0–6606.4) |
| Median hemoglobin, g/dl (range) | 11.8 (7.2–16.5) | 13.1 (7–16.6) |
| Median lactate dehydrogenase, IU/l (range) | 227 (84–5125) | 185 (60–871) |
| Median alkaline phosphatase, IU/l (range) | 134 (20–4896) | 91 (21–3056) |
A total of 2267 patients in COU-AA-301 and COU-AA-302 received prednisone 5 mg BID for a median of 8.3 mo (range 0.1–34.9 mo), which represents 2006 patient-years of prednisone exposure, defined as the sum of the number of years for which each patient in a study population has been treated with prednisone.
3.1. Safety
The safety populations in COU-AA-301 and COU-AA-302 included all randomized patients who received any study medication (N = 2267). The overall incidence of CA-AEs observed in patients in COU-AA-301 and COU-AA-302 is shown in Table 2.
Table 2.
All corticosteroid-associated adverse events in COU-AA-301 and COU-AA-302 (N = 2267)
| Patients, n (%)
|
||
|---|---|---|
| Any grade | Grade ≥3 | |
| Any corticosteroid adverse event | 558 (24.6) | 103 (4.54) |
| Hyperglycemia | 168 (7.4) | 48 (2.0) |
| Weight increase | 97 (4.3) | 0 |
| Ecchymosis | 66 (2.9) | 0 |
| Rib fracture | 53 (2.3) | 2 (0.1) |
| Cushingoid state | 33 (1.5) | 0 |
| Cataract | 30 (1.3) | 10 (0.4) |
| Diabetes mellitus | 26 (1.1) | 8 (0.4) |
| Skin atrophy | 23 (1.0) | 0 |
| Hematochezia | 20 (0.9) | 2 (0.1) |
| Purpura | 19 (0.8) | 1 (<0.1) |
| Osteoporosis | 20 (0.9) | 2 (0.1) |
| Myopathy | 18 (0.8) | 2 (0.1) |
| Spinal compression fracture | 14 (0.6) | 3 (0.1) |
| Gastrointestinal hemorrhage | 10 (0.4) | 7 (0.3) |
| Osteopenia | 10 (0.4) | 0 |
| Melena | 9 (0.4) | 3 (0.1) |
| Adrenal insufficiency | 8 (0.4) | 3 (0.1) |
| Wrist fracture | 8 (0.4) | 1 (< 0.1) |
| Hip fracture | 5 (0.2) | 3 (0.1) |
| Cataract operation | 4 (0.2) | 2 (0.1) |
| Hematemesis | 4 (0.2) | 1 (<0.1) |
| Lumbar vertebral fracture | 4 (0.2) | 1 (<0.1) |
| Spinal fracture | 4 (0.2) | 2 (0.1) |
| Diabetes mellitus (inadequate control) | 4 (0.2) | 0 |
| Skin fragility | 4 (0.2) | 0 |
| Feces discolored | 4 (0.2) | 0 |
| Cervical vertebral fracture | 3 (0.1) | 2 (0.1) |
| Gastric ulcer | 3 (0.1) | 1 (<0.1) |
| Osteoporotic fracture | 3 (0.1) | 1 (<0.1) |
| Thoracic vertebral fracture | 3 (0.1) | 0 |
| Impaired healing | 3 (0.1) | 0 |
| Upper gastrointestinal hemorrhage | 2 (0.1) | 2 (0.1) |
| Glucose tolerance impaired | 2 (0.1) | 1 (<0.1) |
| Skin hemorrhage | 2 (0.1) | 0 |
| Cushing’s syndrome | 2 (0.1) | 0 |
| Femoral neck fracture | 1 (<0.1) | 1 (<0.1) |
| Central obesity | 1 (<0.1) | 0 |
| Hemorrhage subcutaneous | 1 (<0.1) | 0 |
| Skin striae | 1 (<0.1) | 0 |
| Blood glucose fluctuation | 1 (<0.1) | 0 |
| Occult blood positive | 1 (<0.1) | 0 |
| Duodenal ulcer | 1 (<0.1) | 0 |
| Gastric hemorrhage | 1 (<0.1) | 0 |
| Gastritis erosive | 1 (<0.1) | 0 |
| Gastritis hemorrhagic | 1 (<0.1) | 0 |
| Peptic ulcer | 1 (<0.1) | 0 |
| Bone loss | 1 (<0.1) | 0 |
| Pituitary-dependent Cushing’s syndrome | 1 (<0.1) | 0 |
| Glycosuria | 1 (<0.1) | 0 |
The overall incidence of any-grade CA-AEs for any P exposure was 24.6%, 25.5%, and 23.3% for all patients, AA +P, and P alone, respectively (Fig. 1A). The two most common any-grade CA-AEs were hyperglycemia (7.4%, 7.8%, and 6.9% for all patients, AA + P, and P alone, respectively) and weight increase (4.3%, 3.9%, and 4.8%). Other any-grade AEs occurring in ≥1% of patients were ecchymosis, rib fracture, Cushingoid state, cataract, diabetes mellitus, and skin atrophy (Table 2).
Fig. 1.
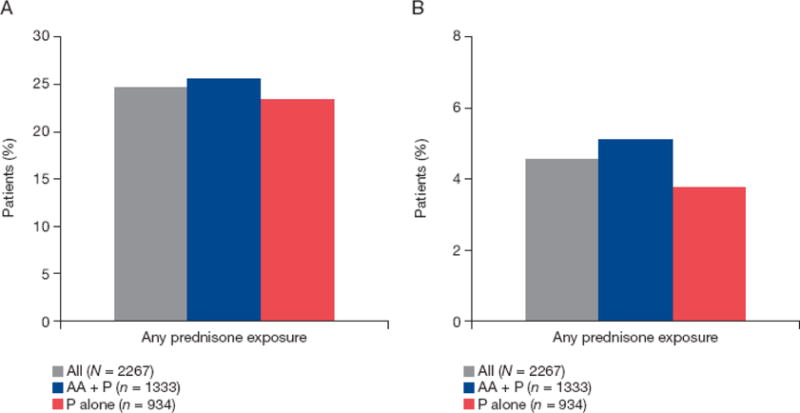
Overall incidence of (A) any-grade and (B) grade ≥3 corticosteroid-associated adverse events. AA = abiraterone acetate; P = prednisone.
The incidence of grade ≥3 CA-AEs for any prednisone exposure was 4.5%, 5.1%, and 3.7% for all patients, AA + P, and P alone, respectively (Fig. 1B). The two most common grade ≥3 CA-AEs were hyperglycemia and cataract. Other grade ≥3 AEs occurring in ≥0.1% of patients are listed in Table 2.
When assessed by duration of exposure in 3-mo intervals for a median of 8.3 mo (range 0.1–34.9 mo), any-grade CA-AEs fluctuated between 0% and 12%, but no discernable trend was observed (Fig. 2A). Grade ≥3 CA-AEs fluctuated between 1% and 2% when assessed by duration of exposure (Fig. 2B). We assessed the incidence of any-grade hyperglycemia and any-grade weight increase, the two most common CA-AEs, by duration of exposure in 3-mo intervals up to ≥30 mo. No discernible trend was observed for overall hyperglycemia (Fig. 3) and weight increase (Fig. 4).
Fig. 2.
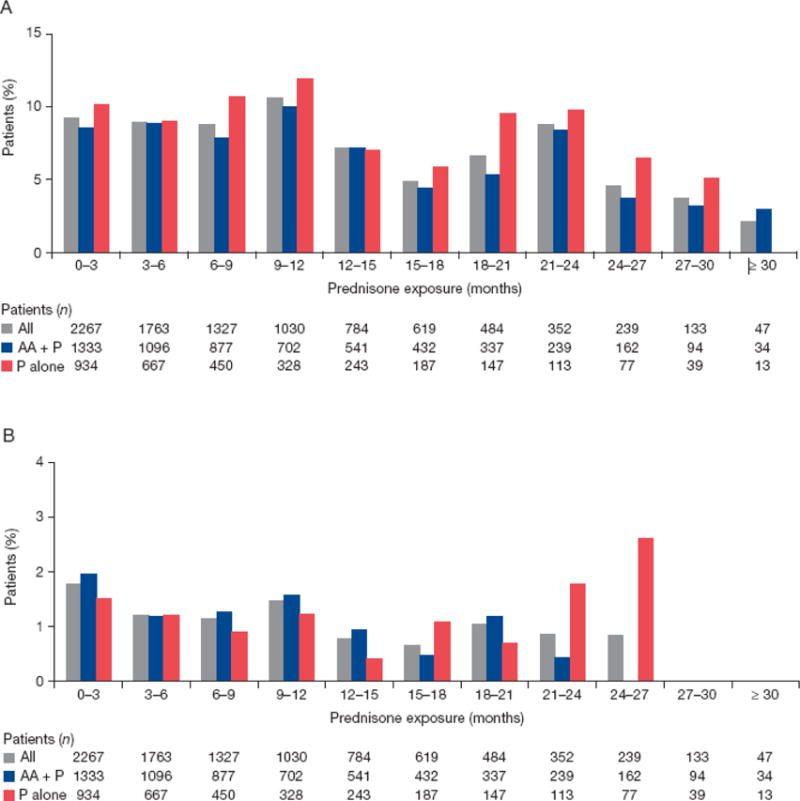
Incidence of new-onset (A) any-grade and (B) grade ≥3 corticosteroid-associated adverse events by exposure. AA = abiraterone acetate; P = prednisone.
Fig. 3.
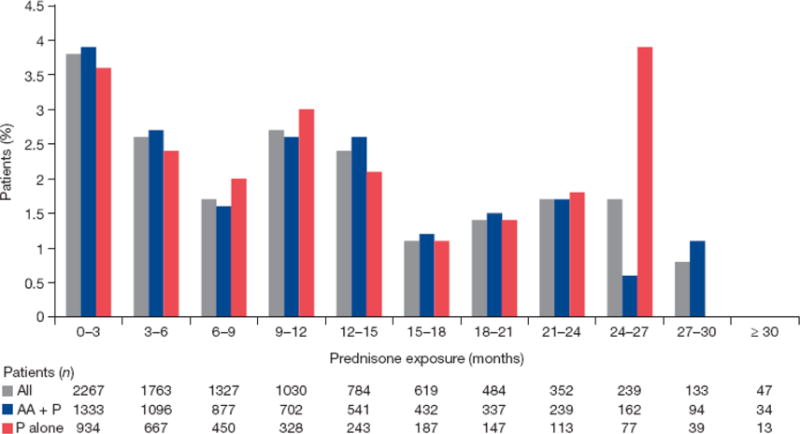
Incidence of any-grade hyperglycemia by exposure. AA = abiraterone acetate; P = prednisone.
Fig. 4.
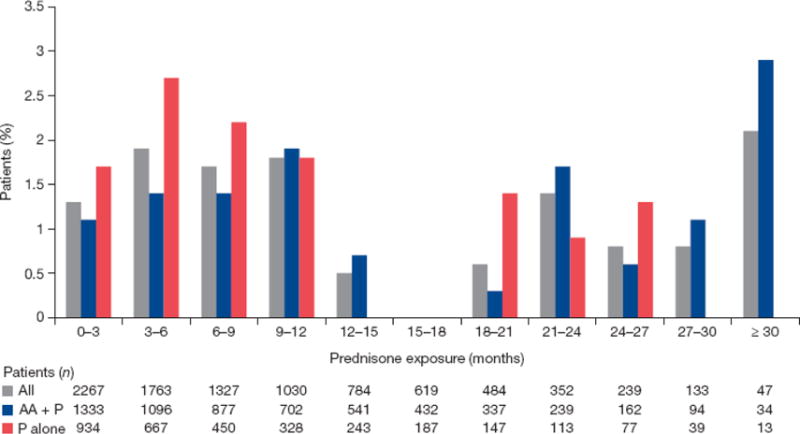
Incidence of grade 1 and grade 2 weight increase by exposure. AA = abiraterone acetate; P = prednisone.
Only grade 1 and grade 2 weight increase AEs were observed in this combined patient population, and most cases (76/97, 78%) were grade 1. There were no incidences of grade ≥3 weight increase. Weight increases of grade 1 (increase from baseline of 5% to <10%) and grade 2 (increase of 10% to <20%) were defined according to the Common Terminology Criteria for Adverse Events (version 4.0). As weight gain is a common concern for patients taking corticosteroids, we evaluated changes in weight over time. The results in Figure 5 show that AA + P had a minimal impact. Furthermore, of the 33 patients with Cushingoid state (all grade 1 or 2), which frequently presents with central adiposity among other phenotypic characteristics, only four had increased weight.
Fig. 5.
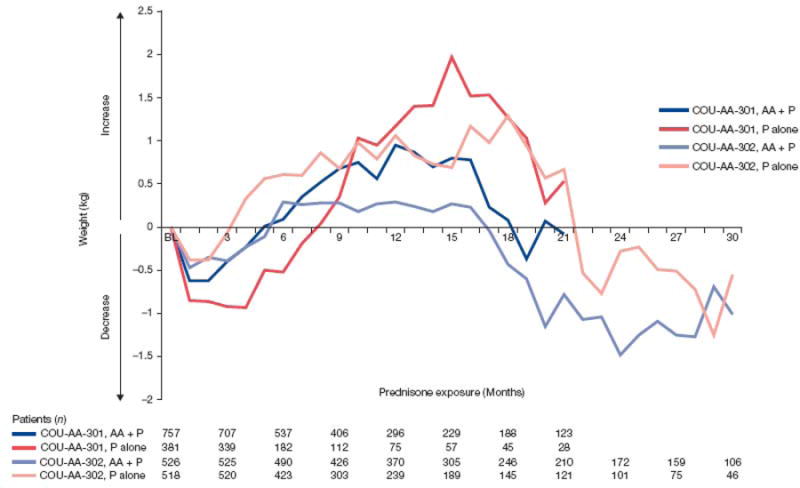
Weight change from baseline in COU-AA-301 and COU-AA-302 by exposure. AA = abiraterone acetate; P = prednisone.
The investigator-reported study discontinuation rate due to CA-AEs was 0.6% (8/1333) for AA + P and 0.3% (3/934) for P alone. Discontinuations in the AA + P group were attributed to hip fracture (n = 1), spinal fracture (n = 2), spinal compression fracture (n = 1), gastrointestinal hemorrhage (n = 2), melena (n = 1), and adrenal insufficiency (n = 1). In the P alone group, skin hemorrhage (n = 1), diabetes mellitus (n = 1), and upper gastrointestinal hemorrhage (n = 1) were cited by the investigator as reasons for discontinuation. One patient in COU-AA-301 had a CA-AE that resulted in death; sponsor assessment of the cause of death was upper gastrointestinal hemorrhage.
4. Discussion
This analysis, which specifically assessed known CA-AEs according to the prednisone label, demonstrates that low-dose P (5 mg BID) given with or without AA to men with mCRPC is associated with low overall incidence of CA-AEs. The frequency of CA-AEs remained low, even with increased duration of exposure to low-dose P. Furthermore, the low rate of discontinuation due to CA-AEs confirms the manageability of these AEs in the few cases in which they do arise. Addition of AA to P did not appear to increase the incidence of CA-AEs, addressing concerns about long-term coadministration of this combination.
There are a few limitations to our study. First, the lack of a placebo-only comparator arm makes it impossible to assess the baseline frequency of these AEs in this population without steroid exposure. This limitation further precludes us from determining whether grade ≥3 AEs in high-risk patients arise from an underlying condition or from low-dose P use. Nevertheless, the absence of an increase in CA-AE frequency over time after ≥30 mo of exposure to prednisone provides strong evidence in support of our conclusions. Second, there were low numbers of evaluable patients at later time points, with <50% of patients remaining by 12 mo and ~10% remaining by 24 mo. Finally, this was a retrospective analysis, and therefore patient physiological status, comorbidities, and other baseline factors that may impact the interpretation of these findings were not prespecified. The ongoing phase 3 Latitude trial (NCT01715285) is evaluating coadministration of abiraterone plus androgen deprivation therapy plus 5 mg prednisone in patients with hormone-naïve de novo metastatic prostate cancer, which will allow prospective assessment of daily low-dose prednisone.
5. Conclusions
Taken together, the results reported here support those of previous studies demonstrating that long-term treatment with abiraterone plus low-dose prednisone is well tolerated. Our results also show that the incidence of CA-AEs in patients with mCRPC after long-term administration of low-dose prednisone is low and manageable.
Long-term treatment with abiraterone acetate plus low-dose prednisone is well tolerated. These results further show that the incidence of corticosteroid-associated adverse events in patients with metastatic castration-resistant prostate cancer after long-term administration of low-dose prednisone is low and manageable.
Acknowledgments
Writing assistance was provided by Lashon Pringle of PAREXEL and was funded by Janssen Global Services LLC.
Funding/Support and role of the sponsor: This study was funded by Janssen Research & Development (formerly Ortho Biotech Oncology Research & Development, unit of Cougar Biotechnology). The sponsor played a role in the design and conduct of the study; in the collection, management, analysis, and interpretation of data; and in the preparation, review, and approval of the manuscript.
Author contributions
Karim Fizazi had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Fizazi, de Bono, Scher, De Porre, Londhe, McGowan, Pelhivanov, Charnas, Todd.
Acquisition of data: Fizazi, Chi, de Bono, Gomella, Miller, Rathkopf, Ryan, Scher, Shore, Bruce Montgomery.
Analysis and interpretation of data: Fizazi, De Porre, Londhe, McGowan, Pelhivanov, Charnas, Todd.
Drafting of the manuscript: All authors.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Londhe.
Obtaining funding: De Porre, Todd.
Administrative, technical, or material support: De Porre, Todd.
Supervision: De Porre.
Other: None.
Financial disclosures
Karim Fizazi certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Karim Fizazi has participated in advisory boards and served as a speaker for Janssen. Kim N. Chi has served as a consultant/advisor to Amgen, Astellas Pharma, Bayer, ESSA, Janssen Pharmaceuticals, Lilly/ImClone, Sanofi, and Takeda; has received honoraria from Astellas Pharma, Janssen Pharmaceuticals, and Sanofi; and has received research funding from Amgen, Astellas Pharma, Bayer, Exelixis, Janssen Pharmaceuticals, Novartis, Oncogenex, Sanofi, Teva, and Tokai Pharmaceuticals. Johann S. de Bono is a paid employee of The Institute of Cancer Research, which has a commercial interest in abiraterone, and has served as a paid consultant for Johnson & Johnson. Leonard G. Gomella has served as a consultant/advisor to Astellas Pharma, Bayer, Dendreon, and Janssen Pharmaceuticals; and has received research funding from Astellas Pharma and Janssen Pharmaceuticals. Kurt Miller has served as a consultant/advisor to and received research funding from Amgen, Bayer, BMS, Ferring, Dendreon, GSK, Astellas Pharma, Janssen Pharmaceuticals, Merck, Novartis, Pfizer, and Roche. Dana E. Rathkopf has served as a consultant/advisor to and received research funding from Janssen Research & Development, Astra Zeneca, Celgene, Ferring, Medication, Millenium/Takeda, and Novartis. Charles J. Ryan has received honoraria from Janssen Research & Development. Howard I. Scher has served as a paid consultant/advisor to Amgen, BIND Pharmaceuticals, Blue Earth Diagnostics, Chugai Academy for Advanced Oncology, Dendreon, Elsevier’s PracticeUpdate Website, Endo/Orion Pharmaceuticals, Enzon, Ferring Pharmaceuticals, Genentech, ImClone, Johnson & Johnson, Light Oncology Sciences, MED IQ, Medivation, Millennium, Novartis, OncologySTAT, Ortho Biotech Oncology Research and Development, Roche, Roche (Genentech), Sanofi-Aventis, and WCG Oncology; has served as an unpaid consultant/advisor to Aragon, Astellas, Astra Zeneca, Bristol-Myers Squibb, Celgene, Endocyte, Exelixis, Foundation Medicine, Genentech, Janssen Pharmaceuticals, Janssen Research (Veridex), Novartis, Pfizer (Biologic), Takeda Millennium, and Ventana (Member of the Roche Group); has received honoraria from Chugai Academy for Advanced Oncology; and has received research funding to the Memorial Sloan Kettering Cancer Center from Agensys, Aragon, BIND Therapeutics, Bristol-Myers Squibb, Exelixis, Guardant Health, Genentech, Innocrin Pharma, Janssen Diagnostics, Janssen Pharmaceuticals, and Medivation. Neal D. Shore is a consultant/advisor to Algeta, Amgen, Bayer, BNI, Dendreon, Ferring, Janssen, Millennium, and Sanofi. Peter De Porre, Anil Londhe, Nonko Pehlivanov, Robert Charnas, and Mary B. Todd are employees of Janssen Research & Development and hold stock options in Johnson & Johnson. Tracy McGowan is an employee of Janssen Scientific Affairs and holds stock options in Johnson & Johnson. Bruce Montgomery has received research funding from Janssen Research & Development.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barrie SE, Potter GA, Goddard PM, Haynes BP, Dowsett M, Jarman M. Pharmacology of novel steroidal inhibitors of cytochrome P450(17) alpha (17 alpha-hydroxylase/C17-20 lyase) J Steroid Biochem Mol Biol. 1994;50:267–73. doi: 10.1016/0960-0760(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 2.Massard C, Fizazi K. Targeting continued androgen receptor signaling in prostate cancer. Clin Cancer Res. 2011;17:3876–83. doi: 10.1158/1078-0432.CCR-10-2815. [DOI] [PubMed] [Google Scholar]
- 3.Potter GA, Barrie SE, Jarman M, Rowlands MG. Novel steroidal inhibitors of human cytochrome P45017 alpha (17 alpha-hydroxylase-C17,20-lyase): potential agents for the treatment of prostatic cancer. J Med Chem. 1995;38:2463–71. doi: 10.1021/jm00013a022. [DOI] [PubMed] [Google Scholar]
- 4.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fizazi K, Scher HI, Molina A, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13:983–92. doi: 10.1016/S1470-2045(12)70379-0. [DOI] [PubMed] [Google Scholar]
- 6.Rathkopf DE, Smith MR, de Bono JS, et al. Updated interim efficacy analysis and long-term safety of abiraterone acetate in metastatic castration-resistant prostate cancer patients without prior chemotherapy (COU-AA-302) Eur Urol. 2014;66:815–25. doi: 10.1016/j.eururo.2014.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–48. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomized, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2015;16:152–60. doi: 10.1016/S1470-2045(14)71205-7. [DOI] [PubMed] [Google Scholar]
- 9.Lafeuille MH, Gravel J, Grittner A, Lefebvre P, Ellis L, McKenzie RS. Real-world corticosteroid utilization patterns in patients with metastatic castration-resistant prostate cancer in 2 large US administrative claims databases. Am Health Drug Benefits. 2013;6:307–16. [PMC free article] [PubMed] [Google Scholar]
- 10.Leppert W, Buss T. The role of corticosteroids in the treatment of pain in cancer patients. Curr Pain Headache Rep. 2012;16:307–13. doi: 10.1007/s11916-012-0273-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Auchus RJ, Yu MK, Nguyen S, Mundle SD. Use of prednisone with abiraterone acetate in metastatic castration-resistant prostate cancer. Oncologist. 2014;19:1231–40. doi: 10.1634/theoncologist.2014-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dorff TB, Crawford ED. Management and challenges of corticosteroid therapy in men with metastatic castrate-resistant prostate cancer. Ann Oncol. 2013;24:31–8. doi: 10.1093/annonc/mds216. [DOI] [PubMed] [Google Scholar]


