Abstract
HIV testing constitutes a key step along the continuum of HIV care. Men who have sex with men (MSM) have low HIV testing rates and delayed diagnosis, especially in low-resource settings. Peer-led interventions offer a strategy to increase testing rates in this population. This systematic review and meta-analysis summarizes evidence on the effectiveness of peer-led interventions to increase the uptake of HIV testing among MSM. Using a systematic review protocol that was developed a priori, we searched PubMed, PsycINFO and CINAHL for articles reporting original results of randomized or non-randomized controlled trials (RCTs), quasi-experimental interventions, and pre- and post-intervention studies. Studies were eligible if they targeted MSM and utilized peers to increase HIV testing. We included studies published in or after 1996 to focus on HIV testing during the era of combination antiretroviral therapy. Seven studies encompassing a total of 6,205 participants met eligibility criteria, including two quasi-experimental studies, four non-randomized pre- and-post intervention studies, and one cluster randomized trial. Four studies were from high-income countries, two were from Asia and only one from sub-Saharan Africa. We assigned four studies a ‘moderate’ methodological rigor rating and three a ‘strong’ rating. Meta-analysis of the seven studies found HIV testing rates were statistically significantly higher in the peer-led intervention groups versus control groups (pooled OR 2.00, 95% CI 1.74–2.31). Among randomized trials, HIV testing rates were significantly higher in the peer-led intervention versus control groups (pooled OR: 2.48, 95% CI 1.99–3.08). Among the non-randomized pre- and post-intervention studies, the overall pooled OR for intervention versus control groups was 1.71 (95% CI 1.42–2.06), with substantial heterogeneity among studies (I2=70 %, p<0.02). Overall, peer-led interventions increased HIV testing among MSM but more data from high-quality studies are needed to evaluate effects of peer-led interventions on HIV testing among MSM in low- and middle-income countries.
Keywords: peers, interventions, HIV testing, MSM, systematic review, meta-analysis
Introduction
Globally, men who have sex with men (MSM) are disproportionately affected by HIV infection (Beyrer et al., 2012; UNAIDS, 2013). Incidence of HIV among MSM remains steadily high, despite declines in the general population (Beyrer et al., 2012; Prejean et al., 2011; UNAIDS, 2013) and increasing benefits of antiretroviral therapy (ART) for the management of HIV (Anglemyer, Horvath, & Rutherford, 2013; Das et al., 2010; Rutherford, 2011). In addition, rates of HIV testing among MSM have stayed low worldwide (Adam et al., 2009; Zablotska et al., 2012), as have their rates of access to HIV prevention and care services (Beyrer et al., 2012). Recent WHO guidelines highlight the need to strengthen HIV programs so that all key populations benefit from advances in HIV prevention and treatment (Hirnschall, Baggaley, & Verster, 2014). In particular, improved outreach efforts to MSM are necessary to achieve the UNAIDS goal of creating an AIDS free generation (UNAIDS, 2013; WHO, 2014).
Engagement in HIV care, including early initiation of and adherence to ART have the potential to improve health outcomes and greatly reduce onward transmission of infection (Cohen et al., 2011; Gardner, McLees, Steiner, Del Rio, & Burman, 2011). To benefit fully from treatment, however, individuals need to first be aware of their HIV status (Ayala et al., 2014; Bickman & Hoagwood, 2010; Rosenberg, Millett, Sullivan, del Rio, & Curran, 2014; Singh et al., 2014; Zanoni & Mayer, 2014). Early diagnosis allows HIV-infected individuals to take steps to protect their partners from infection, and early treatment can lower viral load and reduce the risk of transmitting HIV (Cohen et al., 2011). Unfortunately, HIV testing and treatment programs often fail to reach MSM and other marginalized groups despite being disproportionately affected by the infection (Adams, 2009; Deblonde et al., 2010; MacKellar, et al., 2005). Therefore, strategies that can be used to effectively reach MSM and optimally engage them in HIV care are critically needed.
Peer-led interventions to promote HIV testing can potentially increase testing rates among MSM. Peer-led HIV interventions typically involve enlisting members of a specific at-risk group to influence and support members maintain healthy sexual behaviors, change risky sexual behaviors, and modify norms in ways conducive to healthier lifestyles (Webel, 2010). Peers are more likely than professionals to influence the behaviors of fellow group members, and also have better access to hidden populations who may have limited interaction with conventional health programs (Simoni, Nelson, Franks, Yard, & Lehavot, 2011). Peers have been deployed to help MSM negotiate complex prevention, care, substance abuse, and social service systems (Bradford, Coleman, & Cunningham, 2007). Peer-based interventions to promote HIV behavioral and clinical outcomes have shown promise, based on recent systematic reviews. For example, peer-led programs have been demonstrated to effectively support adherence to ART and sustain retention in care over time (Genberg et al., 2016). Peer-based programs can effectively reduce the incidence of condomless sex with new partners (Ye et al., 2014). To date, there is no known review that systematically identifies and synthesizes evidence on effectiveness of peer-led interventions to improve HIV testing among MSM. Accordingly, we aimed to fill this gap by conducting a systematic review and meta-analysis to examine interventions that have used peers to facilitate and improve HIV testing among MSM. We focused on the era of combination ART and examined studies published in or after 1996.
Methods
This systematic review is written in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Moher, Liberati, Tetzlaff, & Altman, 2009). It also abides by Cochrane Collaboration guidelines, including procedures for defining the review question a priori, searching for studies, selecting studies, extracting data, appraising the risk of bias in included studies, and analyzing data (Higgins & Green, 2008).
Eligibility criteria
Studies were selected if they met the following inclusion criteria: 1) randomized or non-randomized controlled trials (RCTs), quasi-experimental intervention studies, pre- and post-intervention studies without control groups, or studies of prospective outcomes/cohort studies; 2) study populations were MSM who are HIV-negative or do not know their HIV status; 3) interventions utilized peers of MSM to increase the uptake of HIV testing; 4) study assessed HIV testing; and 5) study was published in or after 1996. We defined peers as demographically-similar counterparts of the target population (e.g., lay persons, community health care workers, opinion leaders, patient advocates, patient expert, patient navigator, peer navigator, and peer volunteer). Studies were excluded if they: 1) focused on the general population and did not present outcome data on MSM, and 2) were published in a language other than English.
Search strategy
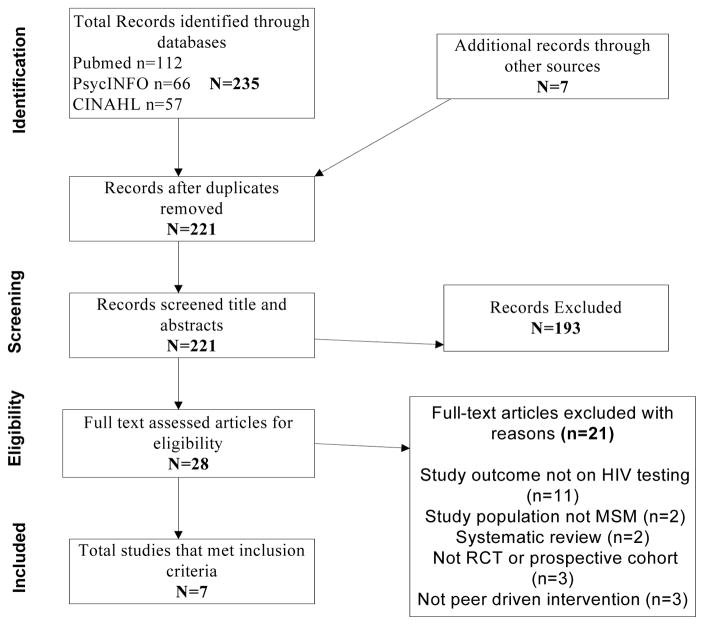
A systematic literature search was conducted using 3 databases: PubMed, PsycINFO and CINAHL. The search included all literature published between 1996 and January 2016. Keywords used included: [(men who have sex with men) OR (MSM) OR (homosexual men) OR (gay men) OR (bisexual men) OR (transgender women) OR (money boy)] AND [(HIV) OR (AIDS) OR testing OR counseling] AND [(peer) OR (opinion leader)]. All publications were exported to an Endnote file (Endnote X7, Thomson Reuters, San Francisco, CA), merged, and duplicates deleted, as shown in Figure 1. Using an a priori screening checklist, we reviewed abstracts and titles. If abstracts were incomplete, we reviewed full texts to determine eligibility. Manuscripts that met inclusion criteria were retained for full analysis.
Figure 1.
Flow chart of article inclusion and exclusion for review.
The initial search of PubMed, PsycINFO, and CINAHL electronic databases yielded 235 entries meeting the predefined inclusion criteria, among which 14 duplicates were identified and removed (Figure 1). Of the remaining 221 studies, 193 were excluded because they did not meet the inclusion criteria, leaving 28 studies for full text review.
Studies excluded after full text review
Among the 28 studies reviewed in full, 21 were excluded. Eleven studies did not have HIV testing as the primary outcome (Duan et al., 2013; Elford, Bolding, & Sherr, 2004; Elford, Sherr, Bolding, Serle, & Maguire, 2002; Hallett, Brown, Maycock, & Langdon, 2007; Hidalgo et al., 2011; Hosek et al., 2015; Jaganath, Gill, Cohen, & Young, 2012; Kegeles, Hays, Pollack, & Coates, 1999; Subramanian et al., 2013; Yan et al., 2014). In two studies, the study population was not MSM (Gutierrez, McPherson, Fakoya, Matheou, & Bertozzi, 2010; Koech et al., 2014). Three studies were excluded because they did not use an RCT or prospective cohort design (Bowles et al., 2008; Ntata, Muula, & Siziya, 2008; Scott et al., 2014), another two because they were systematic reviews and meta-analyses (Stromdahl et al., 2015; Ye et al., 2014), and three because they were not peer-driven interventions (Fernandez-Balbuena et al., 2014; Outlaw et al., 2010; Prejean et al., 2011). The 7 studies that met all eligibility criteria are described in Table 1.
Table 1.
Description of the seven studies that met eligibility criteria for inclusion in the review and meta-analysis.
| Author/Year | Country and study period | Study design | Sample and sample size | Intervention | Comparison | Outcome measures | Main finding | Months of follow-up |
|---|---|---|---|---|---|---|---|---|
| Elford 2001 | London, UK; 1997–1999 | RCT | n=1000 gay men | Peers recruited gay men from a gym | Gym with no peer educators | Condomless anal intercourse and ever tested for HIV | % of men ever-tested for HIV increased from 73.0% at baseline to 79.6 % follow-up (p=0.002) | 18 |
| Golden 2006 | King County, Washington State, US; 2002–2004 | Pre/post | n=781 MSM | Peers were trained and provided with incentives to refer MSM to care for HIV testing | No comparison | New cases of HIV | Intervention group were not more likely to have been previously tested for HIV at follow up compared with baseline (83% vs 89%, p=0.37) | Unspecified |
| Erausquin 2009 | Los Angeles, California, US; 2003–2004 | Pre/post | n=95 young Latino MSM | Trained outreach volunteers that shared characteristics with the target population distributed bilingual outreach cards to encourage young Latino MSM to test for HIV | No comparison | HIV testing and self-reported sexual risk behaviors | At post-intervention, there was more HIV testing among young Latino MSM participants compared to baseline | 12 |
| Wilton 2009 | New York, US; 2005–2006 | Quasi-randomized trial | 338 African American MSM, 18 years and older, residing in New York. Randomly assigned to intervention (n=164) or comparison (n=174). | 3MV, a small group intervention to address factors influencing the HIV/STI risk and protective behaviors. 6 sessions lasting 2–3 hours. Two trained Black MSM peers co-facilitated the sessions | Assigned to delayed 3MV comparison (wait list) | Sexual risk behaviors, and HIV and STI testing | At the 6-month follow-up 3MV participants (intervention group) had an 81% greater odds of testing for HIV than comparison participants (OR; 1.81, 95% CI 1.08–3.01) | 6 |
| Geibel 2012 | Coastal Kenya; 2002–2009 | Pre/post | n=1026 male sex workers | 40 peer educators (male sex workers or non-sex worker MSM familiar with the sex worker environment) were trained in HIV prevention. | No comparison | HIV knowledge and condomless sexual behavior | Intervention group were more likely to have ever been tested for HIV (87.2% vs 60.9%), aOR: 4.37, 95% CI 2.04–9.36 | 12 |
| Ko 2013 | Taiwan; Apr–Sep 2011 | Quasi-randomized trial | Internet-using MSM, aged 18 years and older, who had sex with a man in the past 12 months. Intervention group n=1037 and control group n=485 | iPOL trained for 12 weeks to disseminate HIV-related information on HIV prevention, strategies for risk reduction, and behavior change. Information was disseminated via the Internet | Website created but no iPOL | HIV testing behavior, risky behaviors | At 6 months follow-up, MSM receiving iPOL interventions were more likely to have tested for HIV (43.9% vs 22.3%, p<0.001) | 6 |
| Young 2015 | Peru; Jan–Dec 2012 | Cluster randomized trial | n=1112 males, ages 18 years or older, who had sex with a man in the past 12 months, HIV negative or serostatus unknown. n=556 assigned to either intervention or control (1:1 ratio) | Peer leaders attended three 3-hour training sessions and each was assigned a Facebook group to train and mentor MSM. Main topic for discussion was HIV prevention and testing | Standard of care, including standard offline HIV prevention available in Peru and participation in Facebook groups (without peer leaders) that provided study updates and HIV testing information | Primary outcome: proportion that received free HIV test at a local community clinic. Secondary outcome: number of requests for HIV testing | 43 participants (17%) in the intervention group and 16 (7%) in the control groups got tested for HIV (aOR: 2.61, 95% CI 1.55–4.38). Odds of requesting a test were 2.79 times higher (95% CI 1.42–5.72) among participants in the intervention group | 12 |
Notes: RCT= randomized controlled trial; 3MV=Many men, Many voices; vs=versus; aOR= Adjusted odds ratio; iPOL=Internet popular opinion leader
Data extraction
For all eligible studies, the following information was extracted: first author, publication year, study country, study design, sample sizes, study durations of follow up, description of interventions, description of the comparison arm, outcome of interest and key findings.
Assessment of methodological quality of included studies
Methodological quality was assessed using the quality assessment tool for quantitative studies developed by the Effective Public Health Practice Project (Thomas, 2003). Studies were assessed for selection bias, study design, confounding, blinding, data collection, withdrawals and drop-outs. Based on ratings for each of the seven components, each study received an overall global quality score of ‘strong’, ‘moderate’, or ‘weak’. In order for a study to be rated as ‘strong’, four of the six quality assessment criteria had to be considered ‘strong’, with no ‘weak’ rating. A ‘moderate’ rating was awarded if less than four criteria were rated ‘strong’ and one criterion was ‘weak’. A ‘weak’ rating of was assigned if two or more criteria were rated ‘weak’. Quality assessment ratings are provided for each study alongside study characteristics in Table 2.
Table 2.
Quality assessment scores for the seven studies included in the review and meta-anlaysis.
| Author/Year | Selection bias | Study design | Confounders | Blinding | Data collection method | Withdrawals and dropouts | Overall quality rating of study |
|---|---|---|---|---|---|---|---|
| Elford et al., 2001 | 2 | 1 (RCT) | 1 | NA | 1 | 2 | Moderate |
| Golden et al., 2006 | 3 | 2 (pre-post) | 1 | NA | 1 | 1 | Moderate |
| Erausquin et al., 2009 | 2 | 2 (pre-post) | 1 | NA | 1 | 3 | Moderate |
| Wilton et al., 2009 | 2 | 1 (quasi-randomized trial) | 1 | NA | 1 | 1 | Strong |
| Geibel et al., 2012 | 3 | 2 (pre-post) | 1 | NA | 1 | 1 | Moderate |
| Ko et al., 2013 | 2 | 2 (quasi-randomized trial) | 1 | NA | 1 | 1 | Strong |
| Young et al., 2015 | 1 | 1 (cluster-RCT) | 1 | NA | 1 | 1 | Strong |
Notes: RCT= randomized control trial, pre-post= pre-and post-intervention studies, NA=not applicable
- 1: Strong
- 2: Moderate
- 3: Weak
- NA: not relevant to study
Statistical analysis
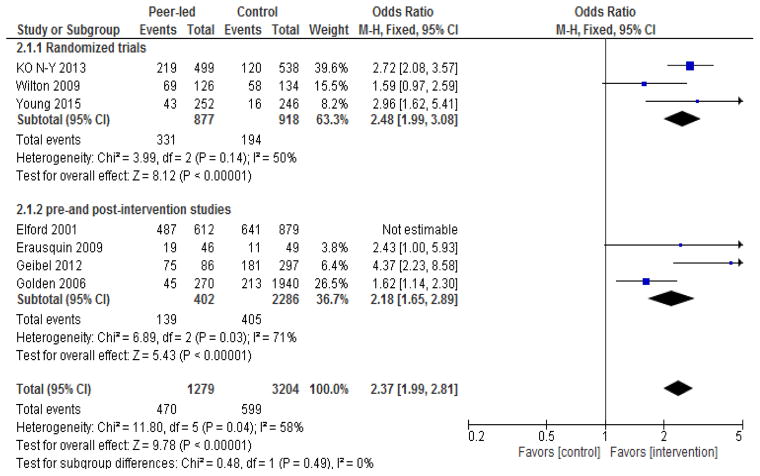
Meta-analysis was performed for the three randomized or quasi-experimental studies and a separate meta-analysis was done for the four pre- and post-intervention studies. HIV testing was the outcome of interest. Meta-analysis was done using RevMan version 5.3 (Cochrane Information Management System). Both random-effect and fixed-effect Mantel-Haenszel models were used to calculate the proportion of MSM testing for HIV and was expressed in terms of the 95% confidence interval (CI) and level of statistical significance. We also evaluated the overall effect size based on all the seven studies. For studies with multiple measurements at different follow-up time points, we used the last follow-up assessment to estimate the overall effect size. We explored sources of heterogeneity by performing subgroup analysis by study design (randomized studies versus pre-post intervention studies), socioeconomic level of setting (high versus low and middle-income country), quality rating (strong versus moderate). The overall effect of peer-led interventions was assessed by the I2 statistic. Pooled odds ratios (ORs) and 95% CIs are presented in Figure 2. To assess the robustness of our estimates, we conducted sensitivity analyses by excluding studies which were identified as outliers.
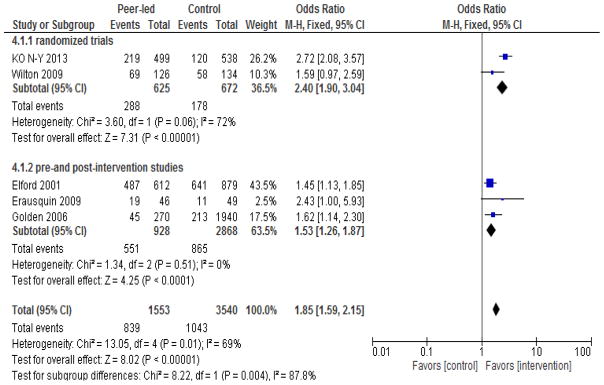
Figure 2.
Study-specific and overall sizes of the effect of peer-led interventions on rate of HIV testing among MSM.
Note: event= number of MSM tested for HIV
Results
Description of the included studies
Among the 7 studies included, 1 was an RCT (Young et al., 2015), 1 was a cluster randomized trial (Young et al., 2015), 2 were quasi-experimental studies (Ko et al., 2013; Wilton et al., 2009; Yan et al., 2014), and 3 were pre- and post-intervention studies (Erausquin et al., 2009; Geibel, King’ola, Temmerman, & Luchters, 2012; Golden et al., 2006) (Table 1). The sample sizes ranged from 95 to 1037. Duration of observation varied from 3 to 18 months after baseline assessment. For studies with control arms, the comparison condition was typically standard of care for HIV prevention. All 7 studies assessed HIV testing uptake among MSM as the primary outcome, though 3 studies measured other outcomes such as knowledge about HIV (Erausquin et al., 2009; Geibel et al., 2012; Wilton et al., 2009). Three studies were conducted in the US (Erausquin et al., 2009; Golden et al., 2006; Wilton et al., 2009), one in the UK (Elford, Bolding, & Sherr, 2001), and three in low- and middle-income countries: Kenya (Geibel, King’ola, Temmerman, & Luchters, 2012), Taiwan (Ko et al., 2013), and Peru (Young et al., 2015).
Methodological appraisal of the included studies
Methodological ratings for the seven studies are shown in Table 2. Three of the seven studies (Ko et al., 2013; Wilton et al., 2009; Young et al., 2015) were assigned a ‘strong’ overall quality rating, and the other four were deemed of ‘moderate’ quality (Elford et al., 2001; Erausquin et al., 2009; S. Geibel et al., 2012; Golden et al., 2006). No study received a ‘weak’ overall rating. Four studies were rated as ‘moderate’ in terms of selection bias (Elford et al., 2001; Erausquin et al., 2009; Ko et al., 2013; Wilton et al., 2009), two were assigned a ‘weak’ rating (Geibel et al., 2012; Golden et al., 2006), whereas one study received a ‘strong’ rating (Young et al., 2015). Most studies got ‘moderate’ or ‘weak’ ratings for selection bias because participants were recruited mostly through establishment-based sampling venues frequented by MSM such as bars, clubs and bathhouses. The one study rated ‘strong’ for selection bias recruited participants via the Internet (Facebook), and the participants were randomly assigned to either a peer-led intervention group or control (Table 2). All the studies scored ‘strong’ ratings for control of confounding as well as for data collection methods. In addition, most studies got ‘strong’ ratings with regard to withdrawals and drop-outs. Five studies described both the numbers and reasons for withdrawals and drop-outs, and only one study did not provide this information (Erausquin et al., 2009).
Effect of peer led interventions on rate of HIV testing among MSM
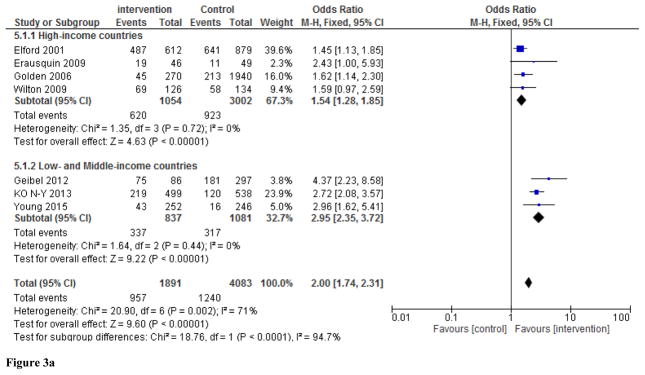
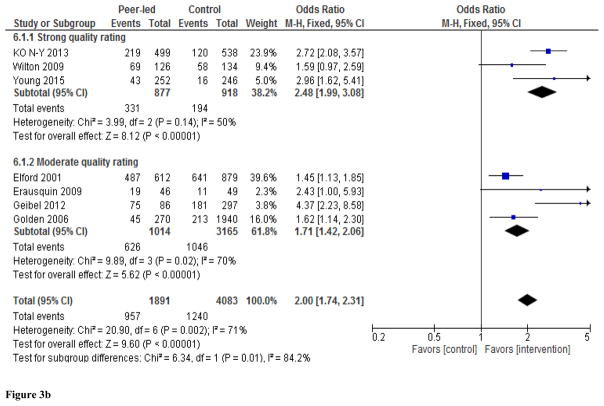
Meta-analysis of the seven studies demonstrated increased uptake of HIV testing among those individuals exposed to peer-led interventions (Figure 2). The overall effect is statistically significant (pooled OR: 2.00, 95% CI 1.74–2.31) with substantial heterogeneity across studies (I2=71%, p<0.002). We used both random-effects and fixed-effects Mantel-Haenszel models, which yielded similar results, so we only present results from the fixed-effects model (Figure 2). Meta-analysis of the two quasi-experimental studies (Ko et al., 2013; Wilton et al., 2009) and one cluster randomized trial (Young et al., 2015) showed that the odds of HIV testing were significantly higher in the peer-led intervention versus control groups (pooled OR: 2.48, 95% CI 1.99–3.08), with low heterogeneity across studies (I2=50 %, p<0.14). Among the pre- and post-intervention studies, the pooled OR was 1.71, 95% CI 1.42–2.06), although there was significant heterogeneity across studies (I2=70 %, p<0.02). Subgroup analyses and sensitivity analyses did not materially alter the results (Figures 3a, 3b, and 4).
Figure 3.
Figure 3a. Subgroup analyses by socio-economic status of study site (high-income versus low-and middle-income country). Values show study-specific and overall sizes of the effect of peer-led interventions on rate of HIV testing among MSM.
Figure 3b. Subgroup analyses by quality rating assigned to study. Values show study-specific and overall sizes of the effect of peer-led interventions on rate of HIV testing among MSM.
Figure 4.
Sensitivity analyses to examine robustness of results after excluding two studies with outliers (Geibel et al. 2012 and Young et al. 2015). Values show study-specific and overall sizes of the effect of peer-led interventions on rate of HIV testing among MSM.
Discussion
This, to our knowledge, is the first systematic review and meta-analysis of the effect of peer-led interventions on the rate of HIV testing among MSM. The review included seven studies conducted in the era of highly active ART, with sites in the US, UK, Kenya, Taiwan, and Peru involving 6205 MSM. Overall, peer-led interventions among MSM were effective in promoting HIV testing. In pooled analysis of seven studies with comparable methods and outcome measures, there was a 2.00 (95% CI 1.74–2.31) increased odds of HIV testing among MSM who were engaged in peer-led interventions compared to counterparts who were not. These findings are particularly compelling in light of renewed calls to improve rates of HIV testing among key populations such as MSM (UNAIDS 2013; WHO, 2014), and the paucity of proven mechanisms to do so.
The current review is consistent with prior meta-analytic reviews showing positive impacts of peer-based interventions for HIV prevention. One meta-analysis demonstrated that peer-led interventions could increase HIV-related knowledge, reduce equipment sharing among injection drug users, and improve condom use (Medley, Kennedy, O’Reilly, & Sweat, 2009). Another one, which included the non-English language and grey literature concluded that peer-led interventions were effective in reducing unprotected anal intercourse among MSM (Ye et al., 2014). An additional systematic review found that peer-led interventions were effective in faciliatating linkage to care for HIV-diagnosed individuals, although the investigators did not find any study focusing exclusively on MSM (Genberg et al., 2016). The current systematic review and meta-analysis complements this growing body of work on the utility of peer-led interventions by adding evidence for the critical outcome of HIV testing among MSM.
Given the levels of stigma and distrust toward the medical community that have been documented among MSM, peer-led interventions might be especially useful for building trust and reaching hidden subgroups that have been alienated from mainstream HIV prevention efforts. Four of the seven peer-led interventions to facilitate HIV testing among MSM that met our eligibility criteria were conducted in developed countries, underscoring the need for further efforts to design and implement more peer-led interventions in low and middle-income countries, which bear the brunt of the epidemic and where barriers to HIV care for MSM may be especially acute (Beyrer, 2012). For instance, we found only one study that examined the involvement of peers to increase HIV testing among MSM in sub-Saharan Africa (Geibel et al., 2012). Given high stigma towards MSM in sub-Saharan Africa, peer-led interventions may be critical for creating enabling and safe testing environments for MSM who may not otherwise seek HIV care in health departments or local clinics.
Three of seven studies in this review received a ‘strong’ rating for methodological quality, and four got a ‘moderate’ rating. Studies generally recruited participants from gay venues or sites of MSM-oriented services or institutions, and were unlikely to include non-gay-identified MSM. This raises the question of whether results from the studied interventions reflect those MSM who are not affiliated with gay or MSM networks and who might have a high likelihood of living with undiagnosed HIV. Notably, two studies used the internet as a strategy for reaching participants, which is a promising approach for engaging MSM who do not frequent gay-centric community venues and who are harder to reach. However, this approach may also fail to reach MSM who may not have access to the Internet. Pre- and post-intervention study designs represented more than half of the included studies, which may compromise the ability to draw inferences about the causal effects of the intervention on HIV testing outcomes. Although meta-analysis of results from both the experimental and observational studies in the review yielded statistically significant findings, further research using rigorous study designs is needed to assess impacts of peer-led HIV testing among MSM in low- and middle-income settings.
This review has some limitations. As in any meta-analysis or systematic review, publication bias is a potential problem. Due to the small number of studies that met eligibility criteria, we were unable to assess for publication bias. According to the Cochrane Collaboration, tests to assess for publication bias should include 10 or more studies. With seven studies, we had insufficient power to distinguish chance from real asymmetry with regard to publication bias (Higgins & Green, 2008). Unlike Ye et al., (2014), our search focused on publications in the English peer-review literature and thus excluded unpublished research or gray literature as well as non-English literature. Although we searched in three databases (PubMed, PsycINFO, and CINAHL), a post-hoc literature search using the Cochrane and ClinicalTrials.org databases yielded no additional studies. Due to our interest in HIV testing strategies in the era of ART, we included evaluations that were conducted after 1996 and thus we may have excluded peer-led MSM testing interventions from the earlier phases of the epidemic. Another limitation of our analysis is the heterogeneity across studies, which may not be accurately reflected in the pooled estimates. Differences in study design, geographical location (country, urban or rural area), and intervention year contributed to the heterogeneity. To address this, we used both fixed-effects and random-effect meta-analysis and stratified by study design, country, and quality rating. Heterogeneity was substantial across the 7 studies largely due to the inclusion of pre- and post-intervention studies. In addition, there is also a lack of consistency and detail in the description of characteristics of study sample and study design across the reviewed studies.
The studies included in our meta-analysis had important design limitations (Table 2). For example, study populations were recruited mainly from MSM-oriented services or institutions, limiting generalizability of these findings to MSM who are not affiliated with MSM networks. Also, randomized trials were of necessity not blinded, raising the possibility of bias. Pre- and post-intervention studies had varying lengths of follow-up and sample sizes, which could affect estimation of the benefits of the interventions. Lastly, these findings might have limited generalizability due to the geographic settings of the primary studies. In spite of these potential limitations, our careful subgroup and sensitivity analyses indicated that the overall results are robust.
In conclusion, we found that peer-led interventions increased the rate of HIV testing among MSM, based on a systematic review and meta-analysis of available literature after a careful search. Efforts to optimize the continuum of HIV care can benefit from peer-led approaches to engage MSM in HIV testing. Peer-led approaches may also be advantageous for other components of the continuum of HIV care, such as linking MSM who are aware of their HIV-positive status to care services (Genberg et al., 2016), and for other hidden at-risk populations. Noteworthy gaps in the published literature include the need for testing these interventions in diverse epidemic contexts, especially in places where MSM experience major barriers to HIV prevention and testing. Innovative use of the Internet and mobile telecommunications devices as a medium of outreach to facilitate the conduct of peer-led HIV testing among MSM is a promising emerging approach. Where possible, researchers should employ high-validity study designs including adequately powered RCTs with longer follow-up periods in order to more accurately assess the effects of peer-led interventions on HIV testing and linkage to care among MSM.
Acknowledgments
Funding:
This work was supported by the Brown/Tufts Fogarty AIDS International Training and Research program grant D43-TW000237 from the NIH-Fogarty International Center, National Institute of Health (NIH) grants NICHD R24-HD077976, NIMH R34-MH106349, and NIAAA U24-AA022000
SS generated the idea, planned and carried out the systematic review, and wrote the initial draft. All authors contributed to the interpretation of the findings and article revisions. All authors approved the final version of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Fogarty International Fellowship or the National Institutes of Health.
References
- Adam PC, de Wit JB, Toskin I, Mathers BM, Nashkhoev M, Zablotska I, … Rugg D. Estimating levels of HIV testing, HIV prevention coverage, HIV knowledge, and condom use among men who have sex with men (MSM) in low-income and middle-income countries. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2009;52:S143–S151. doi: 10.1097/QAI.0b013e3181baf111. [DOI] [PubMed] [Google Scholar]
- Anglemyer A, Horvath T, Rutherford G. Antiretroviral therapy for prevention of HIV transmission in HIV-discordant couples. JAMA. 2013;310(15):1619–1620. doi: 10.1001/jama.2013.278328. [DOI] [PubMed] [Google Scholar]
- Ayala G, Makofane K, Santos GM, Arreola S, Hebert P, Thomann M, … Do TD. HIV Treatment Cascades that Leak: Correlates of Drop-off from the HIV Care Continuum among Men who have Sex with Men Worldwide. Journal of AIDS and Clinical Research. 2014;5(331):2. [Google Scholar]
- Beyrer C, Baral SD, van Griensven F, Goodreau SM, Chariyalertsak S, Wirtz AL, Brookmeyer R. Global epidemiology of HIV infection in men who have sex with men. Lancet. 2012;380(9839):367–377. doi: 10.1016/s0140-6736(12)60821-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyrer C, Sullivan PS, Sanchez J, Dowdy D, Altman D, Trapence G, … Mayer KH. A call to action for comprehensive HIV services for men who have sex with men. Lancet. 2012;380(9839):424–438. doi: 10.1016/S0140-6736(12)61022-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickman L, Hoagwood KE. Introduction to special issue. Administration and Policy in Mental Health and Mental Health Services Research. 2010;37(1–2):4–6. doi: 10.1007/s10488-010-0289-9. [DOI] [PubMed] [Google Scholar]
- Bowles KE, Clark HA, Tai E, Sullivan PS, Song B, Tsang J, … Heffelfinger JD. Implementing rapid HIV testing in outreach and community settings: results from an advancing HIV prevention demonstration project conducted in seven U.S. cities. Public Health Rep. 2008;123(Suppl 3):78–85. doi: 10.1177/00333549081230S310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford JB, Coleman S, Cunningham W. HIV System Navigation: an emerging model to improve HIV care access. AIDS Patient Care and STDS. 2007;21(Suppl 1):S49–58. doi: 10.1089/apc.2007.9987. [DOI] [PubMed] [Google Scholar]
- Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, … Pilotto JH. Prevention of HIV-1 infection with early antiretroviral therapy. New England Journal of Medicine. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, … Fleming TR. Prevention of HIV-1 infection with early antiretroviral therapy. New England Journal of Medicine. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M, Chu PL, Santos GM, Scheer S, Vittinghoff E, McFarland W, Colfax GN. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS One. 2010;5(6):e11068. doi: 10.1371/journal.pone.0011068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deblonde J, De Koker P, Hamers FF, Fontaine J, Luchters S, Temmerman M. Barriers to HIV testing in Europe: a systematic review. European Journal of Public Health. 2010;20(4):422–432. doi: 10.1093/eurpub/ckp231. [DOI] [PubMed] [Google Scholar]
- Duan Y, Zhang H, Wang J, Wei S, Yu F, She M. Community-based peer intervention to reduce HIV risk among men who have sex with men in Sichuan province, China. AIDS Educ Prev. 2013;25(1):38–48. doi: 10.1521/aeap.2013.25.1.38. [DOI] [PubMed] [Google Scholar]
- Elford J, Bolding G, Sherr L. Peer education has no significant impact on HIV risk behaviours among gay men in London. AIDS. 2001;15(4):535–538. doi: 10.1097/00002030-200103090-00018. [DOI] [PubMed] [Google Scholar]
- Elford J, Bolding G, Sherr L. Popular opinion leaders in London: a response to Kelly. AIDS Care. 2004;16(2):151–158. doi: 10.1080/09540120410001640995. [DOI] [PubMed] [Google Scholar]
- Elford J, Sherr L, Bolding G, Serle F, Maguire M. Peer-led HIV prevention among gay men in London: process evaluation. AIDS Care. 2002;14(3):351–360. doi: 10.1080/09540120220123739. [DOI] [PubMed] [Google Scholar]
- Erausquin JT, Duan N, Grusky O, Swanson AN, Kerrone D, Rudy ET. Increasing the reach of HIV testing to young Latino MSM: results of a pilot study integrating outreach and services. Journal of Health Care for the Poor and Underserved. 2009;20(3):756–765. doi: 10.1353/hpu.0.0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Balbuena S, de la Fuente L, Hoyos J, Rosales-Statkus ME, Barrio G, Belza MJ. Highly visible street-based HIV rapid testing: is it an attractive option for a previously untested population? A cross-sectional study. Sex Transm Infect. 2014;90(2):112–118. doi: 10.1136/sextrans-2013-051234. [DOI] [PubMed] [Google Scholar]
- Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clinical Infections Diseases. 2011;52(6):793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geibel, King’ola N, Temmerman M, Luchters S. The impact of peer outreach on HIV knowledge and prevention behaviours of male sex workers in Mombasa, Kenya. Sexually Transmitted Infections. 2012;88(5):357–362. doi: 10.1136/sextrans-2011-050224. [DOI] [PubMed] [Google Scholar]
- Geibel S, King’ola N, Temmerman M, Luchters S. The impact of peer outreach on HIV knowledge and prevention behaviours of male sex workers in Mombasa, Kenya. Sexually Transmitted Infections. 2012;88(5):357–362. doi: 10.1136/sextrans-2011-050224. [DOI] [PubMed] [Google Scholar]
- Genberg BL, Shangani S, Sabatino K, Rachlis B, Wachira J, Braitstein P, Operario D. Improving engagement in the HIV care cascade: A systematic review of interventions involving people living with HIV/AIDS as peers. AIDS and Behavior. 2016:1–12. doi: 10.1007/s10461-016-1307-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden MR, Gift TL, Brewer DD, Fleming M, Hogben M, St Lawrence JS, … Handsfield HH. Peer referral for HIV case-finding among men who have sex with men. AIDS. 2006;20(15):1961–1968. doi: 10.1097/01.aids.0000247118.74208.6a. [DOI] [PubMed] [Google Scholar]
- Gutierrez JP, McPherson S, Fakoya A, Matheou A, Bertozzi SM. Community-based prevention leads to an increase in condom use and a reduction in sexually transmitted infections (STIs) among men who have sex with men (MSM) and female sex workers (FSW): the Frontiers Prevention Project (FPP) evaluation results. BMC Public Health. 2010;10:497. doi: 10.1186/1471-2458-10-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett J, Brown G, Maycock B, Langdon P. Changing communities, changing spaces: the challenges of health promotion outreach in cyberspace. Promot Educ. 2007;14(3):150–154. [PubMed] [Google Scholar]
- Hidalgo J, Coombs E, Cobbs WO, Green-Jones M, Phillips G, 2nd, Wohl AR, … Fields SD. Roles and challenges of outreach workers in HIV clinical and support programs serving young racial/ethnic minority men who have sex with men. AIDS Patient Care STDS. 2011;25(Suppl 1):S15–22. doi: 10.1089/apc.2011.9880. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. Vol. 5. Wiley Online Library; 2008. [Google Scholar]
- Hirnschall G, Baggaley R, Verster A. WHO. Consolidated guidelines on HIV prevention, diagnosis, treatment and care for key populations. Paper presented at the Proceedings of the International AIDS Conference.2014. [Google Scholar]
- Hosek SG, Lemos D, Hotton AL, Fernandez MI, Telander K, Footer D, Bell M. An HIV intervention tailored for black young men who have sex with men in the House Ball Community. AIDS Care. 2015;27(3):355–362. doi: 10.1080/09540121.2014.963016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaganath D, Gill HK, Cohen AC, Young SD. Harnessing Online Peer Education (HOPE): integrating C-POL and social media to train peer leaders in HIV prevention. AIDS Care. 2012;24(5):593–600. doi: 10.1080/09540121.2011.630355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegeles SM, Hays RB, Coates TJ. The Mpowerment Project: a community-level HIV prevention intervention for young gay men. Am J Public Health. 1996;86(8):1129–1136. doi: 10.2105/ajph.86.8_pt_1.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegeles SM, Hays RB, Pollack LM, Coates TJ. Mobilizing young gay and bisexual men for HIV prevention: a two-community study. Aids. 1999;13(13):1753–1762. doi: 10.1097/00002030-199909100-00020. [DOI] [PubMed] [Google Scholar]
- Koech E, Teasdale CA, Wang C, Fayorsey R, Alwar T, Mukui IN, … Abrams EJ. Characteristics and outcomes of HIV-infected youth and young adolescents enrolled in HIV care in Kenya. Aids. 2014;28(18):2729–2738. doi: 10.1097/qad.0000000000000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko NY, Hsieh CH, Wang MC, Lee C, Chen CL, Chung AC, Hsu ST. Effects of Internet popular opinion leaders (iPOL) among Internet-using men who have sex with men. Journal of Medical Internet Research. 2013;15(2):e40. doi: 10.2196/jmir.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKellar DA, Valleroy LA, Secura GM, Behel S, Bingham T, Celentano DD, … Thiede H. Unrecognized HIV infection, risk behaviors, and perceptions of risk among young men who have sex with men: opportunities for advancing HIV prevention in the third decade of HIV/AIDS. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2005;38(5):603–614. doi: 10.1097/01.qai.0000141481.48348.7e. [DOI] [PubMed] [Google Scholar]
- Medley A, Kennedy C, O’Reilly K, Sweat M. Effectiveness of peer education interventions for HIV prevention in developing countries: a systematic review and meta-analysis. AIDS Education and Prevention. 2009;21(3):181. doi: 10.1521/aeap.2009.21.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of Internal Medicine. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- Ntata PR, Muula AS, Siziya S. Socio-demographic characteristics and sexual health related attitudes and practices of men having sex with men in central and southern Malawi. Tanzan J Health Res. 2008;10(3):124–130. doi: 10.4314/thrb.v10i3.14351. [DOI] [PubMed] [Google Scholar]
- Outlaw AY, Naar-King S, Parsons JT, Green-Jones M, Janisse H, Secord E. Using motivational interviewing in HIV field outreach with young African American men who have sex with men: a randomized clinical trial. Am J Public Health. 2010;100(Suppl 1):S146–151. doi: 10.2105/ajph.2009.166991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prejean J, Song R, Hernandez A, Ziebell R, Green T, Walker F, … Lansky A. Estimated HIV incidence in the United States, 2006–2009. PLoS ONE. 2011;6(8):e17502. doi: 10.1371/journal.pone.0017502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg ES, Millett GA, Sullivan PS, del Rio C, Curran JW. Understanding the HIV disparities between black and white men who have sex with men in the USA using the HIV care continuum: a modelling study. Lancet HIV. 2014;1(3):e112–e118. doi: 10.1016/S2352-3018(14)00011-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford GW. Antiretroviral therapy and the prevention of sexually transmitted HIV infection. BMJ. 2011:343. doi: 10.1136/bmj.d7796. [DOI] [PubMed] [Google Scholar]
- Scott H, Pollack L, Rebchook G, Huebner D, Peterson J, Kegeles S. Peer Social Support is Associated with Recent HIV Testing Among Young Black Men Who Have Sex with Men. AIDS and Behavior. 2014;18(5):913–920. doi: 10.1007/s10461-013-0608-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni JM, Nelson KM, Franks JC, Yard SS, Lehavot K. Are peer interventions for HIV efficacious? A systematic review. AIDS and Behavior. 2011;15(8):1589–1595. doi: 10.1007/s10461-011-9963-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Bradley H, Hu X, Skarbinski J, Hall HI, Lansky A. Men living with diagnosed HIV who have sex with men: progress along the continuum of HIV care—United States, 2010. MMWR Morbidity and Mortality Weekly Report. 2014;63(38):829–833. [PMC free article] [PubMed] [Google Scholar]
- Stromdahl S, Hickson F, Pharris A, Sabido M, Baral S, Thorson A. A systematic review of evidence to inform HIV prevention interventions among men who have sex with men in Europe. Euro Surveill. 2015;20(15) doi: 10.2807/1560-7917.es2015.20.15.21096. [DOI] [PubMed] [Google Scholar]
- Subramanian T, Ramakrishnan L, Aridoss S, Goswami P, Kanguswami B, Shajan M, … Paranjape RS. Increasing condom use and declining STI prevalence in high-risk MSM and TGs: evaluation of a large-scale prevention program in Tamil Nadu, India. BMC Public Health. 2013;13:857. doi: 10.1186/1471-2458-13-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas H. Effective Public Health Practice Project. McMaster University; Toronto: 2003. Quality assessment tool for quantitative studies. [Google Scholar]
- UNAIDS. Report on Global AIDS Epidemic. 2013. [Google Scholar]
- UNAIDS. Getting to Zero, 2011–2015 Strategy. 2013. [Google Scholar]
- Webel AR. Testing a peer-based symptom management intervention for women living with HIV/AIDS. AIDS Care. 2010;22(9):1029–1040. doi: 10.1080/09540120903214389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Global update on HIV treatment 2013: results, impact and opportunities. Geneva, Switzerland: 2014. 2013. [Google Scholar]
- Wilton L, Herbst JH, Coury-Doniger P, Painter TM, English G, Alvarez ME, … Johnson WD. Efficacy of an HIV/STI prevention intervention for black men who have sex with men: findings from the Many Men, Many Voices (3MV) project. AIDS & Behavior. 2009;13(3):532–544. doi: 10.1007/s10461-009-9529-y. [DOI] [PubMed] [Google Scholar]
- Yan H, Zhang R, Wei C, Li J, Xu J, Yang H, McFarland W. A peer-led, community-based rapid HIV testing intervention among untested men who have sex with men in China: an operational model for expansion of HIV testing and linkage to care. Sexually Transmitted Infections. 2014;90(5):388–393. doi: 10.1136/sextrans-2013-051397. [DOI] [PubMed] [Google Scholar]
- Ye S, Yin L, Amico R, Simoni J, Vermund S, Ruan Y, … Qian H-Z. Efficacy of Peer-Led Interventions to Reduce Unprotected Anal Intercourse among Men Who Have Sex with Men: A Meta-Analysis. PLoS One. 2014;9(3):e90788. doi: 10.1371/journal.pone.0090788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SD, Cumberland WG, Nianogo R, Menacho LA, Galea JT, Coates T. The HOPE social media intervention for global HIV prevention in Peru: a cluster randomised controlled trial. Lancet HIV. 2015;2(1):e27–e32. doi: 10.1016/S2352-3018(14)00006-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SD, Cumberland WG, Nianogo R, Menacho LA, Galea JT, Coates T. The HOPE Social Media Intervention for Global HIV Prevention: A Cluster Randomized Controlled Trial in Peru. Lancet HIV. 2015;2(1):e27–e32. doi: 10.1016/s2352-3018(14)00006-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zablotska I, Holt M, De Wit J, McKechnie M, Mao L, Prestage G. Gay men who are not getting tested for HIV. AIDS and Behavior. 2012;16(7):1887–1894. doi: 10.1007/s10461-012-0184-3. [DOI] [PubMed] [Google Scholar]
- Zanoni BC, Mayer KH. The adolescent and young adult HIV cascade of care in the United States: Exaggerated health disparities. AIDS Patient Care and STDS. 2014;28(3):128–135. doi: 10.1089/apc.2013.0345. [DOI] [PMC free article] [PubMed] [Google Scholar]