Abstract
Purpose
Fear of needles develops at approximately five years of age, and decreases compliance with healthcare. We sought to examine the relationship of preschool vaccine history, parent and preadolescent needle fear, and subsequent compliance with optional vaccines.
Methods
As part of a private practice randomized controlled trial, parents and 10–12 year olds rated needle anxiety on a 100mm visual analog scale. This follow-up cohort study compared their needle anxiety to previous vaccination records, including number of vaccinations between ages four and six years (total and same-day maximum), and subsequent initiation of the HPV vaccine through age 13.
Results
Of the 120 preadolescents enrolled between 4.28.09 and 1.19.2010, 117 received preschool vaccinations between ages four and six years. The likelihood of being in the upper quartile of fear (VAS ≥ 83) five years later increased with each additional same-day injection (OR=3.108 p= 0.0100 95%CI=1.311,7.367), but was not related to total lifetime or total four-to-six year injections. Only 12.5% (15) of parents reported anxiety about their preadolescents’ vaccines (VAS>50). Parent and child anxiety was weakly correlated (r=0.15). Eight children in the upper fear quartile began their HPV series (26.67%) compared to 14 in the lower quartile (48.28% VAS<32) (OR 2.57, p=0.0889, 95%CI 0·864–7·621); there was no difference in HPV uptake between upper and lower quartile of parent anxiety.
Conclusions
The more same-day preschool injections between 4–6 years of age, the more likely a child was to fear needles five years later. Preadolescent needle fear was a stronger predictor than parent vaccine anxiety of subsequent HPV vaccine uptake.
Introduction
Fear of needles impacts parents’ willingness to have children vaccinated1–3 and affects adults’ subsequent healthcare experiences.4–6 According to hospitalized children, needle punctures are their greatest source of pain next to their disease.7 The effects of untreated needle pain are remembered even by preverbal children,8,9 and may amplify with age: up to 15% of geriatric patients refuse flu shots due to injection fear,10 and 28% of HIV patients delayed being tested.11 Young adults delay or may not seek medical treatment,12 and almost 40% of adults refused blood sampling due to reported fear of needle pain.13
Despite these associations between fear of needles and health behaviors, prospective research into the cause, incidence and impact of needle fear is scant. When the incidence of needle phobia in the general population was described in 1995, 10% of adults and 25% of children reported a moderate to severe fear of needles.14 In 2012, Taddio et al reported a 2.5 fold increase: 23% of adults and 63% of children.3 The cause of this increase has not been explained. Retrospective publications have hypothesized that experiences between ages four and six years result in an acquired fear of needles.15 The blood donation literature supports that needle-associated vasovagal responses16 are primarily predicted by acquired needle fear and lead to decreased blood donation,17–19 and recent work suggests vaccines play a role in the development of fear.5,6
In a previous study we collected parent anxiety and child fear of impending vaccinations at a 10–12 year routine pediatric visit.20 For the current study, we compared these responses with previous vaccination history and subsequent vaccination records through age 13 to evaluate the impact of fear on the subsequent decision to initiate the HPV series. Our aim was to determine if more vaccinations at one time and younger initiation of the childhood vaccines would be associated with greater fear of needles at age 10–12 years. In addition, we hypothesized that greater needle fear would be associated with reduced initiation of HPV vaccination.
Methods
This cohort study included preadolescents enrolled at a private pediatric office in Atlanta Georgia in the United States between 4.28.09 and 1.19.2010 as part of a randomized controlled clinical trial NCT00910611 supported by the NIH/SBIR Grant 4R44HD056647-02. The current study evaluated the cohort’s immunization records through 10.31.13; both studies were approved by the IRB of Georgia State University. For the initial recruitment, all children ages 10–12 years of age presenting to their pediatrician to receive scheduled required intramuscular (IM) vaccinations were eligible for inclusion. Patients were excluded if there was no caregiver available to give informed consent, there were clear cognitive impairments affecting children’s ability to understand or communicate the measures used in the study, or parents or children were unable to speak English.
After written informed consent and assent were obtained from the parent and child respectively, the accompanying caregiver provided information about their relation to child, sex, age, race, and maternal level of education, and child’s date of birth, sex, race, relevant medical and vaccine history. Prior to randomization, children and parents heard a script explaining how 100mm Visual Analog Scales (VAS) were used. Parents indicated their child’s historical anxiety with vaccination on a 100mm linear VAS between 0 = “usually not at all upset” to 100 = “usually extremely upset,” and their own anxiety about the child’s vaccinations that day. Children indicated with a vertical mark on a separate VAS how fearful they were about their shots on that day, between not at all anxious and most anxiety possible. While “fear” is the technical academic term used to describe justified anxiety with impending pain, we chose to use the less loaded words “anxiety” and “nervousness” in the data collection instrument. Parents and children were blinded to the others’ response.
Immunization history was obtained from the Georgia Registry of Immunization Transactions and Services (GRITS), a state-wide computer database populated by the pediatric offices. Twelve charts were initially missing vaccination data and required additional follow up to verify the records. When state data was missing, researchers verified vaccines and dates with the primary care physician. When the primary care physician was unable to verify whether a patient received a vaccine or the date, parents were contacted directly to confirm.
Statistical Analysis
The sample size was predetermined for the three arm controlled trial of a pain relief device; all recruited subjects were eligible for inclusion in the current cohort. Presumptive predictors of preadolescent fear included parental anxiety, the greatest number of single-day injections between age four and six, age in months at that time, the total number of childhood vaccinations and total between age four to six. Quantitative variables were categorized into quartiles, and adjacent categories combined when similar. Cochran-Mantel-Haenszel Statistics were used to calculate odds ratios and assess for significance of relationships and dose-response. Relationships between child fear and parent anxiety were examined using scatterplots, Pearson’s correlation coefficient, histograms and paired mean analyses. Number of injections as a predictor of child fear was modeled using linear and logistic regression. Data were analyzed with SAS 9.4 (Cary, North Carolina).
Results
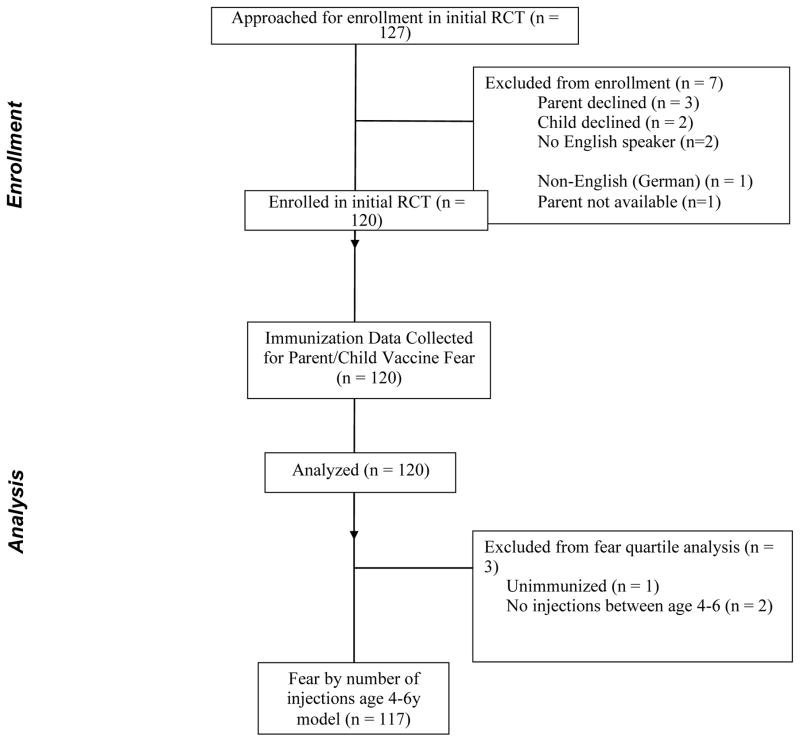
120 children aged 10 – 12 years were enrolled between April 28 2009 and January 19 2010. (Figure 1) One patient was previously unvaccinated, and two patients were not vaccinated within the four to six year age range; these were excluded from further prospective fear quartile analysis.
Figure 1.
Consort Diagram
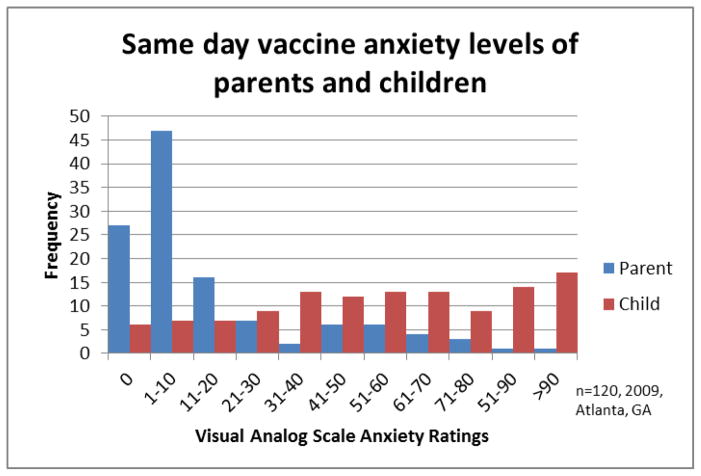
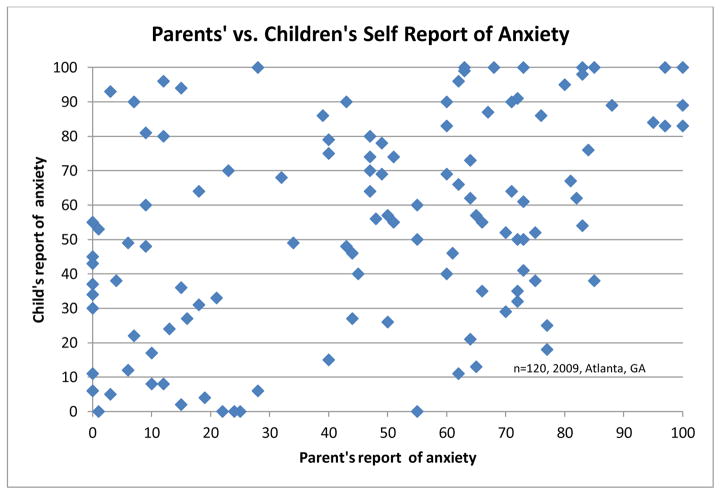
There were no demographic differences between preadolescents in the low, middle two, and upper quartiles of fear. (Table 1) Parents were much less likely to be anxious about their child’s vaccinations that day than their children. (Figure 2) The majority of parents (63%) indicated an anxiety about their child’s vaccines of 10mm or less, with 27 parents endorsing 0/100mm. Only 15/120 parents (12.5%) indicated an anxiety of 50mm or greater. In addition, parent anxiety was weakly correlated to child fear (r=0.15 p=.0150)(Figure 3).
Table 1.
Continuous and Categorical Demographic Variables by Self-expressed Needle Fear
| Quartiles of Self Reported Fear by Visual Analog Scale | |||
|---|---|---|---|
| Low Fear VAS 0–32 (n = 31) | Middle Quartiles VAS 33–82 (n = 62) | High Fear VAS 83–100 (n = 27) | |
| Child Age (Median, (95% CI)) | 132 (124.2–133.8) | 132 (127.58–131.02) | 132 (127.1–132.7) |
| Child Sex (% Male) | 51.61% | 50% | 37.04% |
| Race and Ethnicity | |||
| % White | 87.10% | 80.65% | 81.48% |
| % Black | 0% | 9.68% | 3.70% |
| % Asian | 3.23% | 1.61% | 3.70% |
| % Hispanic or Latino | 3.23% | 4.84% | 3.70% |
| % More than 1 race | 3.23% | 6.45% | 7.41% |
| Mother’s Age (Mean; SD) | 44.3 (5.06) | 44.1 (4.12) | 44 (4.23) |
| Mother’s highest education (Mean; SD) | 18 (9.89) | 18.88 (12.91) | 18.25 (10.87) |
| Parent Pre-Procedure Anxiety (Median; 95% CI) | 6 (8.24–23.68) | 6 (9.88–20.82) | 6 (7.67–24.53) |
| Accompanying Parent Sex (% Male) | 12.90% | 9.68% | 11.11% |
| Total number injections since birth | 19.47 (4.75) | 19.8 (5.11) | 19.58 (5.03) |
| Total number of visits with injections | 10.52 (3.02) | 10.05 (3.08) | 10.36 (3.05) |
| Negative medical experience per parent | 19.35% | 19.35% | 22.22% |
| History vasovagal experience | 0% | 1.61% | 0% |
Low Fear:
n = 9 patients not included in mother’s age, n = 1 patient did not have information for total injections or visits, n = 1 patient did not include ethnicity, n= 2 patients did not include race
Middle Quartiles:
n = 19 patients did not include mother’s age, n = 1 patient did not include mother’s highest education, n = 1 patient did not include race, n = 1 patient did not include ethnicity
High Fear:
n = 9 patients not included in mother’s age, n = 1 patient did not include race
Figure 2.
Vaccine anxiety of parents and vaccination fear of children at age 10–12
Parents (blue) and children (red) rated their anxiety on a VAS with the prompt “How anxious are you about your (your child’s) injections today?” Anchors were “Not at all anxious” and “Most anxiety possible”. Parent and child anxiety correlated poorly, with the mode pediatric response being “most anxiety possible” and mode parent response least anxiety possible.
Figure 3.
Parent’s assessment of their child’s anxiety compared to child’s report.
Parent and child anxiety was poorly correlated, suggesting that older children’s anxiety is not linked to parental anxiety at the time of vaccination.
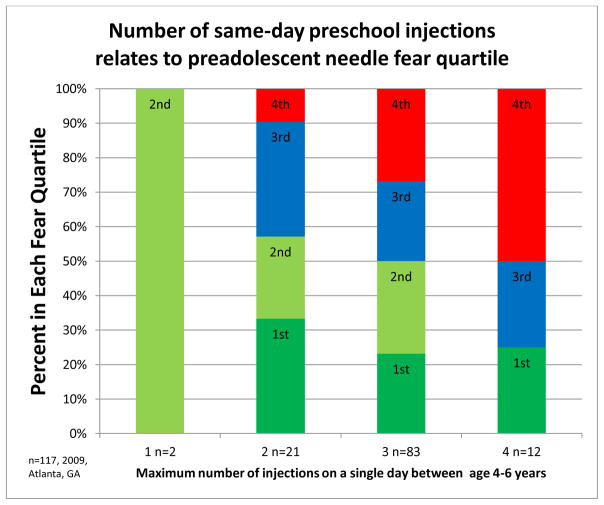
The median endorsement of fear for the children was 56/100. The likelihood of children being in the upper quartile of fear (VAS ≥ 83) was related to the number of previous vaccinations on one day in a dose response manner,(Figure 4) however the age or injection relationship did not fit a linear model (r=.19724 p=.0330). No child receiving one injection was in the upper fear quartile, 2(9.5%) with two, 22(26.8%) with three, and 6(50%) of children who received four vaccinations on the same day (p=0.0387). For every 1 additional same-day injection, the likelihood of being in the highest fear group five years later increased (OR=3.108 p= 0.0100 95%CI=1.311,7.367).
Figure 4.
Quartile of needle fear at age 10–12 compared to maximum number of same day injections at age 4–6 years.
Fear of injections at age 10–12 related to the maximum number of injections children received on the same day during the preschool vaccination period.
The correlation between parents’ VAS assessment of “How does your child usually react to vaccinations?” (not at all to extremely upset) and the child’s reported fear of their vaccinations was r=0.45 p=<.0001, indicating moderate correlation. 53% of parents underestimated anxiety (mean 32mm), 13% were within +/−5mm of their child’s stated anxiety, and 34% overestimated (mean 25.9mm). A paired means analysis showed a significant difference in the parents’ historical estimation of their child’s anxiety and their child’s anxiety report (p=0.0085).
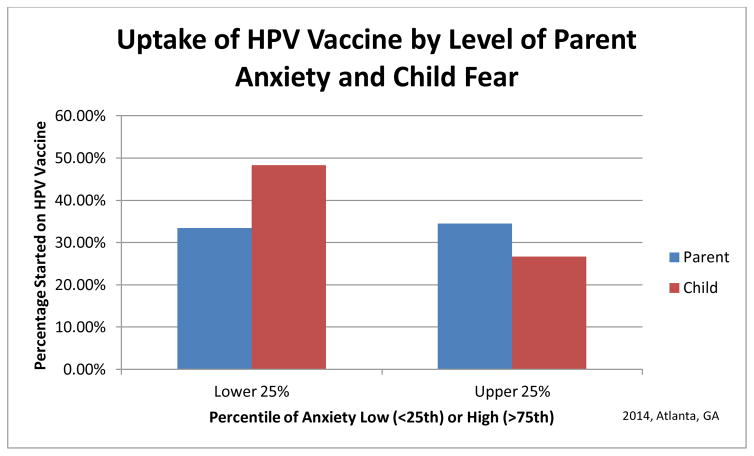
The total number of prior vaccinations over a child’s lifetime was not related to fear at age 10–12 years (r=0.11) or initiation of HPV vaccination. HPV initiation for children whose parents were in the lower or upper quartile of vaccine anxiety did not differ. However, 26.7% of children in the upper quartile of needle anxiety (≥83 on VAS) began their HPV series compared to 48.3% of those in the least anxious quartile (<32 on VAS) (OR 2.57, p=0.0889, 95%CI 0.864–7.621) (Figure 5).
Figure 5.
Subsequent initiation of HPV Vaccine by high and low quartiles of parent anxiety and child fear.
Initiation of the HPV vaccine series was more strongly influenced by the child’sfear of needles than the parent’s anxiety about vaccination.
Discussion
This study found that preadolescent fear related to childhood single-day injection history in a dose-dependent manner, but the infant and total number of childhood vaccinations did not predict fear. Parents of preadolescents underestimated their children’s anxiety, and parent and child anxiety correlated poorly: parents skewed toward “not anxious” while the preadolescents skewed to the “most anxiety possible”. Preadolescents’ needle fear was a stronger predictor of subsequent uptake of the HPV vaccine than parent vaccine anxiety.
Needle fear is associated with decreasing adherence to healthcare, but the genesis of this fear is unclear.21 Retrospective studies cite a traumatic, directly conditioned needle event at an average age of 5.5 years;14 more same-day vaccinations or needle attempts increase distress ratings.22–24 To our knowledge, however, no study has correlated vaccination experiences with later fear, thus a target intervention to slow the rapid rise of needle phobia is debatable: is it the total number, the age at vaccination, parental anxiety, injection pain, or the intensity of the event?5,6
From a theoretical standpoint, the greater fear in those who had more same-day injections supports the direct conditioning model of fear acquisition, and may relate to the growing body of literature relating adverse childhood events to future health outcomes. This and the seeming resilience in children who got one injection multiple times supports exposure desensitization therapies for needle fear reduction.25
Although early infant pain experiences matter,8,9 our data support that preadolescent vaccination fear is primarily established in the 4–6 year period and is not related to infant vaccinations. This construct supports current theory that long term memory is established after 21 months,26 and could explain new research showing optimally treating infant vaccination pain didn’t alter their pain response as toddlers.27
Previous research in non-adherent adolescents and adults found pain mitigation reduces fear and improves adherence for bicillin injections;28 following up previous randomized immunization pain reduction studies29,30 could evaluate pain reduction’s impact on subsequent needle fear. One study found distraction caused children undergoing venipuncture to perceive the experience as “better than previous” venipuncture procedures.31 Distraction is inexpensive; following up distraction immunization studies32 could demonstrate whether using coping skills impact long term needle anxiety.
Since 1983, over 20 potential new vaccinations have been recommended for young children in developed countries. While parents indicate they consider 3 or more injections on one day to be excessive,2,33 almost all patients in our study received this many on one day at some point in their lives. Altering the vaccination schedule carries with it the inherent risk of patients not returning for vaccinations, a risk that could outweigh the theoretical later health benefit of reducing adult needle fear. Our study did not find a decrease in total vaccine uptake when patients received fewer vaccines spaced out over separate visits.
Previous studies by Racine et al have indicated that parent’s anxiety predicts the reactions to vaccinations in patients younger than two.34,35 Our study revealed that parents’ and preadolescent children’s anxiety were poorly correlated (r=0.15); while only 12.5% of parents indicated an anxiety above 50mm, over half (55%) of the children endorsed needle fear above 50mm. This parental difference could be regional or generational, or simply that parents of older children are less anxious.
The 12.5% ‘anxious parent’ finding indicates it may be possible to quickly screen for vaccine-reluctant parents. Coupled with a study by Rikert et al reporting a similar 15% of vaccine-anxious parents,36 this suggests focusing pediatrician vaccination interventions on a subset of parents identified by a simple VAS may be effective.
The study was underpowered to evaluate whether children’s fear of needlesimpacts uptake of the HPV vaccine, however parental vaccine anxiety was less related to HPV initiation than their child’s needle fear. While parents’ attitudes toward vaccines in general is correlated with greater HPV uptake,37 previous HPV adherence interventions have targeted parents with little impact.38 Interestingly, we did note a trend that patients who took nasal FluMist® were more likely to get the HPV vaccine that day, whereas those taking injected flu vaccine did not. The willingness of adolescents to receive multiple injections on one day, and ability to influence their parents’ intention for vaccination, are areas of future research.
Taddio has shown that needle fear impacts parents’ willingness to vaccinate their children.3 As more vaccines become available, and as parental reluctance to fully vaccinate becomes a problem, understanding needle fear is of critical public health importance. Our results support the theory that fear established in childhood persists. Future interventional studies to reduce needle fear should focus on making the 4–6 year vaccinations less traumatic. Following up previous immunization pain reductions studies, larger cohort studies to evaluate the effect of age and number, and intervention trials to relieve the 63% who are now fearful are all avenues of valuable research. Our study suggests that randomizing patients to low-needle intensity schedules would not jeopardize vaccine schedule compliance, making a potential multi-arm trial possible. Until we develop patch, microneedle, or effective sublingual or intranasal options, we must consider the long term effects of iatrogenic pediatric needle pain.
Limitations
We were unable to evaluate whether low-fear subjects in our study had interventions to reduce preschool injection pain or mitigate the intensity of multiple injections. The lack of a direct correlation between number of injections and fear demonstrates that fear acquisition and resilience are multifactorial.
Because of the small sample size, a difference of one child in a fear quartile made a large difference. For example, if one fewer child receiving 4 injections had high fear, 42% would have been in the high fear quartile rather than 50%. While still statistically significant, this illustrates that drawing strong conclusions about pre-teens’ influence on HPV uptake would be premature. Our cohort was too small and had insufficient variability to test our hypothesis that 4-year old vaccination resulted in greater preadolescent fear than age 5.
The sample was from a higher socioeconomic status practice. While those with greater education had a nonsignificant likelihood of children in the lower fear group, these findings may not apply to the general population.
Acknowledgments
Amy Baxter MD conceived the study, wrote the protocol, and wrote the NIH grant that provided the data, and wrote the manuscript. Lindsey Cohen PhD edited the study and protocol, submitted this cohort study to the Georgia State IRB, and coordinated the team collecting the data. He also edited the manuscript. Louise Lawson PhD edited the protocol, analysed the data, and edited the manuscript. Mark Burton collaborated on the protocol, ran the survey data collection, and edited the manuscript. Anaam Mohammed collected and analyzed the GRITS data and edited the manuscript. We would like to acknowledge Carl Von Baeyer PhD for his thoughtful review and support. No one received compensation for their contribution.
Footnotes
Conflict of Interest: Dr. Baxter invented Buzzy and is the CEO of MMJ Labs, the manufacturer of Buzzy®. This conflict of interest was disclosed to participants in the informed consent. After orientation of enrollers employed by the hospital, Dr. Baxter was not present for data collection. Dr. Cohen, who has no financial conflict of interest to disclose, recruited study coordinators.
Financial Disclosure: Funding for this study was provided to MMJ Labs by the National Institutes of Health 4R44HD056647-02
Declaration of Interests
The initial NIH grant supported research and development of a needle pain device created by Amy Baxter MD. Dr. Baxter created a company to manufacture and market the device, and has subsequent conflict of interest; this study does not refer to the device. Lindsey Cohen PhD, M. Louise Lawson PhD, and Mark Burton have no conflicts of interest. Anaam Mohammed worked as a study coordinator for Dr. Baxter when she ran a pediatric emergency research division but otherwise has no conflict of interest. All funding for the study was provided by the NIH grant 4R44HD056647-02.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Froehlich H, West DJ. Compliance with hepatitis B virus vaccination in a high-risk population. Ethn Dis. 2001;11:548–53. [PubMed] [Google Scholar]
- 2.Meyerhoff AS, Weniger BG, Jacobs RJ. Economic value to parents of reducing the pain and emotional distress of childhood vaccine injections. Pediatr Infect Dis J. 2001;20:S57–62. doi: 10.1097/00006454-200111001-00009. [DOI] [PubMed] [Google Scholar]
- 3.Taddio A, Ipp M, Thivakaran S, et al. Survey of the prevalence of immunization non-compliance due to needle fears in children and adults. Vaccine. 2012;30:4807–12. doi: 10.1016/j.vaccine.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 4.France CR, France JL, Wissel ME, Ditto B, Dickert T, Himawan LK. Donor anxiety, needle pain, and syncopal reactions combine to determine retention: a path analysis of two-year donor return data. Transfusion. 2013;53:1992–2000. doi: 10.1111/trf.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McMurtry CM, Pillai Riddell R, Taddio A, et al. Far From “Just a Poke”: Common Painful Needle Procedures and the Development of Needle Fear. Clin J Pain. 2015;31:S3–11. doi: 10.1097/AJP.0000000000000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noel M, Taddio A, McMurtry CM, Chambers CT, Pillai Riddell R, Shah V. HELPinKids&Adults Knowledge Synthesis of the Management of Vaccination Pain and High Levels of Needle Fear: Limitations of the Evidence and Recommendations for Future Research. Clin J Pain. 2015;31:S124–31. doi: 10.1097/AJP.0000000000000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kortesluoma RL, Nikkonen M. ‘I had this horrible pain’: the sources and causes of pain experiences in 4- to 11-year-old hospitalized children. Journal of child health care: for professionals working with children in the hospital and community. 2004;8:210–31. doi: 10.1177/1367493504045822. [DOI] [PubMed] [Google Scholar]
- 8.Taddio A, Katz J, Ilersich AL, Koren G. Effect of neonatal circumcision on pain response during subsequent routine vaccination. Lancet. 1997;349:599–603. doi: 10.1016/S0140-6736(96)10316-0. [DOI] [PubMed] [Google Scholar]
- 9.Taddio A, Shah V, Gilbert-MacLeod C, Katz J. Conditioning and hyperalgesia in newborns exposed to repeated heel lances. JAMA. 2002;288:857–61. doi: 10.1001/jama.288.7.857. [DOI] [PubMed] [Google Scholar]
- 10.Iwasa T, Wada K. Reasons for and against receiving influenza vaccination in a working age population in Japan: a national cross-sectional study. BMC public health. 2013;13:647. doi: 10.1186/1471-2458-13-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spielberg F, Branson BM, Goldbaum GM, et al. Overcoming barriers to HIV testing: preferences for new strategies among clients of a needle exchange, a sexually transmitted disease clinic, and sex venues for men who have sex with men. J Acquir Immune Defic Syndr. 2003;32:318–27. doi: 10.1097/00126334-200303010-00012. [DOI] [PubMed] [Google Scholar]
- 12.Vika M, Raadal M, Skaret E, Kvale G. Dental and medical injections: prevalence of self-reported problems among 18-yr-old subjects in Norway. Eur J Oral Sci. 2006;114:122–7. doi: 10.1111/j.1600-0722.2006.00335.x. [DOI] [PubMed] [Google Scholar]
- 13.Wong ML, Chia KS, Yam WM, Teodoro GR, Lau KW. Willingness to donate blood samples for genetic research: a survey from a community in Singapore. Clin Genet. 2004;65:45–51. doi: 10.1111/j..2004.00192.x. [DOI] [PubMed] [Google Scholar]
- 14.Hamilton JG. Needle phobia: a neglected diagnosis. J Fam Pract. 1995;41:169–75. [PubMed] [Google Scholar]
- 15.Du S, Jaaniste T, Champion G, Yap C. Theories of fear acquisition: The development of needle phobia in children. Pediatric Pain Letter. 2008;10:13–7. [Google Scholar]
- 16.Deacon B, Abramowitz J. Fear of needles and vasovagal reactions among phlebotomy patients. J Anxiety Disord. 2006;20:946–60. doi: 10.1016/j.janxdis.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Olatunji BO, Etzel EN, Ciesielski BG. Vasovagal syncope and blood donor return: examination of the role of experience and affective expectancies. Behav Modif. 2010;34:164–74. doi: 10.1177/0145445510362576. [DOI] [PubMed] [Google Scholar]
- 18.France CR, France JL, Frame-Brown TA, Venable GA, Menitove JE. Fear of blood draw and total draw time combine to predict vasovagal reactions among whole blood donors. Transfusion. 2015 doi: 10.1111/trf.13264. [DOI] [PubMed] [Google Scholar]
- 19.France CR, France JL, Himawan LK, et al. How afraid are you of having blood drawn from your arm? A simple fear question predicts vasovagal reactions without causing them among high school donors. Transfusion. 2013;53:315–21. doi: 10.1111/j.1537-2995.2012.03726.x. [DOI] [PubMed] [Google Scholar]
- 20.Baxter AL, Cohen LL, Weisman SJ, Lawson ML. Pediatric Academic Societies. Boston, MA: Apr 29, 2012. Does a vibrating cold device decrease pre-adolescent immunization pain? [Google Scholar]
- 21.Spagrud LJ. Investigating the Relationship between Children’s Self-Reported Coping Strategies and Repeated Needle Pain [Graduate] Saskatoon: University of Saskatchewan; 2008. [Google Scholar]
- 22.Courtois E, Cimerman P, Dubuche V, et al. The burden of venipuncture pain in neonatal intensive care units: EPIPPAIN 2, a prospective observational study. International journal of nursing studies. 2016;57:48–59. doi: 10.1016/j.ijnurstu.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 23.Sanger R, Behre U, Krause KH, et al. Booster vaccination and 1-year follow-up of 4-8-year-old children with a reduced-antigen-content dTpa-IPV vaccine. European journal of pediatrics. 2007;166:1229–36. doi: 10.1007/s00431-006-0403-x. [DOI] [PubMed] [Google Scholar]
- 24.Franck LS, Berberich FR, Taddio A. Parent participation in a childhood immunization pain reduction method. Clinical pediatrics. 2015;54:228–35. doi: 10.1177/0009922814561593. [DOI] [PubMed] [Google Scholar]
- 25.McMurtry CM, Taddio A, Noel M, et al. Exposure-based Interventions for the management of individuals with high levels of needle fear across the lifespan: a clinical practice guideline and call for further research. Cognitive behaviour therapy. 2016;45:217–35. doi: 10.1080/16506073.2016.1157204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liston C, Kagan J. Brain development: memory enhancement in early childhood. Nature. 2002;419:896. doi: 10.1038/419896a. [DOI] [PubMed] [Google Scholar]
- 27.Taddio A, Riddell RP, Ipp M, et al. A longitudinal randomized trial of the impact of consistent pain management for infant vaccinations on future vaccination distress. The journal of pain: official journal of the American Pain Society. 2017 doi: 10.1016/j.jpain.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Russell KA, Nicholson R. Reducing the pain of Benzathine Penicillin Injections in the Rheumatic Fever population of CMDHB. 6th Biennial Conference of the New Zealand Pain Society; Wellington, New Zealand: Hospital Play Specialists Association; 2012. [Google Scholar]
- 29.Cassidy KL, Reid GJ, McGrath PJ, Smith DJ, Brown TL, Finley GA. A randomized double-blind, placebo-controlled trial of the EMLA patch for the reduction of pain associated with intramuscular injection in four to six-year-old children. Acta Paediatr. 2001;90:1329–36. doi: 10.1080/080352501317130416. [DOI] [PubMed] [Google Scholar]
- 30.Berberich FR, Landman Z. Reducing immunization discomfort in 4- to 6-year-old children: a randomized clinical trial. Pediatrics. 2009;124:e203–9. doi: 10.1542/peds.2007-3466. [DOI] [PubMed] [Google Scholar]
- 31.Inal S, Kelleci M. Distracting children during blood draw: looking through distraction cards is effective in pain relief of children during blood draw. International journal of nursing practice. 2012;18:210–9. doi: 10.1111/j.1440-172X.2012.02016.x. [DOI] [PubMed] [Google Scholar]
- 32.Cohen LL, Rodrigues NP, Lim CS, et al. Automated parent-training for preschooler immunization pain relief: a randomized controlled trial. Journal of pediatric psychology. 2015;40:526–34. doi: 10.1093/jpepsy/jsu162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaaijk P, Kleijne DE, Knol MJ, Harmsen IA, Ophorst OJ, Rots NY. Parents’ attitude toward multiple vaccinations at a single visit with alternative delivery methods. Human vaccines & immunotherapeutics. 2014;10:2483–9. doi: 10.4161/hv.29361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Racine NM, Pillai Riddell RR, Flora DB, Taddio A, Garfield H, Greenberg S. Predicting preschool pain-related anticipatory distress: the relative contribution of longitudinal and concurrent factors. Pain. 2016 doi: 10.1097/j.pain.0000000000000590. in press. [DOI] [PubMed] [Google Scholar]
- 35.Racine NM, Riddell RR, Khan M, Calic M, Taddio A, Tablon P. Systematic Review: Predisposing, Precipitating, Perpetuating, and Present Factors Predicting Anticipatory Distress to Painful Medical Procedures in Children. J Pediatr Psychol. 2016;41:159–81. doi: 10.1093/jpepsy/jsv076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rickert VI, Rehm SJ, Aalsma MC, Zimet GD. The role of parental attitudes and provider discussions in uptake of adolescent vaccines. Vaccine. 2015;33:642–7. doi: 10.1016/j.vaccine.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 37.Rosenthal SL, Rupp R, Zimet GD, et al. Uptake of HPV vaccine: demographics, sexual history and values, parenting style, and vaccine attitudes. J Adolesc Health. 2008;43:239–45. doi: 10.1016/j.jadohealth.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 38.Fishman J, Taylor L, Frank I. Awareness of HPV and Uptake of Vaccination in a High-Risk Population. Pediatrics. 2016:138. doi: 10.1542/peds.2015-2048. [DOI] [PubMed] [Google Scholar]