Abstract
Purpose
Prostate cancer is dependent on androgen receptor (AR) activation. Optimal AR antagonism may effectively cytoreduce local disease and suppress or eliminate micrometastases. We evaluated neoadjuvant therapy prior to prostatectomy with the potent AR antagonist enzalutamide (enza) either alone or in combination with dutasteride and leuprolide (enza/dut/LHRHa).
Experimental Design
Forty-eight of 52 men with intermediate or high-risk localized prostate cancer proceeded to prostatectomy after neoadjuvant enza or enza/dut/LHRHa for 6 months. We assessed pathologic complete response (pCR), minimal residual disease (MRD; ≤3 mm maximum diameter of residual disease), residual cancer burden (RCB), and expression of prostate-specific antigen (PSA) and serum and tissue androgen concentrations. We compared the proportion of patients with pCR in each treatment arm with a historical control rate of 5%, based on previous reports of flutamide with LHRHa.
Results
In the enza arm, none of the 25 patients achieved pCR or MRD. In the enza/dut/LHRHa arm, one of 23 patients (4.3%) achieved pCR and three of 23 (13.0%) achieved MRD. Median RCB was higher in the enza arm than in the enza/dut/LHRHa arm (0.41 cm3 vs. 0.06 cm3, respectively). Tissue testosterone and dihydrotestosterone levels correlated with RCB. No adverse events leading to study drug discontinuation were reported.
Conclusions
Combination therapy with enza/dut/LHRHa resulted in pCR and MRD rates comparable to historical controls. Evidence of continued AR activity in residual tumor suggests that AR signaling may contribute to survival. Strategies to more effectively ablate AR activity are warranted to determine if more substantial antitumor effects are observed.
Keywords: enzalutamide, dutasteride, neoadjuvant, CL01 PHASE I – III CLINICAL TRIALS: Phase I–III Trials_Genitourinary cancers: prostate, ET02 MECHANISMS OF DRUG ACTION/NEW MOLECULAR TARGETS/THERAPEUTICS: Cellular responses to anticancer drugs
Introduction
Therapeutic approaches that inhibit androgen receptor (AR) activity are a critical component of effective treatment of castration-sensitive and castration-resistant prostate cancer (CRPC) (1, 2). It is clear that a common resistance mechanism to androgen deprivation therapy (ADT) is mediated by persistent AR signaling through mechanisms that include the import and metabolism of androgens and increased AR expression, even in the setting of low serum ligand levels (3–5). Agents that further inhibit androgen synthesis and AR signaling include abiraterone acetate (a direct inhibitor of tissue steroidogenesis and AR antagonist) and enzalutamide (enza), a small molecule inhibitor of the ligand-binding domain of the AR, AR translocation and DNA binding (1, 2, 6, 7). Potent AR inhibitors such as enza induce tumor regression and growth suppression even after the failure of approaches that produce castrate serum androgen levels, such as the administration of luteinizing hormone-releasing hormone analogs (LHRHa). Direct blockade of the AR with enza has the potential to ablate AR signaling and induce tumor cell death with or without ADT (8). Phase III studies of enza in metastatic CRPC have provided evidence for clear survival advantages and confirm the relevance of targeting AR even in late-stage prostate cancer (2, 7).
Evaluating therapeutics in the neoadjuvant setting prior to prostatectomy provides an opportunity to quantitate the impact of agents on tumor volume and the potential to cytoreduce or eliminate systemic micrometastatic disease. It also facilitates studies to determine mechanisms of resistance in residual tumor. Previous neoadjuvant studies using ADT in localized prostate cancer found limited effects on tumor reduction and relapse rates (9, 10). Subsequent studies with combinations of agents targeting AR in localized prostate cancer signaling have shown higher rates of complete pathological response (pCR) in small phase II studies (11). This preliminary study was designed to determine if enza alone or in a unique combination with leuprolide, an LHRHa, and dutasteride, a 5α-reductase inhibitor that prevents conversion of testosterone into the more potent androgen dihydrotestosterone (DHT) (12) would result in an improvement of pCR compared with those previously reported in the literature. Additional exploratory analyses examined surrogates for AR signaling tissue in order to identify potential mechanisms of sensitivity and resistance.
Patients and Methods
Patients
This was a randomized, open-label, parallel-group, noncomparative study (clinicaltrials.gov ID NCT01547299). All procedures were approved by the institutional review boards of University of Washington, Dana-Farber/Harvard Cancer Care, Princess Margaret Cancer Centre, and University of British Columbia, and all subjects signed written informed consent. Eligible men had surgically resectable, localized adenocarcinoma of the prostate (T1c–T3, N0/NX, M0) with at least three biopsy cores positive for tumor, and a baseline Gleason score ≥7 or prostate-specific antigen (PSA) >10 ng/mL. Patients were excluded if they had: a positive bone scan and computed tomography or magnetic resonance imaging scan of the abdomen/pelvis; received prior therapy for prostate cancer, including drugs affecting androgen metabolism, within 6 months of enrollment; or a history of thrombosis, seizure or cerebrovascular disease, ventricular arrhythmia, bradycardia, heart block, uncontrolled hypertension, unstable angina or heart failure, or another malignancy other than nonmelanoma skin cancer or superficial bladder cancer. Men were required to have a serum testosterone ≤50 ng/dL below the institutional lower limit of normal, as well as normal blood counts, creatinine, and transaminases.
Study procedures
Prior to radical retropubic prostatectomy, patients were randomized 1:1 to 6 months of neoadjuvant therapy with either enza alone (160 mg daily) or enza (160 mg daily) with leuprolide (22.5 mg once every 3 months) and dutasteride (0.5 mg daily) (enza/dut/LHRHa). Patients were stratified by baseline risk category (intermediate risk vs. high risk). Intermediate risk was defined as having any one of the following: clinical stage T2b or T2c, baseline PSA of 10–20 ng/mL, or Gleason score by local pathology of 7 (4 + 3). High risk was defined as having any one of the following: clinical stage T3, baseline PSA >20 ng/mL, or Gleason score of 8–10.
Outcome variables
The primary objective was to assess the pCR rate, defined as the absence of morphologically identifiable carcinoma in the prostatectomy specimen. Secondary objectives included: analysis of cases with ≤3 mm maximum diameter of residual tumor (minimal residual disease [MRD]), presence of positive surgical margins, nodal involvement, expression of AR, glucocorticoid receptor (GR), PSA, tissue and serum androgens, and safety and tolerability. Exploratory analyses of residual cancer burden (RCB), with MRD defined as <0.25 cm3 of residual tumor (11, 13), were also performed.
Tissue acquisition and determination of tumor volume
A detailed standard operating procedure for acquisition and processing of prostatectomy specimens was developed and followed at all sites. Endpoints were evaluated by independent central pathology review. RCB was calculated by using three-dimensional volume estimation based on the largest cross-sectional tumor dimension and number of cross-sections involved by tumor, corrected for tumor cellularity (13).
Steroid measurements
Serum and tissue androgen levels were determined by liquid chromatography-tandem mass spectrometry in a blinded manner, using methods previously described (14). Steroids analyzed included DHT, testosterone, pregnenolone, progesterone, androstenedione (AED), and dehydroepiandrosterone (DHEA). Lower limits of detection for steroids in serum were 0.49 pg/sample for testosterone, progesterone, and AED, 0.98 pg/sample for DHT and pregnenolone, and 3.9 pg/sample for DHEA. Lower limits of detection in tissue were 0.49 pg/sample for testosterone, progesterone, and AED, 0.98 pg/sample for DHT and pregnenolone, and 15.6 pg/sample for DHEA.
Immunohistochemistry
Immunohistochemistry (IHC) was performed in a College of American Pathologists–certified diagnostic IHC laboratory according to standardized protocols. In brief, serial unstained sections were deparaffinized on an automated immunostainer (Bond III™, Leica Biosystems, Buffalo Grove, Illinois) using Bond Dewax Solution. After dewaxing, dehydrating, and rinsing multiple times, antigen retrieval was performed using either epitope retrieval solution 1 (pH 8.0) or epitope retrieval solution 2 (pH 9.0) for 20 minutes. A peroxidase blocking solution was applied to neutralize endogenous peroxidase, followed by several rinses with Bond Wash Solution. Slides were incubated for 20 minutes with either mouse monoclonal antibodies against N-terminus AR (clone F39.4.1, 1:200, Biogenex, Fremont, California), N-terminus nuclear receptor subfamily 3 group C member 1 (glucocorticoid receptor [GR]; clone 4H2, 1:25, Novocastra/Leica Biosystems), marker of proliferation Ki-67 (Ki-67; clone MIB-1, 1:200, cat. M7240, Dako, Carpinteria, California), or a rabbit polyclonal antibody against kallikrein related peptidase 3 (PSA; 1:8000, cat. A0562, Dako). Slides were then rinsed multiple times with Bond Wash Solution and incubated for 8 minutes with polymer antimouse or antirabbit poly-HRP-immunoglobulin and polymer detection reagent (Bond Polymer Refine Detection kit, Leica Biosystems). After multiple rinses, slides were incubated with 3,3′-diaminobenzidine tetrahydrochloride for 10 minutes and counterstained with hematoxylin. Staining was assessed in a blinded manner for intensity level (0, no stain; 1+, weak stain; 2+, intermediate; 3+, intense stain) and proportion of positive cells (categorized as none, <10%, 10%–50%, or >50%).
Statistical analyses
Statistical analyses were performed by treatment arm using all patients with evaluable data (e.g., pCR and surgical outcomes among those who had a prostatectomy, and PSA reduction among those with a baseline and at least one post-baseline PSA assessment). For safety analyses, all patients who received at least one dose of study drug were evaluated.
The proportion of patients with pCR was compared for each treatment arm against a 5% historical control pCR rate using exact binomial P values, each of which were tested with a one-sided type I error of 0.025. The historical control rate was based on studies using leuprolide and flutamide with pCR rates of 4%–9% (9, 10). The proportion of patients with intense IHC staining were compared between treatment arms using Fisher’s exact test (9, 15). Comparisons of serum androgen levels and tissue androgen levels between the two treatment arms were carried out using the nonparametric Mann-Whitney test. All correlations were based on the Spearman rank correlation. P values were exploratory only; there was no adjustment for multiple testing.
The planned sample size was 20 to 25 patients per arm, with an option to replace patients who have not received an adequate course of therapy. With 20 patients in the enza arm, the 2-sided exact binomial 95% confidence interval corresponding to an observed pCR rate of 20% (4/20 patients) is (5.73%, 43.66%). Similarly, with 20 patients in the enza/dut/LHRHa arm, the 2-sided exact binomial confidence interval corresponding to an observed pCR rate of 25% (5/20 patients) is (8.66%, 49.10%). Both scenarios would yield a lower bound of the 95% confidence interval larger than the estimated historical control rate of 5%. The two arms of the trial were not intended to be compared against each other.
Results
Patient characteristics
Fifty-two patients with intermediate- to high-risk prostate adenocarcinoma were enrolled (27 in the enza arm and 25 in the enza/dut/LHRHa arm). Baseline characteristics were well balanced between the two treatment arms (Table 1). Four patients discontinued therapy prior to prostatectomy to undergo prostatectomy or radiation outside of the study, not resulting from toxicity, and 48 patients underwent radical prostatectomy (25 from the enza arm and 23 from the enza/dut/LHRHa arm).
Table 1.
Baseline characteristics
| Enza (n = 27) | Enza/dut/LHRHa (n = 25) | |
|---|---|---|
| Median age, y (range) | 61.0 (47.0–75.0) | 60.0 (46.0–74.0) |
| High risk,a n (%) | 21 (77.8) | 20 (80.0) |
| Intermediate risk,b n (%) | 6 (22.2) | 5 (20.0) |
| Biopsy Gleason score, n (%) | ||
| 7 | 11 (40.7) | 9 (36.0) |
| 8 | 8 (29.6) | 9 (36.0) |
| ≥9 | 8 (29.6) | 7 (28.0) |
| Median PSA, μg/mL (min, max) | 10.9 (3.8, 61.1) | 12.8 (0.6, 61.1) |
| Clinical stage, n (%) | ||
| T1 | 8 (29.6) | 6 (24.0) |
| T2 | 13 (48.1) | 13 (52.0) |
| T3 | 6 (22.2) | 6 (24.0) |
T3 clinical stage or Gleason score ≥8 or PSA ≥20 ng/mL.
T2b or T2c clinical stage or Gleason score of 7 or PSA ≥10 and <20 ng/mL.
Pathologic outcomes
The majority of patients had ypT3 pathologic staging at the time of prostatectomy (72% of patients in the enza arm and 61% of patients in the enza/dut/LHRHa arm; Table 2). Zero of 25 patients in the enza arm and one of 23 patients (4.3%) in the enza/dut/LHRHa arm achieved a pCR (Table 2). Neither treatment arm demonstrated a significantly higher pCR rate compared with the historical control rate of 5% (9, 10). Median RCB was 0.41 cm3 in the enza arm and 0.06 cm3 in the enza/dut/LHRHa arm (Table 2). RCB volumes for each treatment arm are presented in Supplementary Figure S1. The proportion of patients who achieved MRD based on RCB volumes (<0.25 cm3) was 36% in the enza arm versus 74% in the enza/dut/LHRHa arm; however, the proportion with lymph node involvement was lower in the enza arm than in the enza/dut/LHRHa arm (4% vs. 26%, respectively; Table 2). MRD, stage ypT2 or lower at prostatectomy, and the proportion of patients with positive margins were similar between the two treatment arms.
Table 2.
Pathologic outcomes
| Enza (n = 25) | Enza/dut/LHRHa (n = 23) | |
|---|---|---|
| pCR, n (%) | 0 (0) | 1 (4.3) |
| MRD (≤3 mm) | 0 (0) | 3 (13.0) |
| Median RCB, cm3 | 0.41 | 0.06 |
| MRD (<0.25 cm3), n (%) | 9 (36.0) | 17 (73.9) |
| Margin positive, n (%) | 4 (16.0) | 5 (21.7) |
| Lymph node positive, n (%) | 1 (4.0) | 6 (26.1) |
| Stage at prostatectomy, n (%) | ||
| ypT2 or lower | 7 (28.0) | 9 (39.1) |
| ypT3 | 18 (72.0) | 14 (60.9) |
| ypT3a | 9 (36.0) | 7 (30.4) |
| ypT3b | 9 (36.0) | 7 (30.4) |
| yN1 | 1 (4.0) | 6 (26.1) |
Tissue and serum concentrations
Comparison of tissue androgens is shown in Table 3 and Supplementary Figure S2. Tissue DHT and testosterone were significantly higher in tumors from the enza arm than in those from the enza/dut/LHRHa arm (mean DHT, 3.34 pg/mg vs. 0.04 pg/mg, and mean testosterone, 0.90 pg/mg vs. 0.18 pg/mg, respectively; P < 0.0001 for both). DHEA was not significantly different between the enza arm and enza/dut/LHRHa (mean, 74.48 vs. 63.79 pg/mg, respectively; P = 0.38), and AED was significantly lower in the enza arm than in the enza/dut/LHRHa arm (mean, 1.06 pg/mg vs. 1.70 pg/mg, respectively; P = 0.03). Trends in day 180 serum levels were similar to the changes in tissue upon prostatectomy, with enza alone resulting in higher serum DHT and testosterone levels than those of the enza/dut/LHRHa arm (Supplementary Fig. S3). Of interest, serum levels of essentially all androgens increased between baseline and day 180 (prior to prostatectomy) in the enza arm, including pregnenolone, progesterone, DHEA, AED, DHT, and testosterone (Supplementary Fig. S3). This pattern was consistent with previous reports that antiandrogens, such as bicalutamide, will abrogate negative feedback to the hypothalamus and consequently increase androgens and estradiol (16).
Table 3.
Tissue hormones and serum PSA kinetics by day 180 (pre-prostatectomy)
| Enza | Enza/dut/LHRHa | P valuea | |
|---|---|---|---|
| Mean DHT, pg/mg (SD) | 3.34 (1.57) | 0.04 (0.01) | <0.001 |
| Mean testosterone, pg/mg (SD) | 0.90 (0.86) | 0.18 (0.13) | <0.001 |
| Mean AED, pg/mg (SD) | 1.06 (1.15) | 1.70 (0.92) | 0.03 |
| Mean DHEA, pg/mg (SD) | 74.48 (40.34) | 63.79 (41.21) | 0.38 |
| Serum PSA at day 180, n | 25 | 24 | |
| Median PSA, μg/L (min, max) | 0.51 (0.02, 7.92) | 0.04 (0.01, 0.53) | <0.001 |
| Serum PSA nadir value, n | 27 | 25 | |
| Median μg/L (min, max) | 0.51 (0.02, 8.80) | 0.04 (0.01, 0.53) | |
| ≥90% decrease in PSA, n (%) | 17 (63.0) | 25 (100.0) | 0.0007c |
| 95% CI,b % | 42.4, 80.6 | 86.3, 100.0 |
Limits of quantification were 0.015 pg/mg for AED and testosterone, 0.03 pg/mg for DHT, and 0.49 pg/mg for DHEA.
Mann-Whitney test adjusted vs. enza.
Clopper-Pearson CI.
Fisher’s exact test.
CI, confidence interval; SD, standard deviation.
Androgen receptor activity
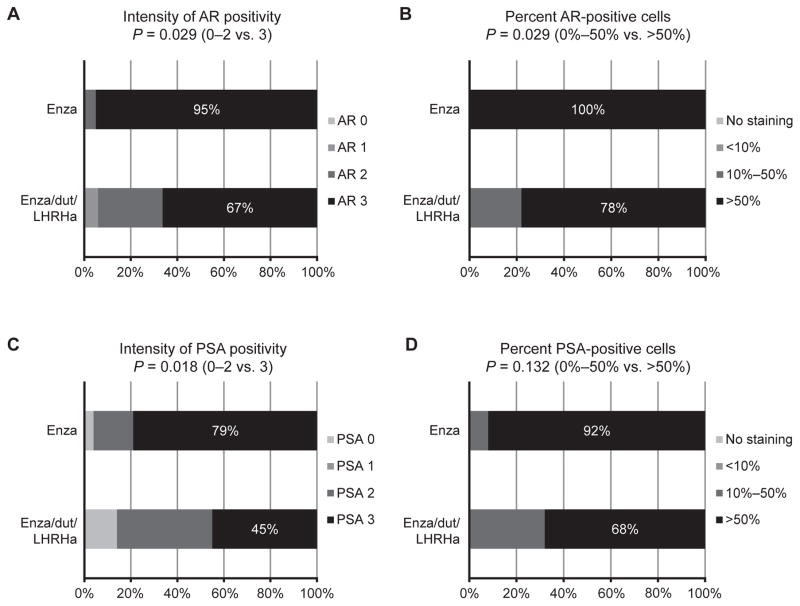
To determine the effect of therapy on AR pathway suppression in each treatment arm, we evaluated epithelial tumor nuclear expression of AR, cytoplasmic expression of PSA, and serum PSA kinetics. Intense and extensive nuclear AR staining remained in the majority of tumor cells in all residual tumors in both arms, suggesting incomplete suppression of AR expression. Treatment with enza alone resulted in a higher proportion of patients with intense (3+) nuclear AR staining than treatment with enza/dut/LHRHa (95% vs. 67%, respectively; P = 0.029; Fig. 1A). The proportion of patients with tumors having >50% of nuclei positive for AR was also statistically higher in tumors from the enza arm than in those from the enza/dut/LHRHa arm (100% vs. 78%, respectively; P = 0.029; Fig. 1B). As a reflection of greater suppression of AR activity in the enza/dut/LHRHa arm, the proportion of patients with intense (3+) cytoplasmic PSA staining was higher in tumors from the enza arm than in those from the enza/dut/LHRHa arm (79% vs. 45%, respectively; P = 0.018; Fig. 1C). The proportion of patients with tumors having >50% of cells positive for PSA was higher in tumors from the enza arm than in those from the enza/dut/LHRHa arm (92% vs. 68%, respectively; P = 0.132; Fig. 1D). Serum PSA kinetics largely reflected the greater suppression of AR activity and lower tissue PSA in the enza/dut/LHRHa arm (Table 3). Nadir PSA was achieved at day 180 and was higher in patients from the enza arm than in those from the enza/dut/LHRHa arm (median PSA, 0.51 μg/L vs. 0.04 μg/L, respectively; P < 0.001). Also, a smaller proportion of patients achieved a >90% decline in PSA from baseline in the enza arm than in the enza/dut/LHRHa arm (63% vs. 100%, respectively; P = 0.0007). Both PSA and Ki-67 are potential indicators of tumor proliferation (17). The percentage of Ki-67–positive nuclei in tumors from the enza versus enza/dut/LHRHa arms was 3% versus 1% (P = 0.012), suggesting greater suppression of proliferation with enza/dut/LHRHa.
Figure 1.
Immunohistochemical expression of the AR and PSA after enza or enza/dut/LHRHa. A tissue microarray comprising cores of cancer tissue from each patient was analyzed for (A) intensity of the majority of stained nuclei for AR and (B) nuclear expression of AR calculated as the total proportion of AR-positive tumor nuclei. Cytoplasmic PSA staining was performed in the same manner (C and D). P values were calculated by the chi-square test.
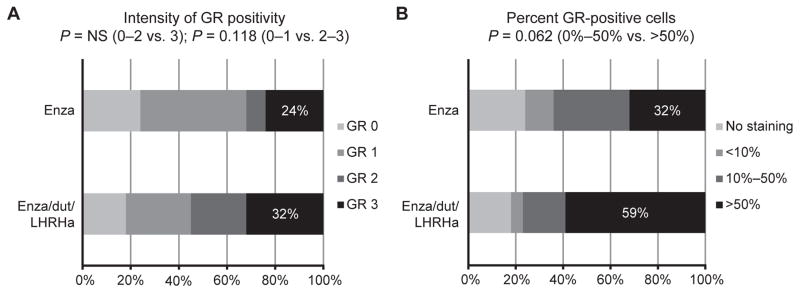
One postulated mechanism of resistance to enza is binding of the GR to a subset of AR target genes and consequent transcription activation (18). Higher GR expression (in both intensity of GR staining and number of positive cells) was observed in the majority of cells in both treatment arms (Fig. 2).
Figure 2.
Immunohistochemical expression of the GR after enza or enza/dut/LHRHa. A tissue microarray comprising cores of cancer tissue from each patient was analyzed for (A) intensity of the majority of stained nuclei for GR and (B) nuclear expression of GR calculated as total proportion of GR-positive tumor nuclei. P values were calculated by the chi-square test.
Correlations with residual cancer burden
Correlations of tissue androgen levels with RCB using both treatment arms combined showed that lower tissue testosterone (Spearman r = 0.41) and tissue DHT (Spearman r = 0.32) were associated with lower RCB. No correlation of GR staining with RCB was observed when assessing both the enza and enza/dut/LHRHa arms combined, although when the analysis was performed in the enza/dut/LHRHa arm alone, there was a significant correlation of higher RCB with a higher proportion of GR-positive tumor nuclei (Spearman r = 0.54; P = 0.04; 0%–10% positive nuclei vs >50% positive nuclei; Supplementary Fig. S4).
Safety
Safety was evaluated in the neoadjuvant and perioperative period, up to 30 days after prostatectomy. Overall, both enza and enza/dut/LHRHa were well tolerated (Table 4). While the most frequent adverse events were generally very similar, in the absence of LHRHa to suppress testosterone and estrogen, gynecomastia and mastodynia were more prominent in the enza arm than in the enza/dut/LHRHa arm (63% vs. 12% and 59% vs. 8%, respectively). Patients in the enza arm experienced fewer hot flushes than those in the enza/dut/LHRHa arm (26% vs. 96%, respectively). There were no instances of significant hepatitis or seizures.
Table 4.
Safety
| Enza (n = 27) | Enza/dut/LHRHa (n = 25) | Total (n = 52) | |
|---|---|---|---|
| Any TEAE, n (%) | 27 (100.0) | 25 (100.0) | 52 (100.0) |
| Grade ≥3 TEAE, n (%) | 3 (11.1) | 6 (24.0) | 9 (17.3) |
| TEAE leading to permanent discontinuation or death, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Most frequent TEAEsa (all grades), n (%) | |||
| Fatigue | 19 (70.4) | 15 (60.0) | 34 (65.4) |
| Hot flush | 7 (25.9) | 24 (96.0) | 31 (59.6) |
| Gynecomastia | 17 (63.0) | 3 (12.0) | 20 (38.5) |
| Insomnia | 6 (22.2) | 9 (36.0) | 15 (28.8) |
| Decreased libido | 4 (14.8) | 8 (32.0) | 12 (23.1) |
| Mastodynia | 16 (59.3) | 2 (8.0) | 18 (34.6) |
| Diarrhea | 5 (18.5) | 6 (24.0) | 11 (21.2) |
| TEAEs of special interest (all grades), n (%) | |||
| AST >2 × ULN | 0 (0) | 0 (0) | 0 (0) |
| ALT >2 × ULN | 0 (0) | 0 (0) | 0 (0) |
| Total bilirubin >2 × ULN | 0 (0) | 0 (0) | 0 (0) |
| Seizure | 0 (0) | 0 (0) | 0 (0) |
Occurring in ≥20% of all patients.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; TEAE, treatment-emergent adverse event; ULN, upper limit of normal.
Discussion
This study was designed to determine if neoadjuvant treatment with enza, a potent inhibitor of AR activity, either as a single agent (noncastrating therapy) or combined with androgen suppression (i.e., enza/dut/LHRHa), would result in a pCR rate assessed at the time of radical prostatectomy that was higher than historical controls of standard ADT prior to prostatectomy. No patients in the enza arm achieved pCR, while one of 23 patients (4.3%) receiving enza/dut/LHRHa achieved pCR as assessed per protocol. Previous studies have reported pCR rates between 0% and 10% using interventions designed to suppress AR signaling (9, 11, 19). The published trials evaluating neoadjuvant ADT enrolled a majority of what today are consider low-risk patients. A historic control for pCR in a majority high-risk population also allowing nodal enlargement up to 15 mm does not exist. When planning the trial, we thought a pCR rate of ≥5% would be favorable. Exploratory pathologic metrics of response, including MRD and RCB, may also be predictors of positive clinical outcomes. We observed that seven of 52 patients had positive lymph nodes (4% in the enza arm and 26% in the enza/dut/LHRHa arm). This rate of nodal positivity was within range of expected given the characteristics typical of high-risk patients. While the increased percentage of node positivity in the enza/dut/LHRHa arm was provocative given that baseline patient characteristics were balanced (see Table 1), the study size overall was small and no definite conclusions could be made. In a future analysis, we plan to molecularly characterize tumor from baseline, radical prostectomy tissue, and nodal disease from exceptional and poor responders to understand drivers of response and resistance.
The neoadjuvant setting provides an opportunity to test therapeutics using tissue and pathologic measures. In prostate cancer there are no clinically validated tissue or pathologic biomarkers. We chose to go beyond standard two-dimensional pathology measurements and explored assessments of tumor response, including cellularity and RCB. A recently reported neoadjuvant study of the use of LHRHa with abiraterone acetate for 6 months demonstrated pCR rates of 4% and 10% depending on the duration of abiraterone acetate treatment (11). That study also demonstrated MRD, defined as a RCB <0.25 cm3, in 44% and 52% of patients after 12 and 24 weeks, respectively. In our study, MRD using the same definition was observed in 36% of patients in the enza arm and 72% in the enza/dut/LHRHa arm, which compared very favorably with that of prior neoadjuvant studies. Finally, median RCB was higher in the enza arm than in the enza/dut/LHRHa arm. However, comparisons of treatment arms were not the intent of this study and are limited by the small sample size, and comparisons with prior studies are hampered by a lack of clear consensus on methods for assessing pCR and RCB in the prostate.
Strong correlations between lower RCB and lower intraprostatic androgens were found, suggesting that lowering serum and prostatic androgens may reduce tumor volume. Reducing natural ligands that compete with enza for AR binding, such as testosterone and DHT (current role of dutasteride), would be expected to enhance the ability of enza to antagonize AR activity. The surrogacy of pCR, downstaging, or RCB after neoadjuvant therapy has been clearly demonstrated in locally advanced breast cancer and other malignancies (20–22). Whether RCB will ultimately be a useful surrogate in prostate cancer requires longer follow-up of patients in this and other studies, as well as larger randomized studies evaluating an optimal regimen once such a regimen is defined in phase II studies.
Tissue assessment of AR signaling in the different treatment arms demonstrated that concentrations of intraprostatic testosterone and DHT, and expression of nuclear AR, and PSA were significantly lower in the enza/dut/LHRHa arm, suggesting that effective inhibition of AR signaling generally requires suppression of tissue androgens. Enza/dut/LHRHa also resulted in a lower median serum PSA nadir and a larger proportion of patients with a >90% PSA decline, consistent with relative tissue AR inhibition. Assessment of GR expression, one potential mechanism of resistance to enza, showed a strong correlation between higher RCB and GR-positive tumor nuclei in the enza/dut/LHRHa arm, which raised the possibility of a relationship between GR-positive tumors and resistance to anti-AR therapy.
The use of enza alone or in the enza/dut/LHRHa combination in a neoadjuvant setting was generally well tolerated, with no safety signal for significant toxicity. As expected in a patient population that had noncastrate levels of testosterone, the proportion of patients experiencing gynecomastia and mastodynia was higher in the enza treatment arm than in prior CRPC studies (2, 7), but was comparable to another study of enza in hormone-naïve prostate cancer (23).
Future correlative analyses will address the variability in responses to neoadjuvant therapy with enza and the mechanisms contributing to treatment resistance. It remains to be determined why a subset of patients had complete or near-complete tumor eradication, whereas substantial residual tumor volumes were found in others. Our results indicate that AR signaling remains active in the majority of surviving tumor cells despite exposure to enza and suppression of the AR ligands testosterone and DHT by leuprolide and dutasteride, respectively. Consequently, treatment failure is likely mediated through continued AR pathway activation, which could occur through a number of mechanisms, including increased expression of the AR or AR splice variants, the activity of androgen synthesis enzymes such as CYP17A or SRD5A, or through alternative signal transduction pathways that may involve other nuclear hormone receptors such as the GR (24–28). More effective AR blockade, potentially through combining ADT with inhibition of CYP17 and direct inhibition of AR with enza or other compounds in development, will be necessary to determine whether complete ablation of the AR pathway is possible and whether this approach will result in a higher rate of complete response. The ongoing analysis of residual tumor clones will provide insights into which agents should be combined with AR targeting in order to optimize tumor killing. As one potential target, if the GR is a relevant pathway of resistance, preemptively targeting the GR pathway may contribute to improved response rates.
Supplementary Material
Statement of Translational Relevance.
Inhibition of androgen receptor (AR) activity is an effective treatment for prostate cancer. We evaluated neoadjuvant therapy prior to prostatectomy with the potent AR antagonist enzalutamide, either alone or in combination with dutasteride and leuprolide. The neoadjuvant setting provides an opportunity to examine the activity of therapies using both tissue and pathologic measures. We found that the combination of enzalutamide with dutasteride and leuprolide resulted in pathologic complete response (pCR) rates similar to previous therapies targeting the AR signaling pathway such as abiraterone acetate. We also found evidence of continued AR activity in residual tumor cells and a correlation between lower residual cancer burden and lower intraprostatic androgen levels, suggesting that AR signaling may contribute to the survival of tumor cells. We consider this another important step toward optimizing both local and systemic therapy for high-risk prostate cancer using translational surrogates as a means of improving therapy.
Acknowledgments
Research support: This work was supported by Medivation, Inc., and Astellas Pharma, Inc., as well as National Institutes of Health (Pacific Northwest Prostate Cancer SPORE P50 CA097186; Dana-Farber Cancer Institute Prostate Cancer SPORE 5 P50 CA090381-10; P01CA163227; P01CA85859), PC093509, and the Prostate Cancer Foundation (YI Award to E.A. Mostaghel and separate Challenge Awards to M.-E. Taplin/B. Montgomery and to P.S. Nelson/S.P. Balk).
The trial was conducted in the Prostate Cancer Clinical Trials Consortium. Medical writing and editorial support funded by Medivation, Inc., and Astellas Pharma, Inc., was provided by Stephanie Vadasz, PhD, and Shannon Davis of Infusion Communications (Haddam, Connecticut).
Footnotes
Conflicts of interest: BM reports research funding from Janssen, Medivation, and Tokai. AMJ reports grant support from Astellas Pharma, Inc. NF reports grant support from Amgen, Janssen, Astellas Pharma Inc., Ferring, Bavarian Nordic, Canadian Cancer Society Research Institute, and Prostate Cancer Canada, personal fees from Amgen, Janssen, Astellas Pharma Inc., Bayer, Sanofi, Abbvie, Ferring, Hybridyne Imaging Technologies, and non-financial support from Apotex. KNC reports personal fees from Astellas Pharma Inc. PK has served on scientific advisory boards for Astellas Pharma Inc., Bayer, Bellicum, BIND Biosciences, Inc., BN Immunotherapeutics, Cristal Therapeutics, Endocyte/TRM Oncology, GTx, DRGT, Ipsen Pharmaceuticals, Jenssen, Metamark, Medivation, Millennium/Prometrika, MorphoSys, MTG Therapeutics, Omnitura, OncoCellMDX, Pfizer, Sotio, Sanofi, Tarveda Therapeutics, and Tokai and reports investment interests in Bellicum, DRGT, Metamark, Placon, and Tarveda Therapeutics. SP has served on a scientific advisory board for ESSA Pharma. PSN reports grant support from Astellas Pharma Inc. and personal fees from Astellas Pharma Inc., Janssen, Roche, and AstraZeneca. AMM reports grant support from GlaxoSmithKline. MET reports grant support and personal fees from Medivation and Astellas Pharma Inc. KW and AH are employees of Medivation. WN was an employee of Medivation at the time of the study. GPH and AK are employees of Astellas Pharma, Inc. MT, MG, GJB, EAM, DWL, MS, BTM, SPB, and RL have nothing to disclose.
References
- 1.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 3.Dutt SS, Gao AC. Molecular mechanisms of castration-resistant prostate cancer progression. Future Oncol. 2009;5:1403–13. doi: 10.2217/fon.09.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Q, Li W, Zhang Y, Yuan X, Xu K, Yu J, et al. Androgen receptor regulates a distinct transcription program in androgen-independent prostate cancer. Cell. 2009;138:245–56. doi: 10.1016/j.cell.2009.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linja MJ, Savinainen KJ, Saramaki OR, Tammela TL, Vessella RL, Visakorpi T. Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer Res. 2001;61:3550–5. [PubMed] [Google Scholar]
- 6.Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–48. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–33. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–90. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gleave ME, Goldenberg SL, Chin JL, Warner J, Saad F, Klotz LH, et al. Randomized comparative study of 3 versus 8-month neoadjuvant hormonal therapy before radical prostatectomy: Biochemical and pathological effects. J Urol. 2001;166:500–6. discussion 506–7. [PubMed] [Google Scholar]
- 10.Soloway MS, Pareek K, Sharifi R, Wajsman Z, McLeod D, Wood DP, Jr, et al. Neoadjuvant androgen ablation before radical prostatectomy in ct2bnxmo prostate cancer: 5-year results. J Urol. 2002;167:112–6. [PubMed] [Google Scholar]
- 11.Taplin ME, Montgomery B, Logothetis CJ, Bubley GJ, Richie JP, Dalkin BL, et al. Intense androgen-deprivation therapy with abiraterone acetate plus leuprolide acetate in patients with localized high-risk prostate cancer: Results of a randomized phase ii neoadjuvant study. J Clin Oncol. 2014;32:3705–15. doi: 10.1200/JCO.2013.53.4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mostaghel EA, Nelson PS, Lange P, Lin DW, Taplin ME, Balk S, et al. Targeted androgen pathway suppression in localized prostate cancer: A pilot study. J Clin Oncol. 2014;32:229–37. doi: 10.1200/JCO.2012.48.6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen ME, Johnston D, Reyes AO, Soto CP, Babaian RJ, Troncoso P. A streamlined three-dimensional volume estimation method accurately classifies prostate tumors by volume. Am J Surg Pathol. 2003;27:1291–301. doi: 10.1097/00000478-200310000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Kalhorn TF, Page ST, Howald WN, Mostaghel EA, Nelson PS. Analysis of testosterone and dihydrotestosterone from biological fluids as the oxime derivatives using high-performance liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2007;21:3200–6. doi: 10.1002/rcm.3205. [DOI] [PubMed] [Google Scholar]
- 15.Soloway MS, Sharifi R, Wajsman Z, McLeod D, Wood DP, Jr, Puras-Baez A. Randomized prospective study comparing radical prostatectomy alone versus radical prostatectomy preceded by androgen blockade in clinical stage b2 (t2bnxm0) prostate cancer. The lupron depot neoadjuvant prostate cancer study group. J Urol. 1995;154:424–8. [PubMed] [Google Scholar]
- 16.Eri LM, Haug E, Tveter KJ. Effects on the endocrine system of long-term treatment with the non-steroidal anti-androgen casodex in patients with benign prostatic hyperplasia. Br J Urol. 1995;75:335–40. doi: 10.1111/j.1464-410x.1995.tb07345.x. [DOI] [PubMed] [Google Scholar]
- 17.Niu Y, Yeh S, Miyamoto H, Li G, Altuwaijri S, Yuan J, et al. Tissue prostate-specific antigen facilitates refractory prostate tumor progression via enhancing ara70-regulated androgen receptor transactivation. Cancer Res. 2008;68:7110–9. doi: 10.1158/0008-5472.CAN-07-6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arora VK, Schenkein E, Murali R, Subudhi SK, Wongvipat J, Balbas MD, et al. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell. 2013;155:1309–22. doi: 10.1016/j.cell.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chi KN, Chin JL, Winquist E, Klotz L, Saad F, Gleave ME. Multicenter phase ii study of combined neoadjuvant docetaxel and hormone therapy before radical prostatectomy for patients with high risk localized prostate cancer. J Urol. 2008;180:565–70. doi: 10.1016/j.juro.2008.04.012. discussion 570. [DOI] [PubMed] [Google Scholar]
- 20.Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25:4414–22. doi: 10.1200/JCO.2007.10.6823. [DOI] [PubMed] [Google Scholar]
- 21.Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–66. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 22.Rodel C, Martus P, Papadoupolos T, Fuzesi L, Klimpfinger M, Fietkau R, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005;23:8688–96. doi: 10.1200/JCO.2005.02.1329. [DOI] [PubMed] [Google Scholar]
- 23.Tombal B, Borre M, Rathenborg P, Werbrouck P, Van Poppel H, Heidenreich A, et al. Long-term efficacy and safety of enzalutamide monotherapy in hormone-naive prostate cancer: 1- and 2-year open-label follow-up results. Eur Urol. 2015;68:787–94. doi: 10.1016/j.eururo.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 24.Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: A mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–54. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mostaghel EA, Marck BT, Plymate SR, Vessella RL, Balk S, Matsumoto AM, et al. Resistance to cyp17a1 inhibition with abiraterone in castration-resistant prostate cancer: Induction of steroidogenesis and androgen receptor splice variants. Clin Cancer Res. 2011;17:5913–25. doi: 10.1158/1078-0432.CCR-11-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu R, Dunn TA, Wei S, Isharwal S, Veltri RW, Humphreys E, et al. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Research. 2009;69:16–22. doi: 10.1158/0008-5472.CAN-08-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun S, Sprenger CC, Vessella RL, Haugk K, Soriano K, Mostaghel EA, et al. Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant. J Clin Invest. 2010;120:2715–30. doi: 10.1172/JCI41824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carver BS, Chapinski C, Wongvipat J, Hieronymus H, Chen Y, Chandarlapaty S, et al. Reciprocal feedback regulation of pi3k and androgen receptor signaling in pten-deficient prostate cancer. Cancer Cell. 2011;19:575–86. doi: 10.1016/j.ccr.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.