Abstract
More than 200 valid Sarcocystis species have been described in the parasitological literature. The developmental life cycle in the intermediate host and definitive host has only been described for a few species. The majority of species have been identified based solely on the presence of the sarcocyst stage in the muscles of the intermediate host. Information on the immune response to infection is limited due to difficulties growing and conducting experimental infections in laboratory animals. One genome has been assembled and only recently have molecular methods been used to examine phylogenetic relationships within, and between, different Sarcocystis species. Sarcocystis parasites are common pathogens infecting a wide range of animals, including humans, and this unit reviews the methods used for isolating infective stages of the parasite from both definitive and intermediate host(s), as well as methods used to initiate cultures from sporocysts and merozoites, and cryopreservation of various Sarcocystis spp. These methods are based on published reports and our experience with Sarcocystis species in cell culture over many years. The information presented is suitable for the efficient culture of many Sarcocystis species, however, some minor modifications may be needed based on the unique developmental patterns of some species.
Keywords: Parasite, Host, Sarcocystis, Isolation, Culture, Cryopreservation
Introduction
Sarcocystis infection is globally distributed and is common in many species of reptiles, birds, and animals, including humans. Species of Sarcocystis have an obligatory predator-prey 2-host life cycle. Asexual stages can only develop in the intermediate host, which is often a prey animal (herbivore or omnivore). Sexual stages only develop in the definitive host, which is typically a carnivore or an omnivore. Intermediate and definitive hosts vary for each species of Sarcocystis. There are more than 200 valid Sarcocystis species which have been described in the literature. The complete life cycle consisting of the identity of the definitive host (or hosts), identity of the intermediate host (or hosts), and description of the asexual and sexual stages in each host is known for only a few species. The methods required to isolate Sarcocystis spp. from nature to expand the parasite in either cell culture or laboratory animals, and to cryopreserve the parasite are either scattered or missing in the literature. The developmental stages for the following species of Sarcocystis (S. speeri, S. falcatula, S. lindsayi, S. neurona, S. cruzi, S. tenella, S. capracanis, Sarcocystis sp. ex Pantherophis alleghaniensis, Sarcocystis sp. ex Accipiter cooperii) have been successfully grown in vitro. Here, we review and describe methods for the isolation, culture, and cryopreservation of various Sarcocystis spp. We believe that this information will be useful for future studies on this group of economically important and biologically diverse parasites.
Basic Protocol 1: Isolation of Sarcocystis sporocysts from the intestine of definitive hosts
Sarcocystis sporocysts after sporulating in the intestinal lamina propria are normally released into the intestinal lumen over several months. As many as 200 million sporocysts can be recovered from the intestinal scrapings of definitive hosts (Dubey, 1980; Ford, 1974). There is little or no immunity capable of limiting the re-excretion of sporocysts (Fayer, 1977; Heydorn and Rommel, 1972; Rommel et al., 1972). Therefore, each meal of infected meat can initiate a new round of production of sporocysts. The structure of the oocyst and sporocysts, traditionally used to determine species of other coccidia, is of little or no taxonomic value in Sarcocystis (Dubey et al., 2016).
Materials
Lab coats, gloves, face mask, face shield
commercial blender or meat grinder
centrifuge
microscope
water bath
refrigerator
surgical scissors
scalpels
forceps
cotton rolls
paper towels
plastic containers with lids
250 ml centrifuge bottles
50 ml conical centrifuge tubes
glass slides
22 mm2 coverslips
Pasteur pipettes
Cheesecloth
90 μm wire mesh sieve
Water
Ethanol
bleach (5.25% sodium hypochlorite)
Hanks’ balanced salt solution (HBSS)
HBSS antibiotic mixture (See in reagents and solutions section)
Euthanatize the definitive host 3 to 7 days after oocysts or sporocysts are first detected in the feces (See Basic Protocol 2 for how to process and visualize sporocysts in the feces of definitive hosts).
Remove the small intestine, cut it lengthwise, and spread over paper towels with the luminal side up.
Lightly scrape the intestinal epithelium with glass slides, remove only the tips of the villi where most sporocysts are concentrated.
Suspend scrapings in 200–500 ml water and then homogenize in a commercial blender at top speed for 1–5 min or more with breaks to prevent frothing.
Centrifuge the homogenate at 800 × g for 10 min in 250 ml centrifuge bottles or 50 ml conical centrifuge tubes.
-
Discard supernatant and resuspend the sediment in 200–500 ml water. Repeat the above process until most sporocysts are released from the host tissue. Concentrate sporocysts by centrifugation and decantation.
To examine sporocysts under microscope, take small drops of sediment suspensions with a Pasteur pipette, then put on a glass slide and cover with a 22 mm2 coverslip. Let the slide-coverslip lie flat for about 5 to 10 min so that the fecal particles can settle and the sporocysts can rise to the top before the slide is examined. Examine the slide at 20X magnification or more. Sarcocystis sporocysts are small (usually 8 × 15 μm, Fig. 1), thin-walled, and of lower density than most other parasite eggs or oocysts; therefore, they lie just beneath the coverslip and are often missed. For example, sporocysts of Sarcocystis spp. from bobcat feces, processed following sugar flotation protocol are shown in Figure 1. Suspend in the sporocysts pellet in HBSS, filter through two layers of cheesecloth or a 90 μm wire mesh sieve then centrifuge liquid again and repeat filtering through two layers of cheesecloth or a 90 μm wire mesh sieve.
Although most sporocysts are concentrated towards the tips of the villus, some are located deep in the mucosa. To obtain these sporocysts, deeply scrape intestine and homogenize intestinal scrapings in water. Alternatively, the entire small intestine can be ground with a commercial meat grinder, mix well in water and filter through two layers of cheesecloth.
-
Emulsify homogenate in 5.25% sodium hypochlorite (bleach) solution (1:1 ratio) in a cold bath for 30 min.
Oocysts or sporocysts are resistant to freezing, high temperature and several disinfectants including bleach (Clorox), (Dubey et al., 2016; Dubey et al., 2002). Centrifuge the homogenate at 800 × g for 10 min and discard supernatant.
Suspend the sediment in water, wash by centrifugation and decantation until the smell of bleach (chlorine) is gone.
-
Suspend sporocyst pellet in HBSS antibiotic mixture, use a haemocytometer (APPENDIX 4A; Stevenson 2006) to count sporocysts, and store collections in refrigerator at 4 °C.
Although survival of Sarcocystis oocysts or sporocysts in different environmental conditions outdoors has not been tested, sporocysts remain viable at 4 °C for months, even at low humidity. Sporocysts stored in HBSS antibiotic mixture at 4 °C remain viable for 12 months or more (Dubey et al., 2016).
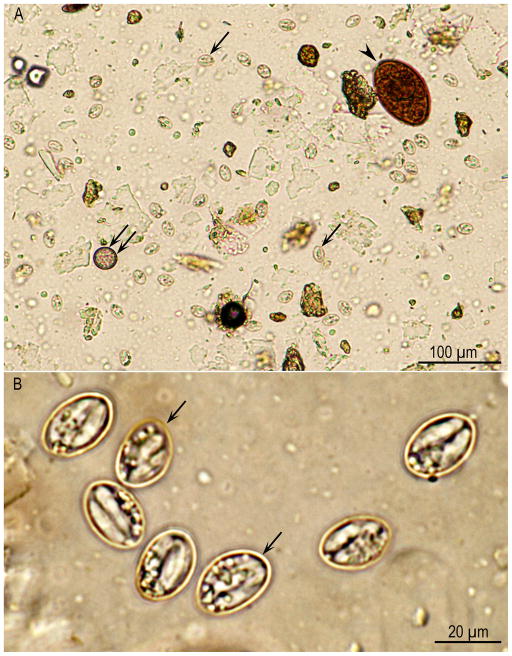
Figure 1.
Sporocysts of Sarcocystis spp. from a bobcat feces, processed following sugar flotation, Unstained. (A) Lower magnification. Note a trematode (Paragonimus sp.) ova (arrowhead), coccidian oocyst (double arrows), and many Sarcocystis sporocysts (arrows) that lie at different focus than the trematode ova. (B) Higher magnification to show Sarcocystis sporocysts (arrows). Sporocysts of many species of Sarcocystis overlap in dimensions (8 × 15 μm).
Basic Protocol 2: Isolation of Sarcocystis sporocysts from the feces of definitive hosts
The number of Sarcocystis sporocysts in feces is usually low; therefore, concentration methods are often necessary to detect sporocysts in feces. Sarcocystis sporocysts can be obtained from feces of definitive hosts by flotation methods used for other coccidia. The specific gravity (SG) of most parasite eggs is <1.18. Either sucrose or salt solutions of specific gravity 1.15 or more can be used to float sporocysts free of fecal debris. Common flotation solutions include saturated sodium chloride (NaCl; SG 1.18), sugar (Sheather’s solution; SG 1.27 to 1.33), sodium nitrate (NaNO3; SG 1.18 to 1.2), magnesium sulfate (MgSO4; SG; 1.2), and zinc sulfate (ZnSO4; SG 1.2). These solutions are effective, easy to make or commercially available, and relatively inexpensive. Sucrose solution is preferred if sporozoite isolation will be attempted, because it is less toxic to sporocysts than salt solutions.
The sucrose solution used in this technique has a specific gravity of 1.15, while parasite eggs and oocysts have a lower specific gravity. This difference in specific gravity causes parasite ova, oocysts, and sporocysts to float to the top where they can be viewed with a light microscope or collected for experimental studies such as excystation for in vitro cultivation, or inoculation in appropriate intermediate host(s) to investigate transmission.
CAUTION: Fecal samples collected from wildlife may contain zoonotic agents, so these preparations should be handled with extra care; wear gloves, lab coat and face mask at all times.
Materials
Lab coats, gloves, face mask, face shield
Centrifuge
Microscope
Shaker
Refrigerator
plastic containers with lids
tongue depressors
cheesecloths
250 ml centrifuge bottles
50 ml conical centrifuge tubes
100, 90, and 45 μm wire mesh sieves
Pasteur pipettes, glass slides
22 mm2 coverslips
Haemocytometer
Water
2M sucrose solution (See in reagents and solutions section)
Hanks’ balanced salt solution (HBSS)
HBSS antibiotic mixture (See in reagents and solutions section)
-
1
Take an aliquot (5–10 g) of stool sample to float in sucrose solution to determine if oocysts/sporocysts are present and estimate the numbers present (few or many).
-
2
Remove extraneous material and soften stool sample by soaking in water in a plastic containers with lid (500 ml in capacity with on lid) for 1 to 2 hrs.
-
3
Homogenize sample using a tongue depressor, place the cover on cup and set sealed cups on the shaker about 200 rpm for 1–2 hrs.
-
4
Re-homogenize sample as much as possible with a tongue depressor and filter through two layers of cheesecloth or a 100 μm wire mesh sieve.
-
5
Place filtrate in 250 ml bottles and centrifuge at 800 × g without brakes for 10 min then gently remove and discard supernatant.
-
6
Add water to sediment to make slurry (1 part of fecal material to four parts of water).
-
7
Pool contents from 250 ml bottles and add 6 parts sucrose solution to 4 parts of filtrate.
-
8
Place the sugar-fecal mixture in 50 ml conical centrifuge tubes and centrifuge at 800 × g for 10 min without brakes.
-
9
To collect sporocysts, aspirate the very top layer (5–10 ml) of the solution with the help of a Pasteur pipette.
-
10
Mix 5 ml aspirate with 45 ml of water, and centrifuge at 800 × g for 10 min without brakes.
-
11
Discard the supernatant and resuspend the final sediment in HBSS antibiotic mixture. This pool would be designated as the “clean pool”.
-
13
Pool the remaining 40–45ml of the sucrose solution with sporocysts left over from step 9 into 50 ml aliquots in a 250 ml bottle, add water to make 1:5 dilutions to wash away the sucrose and centrifuge at 800 × g for 10 min without brakes as in steps 10 and 11.
-
12
Centrifuge, pellet and pool these sporocyst collections. This pool is designated as the “dirty pool”. The oocysts/sporocyst mixture can be further cleaned up by filtering sequentially through 90 then 45 micron wire mesh sieves and/or gradient purification (Elsheikha et al., 2003a).
Examine sporocysts under microscope (See Basic Protocol 1: Step 6).
-
13
Use a haemocytometer to counts sporocysts (APPENDIX 4A; Stevenson 2006).
-
14
Store sporocyst collections in HBSS antibiotic mixture in refrigerator at 4 °C.
Basic Protocol 3: Isolation of Sarcocystis sporocysts from water samples
Examination of water contamination with Sarcocystis sporocysts is essential to prevent infection. Definitive hosts can excrete millions of sporocysts that can be excreted for months in their feces.
Materials
Lab coats, gloves, face mask, face shield
Centrifuge
Microscope
Refrigerator
250 ml centrifuge bottles
50 ml conical centrifuge tubes
Pasteur pipettes
glass slides
22 mm2 coverslips
Water
2M sucrose solution
HBSS antibiotic mixture (See in reagents and solutions section)
CAUTION: Water samples collected from the environment may contain zoonotic oocysts and sporocysts, so these preparations should be handled with extra care; wear gloves, lab coat and face mask at all times.
Centrifuge samples for 10 min at 800 × g with no brakes in 250 ml bottles.
Pour off supernatant, add 50 ml of sucrose solution into sediment, mix well and centrifuge for 10 min at 800 × g.
Pipette 5 ml of sucrose material from the very top into a 50 ml tube, add 45 ml of water and centrifuge again for 10 min at 800 × g.
Pour off supernatant; add 3–5 ml of HBSS antibiotic mixture.
Examine for oocysts/sporocysts by placing small drops on glass slide using 22 mm2 coverslip under microscope (See Basic Protocol 1: Step 6).
Store sporocyst collections in refrigerator at 4 °C.
Basic Protocol 4: Sporocyst excystation and in vitro culture of merozoites
One of the most important preliminary steps to achieve a successful in vitro culture of merozoites is to obtain a high rate of sporozoite excystation from sporocysts. Sporozoites are the infective form released from sporocysts that develop into a merozoite, the replicative form of the parasite. After sporocysts have been isolated from the definitive host, purified, and concentrated, they can be stored until use at 4 °C in HBSS with antibiotics (Leek and Fayer, 1979). Several factors are important in storing and successfully excysting sporocysts, and these conditions may differ with different species of Sarcocystis. For example S. cruzi sporocysts (and most likely others) should not be stored in aqueous K2Cr2O7, which is routinely used to store Eimeria oocysts, because it has a deleterious effect on excystation of Sarcocystis (Cawthorn et al., 1986; Leek and Fayer, 1979). McKenna and Charleston, (1992) reported no excystation of S. gigantea sporocysts after a 5 day exposure in 2% sulfuric acid solution (commonly used to store oocysts of Toxoplasma gondii and Cystoisospora species) compared with 85% excystation by storage in tap water, and 15% excystation after storage in 2.5% K2Cr2O7.
Pre-treatment of sporocysts with sodium hypochlorite (NaOCl) helps by removing contaminating microrganisms from sporocysts and in subsequent excystation. Traditionally, 5.25% NaOCl (available commercially as undiluted laundry bleach) has been used for this purpose and the treatment was done in an ice bath to avoid the deleterious effect of heat generated during the procedure. Horn et al., (1991) reported higher efficiency of excystation of S. gigantea, S. tenella, S. arieticanis, and S. hircicanis using 6 to 8 % NaOCl at room temperature for 20 min instead of an ice bath. The NaOCl treatment is thought to remove the outer lipid layer of the Sarcocystis sporocysts. Incubation in 1–3% formic acid for 24–72 hrs at 4 °C has been reported to enhance excystation (Sanft, 1990). The mechanism of excystation for the different Sarcocystis species is not fully understood. Trypsin required for the excystation of Eimeria species is not necessary for Sarcocystis whereas bile is important (Fayer and Leek, 1973). Also, different concentrations (5 to 15%) of bovine bile have been used for excystation of sporocysts. Treatment of the sporocysts with a reducing agent like 0.02 M Cysteine hydrochloride for 18 hrs may also improve the rate of excystation (Leek and Fayer, 1979). Cysteine HC1 and NaOCl evidently have similar beneficial effects on excystation, but NaOCl acts more rapidly (30 min vs. 18 hrs) and it must be used anyway to disinfect the sporocysts.
Materials
Lab coats, gloves
Centrifuge
Microscope
microbiological safety cabinet
15 and 50 ml conical centrifuge tubes
nylon wool column
hemocytometer
cell culture flasks
sodium hypochlorite NaOCl (i.e., Clorox®, Purex®)
normal saline (See in reagents and solutions section) or phosphate buffered saline (PBS)
HBSS
trypsin or chymotrypsin
bile or bile salt (i.e., sodium taurocholate)
cell culture media (See in reagents and solutions section)
serum
L-glutamine
Dihydrostreptomycin
penicillin G
cryo-preservative medium (solution C) (See Basic Protocol 9)
Table 1.
In vitro culture of Sarcocystis spp. gamonts.
| Species | Host | Type of cells successful | Type of cells unsuccessful | References |
|---|---|---|---|---|
| S. muris | Mouse | MDCK, FE, MF | Not stated | (Entzeroth, 1985; Entzeroth and Chobotar, 1989) |
| S. muris | Mouse | CL, DK | HF, SK | (Becker et al., 1979) |
| S. suihominis | Pig | HF | CL, DK, SK | |
| S. tenella | Sheep | DK | HF, SK, CL | |
| S. capracanis | Goat | DK | HF, SK, CL | |
| Sarcocystis sp. | Grackle | MDCK, EBK, EBTr, ETK, ECM | ECK | (Fayer, 1970; Fayer, 1972) |
| S. suihominis | Pig | HEI, HSF | - | (Mehlhorn and Heydorn, 1979) |
MDCK: Madin-Darby canine kidney; FE: feline embryo; CL: cat lung; DK: dog kidney; SK: swine kidney; MF: mouse fibroblasts; HF: human fibroblasts; EBK: embryonic bovine kidney; EBTr: embryonic bovine trachea; ECK: embryonic chicken kidney; ECM: embryonic chicken muscle; ETK: embryonic turkey kidney; HEI: human embryonic intestine; HSF: human skin fibroblasts.
Table 2.
Species of Sarcocystis schizonts cultivated in vitro*.
| Host | Species | Type of cells successful | Type of cells unsuccessful | Schizonts days | References |
|---|---|---|---|---|---|
| Ox | S. cruzi | BM, CPA | Mouse macrophages | 18-1, 320 | (Andrews et al., 1990; Speer and Burgess, 1988; Speer et al., 1986b) |
| Ox | S. hirsuta | BPE | BM | 14-62 | (Cawthorn et al., 1990) |
| Rat | S. singaporensis | Rat brain endothelial cells, rat pneumonocytes | Hepatoma, fibroblats, myoblasts | 3-18 | (Jäkel et al., 1997; Jäkel et al., 2001) |
| Sheep | S. tenella | BM, CPA | MDBK, OM | 14-50 | (Speer et al., 1986a) |
| Goat | S. capracanis | BM | MDBK, OM, CPA | 60-100 | (Speer et al., 1986a) |
| Budgerigar-Didelphis virginiana | S. falcatula | BT | - | 3-4 | (Lindsay et al., 1999) |
| Budgerigar-Didelphis albiventris | S. falcatula | BM | - | 7 | (Dubey et al., 1999) |
| Budgerigar-Didelphis marsupialis | S. falcatula | CV-1, BT | EK | 4 | (Dubey et al., 2001) |
| Budgerigar-Didelphis albiventris | S. falcatula | BT, Vero | ED, Hep-2 | 4-6 | (Elsheikha et al., 2003b) (510) |
| Budgerigar-Didelphis albiventris | S. lindsayi | EK, BT, CV-1 | - | 2-3 | (Dubey et al., 2000b) |
| Mouse-Didelphis virginiana | S. speeri | BM, EK | CV-1 | 3 | (Dubey et al., 2000c) |
| Mouse-Didelphis marsupialis | S. speeri | BT | - | 15 | (Dubey et al., 2000a) |
BM: bovine monocytes; BPE: bovine pulmonary endothelial; BT: bovine turbinates; CPA: cardiopulmonary endothelial cells; CV-1: African green monkey kidney; ED: equine dermal; EK: equine kidney; Hep-2: human hepatocytes; MDBK: Madin-Darby bovine kidney.
Unlike these species S. neurona schizonts have been cultivated in many cell lines and from many hosts (Reviewed in Dubey et al., 2015).
The following procedure is based on published reports and our experience with Sarcocystis species (S. neurona, S. speeri, S. falcatula, S. lindsayi, S. cruzi, S. tenella, S. capracanis, Sarcocystis sp. ex Pantherophis alleghaniensis, Sarcocystis sp. ex Accipiter cooperii) in cell culture over many years.
Treat the sporocyst pellet with 2.6% (v/v) NaOCl at 4 °C for 30 min to sterilize the inoculum.
Wash the sporocysts several times (at least 4 times) by centrifugation and decantation at 800 × g for 10 min in 50 ml conical centrifuge tubes in normal saline or culture medium (i.e. RPMI 1640 containing 10% (v/v) FBS serum) until the odor of NaOCl can no longer be detected or until the pH color indicator in the culture medium remains unchanged after centrifugation.
-
Suspend the sporocyst pellet in excysting fluid and incubate at 37 °C for 1.5 to 4 hrs.
The excysting fluid should consist of a balanced salt solution (i.e., HBSS, pH 7.2 to 7.4) containing 2% (w/v) trypsin or chymotrypsin (activity of 1:250 or 1:300) and 5 to 10% bile or 2.5 to 5% (w/v) bile salt (i.e., sodium taurocholate obtained from the appropriate intermediate host such as bovine, ovine, etc.). -
Remove the excysting fluid by washing the excysted sporozoites at least twice in culture medium containing 10% (v/v) serum, and resuspend in 5 to 10 ml culture medium.
To remove debris and sporocyst walls, the sporozoites may be passed through a nylon wool column (Larsen et al., 1984). Too much debris and sporocyst walls in the inoculum may have a deleterious effect on parasite development (Speer and Dubey, 1986). Count the number of sporozoites with a hemocytometer and resuspend them at the appropriate concentration (See Step 6) in culture medium containing 5% (v/v) fetal bovine serum (FBS), 2 mM l-glutamine, 50 μg/ml dihydrostreptomycin, 50 Unit/ml penicillin G.
-
Inoculate 2 to 5 × 102 sporozoites per square centimeter of the monolayer of the cell line to be tested and incubate at 37 °C in 5% CO2.
Schizonts of only a few species of Sarcocystis have been grown in cell culture and the selection of the cell line for culture is an important consideration. The cell line tested for its ability to support growth of the parasite should be based on the cell or tissue in which the parasite develops in vivo. For example, if schizonts develop in vivo in mouse hepatocytes, then a mouse liver epithelial cell line should be tested. We typically examine in vitro development in African green monkey kidney (CV-1) cells. Remove the culture medium at 1 day, and then replace it with culture medium containing 2% FBS at 3 to 6 day intervals after sporozoite inoculation.
Examine flasks daily for 21 days and then every other day thereafter.
-
Once merozoites are observed in inoculated cultures, use the infected medium to infect other flasks of cells to keep the cultures growing.
The infected cell cultures might require maintenance for long periods to allow sufficient time for the parasites to undergo one or more generations of schizogony. Long cultivation periods can be achieved by varying the FBS concentration in the culture medium. As the cell culture becomes sparsely populated due to parasite development and lysis of the cell line (usually first observed at 24 to 28 days post inoculation), then treat the cells with cell culture medium containing 10 to 20% FBS, which can then be reduced to 2% FBS once the monolayer has re-established itself. Once merozoites are observed in cell culture (living cultures), additional sub-passage can be also done into fresh monolayer cell flasks and coverslips to study in vitro developmental stages at various intervals by fixing and staining methods (See Basic Protocol 8). Harvest parasites from the infected medium and replace with maintenance medium every 4–5 days.
Centrifuge harvested parasites at 800 × g for 5 min, and discard the supernatant.
Finally resuspend the pellet containing parasites in PBS or saline antibiotic solution, and store at −70 °C for DNA, RNA, or protein extraction.
Living cultures can be frozen to reuse in the future by following the protocol of cryopreservation (See Basic Protocol 8).
Sarcocystis schizonts multiply asynchronously by endopolygeny, a process where the nucleus becomes multilobed but lobes remain interconnected. The merozoites are formed at the periphery. Additionally, a new generation of schizonts can be formed without leaving the host cell. Please refer to Figure 2, most of the developmental stages can be seen in one field.
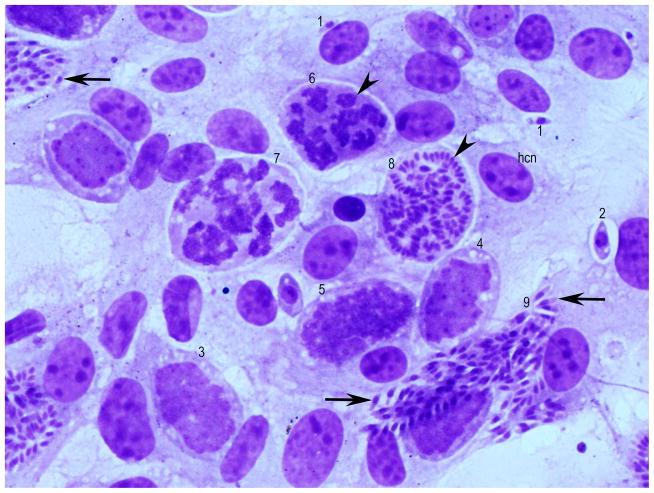
Figure 2.
Developmental stages of Sarcocystis falcatula schizonts in bovine turbinate cells, 5 days after seeding with merozoites. Giemsa stain. hcn = host cell nucleus. Different developmental stages can be seen in 1 field. Stages (1–9) in presumed order of development. Intracellular merozoite (1) with a single nucleus, earliest schizont (2) with a prominent nucleus and 1 nucleolus, immature schizonts with several nucleoli (arrowhead) (3, 4, 5), schizont with differentiation of nuclear lobes (arrowhead) (6). Thereafter the nucleus has become lobulated (7). In (8) ends of merozoites are forming (arrowhead). In (9) the merozoites have formed (arrows).
Basic Protocol 5: Examination of muscles for sarcocysts
Sarcocysts can be detected in intermediate host by gross inspection, examination of unstained squash preparations, histologic examination, or by a digestion method. Each of these methods has its advantages and disadvantages.
Materials
Lab coats, gloves, face mask
Centrifuge
Microscope
surgical scissors
scalpels
forceps
containers with lids
paper towels
normal saline (see in reagents and solutions section)
water
50 ml conical centrifuge tubes
eppendorf tubes
histology cassettes
Pasteur pipettes
glass slides
22 mm2 coverslips
saline antibiotic solution or HBSS antibiotic mixture (See in reagents and solutions section)
10% (v/v) buffered formalin solution (See in reagents and solutions section)
glutaraldehyde buffer (2.5–3% glutaraldehyde, 0.05M NaCacodylate, 0.05M CaCl2 (pH=7)
Examination of unstained squashes
Examination of unstained squashes is useful to study sarcocyst structure. By this method one can examine relatively more tissue than by histologic examination.
-
1a
To examine muscle squashes for microscopic sarcocysts remove fat and connective tissue from the muscles. Cut small portion of muscle tissue in the direction of the muscle fiber run. Put on a glass slide and cover with a 22 mm2 coverslip.
-
2a
Press the coverslip using another clean slide with the help of thumb. Remove the top clean slide, and examine the muscle squash under microscope at magnification of 10 × or more.
Once sarcocyst(s) are observed in the tissue squash, individual sarcocysts can be isolated mechanically under microscope for DNA isolation and for electron microscope processing. Shape and size of microscopic sarcocysts may vary with species of Sarcocystis and with stage of sarcocysts.
Examination of infected organs
To observe merozoites in the brain, liver or other organs of infected intermediate hosts, the following method can be used.
-
1b
Isolate a small piece (1–5mm) of brain (preferentially cerebellum) or liver or other organ.
-
2b
Squash on glass slides with the help of forceps.
-
3b
Mix squashed tissue with 2–3 drops of normal saline, and examine microscopically at magnification of 20 × or more for Sarcocystis merozoites.
Sarcocystis merozoites are small (usually 5 – 15 μm, crescent or sausage shaped). Shape and size of merozoites may vary with each species of Sarcocystis.
Histological examination
Histologic examination of H and E stained muscle is a very common methodology to study sarcocyst morphology. However, this method is not as sensitive as the other methods above because only a small amount of tissue can be sampled on a slide. Histologically, the sarcocyst wall may be smooth, striated, or hirsute or may possess complex branched protrusions (Dubey et al. 2016). Internally, groups of bradyzoites may be divided into compartments by septa that originate from the sarcocyst wall or may not be compartmentalized. When present, septa are usually less than 2 μm thick, but sometimes they are indistinct or not found (Dubey et al., 1988; Kan and Dissanaike, 1976). The structure of the parasites within the sarcocysts varies with the maturation of the sarcocyst. Within the sarcocysts, metrocytes are generally located in the cortex, whereas bradyzoites are located in the medulla. In old, large sarcocysts, bradyzoites near the center of the sarcocyst are sometimes degenerate and are replaced by granules or globules.
-
1c
Fix a portion of the tissue samples (mainly muscles of the heart, tongue, esophagus, diaphragm, skeletal muscles, and muscles of the intestine) in 10% buffered neutral formalin (See in reagents and solutions section).
For ultrastructure of sarcocysts by transmission electron microscope (TEM) and scanning electron microscope (SEM), fixation of fresh tissues in glutaraldehyde or other recommended buffer is preferred. Fixation of fresh tissues for at least 18–24 hrs in duration is optimal. -
2c
Cut the fixed tissue samples into sections (2.5 x 0.7 cm), place in cassettes, embed in paraffin, and section 5 μm thick.
-
3c
Stain tissue sections with hematoxylin and eosin (H and E) or other stains.
-
4c
Observe using light microscopy.
In histologic sections stained with hematoxylin and eosin (H and E), metrocytes are paler than bradyzoites. The bradyzoites contain prominent amylopectin granules that stain bright red with PAS (Periodic Acid-Schiff) stain. In smears stained with Giemsa, metrocytes are oval to round in shape, and pink/magenta in color.
Basic Protocol 6: Tissue digestion to detect Sarcocystis infection by releasing bradyzoites from sarcocysts
Digestion of host tissue is the most sensitive method to detect Sarcocystis infection and is recommended if only a few sarcocysts are present. By this method one may detect even a few sarcocysts in 50 g of tissue because several hundreds or thousands of bradyzoites are released from each sarcocyst as the host tissue and sarcocysts are digested. Resuspend the bradyzoite pellet in HBSS antibiotic mixture and store the suspension in a refrigerator at 4 °C. Bradyzoites survive in the digestive fluid and remain viable. The bradyzoites can be used for antigen preparation, infecting definitive hosts, cryopreservation and molecular PCR methods to determine the species of Sarcocsytis. Disadvantages of this method are (1) it is not possible to distinguish species of Sarcocystis microscopically because the sarcocyst wall is dissolved, (2) metrocytes are not resistant to digestion; therefore, immature sarcocysts may not be detected, and (3) it may not be possible to detect sarcocysts with relatively small bradyzoites (5 to 6 μm).
Materials
Lab coats, gloves, face mask
centrifuge
microscope
commercial blender or meat grinder
magnetic stirrer or shaking water bath
surgical scissors
scalpels
forceps
containers with lids
250 ml centrifuge bottles
50 ml conical centrifuge tubes
eppendorf tubes
Pasteur pipettes
glass slides
22 mm2 coverslips
Cheesecloths
paper towels
water
normal saline (see in reagents and solutions section)
digestion fluids; 1% trypsin solution at pH 7.4 or HCl-pepsin solution (See in reagents and solutions section)
1.2% sodium bicarbonate (pH 8.3)
phenol red
saline antibiotic solution or HBSS antibiotic mixture (See in reagents and solutions section)
cryo-preservative medium (solution C) (See Basic Protocol 9)
To digest muscle tissues, remove extraneous material by washing with water. Trim fat and connective tissue, cut small pieces (1–2 cm) of the muscles, and weigh (50 g).
Homogenize muscle in the commercial blender for 15–30 sec at low speed without normal saline. Add normal saline and homogenize at top speed for 2 min or more.
-
Incubate above homogenate in 10 volumes of digestive fluid containing either 1% trypsin solution at pH 7.4 or HCl-pepsin solution for 30 min to 1 hr at 37 °C on a magnetic stirrer (or in shaking water bath) (See Basic Protocol 7) (Dubey, 2010).
To obtain intact sarcocysts, digestion of a small amount of tissue for only 8–10 min is suggested (Dubey et al., 2016). Filter the digest through two layers of cheesecloth and centrifuge at 800 × g for 10 min and discard supernatant.
Wash the sediment to stop further digestion by repeating centrifugation and decantation after neutralizing the sediment with 1.2% sodium bicarbonate (pH 8.3) plus phenol red (as a pH indicator), then add normal saline to fill the tube.
Centrifuge above solution at 800 × g for 10 min in 50 ml conical centrifuge tubes. Discard supernatant and resuspend the pellet in 5–10 ml saline antibiotic solution.
Take small drops of digest suspension with a Pasteur pipette, put on a glass slide and cover with a 22 mm2 coverslip. Examine the slide for bradyzoites under microscope at 20X magnification or more. Sarcocystis bradyzoites are small (usually 5 – 15 μm, crescent or lanceolate shaped).
Store bradyzoite suspensions in saline antibiotic solution or HBSS antibiotic mixture in the refrigerator at 4 °C or cryopreserve using the freezing protocol (See Basic Protocol 9).
Basic Protocol 7: Isolation, purification, and cryopreservation of infectious bradyzoites
Bradyzoites are released from sarcocysts by incubation in digestive fluid (trypsin or acid pepsin). The sarcocyst wall is dissolved by digestive fluid, but the released bradyzoites survive in it. The number of sarcocysts in different tissues may vary among animals; heart and tongue are the most heavily infected organs (Dubey et al 2016). The following method is useful for obtaining bradyzoites in sufficient quantity for biological and molecular characterization (Farooqui et al., 1987; Lunde and Fayer, 1977).
Materials
Lab coats, gloves, face mask
Centrifuge
magnetic stirrer or shaking water bath
microscope
commercial blender or meat grinder
surgical scissors
scalpels
forceps
containers with lids
250 ml centrifuge bottles
50 ml conical centrifuge tubes
eppendorf tubes
Pasteur pipettes
glass slides
22 mm2 coverslips
Cheesecloths
paper towels
water
normal saline
digestion fluids; 1% trypsin solution at pH 7.4 or HCl-pepsin solution
1.2% sodium bicarbonate (pH 8.3)
phenol red
saline antibiotic solution, or HBSS antibiotic mixture (See reagents and solutions section)
isotonic Percoll solution (mix 9 parts of Percoll + 1 part 9% NaCl solution)
cryo-preservative medium (solution C) (See Basic Protocol 9)
Select only heavily infected tissues by microscopic examination of unstained squash preparations (esophagus, tongue, and heart or leg muscle) (See Basic Protocol 5, steps 1a–2a).
Remove as much connective tissue and fat as possible.
Grind or homogenize 50 g muscle in a commercial meat grinder or blender or mince into small pieces as per Basic Protocol 6.
Resuspend ground or homogenate or minced meat in 100 ml of fresh, pre-warmed (37 °C) digestion fluid; 1% trypsin solution or HCl-pepsin solution.
-
Incubate the suspension of meat-digestion fluid at 37 °C on a magnetic stirrer (or in shaking water bath) for approximately 10 min or longer.
The objective is to release bradyzoites from sarcocysts that were broken during grinding, homogenization, and this method is best suited for heavily infected tissues. Lightly infected tissues may have to be digested longer in digestion fluid, and in that case, the digestion fluid should be isotonic (Farooqui et al., 1987). Strain the digested solution through two layers of cheesecloth to remove large particles. Squeeze the liquid portion from the digest from the cheesecloth.
Centrifuge digest in the conical tube for 10 min at 800 × g.
Discard the supernatant and carefully detach debris with Pasteur pipette. Clean bradyzoites stay at the bottom of the tube.
Resuspend the sediment in normal saline or HBSS at pH 7.4, preferably at 5 to 10 °C.
-
Centrifuge at 800 × g for 10 min and discard the supernatant.
The pellet has 2 layers, the whitish bradyzoite layer at the bottom and a brownish host tissue layer on the top. Remove host tissue by gently aspirating it from the whitish layer. Clean separation is not always possible at this step. -
Resuspend pellet in 5–10 ml saline antibiotic solution or HBSS antibiotic mixture. Examine microscopically for bradyzoites (generally crescent or lanceolate shaped) or metrocytes (globular) (See Basic Protocol 6: Step 7).
If clean separation of bradyzoites is observed, then go to step 17 to store. If host cell contamination is found, then continue with the next step to further clean bradyzoites. Thoroughly mix the concentrated bradyzoite suspension with isotonic Percoll solution, one part of bradyzoite suspension with two parts of isotonic Percoll.
Centrifuge Percoll bradyzoite suspension at 800 × g or more for 10 min. The bradyzoites settle at the bottom and host tissue floats in Percoll.
Pour off Percoll and wash bradyzoite pellet three times in normal saline or HBSS by centrifugation and decantation.
-
Finally, microcentrifuge clean bradyzoites in a small 1.5ml conical eppendorf tube at 2000 rpm for 2 min to pellet bradyzoites. Aspirate supernatant using a pipette.
Bradyzoites recovered by the above procedure remain viable. Store bradyzoite collections in saline antibiotic solution or HBSS antibiotic mixture in the refrigerator at 4 °C or freeze using the protocol for cryopreservation (See Basic Protocol 9).
Basic Protocol 8: In vitro cultivation of Sarcocystis spp
Two factors determine the success of in vitro growth experiments. First, a large supply of sterile stage-specific parasites (sporozoites, merozoites) must be readily available. Second, the selection of the cell line to be tested for its ability to support growth of the parasite should be based on the cell or tissue in which the parasite develops in vivo. For example, if schizonts develop in vivo in mouse hepatocytes, then a mouse liver epithelial cell line should be tested. Because other coccidia have been shown to readily develop in some specific cell types, such as kidney cells, even though they do not normally develop in such cells in vivo (Speer and Dubey, 1986), the literature on in vitro cultivation of coccidia should serve as a guide for possible cell types to test for in vitro growth of Sarcocystis spp. Numerous cell lines that support in vitro growth of Sarcocystis spp. are summarized in Tables 1 and 2 (Dubey et al., 2016). For example, developmental stages during in vitro culture of S. falcatula are shown in Figure 2.
The following method is useful to study in vitro development, and to obtain a large number of parasites from many species of Sarcocystis directly from sporozoites released from sporocysts and by feeding sporocysts to knockout (KO) mice or other intermediate hosts, then in vitro cultivation of infected host tissues.
All cell culture should be performed in microbiological safety cabinet using aseptic techniques to ensure sterility. All media, supplement and reagents must be sterile to prevent microbial growth in the cell culture. Some reagents and supplements will require filter sterilization if they are not provided sterile. Before starting, check the information given with the cell line to identify what media type, additives and recommendations should be used.
Materials
Lab coats, gloves
Centrifuge
Microscope
microbiological safety cabinet
sterile 15 and 50 ml conical centrifuge tubes
5 or 10 ml syringe
18, 20 and 23 gauge needles
sterile 2 mm glass beads
sterile Pasteur pipettes
glass slides
22 mm2 coverslips
Cell culture flasks or 6 welled cell culture plates
cell culture media (See in reagents and solutions section)
serum
antibiotics
ethanol (200 proof)
100% methanol
HBSS
normal saline (See in reagents and solutions section)
saline antibiotic solution or HBSS antibiotic mixture (See in reagents and solutions section)
10% (v/v) buffered formalin solution (See in reagents and solutions section)
Giemsa stain
Permount
Thaw a vial of the target cell line for propagating the Sarcocystis parasites; for example, the African green monkey kidney (CV-1) cell line supports the growth of most Sarcocystis spp. that have so far been cultured successfully (ATCC CCL-70, Manassas, Virginia, USA).
Grow host cells in 25 cm2 cell culture flasks in RPMI 1640 cell culture medium containing 100 IU penicillin/ml, 100μg/ml streptomycin/ml and 10% (v/v) fetal bovine serum (FBS).
Incubate culture flasks at 37 °C in incubators containing 5% CO2.
Maintain host cells in the same medium except at a concentration of FBS 2%.
If sporozoites were released from sporocysts according to Basic Protocol 4, then go to step 8. If the source of Sarcocystis parasites is from infected host tissues, then continue with the next step.
Place infected tissue in 3 ml of HBSS, homogenize and pass sequentially through 18, 20 and 23 gauge needles using a sterile syringe into a 15 ml screw cap centrifuge tube.
Add 2 mm sterile glass beads up to about 0.5 ml in the tube and vortex the sample for about 10 seconds or more.
Examine the unstained sample for the presence and amount of merozoites using a light microscope similar to bradyzoites (See Basic Protocol 6: Step 7). Sarcocystis merozoites are a little longer than bradyzoites (usually 5 x 20 μm, crescent or sausage shaped).
Inoculate samples (about 0.5 ml/flask) onto each of the monolayers of CV-1 cells in 25 cm2 flasks.
Remove inoculums after 2 hrs (or overnight), and add 5 ml of maintenance medium.
Examine flasks daily for 21 days and then every other day thereafter.
Once merozoites are observed in inoculated cultures, use the infected media to infect other flasks of clean cells to keep the cultures growing.
Harvest parasites from the infected media and replace with maintenance medium every 4–5 days.
Centrifuge the harvested parasites at 800 × g for 5 min, and discard the supernatant.
Resuspend the pellet containing parasites in saline antibiotic solution or HBSS antibiotic mixture.
Living cultures can frozen by following the protocol for cryopreservation (See Basic Protocol 9).
Alternate Protocol 1: Cell culture in a 6 or 24 well tissue culture grade plate
The following method is useful for studying the in vitro developmental stages of the parasite using cells grown on sterile 22 mm2 glass coverslips in 6 or 24 welled cell culture plates.
Immerse the coverslips in ethanol (200 proof), flame them briefly, let cool, then set coverslip inside the 6-well; do it one by one.
When splitting the cells, be sure to homogenize the solution prior to distributing into each well.
Change the medium the same way as the above regular culture method.
Inoculate merozoites resuspended in maintenance medium onto the cells in wells containing coverslips.
Remove the inoculum 24 hrs post-inoculation (PI) and replace with maintenance medium.
Remove one or two coverslips at 2 to 8 days PI and process for light microscopic examination after staining with Giemsa. Do at various intervals to study in vitro stages of parasites.
Coverslip staining with Giemsa
Take out the 22 mm2 coverslip from the well using small forceps and/or a bent needle; be careful not to scratch the cell off the surface.
Let it air dry (you can place it on a small Petri dish, and leave inside the hood for a while).
Fix the coverslip in 10% (v/v) buffered formalin solution (See in reagents and solutions section) for 10 min.
Post-fix in 100% methanol for 10 min and let it air dry.
Stain with Giemsa (1:25) for 45 min or more.
Rinse with water (2–3 times).
Let it air dry.
Place coverslip (cells facing down) on a glass slide with a drop of Permount for microscopic examination and photograph.
Basic Protocol 9: Cryo-preservation
Sarcocystis spp. merozoites, sporozoites, tissue cysts or digested and released bradyzoites can be preserved by freezing methods described for other coccidian parasites. It is advisable to start with high numbers (100,000 or more per ml) of the parasite stages in the inoculum because not all organisms survive during freezing. The survival of intracellular merozoites in liquid nitrogen is better than extracellular but free bradyzoites and sporozoites survive better than intact tissue cysts and sporocysts. For cryopreservation of sporozoites, tissue cysts or digested and released bradyzoites, process the sample the same as that of an infected cell culture with merozoites.
Materials
Lab coats, gloves
Centrifuge
Microscope
microbiological safety cabinet
sterile 15 and 50 ml conical centrifuge tubes
cell culture media (See in reagents and solutions section)
bovine serum albumin (BSA)
Dimethyl sulfoxide (DMSO)
0.22 μm syringe filter
10 or 20 ml syringe
cell scrappers
cryovials
Mr. Frosty or similar freezing box
Freezer
liquid nitrogen storage tank
Prepare the complete tissue culture medium (TCM) consisting of RMPI-1640, 2 mM L-glutamine, 10 mM HEPES, 1 mM sodium pyruvate, 0.05 mM 2-mercaptoethanol, non essential amino acids, 100 U/ml penicillin G, 100 μg/ml streptomycin and 20% fetal bovine serum (previously heat inactivated at 56 °C for 30 min).
Prepare a stock solution of 16% fetal bovine serum (FBS) in TCM (Solution A) and a 50% concentration of DMSO in TCM (Solution B) followed by filtration through a 0.22 μm syringe filter if they are not provided sterile. Mix equal volumes of solution A and B to produce solution C.
-
Harvest parasites (preferentially from infected cells) by scraping culture flasks that are heavily infected (approximately 50% of host cells in the monolayer are visibly infected or destroyed) with a cell scraper.
Try to cryopreserve parasites from infected tissues on their first (primary) isolation to minimize the effect of sub-passage in the laboratory. After ascertaining the presence of Sarcocystis spp. in biopsy or infected mouse tissue, grind the tissue in 10% cell culture growth medium (1 brain/ml), dilute with equal volume of cryopreserving medium (solution C) and process as with infected cell culture. Transfer the cell suspension into a sterile tube.
Centrifuge at 800 x g for 10 min at 12 °C, and decant old medium.
Resuspend the pellet in 1.5 ml of complete tissue culture medium (TCM).
Add equal volume of solution C (25% DMSO in TCM + 8% BSA in TCM). Thus, the final concentration of DMSO is 12.5%.
Mix well and distribute into cryovials (2–3 one ml aliquots per 25 cm2 flask is recommended).
Incubate the above suspension for 1 hr at room temperature to allow DMSO to penetrate into the cells.
Freeze samples in Mr. Frosty or similar freezing box which approximates 1 °C/min for 65–70 min. The intermediate temperature should be in the range of −40 °C to −50 °C (Or store the box at −70 °C for overnight).
Transfer frozen cryovials, and plunge into liquid nitrogen storage tank.
Support Protocol: Thaw/Dilution
To revive the culture, remove cryovial from liquid nitrogen and thaw quickly in a 37 °C water bath. Allow sample to thaw completely without attaining 37 °C before infecting animal or cell culture. If the monolayer is the same cell type as the cells that were frozen, wait approximately 6 hrs before changing the medium. If inoculating a different host cell line, remove the supernatant after 1 hr, wash the monolayer (2–3 times) with a balance salt solution or cell culture medium and add fresh medium.
REAGENTS AND SOLUTIONS
2M sucrose solution
To a container, add in sequence:
800 ml of water to make 1000 ml solution at the end.
684.6 g of sucrose in 4 batches (MW 342).
5 ml of liquid phenol if the solutions need to be stored for more than few days to prevent fungal growth.
Procedure
Place the container on a hot plate (low heat setting) with magnetic stirrer on at low speed setting.
Add 800 ml of water.
Add sucrose one batch at a time to the hot water. Leave it mixing using a magnetic stirring bar until all sucrose goes into solution then add more.
Keep the solution continuously mixing until all sucrose goes into solution.
After the sucrose is completely added continue mixing the solution on the hot plate for 4 hrs. Make sure the heat is not too high Do not let the solution boil.
Add 5 ml of liquid phenol and adjust final volume to 1000 ml by adding water.
Turn off the heat and continue mixing the sucrose solution for few more hrs.
The sucrose solution is ready for use.
Store in refrigerator at 4 °C.
Normal saline, 0.85% sodium chloride (NaCl)
Dissolve 8.5 g NaCl (MW 58.44) in 700 ml water.
Add water to bring total solution volume to 1000 ml.
Store in refrigerator at 4 °C.
Saline antibiotic solution (2X)
For 100 ml preparation
Calculate penicillin G concentration (2000 units per ml), weigh powder of penicillin G, and dissolve it in 90 ml sterile normal saline.
Calculate streptomycin concentration (200 μg per ml), add in above solution.
Add more sterile normal saline to make the final 100 ml solution.
This saline antibiotic solution (2X) should be mixed to use equal volume of sample to the final concentration (1X); 1000 units penicillin G and 100 μg of streptomycin per ml.
One liter of antibiotic solution can be prepared and stored at −20 °C in 100 ml aliquots. Thaw each aliquot as needed.
HBSS antibiotic mixture
Prepare HBSS antibiotic mixture (penicillin G, 10,000 units; streptomycin, 10 mg; Fungizone, 0.05 mg; and Mycostatin, 500 units/ml [mixture = PSFM] in HBSS). One liter of antibiotic solution can be prepared and stored at −20 °C in 100 ml aliquots. Thaw each aliquot as needed.
HCl-pepsin solution
Dissolve 2.5g of Pepsin into 200 ml of water
Add 10g NaCl (Reagent grade) to pepsin solution
Centrifuge to remove undissolved pepsin
Add 28 ml of concentrated HCl (6 N Reagent grade),
Dilute with water to make 1000 ml HCl-pepsin solution
Cell culture media
10% cell culture growth medium
Aseptically remove 60 ml medium from 500 ml bottle of RPMI 1640 medium and discard.
Add 50 ml fetal bovine serum (FBS).
Add 5 ml sodium pyruvate + 2-mercaptoethanol (2-ME, 100 X supplement).
Add 5 ml penicillin/streptomycin (10000 unit/100000 μg/ml; 100 X solutions).
Add 0.5 ml amphotericin B (250 μg/ml, fungizone).
2% Cell culture maintenance medium
Aseptically remove 20 ml medium from 500 ml bottle of RPMI 1640 medium and discard.
Add 10 ml FBS.
Add 5 ml sodium pyruvate + 2-mercaptoethanol (2-ME, 100 X supplement).
Add 5 ml penicillin/streptomycin (10000 unit/100000 μg/ml; 100 X solution).
Add 0.5 ml amphotericin B (250 μg/ml, fungizone).
Sodium pyruvate + 2-ME solution (100 X cell culture medium supplement)
Remove 5 ml medium from 500 ml bottle of RPMI 1640 medium and place in a 15 ml tube.
Add 5 ml penicillin/streptomycin to the tube.
Add 0.06 g sodium pyruvate to the tube.
Heat this tube in a 37 °C water bath to dissolve.
Add 175 μl 2-ME (in fume hood).
Sterilize this through a 0.22 μm syringe filter.
Add back to the 500 ml bottle of RPMI 1640 medium.
2X Medium for freezing cell stocks in liquid nitrogen
15 ml of fetal bovine serum.
10 ml of DMSO.
25 ml of 10% cell culture growth medium.
10% (v/v) Neutral buffered formalin solution
Place a container with magnetic on stirrer at low speed setting.
Add 900 ml water.
Weigh both sodium phosphates salts; 4g/L NaH2PO4 (monobasic) and 6.5g/L Na2HPO4 (dibasic/anhydrous).
Add salt slowly with continuous mixing until all salt goes into solution.
After the salt has been completely added, continue mixing the solution for 2 hrs.
Add 100 ml formalin (37–40% stock solution) and allow a few more hrs of mixing to yield a homogeneous solution.
The solution is ready for use.
Store at room temperature (indefinitely).
COMMENTARY
Background Information
There are numerous reasons for isolating parasites from their hosts. They can be used for immunofluorescence antibody test slides, antigen for antibody production, for molecular and biochemical characterizations or other such procedures that require whole parasite preparations. For whatever use, whole parasite preparations should be essentially free from contaminating particles of the host animal (Dubey et al. 2016).
The procedures for isolating and culturing Sarcocystis species are particularly useful in elucidating the biochemistry, as well as the mechanisms of immunity and pathogenesis, especially because the parasite develops in vitro, as well as in vivo, in cell types (i.e., endothelial and mononuclear cells) that have been shown to participate in other immunologic and pathologic mechanisms (Pober et al., 1986; Sobel and Colvin, 1986; Sobel et al., 1986). With minor modifications (i.e., selecting the appropriate cell line) this system may prove useful for culturing other species of Sarcocystis.
Sporocyst formation and their isolation from definitive hosts
The definitive host becomes infected after ingesting muscular or neural tissue containing mature sarcocysts. Bradyzoites are liberated from the sarcocyst by digestion in the stomach and intestine. In the acid pH of the stomach, the bradyzoites are activated and become motile while proteolytic enzymes digest the sarcocyst wall. The released bradyzoites move actively on the epithelial surface and penetrate the epithelium entering the lamina propria of the small intestine. Inside the definitive host cells bradyzoites transform into either male (micro) or female (macro) gamonts. It is presently not known why some bradyzoites become microgamonts and others become macrogamonts. Microgamonts produce flagellated microgametes that fertilize a macrogamont. The entire process of gametogony and fertilization can be completed within 24 hrs, but it is asynchronous. Thus, gamonts and oocysts can be found at the same time. The location of gametogony and the type of host cell parasitized varies with the species of Sarcocystis and the stage of gametogenesis. After fertilization, a wall develops around the zygote and an oocyst is formed (Sheffield and Fayer, 1980). Young oocysts (unsporulated) contain a large nucleus with 1 or 2 prominent nucleoli and there are several PAS-positive granules in the cytoplasm. Sporulated oocysts are generally colorless, thin-walled (<1 μm), and contain 2 elongate sporocysts. Fully developed sporocysts each contain 4 sporozoites. Because sporozoites are flexed inside the sporocyst, all 4 sporozoites are not often seen in a single plane of focus. Sporulation is asynchronous, unsporulated and sporulated oocysts are found simultaneously. The oocyst wall is thin and often ruptures while the oocyst is migrating from the lamina propria to the lumen of the intestine. Free sporocysts, released into the intestinal lumen are passed in feces. Occasionally unsporulated and partially sporulated oocysts are excreted in feces. The prepatent and patent periods vary, but for most Sarcocystis species, oocysts are first excreted in the feces between 7 and 14 days after an appropriate definitive host ingests sarcocysts. Because Sarcocystis oocysts and sporocysts develop in the lamina propria and are not directly in the intestinal epithelium, they can be excreted over a period of many months (Dubey et al., 2016). A definitive host may harbor more than one species of Sarcocystis and different definitive hosts can be involved in the transmission of the same Sarcocystis species (Dubey et al., 2016). Unlike many other species of coccidia, Sarcocystis are passed in feces in their infective form and are not dependent on weather conditions for sporulation. Sporocysts or oocysts remain viable for many months in the environment (McKenna and Charleston, 1994; Savini et al., 1996). Oocysts or sporocysts are resistant to freezing and disinfectants and can survive in the refrigerator at 4 °C for months, even at low humidity (Barutzki et al., 1981; Dubey et al., 2016; McKenna and Charleston, 1992; Savini et al., 1996). Sporocysts can be killed by drying and by exposure to a temperature of 56 °C for 10 min (Savini et al., 1996).
Sarcocyst formation and their isolation from intermediate hosts
The intermediate host becomes infected by ingesting sporocysts contaminating food and/or water. Sporozoites excyst from sporocysts in the small intestine. The fate of the sporozoite from the time of excystation until its initial development in the blood vessels, in mesenteric lymph nodes or other organs is not known. The number of generations of schizogony and the type of host cell may vary with each species of Sarcocystis, but trends are apparent. Sarcocysts (in Greek, Sarkos = flesh, kystis = bladder) are the terminal asexual stage found encysted, primarily in striated muscles of mammals, birds, and poikilothermic animals (intermediate hosts). Sarcocysts generally become infectious around 75 days post inoculation, but there is considerable variation among species of Sarcocystis. Schizonts and immature sarcocysts containing only metrocytes are not infectious for the definitive host. Metrocytes (mother cells) are a globular stage of the parasite that produces two progeny by an internal form of multiplication called endodyogeny. After several generations, some of the metrocytes, through the process of endodyogeny, produce crescent or lanceolate-shaped zoites called bradyzoites. Sarcocysts may persist for the life of the host, but many begin to disappear after 3 months. Live sarcocysts are probably fluid filled with metrocytes and bradyzoites moving within the fluid. The number and distribution of sarcocysts throughout the body vary greatly from host to host. Factors affecting the number and distribution of sarcocysts include the number of organisms (sporocysts) ingested, the species of Sarcocystis, the species of host, and the immunological state of the host. Although most sarcocysts develop in striated muscles of the heart, tongue, esophagus, diaphragm, and skeletal muscles, some sarcocysts have been found in brain and smooth muscles of the intestine. Sarcocysts in brain are often round, and smaller in size than in skeletal muscle. Additionally, sarcocysts in myocardium are smaller than those in skeletal muscle. Microscopic sarcocysts vary from very long and narrow to short and wide (Fig. 3A). Only a few species of Sarcocystis form macroscopic sarcocysts. Macroscopic sarcocysts, which are nearly always in skeletal muscles or esophageal muscles, appear filamentous (e.g., S. muris), like rice grains (e.g., S. rileyi), fusiform (e.g., S. fusiformis) or globular (e.g., S. gigantea) (Dubey et al. 2016) (Fig. 3B). For example, a microscopic sarcocyst in the skeletal muscle of a naturally infected bobcat and several macroscopic sarcocysts on the surface of the esophagus of naturally infected water buffalo are shown in Figure 3.
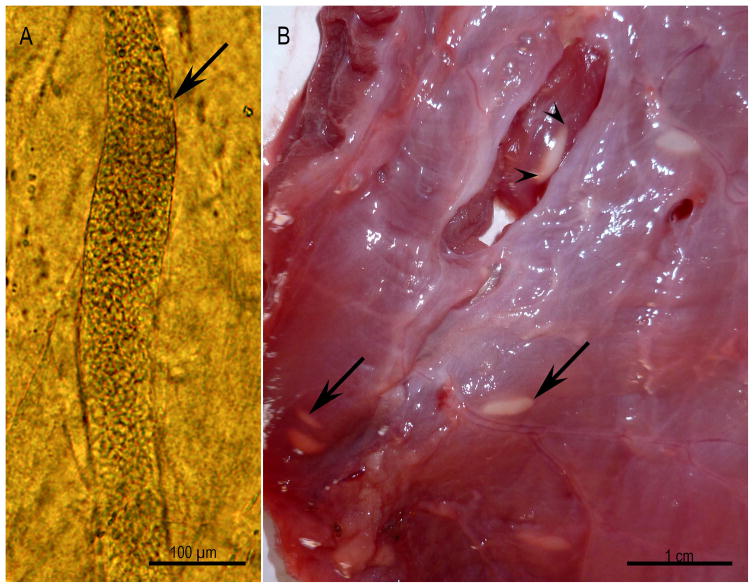
Figure 3.
Microscopic and macroscopic sarcocysts, Unstained. (A) Microscopic sarcocyst (arrow) in the skeletal muscle of a naturally infected bobcat. (B) Macroscopic sarcocysts (arrows) on the surface of the esophagus of naturally infected water buffalo. Note the cysts are covered with fascia (arrow) and one of them is exposed (arrowheads) (Courtesy of Dr. M. A. Hilali, Cairo University, Egypt).
Sarcocysts are always located within a parasitophorous vacuole (PV) in the host cell cytoplasm; often not visible under the light microscope. More than 1 sarcocyst may be found in 1 host cell. The sarcocyst consists of a cyst wall that surrounds the metrocyte and bradyzoite stages. The structure and thickness of the cyst wall varies among species of Sarcocystis and within each species as the sarcocyst matures. A connective tissue wall (secondary sarcocyst wall) surrounds the S. gigantea, S. mucosa, S. hardangeri, and S. rangiferi sarcocysts (Gjerde, 1985a; Gjerde, 1985b; Jakes, 1998; Matuschka et al., 1987; Mehlhorn and Scholtyseck, 1973). The ultrastructure of sarcocysts, especially the cyst wall, is major a criterion in Sarcocystis taxonomy (Dubey et al. 2016).
In vitro development of Sarcocystis spp
The following development stages have been described during in vitro culture of S. cruzi (and most likely others). Soon after inoculation, sporozoites penetrate cells and gradually transform into ovoid or spheroidal schizonts each with an irregularly shaped nucleus containing small nucleoli and small cytoplasmic granules (approximately 2 to 3 weeks) present in the schizont cytoplasm. Ultrastructural studies of infected bovine monocytes (BM) and cardiopulmonary aortic endothelial cells (CPA) have shown that sporozoites, schizonts, and merozoites of S. cruzi are located free in the host cell cytoplasm (i.e. the parasites are not surrounded by a parasitophorous vacuole) (Speer and Burgess, 1987; Speer and Dubey, 1986). More advanced schizont stages appear to have numerous nuclei and nucleoli (ultrastructural studies have shown that the nucleus is highly lobulated, but by light microscopy the lobes often appear as separate nuclei). Eventually, these schizonts form large structures in which merozoites bud radially from 8 to 12 residual bodies. Large mature schizonts contain 150 to 350 merozoites and are present at 2.5 to 10 weeks or more after sporozoite inoculation. Small S. cruzi schizonts contain 36 to 100 merozoites and are present at 16 to 138 days or more after sporozoite inoculation, but are the most numerous at 30 to 52 days. Several generations of small schizonts occur during this time, but the precise number of generations cannot be determined because sarcocyst formation does not occur in cell cultures. Merozoites are active and frequently can be observed to glide rapidly through the host cell cytoplasm, egress through the host cell plasmalemma, and penetrate adjacent host cells. Sarcocystis merozoites are a little longer than bradyzoites (usually 5 x 20 μm, crescent or sausage shaped). Shape and size of merozoites may vary with each species of Sarcocystis. Experimentally, merozoites (from cell culture) of certain species (e.g. S. neurona) are infectious to immunodeficient mice, but bradyzoites do not infect mice or cell lines (Dubey et al., 2013).
Critical Parameters/Troubleshooting
Percoll is very well suited for the bradyzoite purification procedure because it is biologically inert and can be easily made isotonic. We have experienced difficulties with commercial pepsin. Some lots of pepsin may contain small amounts of insoluble materials that migrate in the Percoll gradient and interfere with the process of obtaining “clean” bradyzoites. They can be distinguished from the bradyzoites by their slight difference in color. These contaminates can be left in the bottom of the centrifuge tube as the bradyzoites are resuspended and drawn off. Another way to solve this problem is to dissolve the pepsin in a small amount (200 ml) of the saline-HCl solution and centrifuge 800 x g for 10 min. The supernatant is then added to the saline-HCl solution. The insoluble particles are left behind and the digestion and percoll gradient for bradyzoite purification can proceed without interference of the insoluble materials found in the commercial pepsin preparations.
Laboratory Safety and Occupational Infection Considerations
Similarly, no accidental laboratory infection with Sarcocystis has been reported, although care should be exercised when working with infected meat products to avoid accidental ingestion. It is not known if laboratory personnel can be accidentally infected through parenteral inoculation of Sarcocystis; nevertheless caution should be exercised when working with feces, tissues, homogenates, and cultures. BSL-2 and ABSL-2 practices, containment, equipment, and facilities are recommended for activities with infective stages of the parasites listed.
Anticipated Results
Parasites in the genus Sarcocystis are extremely prevalent in nature and capable of establishing infection in essentially all vertebrates, including birds, reptiles, fish, and animals. However, in many of these hosts, the parasites are strictly enteric, or produce very few tissue cysts, making them difficult to detect. The protocols in this unit that take advantage of the parasite’s unique cell biological properties (their resistance to proteases and chemicals such as dilute bleach, for example) to facilitate the processing of large volumes of tissue, environmental, or fecal preparations for the sensitive detection of these parasites. Although not all samples containing Sarcocystis spp. will give rise to successful cultures, the protocols in this unit should allow for the sensitive detection of the parasite, either by histology, microscopy, or molecular PCR. The techniques highlighted have been optimized not only for detection, but also for the isolation, growth, purification, and cryopreservation of a wide variety of Sarcocystis parasites from tissues, fecal preparations, and environmental sources. This should facilitate the identification, biochemical, histological, ultrastructural, molecular, phylogenetic, and cell biological characterization of different life cycle stages of the parasite. For those parasites identified infecting uncharacterized hosts, it may prove difficult to establish cultures for expansion of the parasites. It may prove prudent to try several different cell lines from various tissues and animal hosts (especially any cell line available from the source vertebrate identified to be infected). Kidney, muscle, monocytic and fibroblast cells have proved to be good target cell types for optimal growth.
Time Considerations
The preparation of 2M sucrose should be done a day in advance, as it can take more than 8 hours to prepare, and it should be used within 1 week of preparation. Sarcocystis parasites often expand slowly in culture, and require constant feeding of the cell lines used to support their growth. It is not a surprising result to see no growth after 30 days in an infected cell line. The evaluation of each culture to check for the presence of Sarcocystis growth is time-consuming and requires examination in situ using an inverted microscope. It may also prove necessary to sample the cell line periodically (after 2 weeks) to assess by molecular methods to determine whether parasites are present in the cell line, if there is no observable growth by microscopic evaluation.
Literature Cited
- Andrews CD, Fayer R, Dubey JP. Continuous in vitro cultivation of Sarcocystis cruzi. J Parasitol. 1990;76:254–255. [PubMed] [Google Scholar]
- Barutzki D, Erber M, Boch J. Möglichkeiten der desinfektion bei kokzidiose (Eimeria, Isospora, Toxoplasma, Sarcocystis) Berl Münch Tierärztl Wochenschr. 1981;94:451–455. [PubMed] [Google Scholar]
- Becker B, Mehlhorn H, Heydorn AO. Light and electron microscopic studies on gamogony and sporogony of 5 Sarcocystis species in vivo and in tissue cultures. Zentralbl Bakteriol Hyg I Abt Orig A. 1979;244:394–404. [PubMed] [Google Scholar]
- Cawthorn RJ, Markham RJF, Hitt ND, Despres D. In vitro cultivation of the vascular phase of Sarcocystis hirsuta (Apicomplexa) Can J Zool. 1990;68:1068–1070. [Google Scholar]
- Cawthorn RJ, Reduker DW, Speer CA, Dubey JP. In vitro excystation of Sarcocystis capracanis, Sarcocystis cruzi and Sarcocystis tenella (Apicomplexa) J Parasitol. 1986;72:880–884. [PubMed] [Google Scholar]
- Dubey JP. Coyote as a final host for Sarcocystis species of goats, sheep, cattle, elk, bison, and moose in Montana. Am J Vet Res. 1980;41:1227–1229. [PubMed] [Google Scholar]
- Dubey JP. Toxoplasmosis of animals and humans. 2. CRC Press; Boca Raton, FL: 2010. [Google Scholar]
- Dubey JP, Calero-Bernal R, Rosenthal BM, Speer CA, Fayer R. Sarcocystosis of animals and humans. 2. CRC Press; Boca Raton, FL: 2016. [Google Scholar]
- Dubey JP, Kerber CE, Lindsay DS, Kasai N, Pena HFJ. The South American opossum, Didelphis marsupialis, from Brazil as another definitive host for Sarcocystis speeri Dubey and Lindsay, 1999. Parasitology. 2000a;121:589–594. doi: 10.1017/s003118200000682x. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Lindsay DS, Rezende PCB, Costa AJ. Characterization of an unidentified Sarcocystis falcatula-like parasite from the South American opossum, Didelphis albiventris from Brazil. J Eukaryot Microbiol. 2000b;47:538–544. doi: 10.1111/j.1550-7408.2000.tb00087.x. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Lindsay DS, Rosenthal BM, Kerber CE, Kasai N, Pena HFJ, Kwok OCH, Shen SK, Gennari SM. Isolates of Sarcocystis falcatula-like organisms from South American opossums Didelphis marsupialis and Didelphis albiventris from São Paulo, Brazil. J Parasitol. 2001;87:1449–1453. doi: 10.1645/0022-3395(2001)087[1449:IOSFLO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Lindsay DS, Speer CA, Fayer R, Livingston CW. Sarcocystis arieticanis and other Sarcocystis species in sheep in the United States. J Parasitol. 1988;74:1033–1038. [PubMed] [Google Scholar]
- Dubey JP, Saville WJ, Sreekumar C, Shen SK, Lindsay DS, Pena HF, Vianna MC, Gennari SM, Reed SM. Effects of high temperature and disinfectants on the viability of Sarcocystis neurona sporocysts. J Parasitol. 2002;88:1252–1254. doi: 10.1645/0022-3395(2002)088[1252:EOHTAD]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Speer CA, Lindsay DS. In vitro cultivation of schizonts of Sarcocystis speeri Dubey and Lindsay, 1999. J Parasitol. 2000c;86:671–678. doi: 10.1645/0022-3395(2000)086[0671:IVCOSO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Sundar N, Kwok OCH, Saville WJA. Sarcocystis neurona infection in gamma interferon gene knockout (KO) mice: Comparative infectivity of sporocysts in two strains of KO mice, effect of trypsin digestion on merozoite viability, and infectivity of bradyzoites to KO mice and cell culture. Vet Parasitol. 2013;196:212–215. doi: 10.1016/j.vetpar.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Venturini L, Venturini C, Basso W, Unzaga J. Isolation of Sarcocystis falcatula from the south American opossum (Didelphis albiventris) from Argentina. Vet Parasitol. 1999;86:239–244. doi: 10.1016/s0304-4017(99)00145-4. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Howe DK, Furr M, Saville WJ, Marsh AE, Reed SM, Grigg ME. An update on Sarcocystis neurona infections in animals and equine protozoal myeloencephalitis (EPM) Vet Parasitol. 2015;209:1–42. doi: 10.1016/j.vetpar.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsheikha HM, Murphy AJ, Fitzgerald SD, Mansfield LS, Massey JP, Saeed MA. Purification of Sarcocystis neurona sporocysts from opossum (Didelphis virginiana) using potassium bromide discontinuous density gradient centrifugation. Parasitol Res. 2003a;90:104–109. doi: 10.1007/s00436-002-0789-y. [DOI] [PubMed] [Google Scholar]
- Elsheikha HM, Saeed MA, Fitzgerald SD, Murphy AJ, Mansfield LS. Effects of temperature and host cell type on the in vitro growth and development of Sarcocystis falcatula. Parasitol Res. 2003b;91:22–26. doi: 10.1007/s00436-003-0902-x. [DOI] [PubMed] [Google Scholar]
- Entzeroth R. Invasion and early development of Sarcocystis muris (Apicomplexa, Sarcocystidae) in tissue cultures. J Protozool. 1985;32:446–453. doi: 10.1111/j.1550-7408.1985.tb04042.x. [DOI] [PubMed] [Google Scholar]
- Entzeroth R, Chobotar B. A freeze-fracture study of the host cell-parasite interface during and after invasion of cultured cells by cystozoites of Sarcocystis muris. Eur J Protistol. 1989;25:89–99. doi: 10.1016/S0932-4739(89)80020-3. [DOI] [PubMed] [Google Scholar]
- Farooqui AA, Adams DD, Hanson WL, Prestwood AK. Studies on the enzymes of Sarcocystis suicanis: Purification and characterization of an acid phosphatase. J Parasitol. 1987;73:681–688. [PubMed] [Google Scholar]
- Fayer R. Sarcocystis: Development in cultured avian and mammalian cells. Science. 1970;168:1104–1105. doi: 10.1126/science.168.3935.1104. [DOI] [PubMed] [Google Scholar]
- Fayer R. Gametogony of Sarcocystis sp in cell culture. Science. 1972;175:65–67. doi: 10.1126/science.175.4017.65. [DOI] [PubMed] [Google Scholar]
- Fayer R. Production of Sarcocystis cruzi sporocysts by dogs fed experimentally infected and naturally infected beef. J Parasitol. 1977;63:1072–1075. [PubMed] [Google Scholar]
- Fayer R, Leek RG. Excystation of Sarcocystis fusiformis sporocysts from dogs. Proc Helminthol Soc Wash. 1973;40:294–296. [Google Scholar]
- Ford GE. Prey-predator transmission in the epizootiology of ovine sarcosporidiosis. Aust Vet J. 1974;50:38–39. doi: 10.1111/j.1751-0813.1974.tb09374.x. [DOI] [PubMed] [Google Scholar]
- Gjerde B. Ultrastructure of the cysts of Sarcocystis rangi from skeletal muscle of reindeer (Rangifer tarandus tarandus) Rangifer. 1985a;5:43–52. doi: 10.1186/BF03546567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjerde B. Ultrastructure of the cysts of Sarcocystis rangiferi from skeletal muscle of reindeer (Rangifer tarandus tarandus) Can J Zool. 1985b;63:2669–2675. doi: 10.1186/BF03546567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydorn AO, Rommel M. Beiträge zum Lebenszyklus der Sarkosporidien. II Hund und Katze als Überträger der Sarkosporidien des Rindes. Berl Münch Tierärztl Wochenschr. 1972;85:121–123. [PubMed] [Google Scholar]
- Horn K, Ono K, Heydorn AO. In vitro excystation and cryopreservation of ovine and caprine Sarcocystis species. J Protozool Res. 1991;1:13–21. [Google Scholar]
- Jäkel T, Henke M, Weingarten B, Kliemt D, Seidinger S. In vitro cultivation of the vascular phase of Sarcocystis singaporensis. J Eukaryot Microbiol. 1997;44:293–299. doi: 10.1111/j.1550-7408.1997.tb05669.x. [DOI] [PubMed] [Google Scholar]
- Jäkel T, Wallstein E, Müncheberg F, Archer-Baumann C, Weingarten B, Kliemt D, Mackenstedt U. Binding of a monoclonal antibody to sporozoites of Sarcocystis singaporensis enhances escape from the parasitophorous vacuole, which is necessary for intracellular development. Infect Immun. 2001;69:6475–6482. doi: 10.1128/IAI.69.10.6475-6482.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakes KA. Sarcocystis mucosa in Bennett’s wallabies and pademelons from Tasmania. J Wildl Dis. 1998;34:594–599. doi: 10.7589/0090-3558-34.3.594. [DOI] [PubMed] [Google Scholar]
- Kan SP, Dissanaike AS. Ultrastructure of Sarcocystis booliati Dissanaike and Poopalachelvam, 1975 from the moonrat, Echinosorex gymnurus in Malaysia. Int J Parasitol. 1976;6:321–326. doi: 10.1016/0020-7519(76)90054-0. [DOI] [PubMed] [Google Scholar]
- Larsen RA, Kyle JE, Whitmire WM, Speer CA. Effect of nylon wool purification on infectivity and antigenicity of Eimeria falciformis sporozoites and merozoites. J Parasitol. 1984;70:597–601. [PubMed] [Google Scholar]
- Leek RG, Fayer R. Survival of sporocysts of Sarcocystis in various media. Proc Helminthol Soc Wash. 1979;46:151–154. [Google Scholar]
- Lindsay DS, Dubey JP, Horton KM, Bowman DD. Development of Sarcocystis falcatula in cell cultures demonstrates that it is different from Sarcocystis neurona. Parasitology. 1999;118:227–233. doi: 10.1017/s003118209800376x. [DOI] [PubMed] [Google Scholar]
- Lunde MN, Fayer R. Serologic tests for antibody to Sarcocystis in cattle. J Parasitol. 1977;63:222–225. [PubMed] [Google Scholar]
- Matuschka FR, Mehlhorn H, Abd-Al-Aal Z. Replacement of Besnoitia Matuschka and Häfner 1984 by Sarcocystis hoarensis. Parasitol Res. 1987;74:94–96. doi: 10.1007/BF00534939. [DOI] [PubMed] [Google Scholar]
- McKenna PB, Charleston WAG. The survival of Sarcocystis gigantea sporocysts following exposure to various chemical and physical agents. Vet Parasitol. 1992;45(1–2):1–16. doi: 10.1016/0304-4017(92)90023-3. [DOI] [PubMed] [Google Scholar]
- McKenna PB, Charleston WAG. The outdoor survival of Sarcocystis gigantea sporocysts. Vet Parasitol. 1994;55:21–27. doi: 10.1016/0304-4017(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Mehlhorn H, Heydorn AO. Electron microscopical study on gamogony of Sarcocystis suihominis in human tissue cultures. Z Parasitenkd. 1979;58:97–113. doi: 10.1007/BF01951336. [DOI] [PubMed] [Google Scholar]
- Mehlhorn H, Scholtyseck E. Elektronenmikroskopische Untersuchungen an Cystenstadien von Sarcocystis tenella aus der Oesophagus-Muskulatur des Schafes. Z Parasitenkd. 1973;41:291–310. [PubMed] [Google Scholar]
- Pober JS, Collins T, Gimbrone J, Libby P, Reiss CS. Inducible expression of class II major histocompatibility complex antigens and the immunogenicity of vascular endothelium. Transplantation. 1986;41:141–146. doi: 10.1097/00007890-198602000-00001. [DOI] [PubMed] [Google Scholar]
- Rommel M, Heydorn AO, Gruber F. Beiträge zum Lebenszyklus der Sarkosporidien. I Die Sporozyste von S tenella in den Fäzes der Katze. Berl Münch Tierärztl Wochenschr. 1972;85:101–105. [PubMed] [Google Scholar]
- Sanft S. Universität Berlin. 1990. In vitro-Exzystation und Lebendkonservierung von Sarkosporidien-Sporozoiten: ein Versuch zur Erhaltung Definierter Sarcocystis-Isolate; p. 69. [Google Scholar]
- Savini G, Robertson ID, Dunsmore JD. Viability of the sporocysts of Sarcocystis cruzi after exposure to different temperatures and relative humidities. Vet Parasitol. 1996;67:153–160. doi: 10.1016/s0304-4017(96)01046-1. [DOI] [PubMed] [Google Scholar]
- Sheffield HG, Fayer R. Fertilization in the coccidia: Fusion of Sarcocystis bovicanis gametes. Proc Helminthol Soc Wash. 1980;47:118–121. [Google Scholar]
- Sobel RA, Colvin RB. Responder strain specific enhancement of endothelial and mononuclear cell Ia in delayed hypersensitivity reactions in (strain 2 x strain 13) F1 guinea pigs. J Immunol. 1986;137:2132–2138. [PubMed] [Google Scholar]
- Sobel RA, van der Veen RC, Lees MB. The immunopathology of chronic experimental allergic encephalomyelitis induced in rabbits with bovine proteolipid protein. J Immunol. 1986;136:157–163. [PubMed] [Google Scholar]
- Speer CA, Burgess DE. In vitro cultivation of Sarcocystis merozoites. Parasitol Today. 1987;3:2–3. doi: 10.1016/0169-4758(87)90087-1. [DOI] [PubMed] [Google Scholar]
- Speer CA, Burgess DE. In vitro development and antigen analysis of Sarcocystis. Parasitol Today. 1988;4:46–49. doi: 10.1016/0169-4758(88)90064-6. [DOI] [PubMed] [Google Scholar]
- Speer CA, Cawthorn RJ, Dubey JP. In vitro cultivation of the vascular phase of Sarcocystis capracanis and Sarcocystis tenella. J Protozool. 1986a;33:486–490. doi: 10.1111/j.1550-7408.1986.tb05647.x. [DOI] [PubMed] [Google Scholar]
- Speer CA, Dubey JP. Vascular phase of Sarcocystis cruzi cultured in vitro. Can J Zool. 1986;64:209–211. [Google Scholar]
- Speer CA, Whitmire WM, Reduker DW, Dubey JP. In vitro cultivation of meronts of Sarcocystis cruzi. J Parasitol. 1986b;72:677–683. [PubMed] [Google Scholar]
- Stevenson B. Common Bacterial Culture Techniques and Media. Current Protocols in Microbiology. 2006:00:4A:A.4A.1–A.4A.8. doi: 10.1002/9780471729259.mca04as00. [DOI] [PubMed] [Google Scholar]