Abstract
Objective
To determine one-year, post-hospital mortality and the predictors of mortality in Tanzanian adults with heart failure (HF) compared to other admitted adults.
Methods
In this prospective cohort study we consecutively enrolled medical inpatients admitted during a 3-month period, screened for HF and followed until 12 months after hospital discharge. Standardized history, physical examination, echocardiography and laboratory investigations were obtained during hospital presentation. The primary outcome was one-year post-discharge mortality. The secondary outcome was in-hospital mortality. Cox regression adjusted for age and sex was used.
Results
During the study period, we enrolled 558 adults; 145 had HF and 107 of these survived until discharge. Patients with HF had a higher one-year post-hospital discharge mortality than all other diagnoses (62/107 (57.9%) vs 150/343 (43.7%), respectively, HR=1.57[1.13–2.18]). In-hospital mortality was similar. Markers of renal disease were more common in adults with HF (40/107 (37.4%) and were the strongest independent predictors of post-hospital mortality: low eGFR (HR=2.94[1.62–5.31]) and proteinuria (HR=2.03, [95%CI 1.13–3.66]). No patients discharged with the combination of low eGFR/proteinuria survived to the one-year endpoint. Of note, 79/145 (54.5%) of adults admitted with HF were newly diagnosed during hospital admission.
Conclusions
Over half of adults discharged with HF died within 12 months after discharge. Adults with HF had higher post-hospital mortality compared to other medical inpatients. Markers of renal disease were the strongest predictor of this mortality. Innovative interventions are needed to reduce post-hospital mortality in adults with HF and should focus on those with renal disease.
Keywords: global health, global disease patterns, heart failure mortality
INTRODUCTION
The global burden of heart failure (HF) is rapidly increasing.1 Heart failure represents up to 3% of all hospital admissions and 11% of all deaths in high-income countries, with these statistics outpacing those of other chronic diseases.2,3 Low and middle-income countries (LMIC) likely bare an even greater burden of HF morbidity, mortality and economic impact.4 In sub-Saharan Africa, HF prevalence is estimated to be 5% and increasing.5 At our own hospital, HF represents 10% of hospital admissions and 10% of in-hospital deaths.6 In addition, primary healthcare resources for managing chronic diseases such as HF are severely limited in sub-Saharan Africa,7,8 with most service provided at hospitals instead of community health facilities.
Although the first-year post-hospital period is known to be one of particularly high risk for HF patients in the U.S. and Europe,9 very little is known about this critical year in LMIC.4 Additionally, while renal dysfunction is common and worsens mortality in adults with HF in high-income countries,10,11 few studies have reported LMIC predictors of poor outcomes in discharged patients. A better understanding of post-hospital outcomes for HF patients as compared to in-hospital, as well as the predictors of mortality, will be critical to designing interventions to improve long-term health in this population.
Therefore, we conducted a prospective cohort study with 12-month follow up to determine the clinical course of adults with HF in Tanzania from the time of hospital admission, through hospital discharge and then for the first year after discharge. Our objectives were: 1) to compare one year post-hospital mortality in adults admitted with HF to adults admitted with other conditions and 2) to determine predictors of post-hospital mortality in adults with HF compared to predictors of in-hospital mortality. We hypothesized that post-discharge HF mortality would be significantly higher than other admitted patients, that outcomes and predictors would differ for in-hospital and post-hospital mortality and that markers of renal dysfunction would be a significant, independent predictor of post-hospital mortality.
METHODS
Study setting
In this prospective cohort study, we consecutively enrolled adults hospitalized on the medical wards of Bugando Medical Centre (BMC). BMC is a public hospital that serves the Lake Victoria region of northwestern Tanzania (population: 13 million). BMC is located in the city of Mwanza, the second largest urban center in Tanzania and the capital of the Mwanza region. BMC has 100 adult medical beds and ~3000 medical hospitalizations per year.
Inclusion & exclusion criteria
Adults (≥18 years) hospitalized in the medical ward were eligible for enrollment. Potential study participants were provided with information regarding the study within 12 hours of hospitalization. All of those who provided informed consent were enrolled. Study participants with multiple hospitalizations to BMC during the study period were only enrolled during their first hospitalization.
All patients enrolled at time of admission were included in the “All Admitted Cohort.” All patients who survived until discharge were included in the “Post-hospital Cohort.” In this manuscript, we have provided data regarding both cohorts in order to describe the entire clinical course of admitted HF patients and for the sake of comparison. Our manuscript, though, focuses on the “Post-hospital HF Cohort” due to absence of published data regarding long-term outcomes for this group as well as the greater opportunity for intervention in this group.
Study procedures
On the day of enrollment, a pre-validated, adapted, translated version of the WHO STEPS questionnaire was administered in Kiswahili by a Tanzanian study investigator. The WHO STEPS questionnaire includes questions regarding prior testing, diagnosis, and treatment for chronic diseases as well as standard protocols for physical examination.12 Heart failure specific questions from the Framingham clinical HF criteria were added in the WHO STEPS questionnaire to screen all admitted adults for clinical HF.
After completing the questionnaire, we conducted a standardized physical examination. Blood pressures were measured by a registered nurse or doctor using a mercury sphygmomanometer according to the WHO STEPS protocol.
Laboratory analysis
At the time of hospitalization, by hospital policy, all medical inpatients were offered voluntary counseling and testing for HIV and underwent measurement of glucose, creatinine, and urine dipstick testing. Serum creatinine levels were measured using a Cobas Integra 400 Plus Analyzer (Roche Diagnostic Limited, Basel, Switzerland). An estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation13 (without ethnic factor) as recommended by international guidelines. Random blood glucose was measured using a finger stick sample (Ascensia Glucometer, Bayer Healthcare, Germany). A urine dipstick was used to test for proteinuria, hematuria, and pyuria (Multistix 10SG, Siemens, USA).
Study Definitions
Clinical HF was defined as the simultaneous presence of at least 2 major Framingham HF criteria or 1 major criterion in conjunction with 2 minor criteria. Patients with clinical HF underwent echocardiography. Echocardiographic confirmation of HF was determined by ejection fraction <50% and/or diastolic dysfunction. These criteria are consistent with current HF study definitions in low and middle income countries.5 Study subjects with diagnoses other than HF were classified as “controls”.
Echocardiography
Echocardiography was performed using a GE VIVID 7 Pro by an expert echocardiographer following American Society of Echocardiography guidelines. LV systolic function was assessed by apical biplane method of discs (modified Simpson’s rule). Patients with EF less than 50% were classified as having systolic dysfunction and LV diastolic dysfunction was diagnosed by the presence of a reduced e’ (e’ < 9 cm/s) and/or an increased E/e’ ratio > 15. Dilated cardiomyopathy (DCM) was defined by the presence of all-chamber or isolated LV dilatation and global hypokinesia in the absence of features of hypertensive heart disease or any other apparent cause of global dilatation and hypokinesia (ie regional wall motion abnormality). Ischemic cardiomyopathy was defined by depressed LV ejection fraction with supportive ECG findings and/or presence of regional wall motion abnormality. Hypertensive HF was defined by symptoms of HF, documented high blood pressure and ECHO finding of LVH or concentric remodeling (e.g. increase in relative wall thickness with normal LVMI), with either systolic or diastolic dysfunction, or both. Valvular HF was defined by HF secondary to primary underlying valvular abnormality (e.g. RHD with classic elbow shaped appearance or degenerative valve disease). HF secondary to HIV infection was defined in patients with HIV with a cardiac condition causing dilated HF. Other conditions, such as hypertrophic obstructive cardiomyopathy and endomyocardial fibrosis, were determined by an expert echocardiographer using ASE guidelines. All diagnoses of HF and determination of HF etiologies were confirmed by two blinded, independent physician reviewers. In the case of disagreement between these two physician reviewers, a certified cardiologist was consulted for final diagnosis.
Discharge diagnoses
Diagnoses were determined at the time of discharge. Since December 2008, our hospital has used a standard list of recommended discharge diagnoses adapted from the WHO’s International Classification of Diseases version 10 (ICD-10). Heart failure patients were discharged with medical outpatient management according to hospital guidelines when determined in “stable discharge condition” by clinical staff. Hospital guidelines for heart failure were specifically written to include medications that are available in the pharmacy of our hospital including beta blockers and ACE inhibitors. Some medications which would be considered part of “optimal treatment” are not available in Tanzania such as digoxin and nephrilysin inhibitors.
HF patients were discharged with optimal medical outpatient management when determined in “stable discharge condition” by clinical staff. This treatment regimen is routinely available in the study outpatient setting. Of note, palliative care in the study setting is provided as an inpatient service only; therefore, no patients were discharged for end-of-life care.
Follow-up of study participants
Three mobile phone numbers were obtained from all participants at discharge. Follow-up phone calls were made at one, three, six, and 12 months. During each call, a standard set of questions were asked in Kiswahili including vital status and primary care clinic attendance. These questions were asked directly to the study participant whenever possible, and alternate phone numbers were used only if the study participant was unavailable or unable to communicate information clearly.
Measures
The primary study outcome was death after hospital discharge. Mortality was classified as in-hospital if it occurred during the index hospitalization and post-hospitalization if it occurred in the year that followed, not including, the index hospitalization.
Data analysis
Data were entered into Microsoft Excel and analyzed using STATA version 11 (StataCorp, College Station, Texas, USA). Categorical variables were described as proportions (percentiles), and continuous variables were described as means (interquartile ranges). For all cross-sectional analyses, a chi-squared test was used for comparing categorical variables and a Wilcoxon rank sum test was used for continuous variables. All available data was included in all calculations. No explanatory variable was missing more than 7 patients except for the urine dipstick results. A two-sided p-value of <0.05 was regarded as statistically significant in all analyses.
Cox regression models adjusted for age and sex were used for all survival analyses to compare outcomes between groups and to determine predictors of in-hospital and 12-month mortality. We adjusted for age and sex since these were thought to be the most important possible confounders for HF outcomes and predictors. Kaplan-Meier survival curves were used to display incident mortality. Study participants who were lost-to-follow-up were censored at the last contact date.
We estimated that one fifth of study participants had HF, that post-hospital mortality would be 50% in the HF group vs 25% in patients admitted with all other diagnoses. Given these assumptions, and allowing for a 10% loss-to-follow-up, we calculated that including 275 consecutively discharged adults would give us >90% power to detect this 25% absolute difference in 12-month, post-hospital mortality.
Ethical issues
Ethical approval was obtained from the ethics committees of BMC (IRB number BREC/001/18/2008), the Tanzanian National Institute of Medical Research and Weill Cornell Medical College. All study participants were informed about the study by a nurse or doctor fluent in Kiswahili and provided written informed consent before participation. Participants also consented to receiving phone calls at either their own mobile phone number or the mobile phone numbers that they provided for friends and relatives. All study procedures were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000.
RESULTS
Enrollment
During the study period from October to December 2014, 670 total patients were admitted to the medical wards of our hospital. All patients were acutely ill and none were admitted for elective reasons. Forty-seven patients admitted with acute infectious diseases (malaria, urosepsis, acute tuberculosis and meningitis) were ineligible for the study. Of the remaining 623, 35 patients were excluded: 12 patients were <18 years of age, 17 patients died prior to enrollment and 6 patients refused informed consent. Therefore, a total of 588/623 (94.4%) of patients were enrolled. Of these 588, 29 (4.6%) and 45 (7.7%) were lost-to-follow up after 6 and 12 months, respectively. Nine of 145 (6.2%) HF and 36/443 (8.1%) of control patients were lost to follow-up in the first year after discharge.
Of the 588 study subjects enrolled, 145 (22.4%) were diagnosed with HF and 443 (75.3%) did not have HF. The in-hospital mortality rates were 38/145 (26.2%) in the HF group and 100/443 (22.6%) in the control group.
Therefore, 107 adults with HF and 343 control subjects continued in the Post-hospital Cohort. A total of 45 HF patients and 193 control patients survived until 365 days post-discharge.
Baseline Characteristics
The characteristics of the two study groups (HF and controls) are described for both the Post-hospital Cohort and All Admitted Patients Cohort in Table 1.
Table 1.
Baseline characteristics of study participants illustrating the differences between patients admitted with HF and all other patients.
| Post-hospital Cohort | All Admitted Cohort | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variable (% or SD) |
HF (n=107) |
Controls (n= 343) |
p-value* | HF (n=145) |
Controls (n=443) |
p-value* |
| Demographic characteristics | ||||||
|
| ||||||
| Male | 41 (38.3%) | 177 (51.6%) | 0.02 | 64 (44.1%) | 241 (54.4%) | 0.03 |
| Age, years# | 50.8 (26) | 44.7 (33) | 0.004 | 52.0 (34) | 45.9 (27) | 0.001 |
|
| ||||||
| Education | ||||||
|
| ||||||
| Did not complete primary education | 48 (44.9%) | 114 (33.3%) | 0.03 | 68 (46.9%) | 153 (34.9%) | 0.03 |
| Completed primary education | 46 (43.0%) | 173 (50.6%) | 57 (39.3%) | 219 (49.9%) | ||
| Completed secondary education or higher | 13 (12.2%) | 55 (16.1%) | 20 (13.8%) | 67 (15.3%) | ||
|
| ||||||
| Occupation | ||||||
|
| ||||||
| Farmer | 54 (50.5%) | 166 (48.5%) | 0.18 | 75 (52.1%) | 207 (47.0%) | 0.06 |
| Petty trader | 21 (19.6%) | 99 (29.0%) | 28 (19.4%) | 135 (30.7%) | ||
| Civil servant, business, or professional | 18 (16.8%) | 46 (13.5%) | 25 (17.4%) | 61 (13.9%) | ||
| Unemployed, retired, or student | 14 (13.1%) | 31 (9.1%) | 16 (11.1%) | 37 (8.4%) | ||
|
| ||||||
| Medical History | ||||||
|
| ||||||
| Current tobacco smoking | 2 (1.9%) | 16 (4.7%) | 0.39 | 5 (3.5%) | 28 (6.3%) | 0.39 |
| Current alcohol use | 4 (3.7%) | 26 (7.7%) | 0.14 | 4 (2.8%) | 42 (9.6%) | 0.01 |
| History of hypertension | 46 (43.0%) | 75 (22.0%) | <0.0001 | 61 (42.1%) | 92 (20.9%) | <0.0001 |
| History of kidney disease | 11 (10.3%) | 8 (2.3%) | <0.0001 | 17 (11.7%) | 8 (1.8%) | <0.0001 |
| History of diabetes | 9 (8.4%) | 28 (8.2%) | 0.83 | 14 (9.7%) | 30 (6.8%) | 0.26 |
|
| ||||||
| Medication Use | ||||||
|
| ||||||
| Herbal or traditional medication | 22 (20.8%) | 66 (19.4%) | 0.68 | 34 (23.6%) | 89 (20.2%) | 0.38 |
| NSAIDS or steroids | 13 (12.3%) | 64 (18.7%) | 0.18 | 16 (11.1%) | 77 (17.4%) | 0.07 |
| Anti-hypertensive | 41 (38.3%) | 67 (19.6%) | 0.001 | 53 (36.6%) | 83 (18.8%) | <0.0001 |
|
| ||||||
| Symptoms on hospitalization | ||||||
|
| ||||||
| Decreased urine output | 28 (26.2%) | 20 (5.6%) | <0.0001 | 41 (28.3%) | 32 (7.3%) | <0.0001 |
| Chest pain | 29 (27.4%) | 43 (12.5%) | <0.0001 | 34 (23.6%) | 53 (12.0%) | 0.001 |
| Shortness of breath | 96 (90.6%) | 54 (15.7%) | <0.0001 | 128 (88.9%) | 83 (18.7%) | <0.0001 |
|
| ||||||
| Framingham HF Criteria | ||||||
|
| ||||||
| Major Criteria | ||||||
| Acute Pulmonary Edema | 56 (65.9%) | 1 (0.3%) | <0.0001 | 67 (59.8%) | 2 (0.5%) | <0.0001 |
| Cardiomegaly | 99 (92.5%) | 60 (17.5%) | <0.0001 | 135 (93.1%) | 85 (19.2%) | <0.0001 |
| Neck Vein Distension | 44 (41.1%) | 2 (0.6%) | <0.0001 | 58 (40.0%) | 2 (0.5%) | <0.0001 |
| PND or Orthopnea | 93 (86.9%) | 18 (5.3%) | <0.0001 | 124 (85.5%) | 27 (6.1%) | <0.0001 |
| Pulmonary Rales | 88 (82.2%) | 21 (6.1%) | <0.0001 | 120 (83.3%) | 41 (9.3%) | <0.0001 |
| S3 Heart Sound | 21 (19.8%) | 11 (3.2%) | <0.0001 | 28 (19.4%) | 11 (2.5%) | <0.0001 |
| Minor Criteria | ||||||
| Bilateral Ankle Edema | 77 (72.6%) | 24 (7.0%) | <0.0001 | 108 (75.0%) | 40 (9.1%) | <0.0001 |
| Dyspnea on Exertion | 96 (90.6%) | 54 (15.7%) | <0.0001 | 128 (88.9%) | 83 (18.7%) | <0.0001 |
| Nocturnal Cough | 83 (77.6%) | 21 (6.1%) | <0.0001 | 112 (77.2%) | 29 (6.5%) | <0.0001 |
| Pleural Effusion | 18 (21.2%) | 5 (1.5%) | 0.58 | 30 (27.0%) | 6 (1.4%) | 0.051 |
| Heart Rate >120 bpm (0) | 7 (6.5%) | 17 (5.0%) | 0.58 | 15 (10.3%) | 29 (6.5%) | 0.13 |
| Meets Framingham criteria for HF | 104 (97.2%) | 6 (1.8%) | <0.0001 | 140 (96.6%) | 42 (9.5%) | <0.0001 |
|
| ||||||
| Signs on physical examination | ||||||
|
| ||||||
| Heart rate (bpm) | 92.1 (25.0) | 91.5 (23.0) | 0.396 | 93.3 (26) | 92.7 (25.1) | 0.318 |
| Systolic blood pressure (mm Hg) | 127.4 (40) | 123.5 (27) | 125.0 (44) | 123.9 (27) | ||
| < 90 | 3 (2.9%) | 2 (0.6%) | 0.04 | 11 (7.7%) | 7 (1.6%) | <0.0001 |
| 90–139 | 69 (65.7%) | 259 (75.7%) | 89 (62.2%) | 330 (74.7%) | ||
| 140–179 | 28 (26.7%) | 63 (18.4%) | 36 (25.2%) | 76 (17.2%) | ||
| > 179 | 5 (4.8%) | 18 (5.3%) | 7 (4.9%) | 29 (6.6%) | ||
| Diastolic blood pressure (mm Hg) | 80.5 (25) | 77.1 (17) | 0.03 | 78.5 (26) | 77.3 (17) | 0.375 |
| Oxygen saturation (%) | 88.7 (18) | 95.1 (2) | <0.0001 | 88.5 (10) | 94.3 (2) | <0.0001 |
| Abdominal obesity | 83 (77.6%) | 219 (63.8%) | 0.01 | 109 (75.2%) | 273 (61.6%) | 0.003 |
| Body mass index (kg/m2) | ||||||
| < 18.5 | 14 (13.1%) | 47 (13.8%) | 0.15 | 16 (11.0%) | 63 (14.3%) | 0.025 |
| 18.5–24 | 58 (54.2%) | 213 (62.5%) | 79 (54.5%) | 277 (63.0%) | ||
| 25–29 | 17 (15.9%) | 52 (15.3%) | 25 (17.2%) | 64 (14.5%) | ||
| ≥ 30 | 18 (16.8%) | 29 (8.5%) | 25 (17.2%) | 36 (8.2%) | ||
|
| ||||||
| Laboratory investigations | ||||||
|
| ||||||
| Random blood glucose (mmol/L) | 7.3 (2.6) | 8.0 (2.8) | 0.562 | 7.4 (2.6) | 8.0 (2.9) | 0.757 |
| Estimated glomerular filtration rate (ml/min/1.73m2) | ||||||
| <45 | 25 (23.4%) | 33 (9.7%) | <0.0001 | 44 (30.3%) | 56 (12.7%) | <0.0001 |
| ≥45 | 82 (76.6%) | 308 (90.3%) | 101 (69.7%) | 384 (87.3%) | ||
| Proteinuria by urinalysis (20) | 25 (23.6%) | 79 (23.0%) | 0.99 | 43 (29.9%) | 122 (28.8%) | 0.80 |
| HIV Status | ||||||
| Positive | 12 (11.2%) | 92 (26.8%) | 0.03 | 14 (9.7%) | 129 (29.1%) | <0.0001 |
| Negative | 92 (86.0%) | 251 (73.2%) | 131 (90.3%) | 344 (77.7%) | ||
HF vs Control for respective Cohort
Maximum number of missing values was 4 for age variable
Compared to control subjects, HF subjects were more female (66/107 (61.7%) vs 188/343 (51.6%), p=0.02, respectively) and older (50.8 vs 44.7 years, p=0.004). Other notable differences were significantly: lower educational status, more known hypertension, more history of renal disease, more patients taking anti-hypertensive medication, more obesity in the HF group. Of note, HF subjects had a statistically greater number of patients with low eGFR (25/107 (23.4%) vs 33/343 (9.7%), p<0.0001) but a similar prevalence of proteinuria compared to controls (25/107 (23.6%) vs 79/343 (23.0%), p=0.99). Additionally, significantly fewer HF patients were HIV infected (12/107 (11.2%) vs 92/343 (26.8%), p=0.03). Of adults with HF, 57/107 (53.3%) were newly diagnosed at the time of hospital admission. Additionally, only 41/107 (38.3%) were currently prescribed any HF medication prior to admission.
At admission, 79/145 (54.5%) of adults with HF were newly diagnosed. Additionally, only 57/145 (39.6%) were currently prescribed any HF medication prior to admission. The most prevalent individual Framingham major and minor criteria present were cardiomegaly and dyspnea on exertion, respectively. Prevalence of all Framingham factors was similar in HF patients not surviving to hospital discharge (Table 1).
Heart Failure Mortality
In the Post-hospital Cohort (Figure 1A), mortality was significantly higher in HF participants as compared to controls, 62/107 (57.9%) vs 150/343 (43.7%) respectively (HR=1.57[1.13–2.18]), p=0.007 by Cox regression adjusted for age and sex). Between HF subtypes, mortality in the year after discharge was not significantly different, p=0.480.
Figure 1.
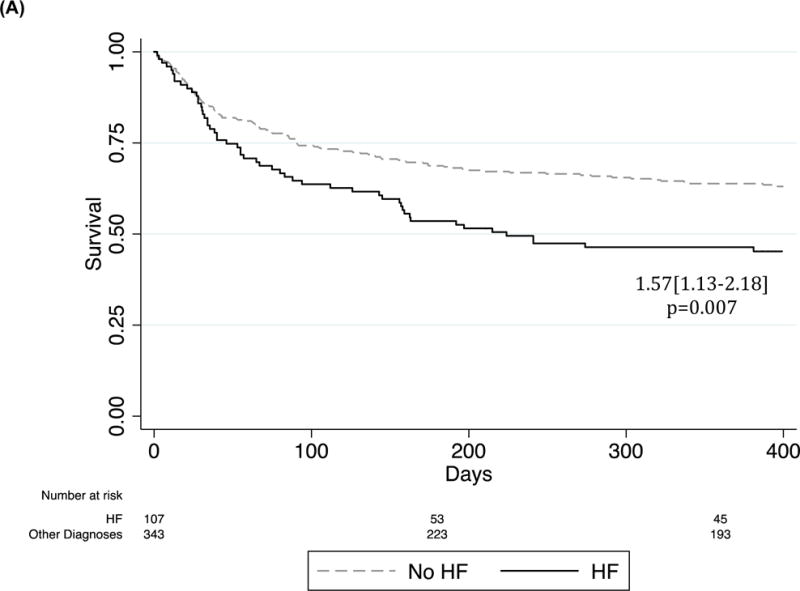
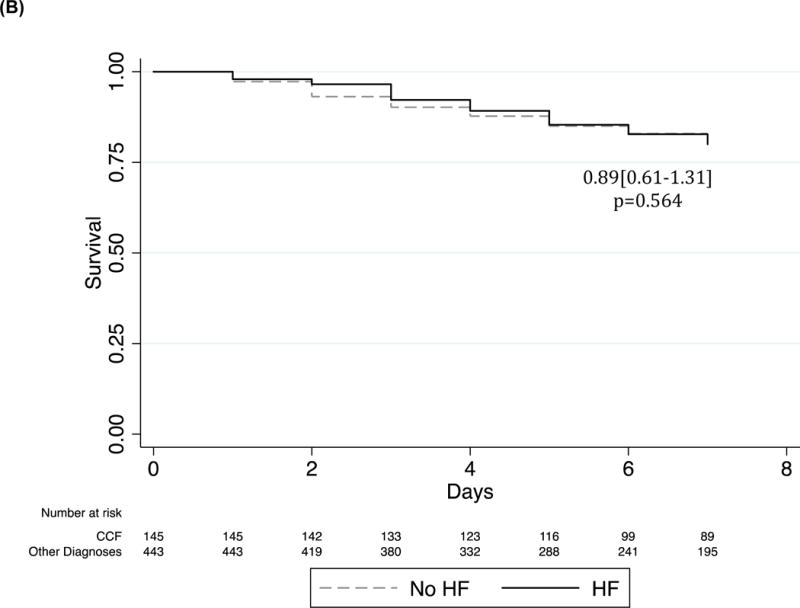
Kaplan Meier survival curve of HF patients as compared to all others admitted. Cox regression adjusted for age and sex. (A) Incidence of post-discharge mortality in the Post-hospital Cohort. (B) Incidence of in-hospital mortality in the All Admitted Cohort.
By comparison, in-hospital mortality was not significantly different between adults with HF and control patients (38/145 (26.2%) vs 100/443 (22.6%), respectively (p=0.564 (Figure 1B). In-hospital mortality was also similar between HF subtypes (p=0.227).
In total, 100 (69.0%) of the 145 enrolled HF participants died within one year of hospital admission compared to 250 (56.4%) of the 443 enrolled control participants (HR=1.45[1.13–1.85]), p=0.004). Of these 100 deaths in the HF group, 38/100 (38.0%) occurred in-hospital and 62/100 (62.0%) occurred in the first year after hospital discharge.
Factors Associated with Heart Failure Mortality
All variables listed in Table 1 were analyzed as possible predictors of post-hospital and in-hospital mortality.
The only significant independent predictors of post-discharge mortality after adjusting for age and sex were eGFR (< or >=45 mL/min/1.73m2) (HR2.94[1.62–5.31], p<0.0001 and proteinuria (HR=2.03[1.13–3.66]), p=0.018) (Figure 2). Additionally, the combination of proteinuria and low eGFR (eGFR <45 mL/min/1.73m2) was strongly associated with HF mortality as compared to controls (HR=1.59[1.25–2.16], p<0.0001) with none of the patients with the low eGFR/positive proteinuria combination surviving at the one-year post-discharge time point.
Figure 2.
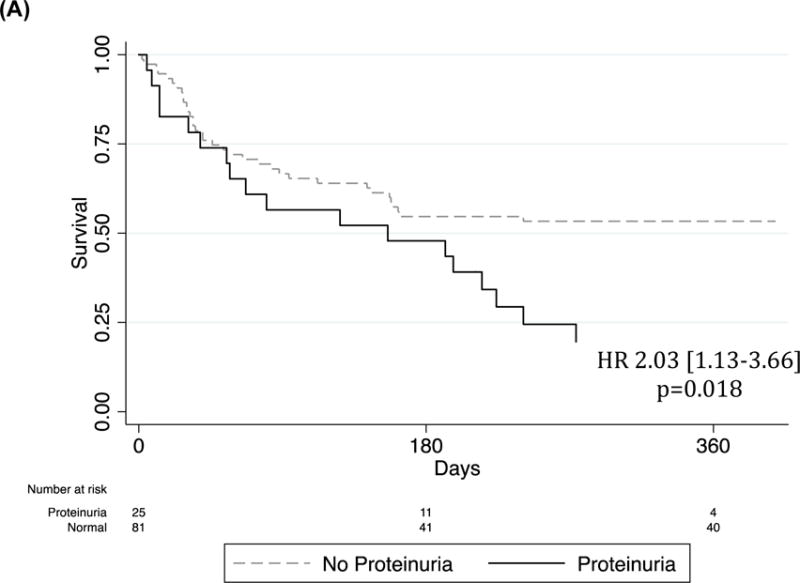
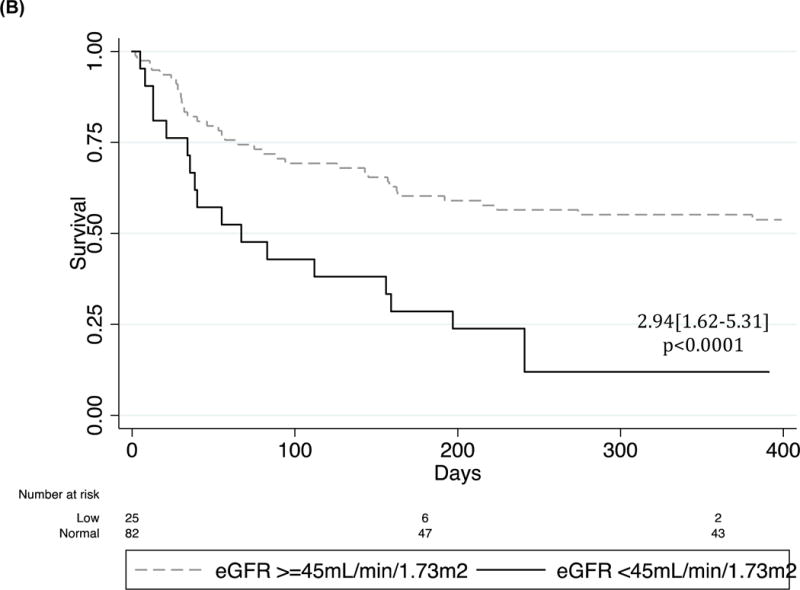
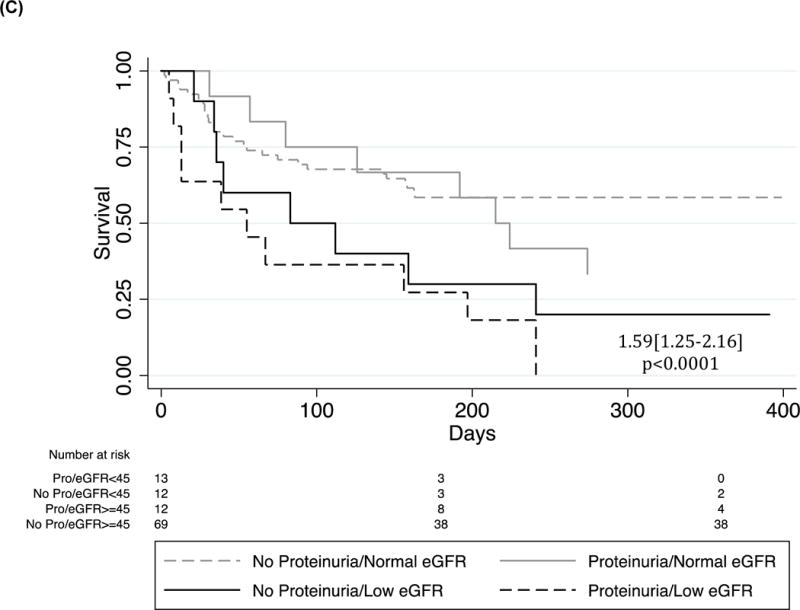
Kaplan Meier survival curves of (A) proteinuria, (B) GFR and (C) proteinuria/GFR combinations in the Post-hospital HF Cohort. Cox regression adjusted for age and sex.
By comparison, Cox regression analysis adjusted for age and sex (Supp Table 1) revealed nine significant predictors of HF mortality in the in-hospital period: heart rate >120 bpm, severe hypotension, low glomerular filtration rate, pleural effusion, proteinuria, sex, diastolic and systolic blood pressure and acute pulmonary edema: (HR[CI]: 3.89[1.61–9.30], 3.85[1.72–8.0], 3.25[1.55–6.70], 3.07[1.38–6.80], 2.03[1.1–3.8], 1.93[1.01–3.70], 0.98[0.97–0.99], 0.59[0.36–0.9], 0.366[0.16–0.82] respectively.
Heart Failure Etiology
Heart failure etiologies and mortality are described in Figure 3. Hypertensive heart disease was the most prevalent HF subtype (45/107 (42.1%)) followed by rheumatic (11/107 (10.3%)), cor pulmonale (11/107 (10.3%)), alcoholic dilated (7/107 (6.5%)), ischemic (6/107 (5.6%)), peripartum and idiopathic cardiomyopathy (5/107 (4.7%)), HIV dilated and tamponade (4/107 (3.7%)), other valvular (3/107 (2.8%)), VSD/ASD and high output (2/107 (1.9%)) and EMF and restrictive cardiomyopathy (1/107 (0.9%)). Total burden of all-cause dilated HF was 21/107 (19.6%) and of all-cause valvular was 14/107 (13.1%). Heart failure etiology prevalence of the All Admitted Cohort was generally similar to those surviving to discharge.
Figure 3.
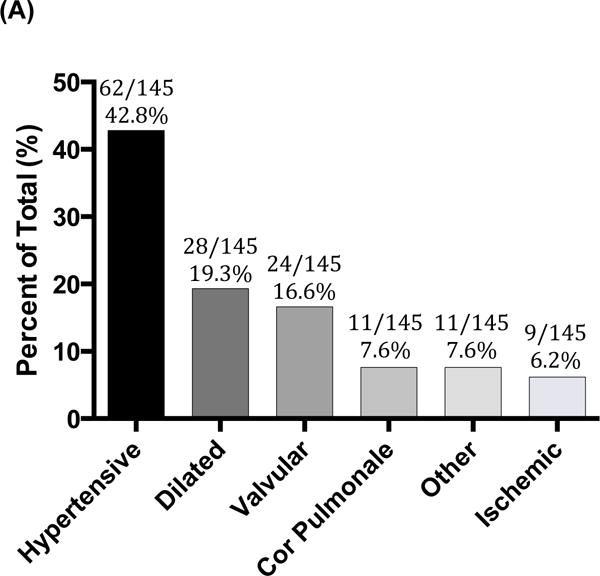
Likely HF etiologies in study subjects admitted with heart failure (n=145) (Figure A). Figure B illustrates the likely etiologies for those adults with dilated cardiomyopathies.
Renal Function in Heart Failure Subgroups
Baseline characteristics of patients with and without low eGFR are described in Supp Table 2. In the Post-hospital Cohort, more adults with HF had an eGFR < 45 ml/min/1.73m2 compared to all other diagnoses (40/107 (37.4%) vs 64/343 (18.7%), p<0.0001). Subgroup analysis of HF patients surviving to hospital discharge showed statistically similar rates of low eGFR, proteinuria and the combination of low GFR/proteinuria across all sub-groups (Supp Table 3A).
For comparison, in the All Admitted Cohort, significantly more of the HF population admitted had a low eGFR as compared to all other diagnoses (67/145 (46.2%) vs 99/443 (22.3%), p<0.0001) and more patients with low eGFR/positive proteinuria (30/145 (20.7%) vs 50/443 (11.3%), p=0.01) as compared to controls. Within the HF group there was a significant difference in number of patients with low eGFR between subgroups, with hypertensive HF having the largest proportion of low eGFR (38/62 (61.3%))(Supp Table 3B). Proteinuria, and the combination of low eGFR/positive proteinuria, were evenly distributed across HF subgroups (p=0.154 and 0.329, respectively).
DISCUSSION
More than half of adults discharged with HF in stable condition from a typical public hospital in Africa had died within 1 year. Greater than 40% of these had died within 6 months. The risk of post-hospital mortality for adults with HF was 70% greater than control adults discharged with other medical conditions and is 3-fold greater than the 20% mortality reported for adults admitted with HF in high-income countries.14–16 This is the first published data to describe the long-term clinical course of African adults admitted and discharged with HF compared to those with other medical conditions. From prior, uncontrolled studies it is difficult to determine if poor post-hospital outcomes for adults with HF was specific to this condition or generally true for all adults discharged from hospitals in Africa. Other recently published data from SSA confirm that long-term outcomes are poor for outpatients with HF as well.17 There is an urgent need for interventions to improve the long-term health of adults with HF in Africa.
The post-hospital period is particularly important for adults with HF in Africa because many adults are first diagnosed with HF during their index hospital admission. In our study, >50% of study subjects admitted with HF were newly diagnosed at the time of admission. Three-fourths of study subjects with HF improved during hospitalization and were discharged in stable condition. A similarly high prevalence of new diagnosis at the time of hospital admission has been reported for other diagnoses such as HIV, hypertension and diabetes.6,18 The delay in diagnosis in HF until the time of the index hospital admission is likely related to the limitations in resources for diagnoses of HF at lower level health facilities.7,8 Therefore, as supported by evidence from the U.S.,19,20 we have identified the post-hospital period as a critical window of interventional opportunity for improving HF outcomes in Africa.
Markers of renal disease (i.e. positive proteinuria and low eGFR) were the only independent predictor of mortality in both the in- and post-hospital periods. These data extend the findings of a recent study of predictors of post-discharge, two-month HF mortality21 reported renal function as an important predictor of mortality. Other data support the strong relationship between renal disease and short-term post-hospital mortality in adults with HF in Africa.17,21 Interestingly, proteinuria was a strong predictor of mortality. This is an especially interesting finding given recent data from the U.S. showing proteinuria is a strong, independent predictor of HF mortality.22 These findings are of dual importance: 1) we believe this group represents an important first-step in intervention of the high HF mortality in SSA, and 2) our data suggest that renal dysfunction in this population may be identifiable with low-cost screening tools (i.e. urine dipstick for proteinuria).
These data have similarities and differences as compared to previous heart failure trials in HIC settings. Compared to major clinical trial of HF in the U.S. and Europe, our study subjects were younger (52 vs. 65 years), more female (56% vs. 29%) and had less presumed ischemic heart disease (6.2% vs. 52%).24–28 These differences in population should be taken into account when efforts are made to generalize clinical trial results from HIC to Africa and indicate a need for clinical trials conducted in Africa. We also report a mortality rate of 65% in the first year post-discharge, a number in-line with that of very early HF trials in HIC settings,28 but much higher than that of those more recently published.29 This is encouraging and suggests that HF outcomes can be improved in LMIC settings. Additionally, we report a 7% loss-to-follow-up rate, which is higher than that of HIC HF trials but is similar to that of previous SSA HF cohorts (7.0% vs 6.9% and 13.8%).17,30
There are limitations to the current study. Most notably, whether the reported mortality associated with renal dysfunction is a result of intrinsic renal disease or secondary to heart failure is unknown. Future studies should include either patients with known baseline renal function or repeat measures of renal function post-discharge.
In summary, we report that 60% of adults discharged from our hospital with HF had died within 1 year. The risk of post-hospital mortality was 70% higher than adults discharged from the same hospital with other diagnoses and 3-fold higher than adults admitted with HF to hospital in high-income countries. Readily available markers of renal disease (such as urine dipstick for protein) are a common and independently predict post-hospital stay mortality. Therefore we conclude that developing and testing interventions to improve post-hospital outcomes for adults admitted with HF will be critical to improve the long-term health of adults with HF in Africa. Clinical this population should address renal disease as important prognostic indicators and possibly a target for intervention in this population.
Supplementary Material
Acknowledgments
We would like to thank Professor Kien Mteta, the Director General of Bugando Medical Centre, for his administrative support in this study.
Funding
This project was supported by National Institutes of Health (NIH) Research Training Grant R25 TW009337, funded by the Fogarty International Center, the NIH Office of the Director, the National Institute of Mental Health, and the National Institute of Diabetes and Digestive and Kidney Diseases. Additionally, This study was supported by grants from the National Institutes of Health Fogarty Foundation (TW000018 and K01 TW010281-01) and the National Institute of Allergy and Infectious Diseases (K24 AI098627).
Footnotes
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Pi??a IL, Trogdon JG. Forecasting the impact of heart failure in the united states a policy statement from the american heart association. Circ Hear Fail. 2013;6:606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cowie MR, Anker SD, Cleland JG, Felker GM, Filippatos G, Jaarsma T, Jourdain P, Knight E, Massie B, Ponikowski P, López-Sendón J. Improving care for patients with acute heart failure Before, during and after hospitalization. 2014 doi: 10.1002/ehf2.12021. [DOI] [PubMed] [Google Scholar]
- 3.Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CSP, Sato N, Shah AN, Gheorghiade M. The global health and economic burden of hospitalizations for heart failure: Lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63:1123–1133. doi: 10.1016/j.jacc.2013.11.053. [DOI] [PubMed] [Google Scholar]
- 4.Callender T, Woodward M, Roth G, Farzadfar F, Lemarie JC, Gicquel S, Atherton J, Rahimzadeh S, Ghaziani M, Shaikh M, Bennett D, Patel A, Lam CS, Sliwa K, Barretto A, Siswanto BB, Diaz A, Herpin D, Krum H, Eliasz T, Forbes A, Kiszely A, Khosla R, Petrinic T, Praveen D, Shrivastava R, Xin D, MacMahon S, McMurray J, Rahimi K. Heart failure care in low- and middle-income countries: a systematic review and meta-analysis. PLoS Med. 2014;11:e1001699. doi: 10.1371/journal.pmed.1001699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dokainish H, Teo K, Zhu J, Roy A, Alhabib KF, Elsayed A, Palileo-Villaneuva L, Lopez-Jaramillo P, Karaye K, Yusoff K, Orlandini A, Sliwa K, Mondo C, Lanas F, Prabhakaran D, Badr A, Elmaghawry M, Damasceno A, Tibazarwa K, Belley-Cote E, Balasubramanian K, Yacoub MH, Huffman MD, Harkness K, Grinvalds A, McKelvie R, Yusuf S. Int J Cardiol. Vol. 204. Elsevier Ireland Ltd; 2016. Heart Failure in Africa, Asia, the Middle East and South America: The INTER-CHF study; pp. 133–141. [DOI] [PubMed] [Google Scholar]
- 6.Peck RN, Green E, Mtabaji J, Majinge C, Smart LR, Downs JA, Fitzgerald DW. Hypertension-related diseases as a common cause of hospital mortality in Tanzania: a 3-year prospective study. J Hypertens. 2013;31:1806–1811. doi: 10.1097/HJH.0b013e328362bad7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peck R, Mghamba J, Vanobberghen F, Kavishe B, Rugarabamu V, Smeeth L, Hayes R, Grosskurth H, Kapiga S. Preparedness of Tanzanian health facilities for outpatient primary care of hypertension and diabetes: A cross-sectional survey. Lancet Glob Heal. 2014;2 doi: 10.1016/S2214-109X(14)70033-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katende D, Mutungi G, Baisley K, Biraro S, Ikoona E, Peck R, Smeeth L, Hayes R, Munderi P, Grosskurth H. Readiness of Ugandan health services for the management of outpatients with chronic diseases. Trop Med Int Heal. 2015;20:1385–1395. doi: 10.1111/tmi.12560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krumholz HM. Post-Hospital Syndrome — An Acquired, Transient Condition of Generalized Risk. N Engl J Med. 2013;368:100–102. doi: 10.1056/NEJMp1212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abraham WT, Adams KF, Fonarow GC, Costanzo MR, Berkowitz RL, Lejemtel TH, Cheng ML, Wynne J. In-hospital mortality in patients with acute decompensated heart failure requiring intravenous vasoactive medications: An analysis from the Acute Decompensated Heart Failure National Registry (ADHERE) J Am Coll Cardiol. 2005:57–64. doi: 10.1016/j.jacc.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 11.Abraham WT, Fonarow GC, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, O’Connor CM, Sun JL, Yancy CW, Young JB. Predictors of In-Hospital Mortality in Patients Hospitalized for Heart Failure. Insights From the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure (OPTIMIZE-HF) J Am Coll Cardiol. 2008;52:347–356. doi: 10.1016/j.jacc.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 12.Riley L, Guthold R, Cowan M, Savin S, Bhatti L, Armstrong T, Bonita R. The world health organization STEPwise approach to noncommunicable disease risk-factor surveillance: Methods, challenges, and opportunities. Am J Public Health. 2016;106:74–78. doi: 10.2105/AJPH.2015.302962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, et al. Heart disease and stroke statistics-2010 update: A report from the american heart association. Circulation. 2010;121:e46–e425. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 15.Marti CN, Georgiopoulou VV, Kalogeropoulos AP. Acute heart failure: Patient characteristics and pathophysiology. Curr Heart Fail Rep. 2013;10:427–433. doi: 10.1007/s11897-013-0151-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J, Normand S-LT, Wang Y, Krumholz HM. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998–2008. JAMA. 2011;306:1669–1678. doi: 10.1001/jama.2011.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Makubi A, Hage C, Lwakatare J, Kisenge P, Makani J, Rydén L, Lund LH. Contemporary aetiology, clinical characteristics and prognosis of adults with heart failure observed in a tertiary hospital in Tanzania: the prospective Tanzania Heart Failure (TaHeF) study. Heart. 2014:1–7. doi: 10.1136/heartjnl-2014-305599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stuart-Clark H, Vorajee N, Zuma S, van Niekerk L, Burch VRP. Stuart_SAfrMedJrnl.pdf. South African Med J. 2012;102:549–553. doi: 10.7196/samj.5615. [DOI] [PubMed] [Google Scholar]
- 19.Hernandez AF, Greiner MA, Fonarow GC, Hammill BG, Heidenreich PA, Yancy CW, Peterson ED, Curtis LH. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303:1716–1722. doi: 10.1001/jama.2010.533. [DOI] [PubMed] [Google Scholar]
- 20.Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30-day rehospitalization: A systematic review. Ann Intern Med. 2011:520–528. doi: 10.7326/0003-4819-155-8-201110180-00008. [DOI] [PubMed] [Google Scholar]
- 21.Sliwa K, Davison BA, Mayosi BM, Damasceno A, Sani M, Ogah OS, Mondo C, Ojji D, Dzudie A, Kouam CK, Suliman A, Schrueder N, Yonga G, Ba SA, Maru F, Alemayehu B, Edwards C, Cotter G. Readmission and death after an acute heart failure event: Predictors and outcomes in sub-Saharan Africa: Results fromthe THESUS-HF registry. Eur Heart J. 2013;34:3151–3159. doi: 10.1093/eurheartj/eht393. [DOI] [PubMed] [Google Scholar]
- 22.Masson S, Latini R, Milani V, Moretti L, Rossi MG, Carbonieri E, Frisinghelli A, Minneci C, Valisi M, Maggioni AP, Marchioli R, Tognoni G, Tavazzi L. Prevalence and prognostic value of elevated urinary albumin excretion in patients with chronic heart failure data from the GISSI-Heart failure trial. Circ Hear Fail. 2010;3:65–72. doi: 10.1161/CIRCHEARTFAILURE.109.881805. [DOI] [PubMed] [Google Scholar]
- 23.Makubi A, Hage C, Sartipy U, Lwakatare J, Janabi M, Kisenge P, Dahlström U, Rydén L, Makani J, Lund LH. Int J Cardiol. Vol. 220. Elsevier Ireland Ltd; 2016. Heart failure in Tanzania and Sweden: Comparative characterization and prognosis in the Tanzania Heart Failure (TaHeF) study and the Swedish Heart Failure Registry (SwedeHF) pp. 750–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor AL, Ziesche S, Yancy C, Carson P, D’Agostino R, Ferdinand K, Taylor M, Adams K, Sabolinski M, Worcel M, Cohn JN. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351:2049–2057. doi: 10.1056/NEJMoa042934. [DOI] [PubMed] [Google Scholar]
- 25.Packer M, Fowler MB, Roecker EB, Coats AJS, Katus HA, Krum H, Mohacsi P, Rouleau JL, Tendera M, Staiger C, Holcslaw TL, Amann-Zalan I, DeMets DL. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: Results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation. 2002;106:2194–2199. doi: 10.1161/01.cir.0000035653.72855.bf. [DOI] [PubMed] [Google Scholar]
- 26.O’Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, Heizer GM, Komajda M, Massie BM, McMurray JJV, Nieminen MS, Reist CJ, Rouleau JL, Swedberg K, Adams KF, Anker SD, Atar D, Battler A, Botero R, Bohidar NR, Butler J, Clausell N, Corbalán R, Costanzo MR, Dahlstrom U, Deckelbaum LI, Diaz R, Dunlap ME, Ezekowitz JA, Feldman D, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365:32–43. doi: 10.1056/NEJMoa1100171. [DOI] [PubMed] [Google Scholar]
- 27.Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJV, Michelson EL, Olofsson B, Östergren J, Yusuf S. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: The CHARM-overall programme. Lancet. 2003;362:759–766. doi: 10.1016/s0140-6736(03)14282-1. [DOI] [PubMed] [Google Scholar]
- 28.The CONSENSUS Trial Study Group. Effects of Enalapril on Mortality in Severe Congestive Heart Failure. N Engl J Med. 1987;316:1429–1435. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 29.McMurray JJV, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR. Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure. N Engl J Med. 2014;371 doi: 10.1056/NEJMoa1409077. 140830040023009. [DOI] [PubMed] [Google Scholar]
- 30.Damasceno A, Mayosi BM, Sani M, Ogah OS, Mondo C, Ojji D, Dzudie A, Kouam CK, Suliman A, Schrueder N, Yonga G, Ba SA, Maru F, Alemayehu B, Edwards C, Davison BA, Cotter G, Sliwa K. The causes, treatment, and outcome of acute heart failure in 1006 Africans from 9 countries. Arch Intern Med. 2012;172:1386–1394. doi: 10.1001/archinternmed.2012.3310. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


