Abstract
Otitis media (OM), inflammation of the middle ear (ME), is a common cause of conductive hearing impairment. Despite the importance of the disease, the aetiology of chronic and recurrent forms of middle ear inflammatory disease remains poorly understood. Studies of the human population suggest that there is a significant genetic component predisposing to the development of chronic OM, although the underlying genes are largely unknown. Using N-ethyl-N-nitrosourea mutagenesis we identified a recessive mouse mutant, edison, that spontaneously develops a conductive hearing loss due to chronic OM. The causal mutation was identified as a missense change, L972P, in the Nischarin (NISCH) gene. edison mice develop a serous or granulocytic effusion, increasingly macrophage and neutrophil rich with age, along with a thickened, inflamed mucoperiosteum. We also identified a second hypomorphic allele, V33A, with only modest increases in auditory thresholds and reduced incidence of OM. NISCH interacts with several proteins, including ITGA5 that is thought to have a role in modulating VEGF-induced angiogenesis and vascularization. We identified a significant genetic interaction between Nisch and Itga5; mice heterozygous for Itga5-null and homozygous for edison mutations display a significantly increased penetrance and severity of chronic OM. In order to understand the pathological mechanisms underlying the OM phenotype, we studied interacting partners to NISCH along with downstream signalling molecules in the middle ear epithelia of edison mouse. Our analysis implicates PAK1 and RAC1, and downstream signalling in LIMK1 and NF-κB pathways in the development of chronic OM.
Author summary
Otitis media (OM) is the most common cause of deafness in children and is primarily characterised by inflammation of the middle ear. It is the most common cause of surgery in children in the developed world, with many children developing recurrent and chronic forms of OM undergoing tympanostomy tube insertion. There is evidence that a significant genetic component contributes towards the development of recurrent and chronic forms of OM. The mouse has been a powerful tool for identifying the genes involved in chronic OM. In this study we identified and characterised edison, a novel mouse model of chronic OM that shares important features with the chronic disease in humans. A mutation in the Nisch gene causes edison mice to spontaneously develop OM following birth and subsequently develop chronic OM, with an associated hearing loss. Our molecular analysis of the mutation reveals the underlying pathological mechanisms and pathways involved in OM in the edison mouse, involving PAK1, RAC1 and downstream signalling in LIMK1 and NF-κB pathways. Identification of the edison mutant provides an important genetic disease model of chronic OM and implicates a new gene and genetic pathways involved in predisposition to OM.
Introduction
Otitis media (OM) is characterised by inflammation of the middle ear (ME), often associated with a conductive hearing impairment, and is the commonest cause of hearing loss in children. It is perceived by many to be a transient affliction that in reality places a substantial social, medical and economic burden on healthcare systems globally [1]. Evidence from studies of the human population suggests that there is a significant genetic component predisposing to the development of recurrent and chronic forms of OM [2,3]. Despite the importance of the disease, many of the genes involved in OM susceptibility have still yet to be identified. At present, the use of mouse models is the most promising method to identify candidate loci underlying susceptibility to OM. Mouse models have highlighted the role of Toll-like receptors (TLRs) in acute OM, in particular the protection against commensal and pathogenic bacteria, and that persistent NF-κB or TGF-β signalling could be two mechanisms leading to the overactive pro-inflammatory response seen in chronic OM [4,5].
The large-scale phenotype-driven mouse ENU (N-ethyl-N-nitrosourea) mutagenesis program at MRC Harwell [6,7] has previously identified two novel mouse mutants, Jeff and Junbo, that develop a conductive hearing loss characterised by ME fluid and mucosal inflammation [8,9]. The Jeff mouse has a mutation in the Fbxo11 gene [10] and the Junbo mouse has a mutation in Evi1 [9]. Studies have revealed that these genes, are involved in signalling of the TGF-β superfamily, via SMAD proteins [11,12]; negatively regulate NF-κB–dependent inflammation [13]; and highlight the role of HIF–VEGF pathways in the underlying genetic and pathophysiological mechanisms that predispose to chronic OM [14].
OM mouse models with single gene mutations have identified a number of genes as candidate susceptibility genes for human OM, including Tlr2, Tlr4, p73, E2f4, Plg, Tgif1, Evi1 and Fbxo11 [4]. These genes identified from mouse models of OM are beginning to be studied in the human population; with significant associations between OM and polymorphisms in FBXO11 [15,16], TLR2 [17] and TLR4 [17–19].
We have identified and characterised a novel OM mouse mutant, edison, from the ENU mutagenesis program at MRC Harwell. Homozygous edison mice spontaneously develop a conductive hearing loss associated with chronic inflammation of the ME, sharing many features with chronic OM in humans. The underlying mutation of this phenotype has been identified as a mutation in the Nisch gene. We have explored the role of Nisch in chronic OM, relating the edison phenotype to the underlying mechanisms of Nisch function. We have utilised double mutants to assess genetic interactions and pathways involved, implicating PAK1 and RAC1, and downstream signalling events in LIMK1 and NF-κB signalling pathways in the development of chronic OM. Overall, the edison mouse highlights a new candidate gene for susceptibility to chronic OM and has provided further insight into the genetic pathways and pathogenic processes involved.
Results
Identification of the edison mutation
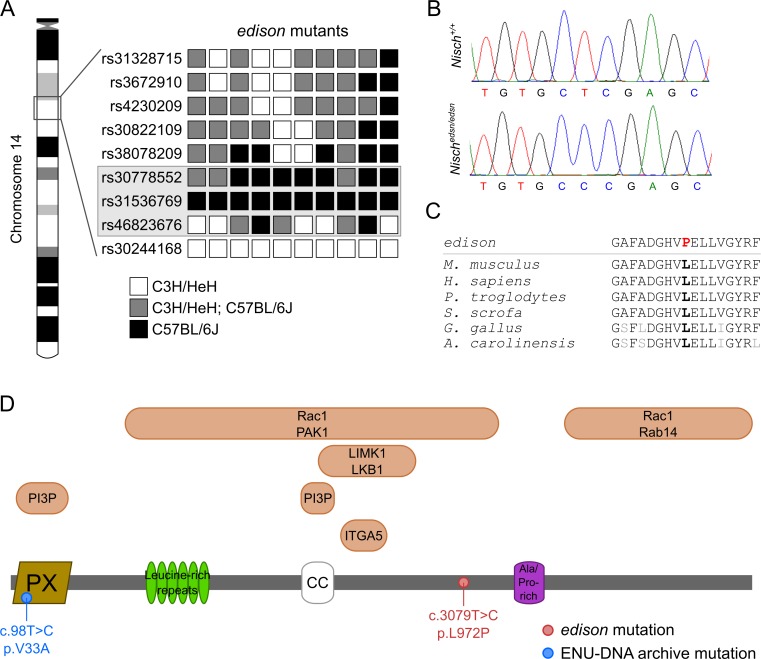
A phenotype-driven ENU mutagenesis screen [20] identified a new recessive mutant, edison (edsn), with hearing loss. Preliminary phenotyping using a click-box test (20 kHz, 90 dB sound pressure level (SPL) tone burst) of an age-matched cohort derived from the founder mouse indicated that 6-week-old (wk) mice demonstrated a reduced startle response. SNP-based linkage analysis and mapping identified an approximately 9 Mb interval on chromosome 14 delineated by marker rs30778552 and rs46823676 containing 119 genes (Fig 1A). Whole-genome sequencing identified 60 ENU-induced, de novo variants within the critical 9Mb interval and importantly only one missense variant was identified. This missense variant was a c.3079T>C substitution in Nischarin (Nisch) (open reading frame of NCBI RefSeq transcript NM_022656.2) that results in a Leu972Pro substitution (Fig 1B). The change occurs in a highly conserved region that has been maintained through evolution (Fig 1C). PROVEAN analysis predicts that this change is ‘‘deleterious” and SIFT predicts that it is ‘‘not tolerated”. No other non-synonymous sequence changes were identified within the minimal interval. The Nisch locus encodes a protein of 1,593 amino acids, coded for by 22 exons. The protein consists of an N-terminal phox homology (PX) domain, six putative leucine-rich repeats (LRRs), a predicted coiled-coil (CC) domain, an alanine/proline-rich region and a long C-terminal region (Fig 1D).
Fig 1. The Nisch gene is mutated in edison mice.
(A) SNP mapping of 10 edsn mutants identified an approximately 9 Mb interval on chromosome 14 delineated by marker rs30778552 and rs46823676. The grayed box indicates the chromosomal interval bearing the edsn mutation. (B) Sequence analysis of the Nisch locus in Nisch+/+ and Nischedsn/edsn DNA. A c.3079T>C transition is detected in Nischedsn/edsn mutants that is not present in Nisch+/+ DNA. (C) Conservation of the mutated leucine residue across species. Mus musculus, ENSMUSG00000021910; Homo sapiens, ENSG00000010322; Pan troglodytes, ENSPTRG00000015001; Sus scrofa, ENSSSCG00000011442; Gallus gallus, ENSGALG00000043825; Anolis carolinensis, ENSACAG00000006939. (D) Schematic of the full length NISCH peptide (1593 amino acids). The molecule consists of a phox-homology (PX) domain, six leucine-rich repeats, a coiled-coil (CC) domain and an alanine/proline-rich region. Both the PX and CC domains of Nischarin are essential for endosomal targeting and interaction with phosphatidylinositol 3-phosphate (PI3P) in PI3P-enriched endosomes. Amino acids 709–807 of Nischarin interact with the integrin α5 (ITGA5) cytoplasmic tail. Both LIMK1 and LKB1 interact with positions 661–869 of Nischarin. Residues 246–1047 of Nischarin interact with PAK1. Rab14 interacts with amino acids 1190–1593. Finally, Rac1 interacts with two regions of Nischarin, amino acids 246–1047 and 1190–1593. The positions of the Nischedsn and NischV33A mutations are also indicated.
DNA and sperm archives derived from ENU mutagenesis programmes [21] were utilised to identify an additional allele at the Nisch locus. We screened ten exons of Nisch employing high resolution melting analysis of ~10,000 mutant mice and identified a c.98T>C substitution resulting in a Val33Ala substitution within a conserved region of the NISCH PX domain. We rederived this second allele, NischV33A, and examined the phenotypes.
Anatomical and histological analysis of edison mutant
Reduced numbers of edison progeny
Heterozygous animals (Nischedsn/+) were intercrossed to generate wild-type control, heterozygous and homozygous (Nischedsn/edsn) populations for the study and the progeny were genotyped. The homozygous mutants were viable, but the frequency of homozygous mutant offspring at weaning age was 15.9% (34 of 214), which was below the expected Mendelian ratio (χ2 test: p = 0.0012). To produce additional homozygous mice for this study, we crossed Nischedsn/+ females with Nischedsn/edsn males. The frequency of surviving Nischedsn/edsn mice at weaning age was 33.0% (69 of 212), again less than the expected Mendelian ratio (χ2 test: p < 0.0001). Around 35% of the Nischedsn/edsn animals from both crosses were missing by weaning age, suggesting embryonic or neonatal lethality. Retarded growth was also observed in edison mice (S1A Fig). We compared weights of Nischedsn/edsn mice to wild-type littermates (S1B Fig) and found male Nischedsn/edsn mice to be 35% smaller (mean weight at 20 wk: Nisch+/+, 43.9 ± 0.72 g, n = 9; Nischedsn/edsn, 27.2 ± 0.82 g, n = 18; Kruskall-Wallis: p < 0.001). Additionally, male Nischedsn/+ mice were found to be 8% smaller than wild-type littermates (mean weight at 20 wk: Nischedsn/+, 38.7 ± 0.57 g, n = 20; Kruskall-Wallis: p < 0.001). Similar observations were seen in female Nischedsn/+ and Nischedsn/edsn mice compared to wild-type littermates (mean weight at 20 wk: Nisch+/+, 42.7 ± 1.02 g, n = 12; Nischedsn/+, 40.2 ± 0.67 g, n = 17; Nischedsn/edsn, 26.6 ± 1.09 g, n = 13; Kruskall-Wallis: Nischedsn/+, p = 0.057; Nischedsn/edsn, p < 0.001).
Mild craniofacial defects in edison mice
We found that all Nischedsn/edsn mice demonstrated a shortened snout compared with wild-type mice, indicating a mild craniofacial abnormality (S1 Fig). To investigate the craniofacial phenotype of mice, we took dorsoventral X-ray images of the skulls and computed cranial measurements (S1C Fig). To determine whether any of the cranial bones showed disproportionate growth, allometric comparisons against skull length were used to normalise the data for differences in body size (S1C and S1D Fig). Evaluation of Nischedsn/edsn and wild-type skulls revealed a significant difference in allometric comparisons between nasal bone/skull length (Kruskal- Wallis, p < 0.001) and frontal bone/skull length (Kruskall-Wallis: p = 0.036). The magnitude of the difference between Nischedsn/edsn and wild-type mice was small (~3%). No other skeletal defects were evident.
To investigate the orientation and morphology of the Eustachian tube (ET) in edison mice, the skulls were stained and the angles between the midline of the skull and the bony part of the left and right ET were measured (S1E and S1F Fig). No statistically significant differences in ET measurements were detected between Nischedsn/edsn and wild-type mice (Kruskall-Wallis: Left, p = 0.848; Right, p = 0.324).
Hearing deficits in Nischedsn/edsn animals due to spontaneous otitis media
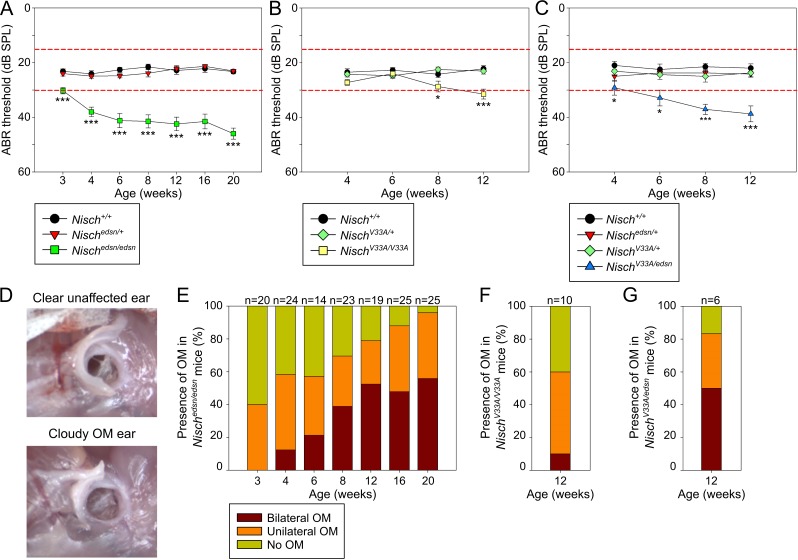
In order to assess the onset and progression of the hearing impairment in edison mice, click-evoked ABR tests to measure auditory function were performed in cohorts of age matched littermates from 3 wk to 20 wk (Fig 2A). Nisch+/+ and Nischedsn/+ mice showed normal ABR thresholds throughout the time course, whereas significantly elevated thresholds were detected in Nischedsn/edsn mice as early as 3 wk and progressively increased with age (mean ABR threshold: Nischedsn/edsn, 3 wk = 30 ± 1 dB SPL, n = 36; 20 wk = 46 ± 2 dB SPL, n = 61; Kruskall-Wallis: p < 0.001). We showed that the mean ABR thresholds were elevated by about 20–30 dB SPL in Nischedsn/edsn animals when compared with wild-type mice, suggesting a conductive hearing loss.
Fig 2. Mild to moderate hearing impairments in Nischedsn/edsn, NischV33A/V33A and Nischedsn/V33A mice due to otitis media.
(A-C) Click-evoked ABR thresholds across a time course show a mild to moderate progressive hearing impairment in (A) Nischedsn/edsn, (B) NischV33A/V33A and (C) NischV33A/edsn mice. Expected ABR threshold range for normal hearing was between 15–30 dB SPL (dashed red lines). * P < 0.05; *** P < 0.001. (A) Nisch+/+, n = 10–33; Nischedsn/+, n = 12–33; Nischedsn/edsn, n = 14–25. (B) Nisch+/+, n = 10; NischV33A/+, n = 13; NischV33A/V33A, n = 10. (C) Nisch+/+, n = 5; Nischedsn/+, n = 6; NischV33A/+, n = 4; NischV33A/edsn, n = 6. Error bars indicate standard error of mean. A Kruskall-Wallis test was performed followed by Dunn’s multiple comparison tests for post-hoc analysis. (D) Visual inspection of the tympanic membrane is a simple method for diagnosing OM. Wild-type (Nisch+/+) mice have no visible fluid behind the tympanic membrane and the malleus is easily recognizable, while affected Nischedsn/edsn mice have fluid behind the tympanic membrane and the malleus is obscured. (E) The incidence of bilateral and unilateral OM increases in prevalence with age in Nischedsn/edsn mice. (F, G) At 12 wk, an increased proportion of (F) NischV33A/V33A and (G) NischV33A/edsn mice display bilateral and unilateral OM.
Visual inspection of the tympanic membrane is a simple method for diagnosing OM. The cloudy appearance of an eardrum is a semi-quantitative measure of bulla fluid accumulation and the incidence of OM was assessed in edison mice (Fig 2D). Nisch+/+ and Nischedsn/+ animals showed a small incidence of unilateral OM (Nisch+/+, 1% unilateral OM, n = 148; Nischedsn/+, 2% unilateral OM, n = 194). In Nischedsn/edsn animals there was a clear trend showing increased prevalence of OM from 3 wk onwards (Fig 2E). At 3 wk, no Nischedsn/edsn mice had bilateral OM, 40% had unilateral OM and 60% had no OM phenotype (Fisher Exact: p = 0.028). The prevalence of OM progressively increased throughout the time course, where at 20 wk 56% of Nischedsn/edsn mice had bilateral OM, 40% had unilateral OM and only 4% displayed no OM phenotype (Fisher Exact: p < 0.001).
In NischV33A/V33A animals, examination of auditory function revealed that mice develop a milder hearing deficit compared to Nischedsn/edsn mice (Fig 2B). Although progressive, the onset of the hearing deficit was observed at 8 wk in NischV33A/V33A animals. Visual inspection of the tympanic membrane was used to assess the incidence of OM in NischV33A/V33A mice (Fig 2F). At 12 wk, NischV33A/V33A mice exhibited significantly increased prevalence of OM compared to wild-type littermates (Fisher Exact: p = 0.011). In NischV33A/V33A mutants, 10% had bilateral OM, 50% had unilateral OM and only 40% displayed no OM phenotype (n = 10). The less significant hearing deficit and limited disease progression suggests that the NischV33A allele is hypomorphic in nature. Compound heterozygotes carrying both Nischedsn and NischV33A alleles showed a similar phenotype to Nischedsn/edsn mice (Fig 2C and 2G). NischV33A/edsn mice develop a spontaneous chronic OM, with an associated elevation in ABR hearing thresholds. The hearing deficit was progressive, with onset recorded at 4 wk in NischV33A/edsn mice. At 12 wk, the incidence of OM in NischV33A/edsn mice was assessed and 50% had bilateral OM, 33% had unilateral OM and only 17% displayed no OM phenotype (n = 6) (Fig 2G). This increased prevalence of OM in NischV33A/edsn mice at 12 wk was significantly different compared to wild-type littermates (Fisher Exact: p = 0.015).
We assessed whether there was a sensorineural element to the hearing loss in Nischedsn/edsn mice by evaluating frequency-specific auditory function and the inner ear morphology (S2 Fig). We tested 5 mice for each genotype from 4 wk to 20 wk for ABR response at 3 frequencies 8, 16, and 32 kHz. Parallel shifts in audiometric profiles across frequencies at the different ages were recorded, consistent with an underlying conductive hearing loss (S2A Fig). We analysed the inner ear morphology from 5 mice for each genotype at 20 wk to examine the structure of the organ of Corti and the sensory cells (S2B–S2D Fig). Histological examination of mid-modiolar sections of the cochlea revealed no obvious abnormalities in Nischedsn/edsn mice. Similarly, ultra-structural analysis of the inner ear with scanning electron microscopy showed no evidence of hair cell damage or hair cell loss, with Nischedsn/edsn mice displaying normal cell and bundle morphology throughout the cochlear turn (basal, mid and apical).
Histological analysis of middle ear demonstrates chronic otitis media
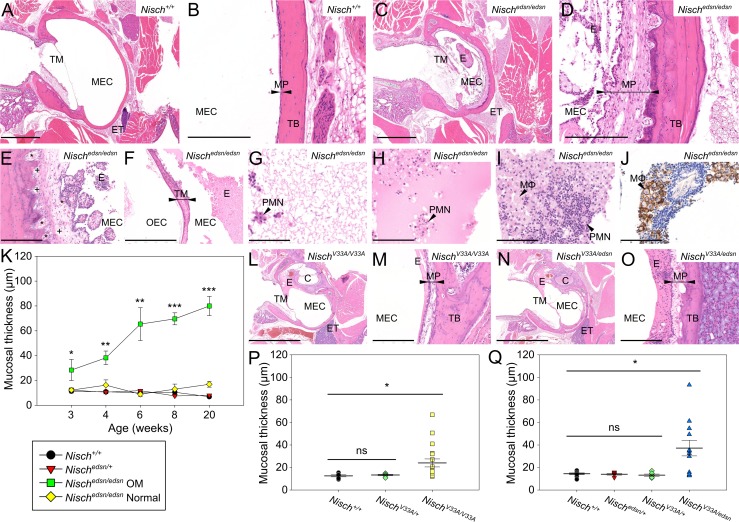
Histological examination was performed to investigate the pathological changes in the ME of edison mice (Fig 3). Wild-type and Nischedsn/+ mice at 20 weeks displayed clear ME cavities, lined with a thin mucoperiosteum (Fig 3A and 3B). Nischedsn/edsn mice develop chronic otitis media with ME cavities filled with a cellular exudate and lined with a thickened mucoperiosteum (Fig 3C and 3D). The mucosal inflammation was diffuse and of moderate severity. Papillary to polypoid exophytic growths were observed projecting into the ME cavity (Fig 3E). The polypoid projections were most likely due to fibroblast stimulation as a consequence of chronic inflammation. There were small accumulations of inflammatory cells and dilated lymphatic and blood vessels in the mucoperiosteum (Fig 3E). In middle ears with the most severe phenotype, there were instances showing inflammation of the tympanic membrane (Fig 3F). There were differing types of ME effusion: a thin watery effusion, a dense granulocyte-rich effusion, or a combination of both (Fig 3G–3I). F4/80 stained sections identified increased macrophage infiltration in the exudates of some Nischedsn/edsn mice, with the presence of enlarged foamy macrophages containing coarsely granular material (Fig 3J).
Fig 3. Middle ear histology indicates chronic otitis media in Nischedsn/edsn mice.
(A-D) H&E stained transverse sections of the MEC and mucoperiosteum, in 20 wk (A, B) Nisch+/+ and (C, D) Nischedsn/edsn mice. Wild-type animals have no inflammation or exudate in the MEC and a thin mucoperiosteum covers the temporal bone. Nischedsn/edsn mice exhibit chronic inflammation with an exudate and thickened mucoperiosteum. (E, F) Additional characteristics of chronic OM in Nischedsn/edsn middle ears; changes include (E) fibrous polyps, with dilated lymphatic (+) and blood vessels (*) in the mucoperiosteum, and (F) fibrous thickening of the tympanic membrane. (G-I) Differing types of middle ear effusion; from (G) a thin watery effusion, (I) a dense granulocyte-rich effusion, or (H) a combination of both. (J) Immunohistochemical staining of a Nischedsn/edsn ear with OM using an F4/80 antibody shows a cellular exudate rich in macrophages (brown). Representative image from four mice (K) Blinded assessment of mean mucosal thickness displays a progressive thickening in Nischedsn/edsn ears with OM, showing significant increases throughout the study. There is no histological difference seen in mean mucosal thickness between Nischedsn/edsn Normal ears and wild-type littermates. The criteria used to impartially categorise the ears into Nischedsn/edsn OM and Nischedsn/edsn Normal was from visualisation of tympanic membrane. Nisch+/+ n = 10–14; Nischedsn/+ n = 10–22; Nischedsn/edsn OM n = 8–16; Nischedsn/edsn Normal n = 3–10. (L, M, N, O) H&E stained transverse sections of the MEC and mucoperiosteum, in 12 wk (L, M) NischV33A/V33A and (N, O) NischV33A/edsn mice, displaying chronic OM in both. (P, Q) Blinded assessment of mean mucosal thickness shows significant increases in both (P) NischV33A/V33A and (Q) NischV33A/edsn mice. Combined data is presented including mice with OM and mice with clear ears. (P) Nisch+/+ n = 6; NischV33A/+ n = 6; NischV33A/V33A n = 18. (Q) Nisch+/+ n = 10; Nischedsn/+ n = 6; NischV33A/+ n = 6; NischV33A/edsn n = 12. C, cochlea; ET, eustachian tube; E, exudate; MΦ, foamy macrophage; MEC, middle ear cavity; MP, mucoperiosteum (arrowheads); PMN, polymorphonuclear cells; TB, temporal bone; TM, tympanic membrane; *, blood vessels; +, lymphatic vessels. A, C, L, N scale bar = 2 mm; F scale bar = 500 μm; B, D, E, M, O scale bar = 200 μm; G-J scale bar = 100 μm. * P < 0.05; ** P < 0.01; *** P < 0.001. Error bars indicate standard error of mean. A Kruskall-Wallis test was performed followed by Dunn’s multiple comparison tests for post-hoc analysis.
To further investigate the ME phenotype in edison, heads were sectioned at 3, 4, 6, 8 and 20 wk. At 3 wk, most Nischedsn/edsn animals displayed a normal wild-type phenotype with clear ears (71%, n = 14 ears), and only three mice had a thin watery effusion (21%, n = 14 ears). At 4 wk, there were five Nischedsn/edsn ears that contained fluid in the ME cavity with a thickened epithelial lining (50%, n = 10 ears). Two ears had a thin watery effusion, and three ears displayed a cellular effusion with the presence of polymorphonuclear cells (PMNs) that are the first responding inflammatory cells that migrate towards the site of inflammation. In 6 wk Nischedsn/edsn animals, four ears showed inflammation in the ME cavity with a thickened epithelial lining (33%, n = 12 ears), with three of these ears also containing a cellular effusion. By 8 wk, the inflammation in Nischedsn/edsn animals had progressed to a chronic inflammation with effusion, as evidenced by a thick cellular fluid in the bulla cavity along with a greatly thickened ME mucosa (67%, n = 12 ears). There was variability in the type of effusion found, where two ears exhibited a serous effusion and in six ears the ME fluid was thick with the presence of macrophages and PMNs. Nischedsn/edsn mice studied at 20 wk displayed a chronic inflammation with effusion, similar to what was observed at 8 wk. Seventeen ears presented a chronic inflammation (77%, n = 22 ears), eleven of which contained fluid. Ten ears exhibited a thick effusion with the presence of macrophages and PMNs and in only one ear a serous exudate was observed. Cellular debris-like aggregates were present in the thick effusions and developing polyps were observed in the ME mucosa. None of the wild-type or Nischedsn/+ mice evaluated displayed signs of OM pathology at any time point.
To quantify the chronic inflammation observed in Nischedsn/edsn mice and assess any subclinical changes in pathology, blinded assessment of the mucoperiosteum thickness was carried out in all edison genotypes from 3 wk to 20 wk (Fig 3K). As there was variation in the penetrance of OM in Nischedsn/edsn mice, the ears were categorised into those that had an OM phenotype and those that did not, Nischedsn/edsn OM and Nischedsn/edsn Normal respectively. The criteria used to impartially categorise the ears into Nischedsn/edsn OM and Nischedsn/edsn Normal was visualisation of tympanic membrane. Nischedsn/edsn Normal ears displayed normal, wild-type ME mucosal morphology of between 9.0 μm to 17.0 μm mucosal thickness, with no significant differences compared to wild-type littermates. In contrast, mucosal thickness observed in Nischedsn/edsn OM ears showed a mucosal morphology that progressively thickened with age. At 3 wk, Nischedsn/edsn OM ears had a mean mucosal thickness of 28.4 ± 8.49 μm and by 20 wk, the mean mucosal thickness had increased to 80.0 ± 7.73 μm. Nischedsn/edsn OM ears showed significantly increased thickening of the ME mucosa at every time point, when compared to Nisch+/+ ears (Kruskall-Wallis: 3 wk, p = 0.013; 4 wk, p = 0.001; 6 wk, p = 0.06; 8 wk, p < 0.001; 20 wk, p < 0.001).
Furthermore, histological analysis confirmed that NischV33A/V33A and NischV33A/edsn ears develop chronic OM with a fluid-filled cavity, lined with thickened mucoperiosteum (Fig 3L–3Q). However, there is a clear phenotypic gradient in the severity of OM seen in NischV33A/V33A, NischV33A/edsn and Nischedsn/edsn mice. At 12 wk, NischV33A/V33A mice had a mean mucosal thickness of 24.0 ± 3.60 μm (Kruskall-Wallis: p = 0.018) and NischV33A/edsn mice had a mean mucosal thickness of 37.2 ± 6.98 μm (Kruskall-Wallis: p = 0.017).
Upregulation of hypoxia and inflammatory genes in middle ear fluid
Venous blood and ear fluid was used to perform RT-qPCR to look for expression changes in inflammatory and hypoxia responsive genes (S3 Fig). Relative to a normoxic baseline control of Nischedsn/edsn white blood cells (WBC), the inflammatory cells that accumulate within the bulla fluids of Nischedsn/edsn mice showed elevated expression of Hif1a (30-fold; t-test: p < 0.001) and the HIF responsive gene Vegfa (87-fold; t-test: p < 0.001). Il1b (15-fold; t-test: p = 0.003) and Tnfa (66-fold; t-test: p < 0.001) are known modulators of Hif1a translation and expression data indicated that they were also elevated in Nischedsn/edsn bulla fluid inflammatory cells relative to WBC (S3 Fig). Additionally expression of Src, which is a known mediator of VEGF signalling, was also significantly elevated in Nischedsn/edsn bulla compared to WBC (24-fold; t-test: p = 0.005). Finally, Evi1 and Fbxo11 were expressed in the bulla fluid of Nischedsn/edsn mice, but only Evi1 (256-fold; t-test: p = 0.006) was expressed at higher levels relative to WBC.
Lung abnormalities
Due to the reduced numbers of Nischedsn/edsn progeny at weaning age, we studied the mice at E16.5, E18.5 and P0 to search for any physiological defect that may contribute to lethality (Fig 4A–4D). In some Nischedsn/edsn mice we observed morphological lung defects including thickened interstitial mesenchyme and smaller airspaces than in wild-type littermates. At E16.5, 33% of Nischedsn/edsn lungs appeared severely affected (n = 6). This was replicated at E18.5 and P0, whereby 30% (n = 10) and 29% (n = 7) of Nischedsn/edsn lungs appeared severely affected respectively. At each time point, the width of airspaces in severely affected Nischedsn/edsn mutants was significantly smaller than wild-type tissue (one-way ANOVA: p < 0.001), whereas no difference in the number of airspaces was observed. The reduced numbers of Nischedsn/edsn progeny are likely a consequence of their lung defect.
Fig 4. Nischedsn/edsn mice display embryonic and adult lung abnormalities.
(A-D) At E16.5, E18.5 and P0, some Nischedsn/edsn embryos display lung abnormalities. H&E stained lung sections from (A) Nisch+/+ and (B) severely affected Nischedsn/edsn mice at P0, show thickened interstitial mesenchyme and smaller airspaces in Nischedsn/edsn lungs. (C) Number of airspaces was counted in three different 6.5 x 105 μm2 regions for all embryos and new born mice, with no significant difference between genotypes. (D) Airspace diameters were measured for 30 airspaces in three different regions for all embryos and new born mice. The mean airspace width in severely affected Nischedsn/edsn animals was significantly smaller. Nisch+/+ n = 6–9; Nischedsn/+ n = 11–15; Nischedsn/edsn n = 6–10; mildly affected Nischedsn/edsn n = 4–7; severely affected Nischedsn/edsn n = 2–3. (E-H) Adult Nischedsn/edsn mice exhibit an emphysema-like phenotype. H&E stained lung sections from (E) Nisch+/+ and (F) Nischedsn/edsn animals at 20 wk, show enlargement of air spaces in Nischedsn/edsn lungs accompanied by disruption of normal alveolar architecture. (G) In Nischedsn/edsn lungs quantification of the number of airspaces indicated a significant decrease compared to wild-type and (H) the mean airspace width in Nischedsn/edsn lungs was significantly larger than in wild-type tissue. Nisch+/+ n = 10; Nischedsn/+ n = 10; Nischedsn/edsn n = 18. (I-M) The severity of the emphysema-like lung phenotype observed in Nischedsn/edsn replicates the severity of the OM phenotype in the middle ear. H&E stained lung sections from Nischedsn/edsn animals at 20 wk show the increasing severity of the emphysema-like phenotype from mice with (I) no OM phenotype, to (J) unilateral OM and to (K) bilateral OM. (L) The mean number of airspaces in Nischedsn/edsn animals with no OM phenotype was significantly increased compared to Nischedsn/edsn animals with bilateral OM. (M) The mean airspace width in Nischedsn/edsn mice with no OM phenotype was significantly smaller than Nischedsn/edsn animals with bilateral OM. Bilateral OM n = 10; Unilateral OM n = 6; Clear n = 2. (N, O) Immunohistochemical staining of lung sections using an F4/80 antibody. Lung sections from (N) Nisch+/+ and (O) Nischedsn/edsn animals at 20 wk stained with F4/80 show collections of enlarged alveolar macrophages (brown) in Nischedsn/edsn lungs compared to wild-type littermates. Representative images from four mice per genotype. N, O scale bar = 200 μm; A, B, E, F, I, J, K scale bar = 100 μm. ns P > 0.05; * P < 0.05; ** P < 0.01; *** P < 0.001. Error bars indicate standard error of mean. Embryonic lung data in panels C and D were analysed by one-way ANOVAs and Holm-Sidak’s multiple comparison procedures for post-hoc testing. Adult lung data (G, H, L, M) was not normally distributed and a Kruskall-Wallis test was performed followed by Dunn’s multiple comparison tests for post-hoc analysis.
Consistent with a number of other adult mouse mutants harbouring defects in embryonic lung generation [22], histological evaluation of adult edison lungs revealed enlargement of the airspaces in Nischedsn/edsn mice, characteristic of an emphysema-like phenotype (Fig 4E–4H). On average, the airspace width in surviving adult Nischedsn/edsn lungs was significantly larger than wild-type tissue (Kruskall-Wallis: p < 0.001). Moreover, in Nischedsn/edsn lungs quantification of the number of airspaces indicated a significant decrease compared to wild-type (Kruskall-Wallis: p < 0.001). Interestingly, the severity of the emphysema-like lung phenotype observed in Nischedsn/edsn replicates the severity of the OM phenotype in the ME (Fig 4I–4M). The mean number of airspaces in Nischedsn/edsn animals with no OM phenotype was significantly higher compared to Nischedsn/edsn animals with bilateral OM (Clear, 200 ± 6.8; Bilateral OM, 104 ± 6.8; Kruskall-Wallis: p < 0.001). Similarly, the average airspace width in Nischedsn/edsn mice with no OM phenotype was significantly smaller than Nischedsn/edsn animals with bilateral OM (Clear, 36.4 ± 1.31 μm; Bilateral OM, 80.4 ± 2.97 μm; Kruskall-Wallis: p < 0.001).
Macrophages are the primary phagocytic cells in the lungs. In wild-type lungs, F4/80-positive cells are generally relatively small and rounded (Fig 4N). In contrast, abnormal accumulations of enlarged alveolar macrophages containing coarsely granular material were observed within the lungs of Nischedsn/edsn mice (Fig 4O).
Genetic interaction of Nisch and Itga5
NISCH binds to the cytoplasmic domain of ITGA5 [23], and is thought to regulate its expression [24]. Cross-talk between VEGF and integrins has been shown to be a critical factor in the regulation of angiogenesis and vascularization [25]. Given these findings we examined the genetic interaction between Nisch and Itga5. Nischedsn/+ mice and Itga5tm1Hyn/+; Nischedsn/+ double heterozygotes were intercrossed to produce Itga5tm1Hyn/+; Nischedsn/edsn littermates for the study, with Itga5tm1Hyn/+; Nisch+/+ and Itga5+/+; Nischedsn/edsn progeny as littermate controls (Fig 5).
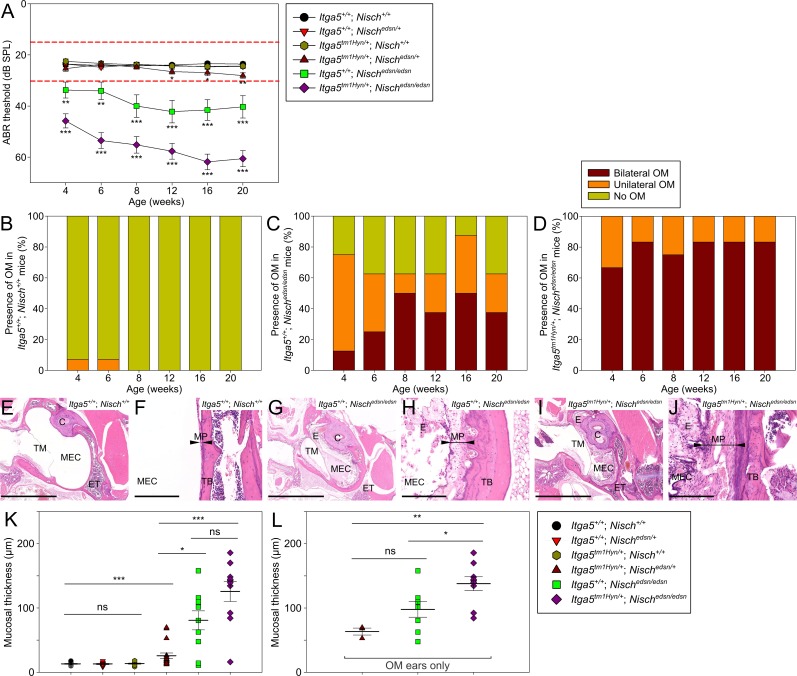
Fig 5. Deficiencies in Nisch and Itga5 exacerbate the otitis media phenotype.
(A) Click-evoked ABR thresholds across a time course show Itga5tm1Hyn/+; Nischedsn/edsn mice exhibit significantly elevated auditory thresholds compared to Itga5+/+; Nischedsn/edsn mice. Additionally, a mild late-onset hearing deficit is observed in Itga5tm1Hyn/+; Nischedsn/+ mice, with onset at 12 wk. Expected ABR threshold range for normal hearing was between 15–30 dB SPL (dashed red lines). Itga5+/+; Nisch+/+ n = 14; Itga5+/+; Nischedsn/+ n = 14; Itga5tm1Hyn/+; Nisch+/+ n = 15; Itga5tm1Hyn/+; Nischedsn/+ n = 13; Itga5+/+; Nischedsn/edsn n = 8; Itga5tm1Hyn/+; Nischedsn/edsn n = 12. (B-D) Visual inspection of the tympanic membrane was used as a semi-quantitative measure for the prevalence of OM. (B) Itga5+/+; Nisch+/+ mice show a very low prevalence of unilateral OM at 4 wk and 6 wk only. (C) Itga5+/+; Nischedsn/edsn mice show a progressive increase in prevalence of OM, whereas (D) Itga5tm1Hyn/+; Nischedsn/edsn mice display a consistently high prevalence of bilateral OM throughout the time course. (E-J) H&E stained transverse sections of the MEC and mucoperiosteum, in 20 wk (E, F) Itga5+/+ Nisch+/+, (G, H) Itga5+/+; Nischedsn/edsn and (I, J) Itga5tm1Hyn/+; Nischedsn/edsn mice. Both Itga5+/+; Nischedsn/edsn and Itga5tm1Hyn/+; Nischedsn/edsn mice demonstrate chronic inflammation with an exudate. Inflammation of mucosa was more severe in sections from Itga5tm1Hyn/+; Nischedsn/edsn ears, with increased polypoid exophytic growths and a thick cellular effusion. (K) Blinded assessment of mean mucosal thickness demonstrates significant increases in Itga5tm1Hyn/+; Nischedsn/+, Itga5+/+; Nischedsn/edsn and Itga5tm1Hyn/+; Nischedsn/edsn mice compared to wild-type. Both Itga5+/+; Nischedsn/edsn and Itga5tm1Hyn/+; Nischedsn/edsn mice exhibit significant increases in mucosal thickness compared to Itga5tm1Hyn/+; Nischedsn/+ mice. Itga5+/+; Nisch+/+ n = 12; Itga5+/+; Nischedsn/+ n = 8; Itga5tm1Hyn/+; Nisch+/+ n = 10; Itga5tm1Hyn/+; Nischedsn/+ n = 18; Itga5+/+; Nischedsn/edsn n = 10; Itga5tm1Hyn/+; Nischedsn/edsn n = 10. (L) To account for the disparities in OM prevalence, the mean mucosal thickness was additionally assessed for OM ears only. Itga5tm1Hyn/+; Nischedsn/edsn OM ears exhibit significant increases in mucosal thickness compared to Itga5+/+; Nischedsn/edsn OM ears. OM only: Itga5tm1Hyn/+; Nischedsn/+ n = 3; Itga5+/+; Nischedsn/edsn n = 8; Itga5tm1Hyn/+; Nischedsn/edsn n = 9. C, cochlea; ET, eustachian tube; E, exudate; MEC, middle ear cavity; MP, mucoperiosteum (arrowheads); TB, temporal bone; TM, tympanic membrane. E, G, I scale bar = 2 mm; F, H, J scale bar = 200 μm. ns P > 0.05; * P < 0.05; ** P < 0.01; *** P < 0.001. Error bars indicate standard error of mean. A Kruskall-Wallis test was performed followed by Dunn’s multiple comparison tests for post-hoc analysis.
Itga5+/+; Nisch+/+, Itga5+/+; Nischedsn/+ and Itga5tm1Hyn/+; Nisch+/+ mice showed normal click ABR thresholds across the study (Fig 5A). Both Itga5+/+; Nischedsn/edsn and Itga5tm1Hyn/+; Nischedsn/edsn mice displayed a progressive hearing loss with onset at 4 wk. Interestingly, Itga5tm1Hyn/+; Nischedsn/edsn mice exhibited significantly elevated auditory thresholds compared to Itga5+/+; Nischedsn/edsn mice, throughout the time course (Kruskall-Wallis: p < 0.01). At 20 wk, Itga5tm1Hyn/+; Nischedsn/edsn animals were recorded with a mean click ABR threshold of 61 ± 3 dB SPL, compared to 40 ± 4 dB SPL for Itga5+/+; Nischedsn/edsn mice. There was a consistently high prevalence of OM in Itga5tm1Hyn/+; Nischedsn/edsn mice compared to Itga5+/+; Nischedsn/edsn animals (Fig 5C and 5D). Itga5tm1Hyn/+; Nischedsn/edsn mice exhibited significantly increased OM prevalence at 4 wk compared to Itga5+/+; Nischedsn/edsn mice (Fisher Exact: p = 0.026). At 4 wk, 67% of Itga5tm1Hyn/+; Nischedsn/edsn mutants had bilateral OM and 33% had unilateral OM (n = 12), whereas in Itga5+/+; Nischedsn/edsn mice at 4 wk, 13% had bilateral OM, 63% unilateral OM and 25% showed no OM phenotype (n = 8). By 20 wk, the difference in OM prevalence observed between Itga5tm1Hyn/+; Nischedsn/edsn and Itga5+/+; Nischedsn/edsn mice was still increased (Fisher Exact: p = 0.051). In Itga5tm1Hyn/+; Nischedsn/edsn mutants at 20 wk, 83% had bilateral OM and 17% had unilateral OM (n = 12). While, in Itga5+/+; Nischedsn/edsn mice at 20 wk, 38% had bilateral OM, 25% unilateral OM and 37% showed no OM phenotype (n = 8). One Itga5tm1Hyn/+; Nisch+/+ mouse (n = 15) displayed unilateral OM at 4 wk, with no other recordings at later time points (S4A Fig).
Histological examination confirmed that Itga5+/+; Nischedsn/edsn and Itga5tm1Hyn/+; Nischedsn/edsn mice develop chronic OM (Fig 5E–5J). Itga5tm1Hyn/+; Nischedsn/edsn mice displayed a more severe mucosal inflammation, with increased polypoid exophytic growths and a thick cellular effusion. Blinded assessment of the mucoperiosteum thickness (Fig 5K) indicated that both Itga5+/+; Nischedsn/edsn and Itga5tm1Hyn/+; Nischedsn/edsn mice had significant mucosal thickening compared to wild-type littermates (Kruskall-Wallis: p = 0.003 and p < 0.001 respectively). Additionally, when only OM ears from Itga5+/+; Nischedsn/edsn and Itga5tm1Hyn/+; Nischedsn/edsn mice were compared (Fig 5L), there was a significant increase in mucosal thickness observed in Itga5tm1Hyn/+; Nischedsn/edsn mice (Itga5+/+; Nischedsn/edsn, 97.8 ± 12.12 μm, n = 8; Itga5tm1Hyn/+; Nischedsn/edsn, 137.9 ± 10.85 μm, n = 9; Kruskall-Wallis: p = 0.026).
Additionally, double heterozygotes (Itga5tm1Hyn/+; Nischedsn/+) exhibited a mild late-onset hearing loss, with onset at 12 wk (Fig 5A). Visualisation of the tympanic membrane showed this mild hearing loss was associated with a late-onset in the prevalence of OM from 12 wk in Itga5tm1Hyn/+; Nischedsn/+ mice (S4B Fig). By 20 wk, Itga5tm1Hyn/+; Nischedsn/+ mice exhibited significantly increased prevalence of OM compared to wild-type littermates (Fisher Exact: p = 0.041). In Itga5tm1Hyn/+; Nischedsn/+ mutants at 20 wk, 31% had unilateral OM and 69% had no OM phenotype (n = 13). Histological examination confirmed that Itga5tm1Hyn/+; Nischedsn/+ mice develop chronic OM (S4D and S4F Fig). The mucosal inflammation was diffuse and of mild severity, with the presence of a cellular effusion. Blinded assessment of the mucoperiosteum thickness indicated that Itga5tm1Hyn/+; Nischedsn/+ mice had significant mucosal thickening compared to wild-type littermates (Fig 5K).
Expression analysis in wild-type, Nischedsn/edsn and Itga5tm1Hyn/+; Nischedsn/edsn mice
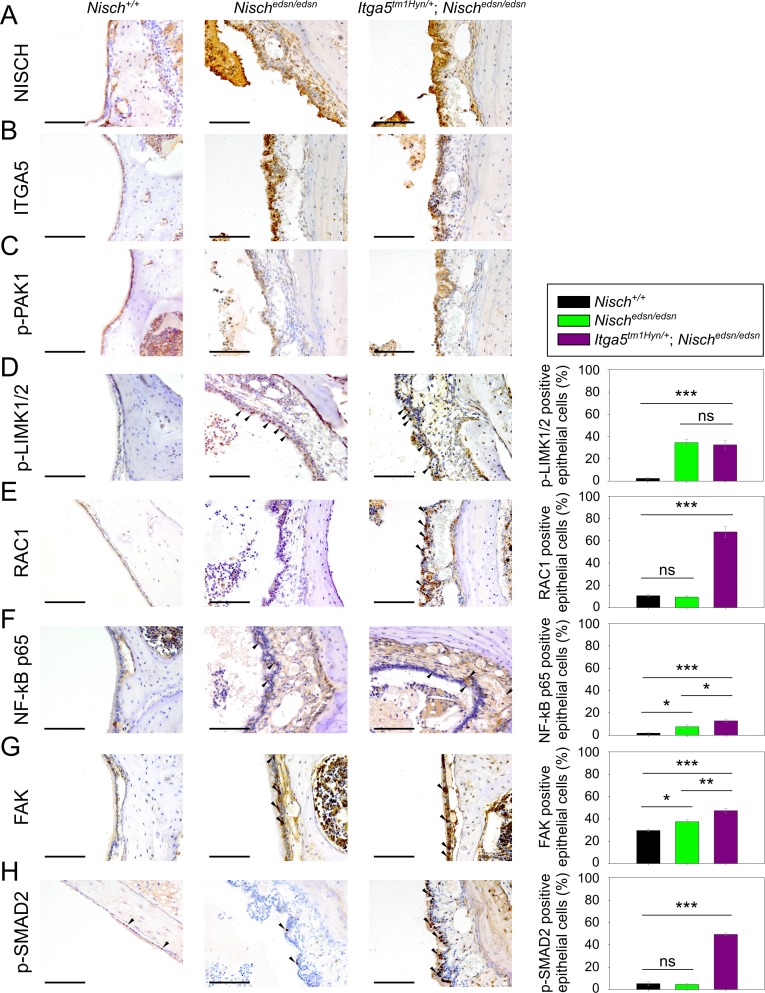
We proceeded to investigate the expression of interacting partners to NISCH, including ITGA5, as well as key downstream effectors in order to relate the underlying mutation to the edison phenotype. In addition to binding ITGA5, NISCH has also been shown to interact directly with PAK1 [23,26], and RAC1 [27]. As well as being a downstream effector of PAK1, LIMK1 [28] also interacts directly with NISCH. In addition PAK controls NF-κB activation [29] and RAC1 leads to activation of NF-κB [30,31]. We thus performed IHC expression analysis of NISCH, ITGA5, phosphorylated-PAK1 (p-PAK1), phosphorylated-LIMK1/2 (p-LIMK1/2), RAC1 and NF-κB p65 on ME epithelia from wild-type, Nischedsn/edsn and Itga5tm1Hyn/+; Nischedsn/edsn mice. In addition, we assessed protein expression in ME epithelia by western blot analysis for each of these proteins.
IHC labelling for NISCH, ITGA5 and p-PAK1 was observed in ME epithelial cells with similar patterns of expression in Nisch+/+, Nischedsn/edsn and Itga5tm1Hyn/+; Nischedsn/edsn mice. No obvious differences in localisation were observed for these three proteins, although staining was stronger in Nischedsn/edsn and Itga5tm1Hyn/+; Nischedsn/edsn mice (Fig 6A–6C). Western analysis of ME epithelial cell lysates showed raised levels of ITGA5 in Nischedsn/edsn mice but not significantly different compared to wild-type (t-test: p = 0.071) and not surprisingly a significant decline in levels between Nischedsn/edsn and Itga5tm1Hyn/+; Nischedsn/edsn mice (t-test: p = 0.020) (Fig 7A). PAK1 protein levels were significantly raised in both Nischedsn/edsn (t-test: p = 0.002), and Itga5tm1Hyn/+; Nischedsn/edsn mice (t-test: p = 0.006) when compared to wild-type, but not between Nischedsn/edsn and Itga5tm1Hyn/+; Nischedsn/edsn mice (t-test: p = 0.282) (Fig 7B).
Fig 6. Protein expression analysis of NISCH interacting partners and downstream pathways in middle ear by immunohistochemistry.
Middle ear sections of Nisch+/+, Nischedsn/edsn and Itga5tm1Hyn/+; Nischedsn/edsn mice at 3 wk, stained with (A) NISCH, (B) ITGA5, (C) p-PAK1, (D) p-LIMK1/2, (E) RAC1, (F) NF-κB p65, (G) FAK and (H) p-SMAD2 antibodies. To quantify the results middle ear epithelial cells in wild-types and mutants were counted in four middle ears from each genotype. Scale bar = 100 μm. ns P > 0.05; * P < 0.05; ** P < 0.01; *** P < 0.001. Error bars indicate standard error of mean. The data was analysed by one-way ANOVAs and Holm-Sidak’s multiple comparison procedures for post-hoc testing.
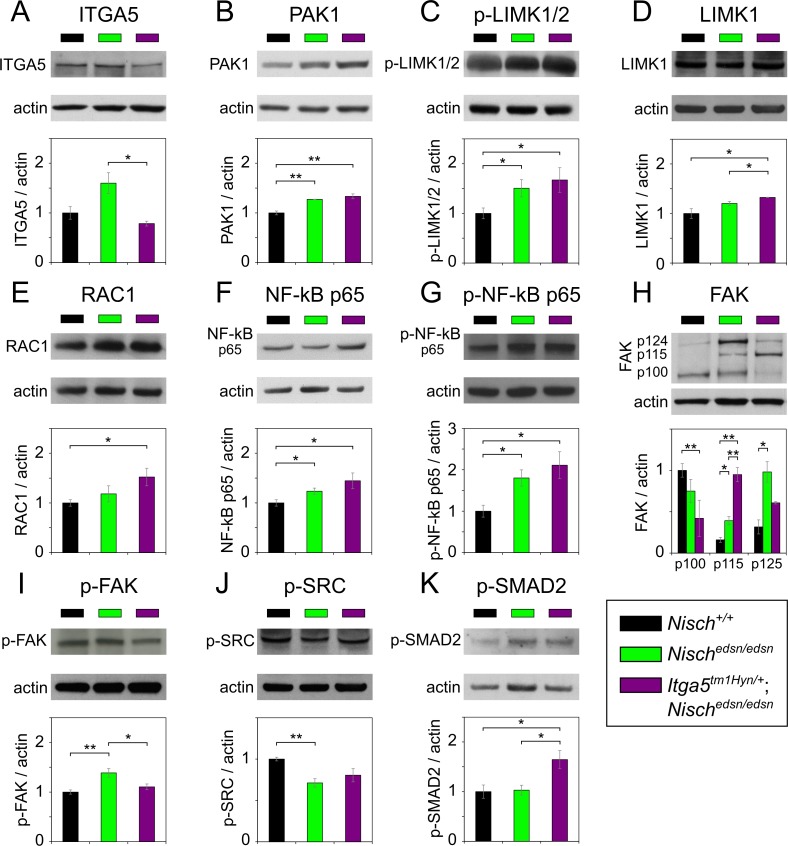
Fig 7. Protein expression analysis of NISCH interacting partners and downstream pathways in middle ear by western blot.
Middle ear epithelial cells extracts of Nisch+/+, Nischedsn/edsn and Itga5tm1Hyn/+; Nischedsn/edsn mice at 8 wk, probed with (A) ITGA5, (B) PAK1, (C) p-LIMK1/2, (D) LIMK1, (E) RAC1, (F) NF-κB p65, (G) p-NFκB p65, (H) FAK, (I) p-FAK, (J) p-SRC and (K) p-SMAD2 antibodies. The results presented are from four independent experiments for all the antibodies except for ITGA5, PAK1, LIMK1, p-NFκB p65 and FAK, where three independent experiments were used to present the data. ns P > 0.05; * P < 0.05; ** P < 0.01; *** P < 0.001. Error bars indicate standard error of mean. A Student’s t-test was performed to analyse the data.
Available antibodies for p-LIMK1 were ineffective. However, we carried out expression analyses using a p-LIMK1/2 and LIMK1 antibody. IHC analysis of p-LIMK1/2 expression in ME epithelial cells revealed nuclear localisation and increased expression from both Nischedsn/edsn and Itga5tm1Hyn/+; Nischedsn/edsn mice (one-way ANOVA: p < 0.001) compared to wild-type. However, no significant difference was observed between ME epithelial cells in Nischedsn/edsn and Itga5tm1Hyn/+; Nischedsn/edsn mice (one-way ANOVA: p = 0.704) (Fig 6D). Similarly, protein levels of p-LIMK1/2 in ME epithelial cells were significantly raised in Nischedsn/edsn (t-test: p = 0.039) and Itga5tm1Hyn/+; Nischedsn/edsn (t-test: p = 0.038) compared to wild-type. No difference was detected between Nischedsn/edsn and Itga5tm1Hyn/+; Nischedsn/edsn samples (t-test: p = 0.598), consistent with the observations from immunohistochemistry (Fig 7C). In addition, levels of LIMK1 in ME epithelial cells were also significantly raised in Itga5tm1Hyn/+; Nischedsn/edsn compared to wild-type (t-test: p = 0.030) and Nischedsn/edsn (t-test: p = 0.041) mice (Fig 7D).
IHC analysis of RAC1 demonstrated nuclear localisation and increased expression from Itga5tm1Hyn/+; Nischedsn/edsn mice compared to wild-type (one-way ANOVA: p < 0.001) (Fig 6E). Moreover, in agreement with these observations, protein levels of RAC1 were significantly raised in Itga5tm1Hyn/+; Nischedsn/edsn mice (t-test: p = 0.033), compared to wild-type (Fig 7E).
IHC analysis of NF-κB p65 expression showed nuclear localisation and raised levels of protein in both Nischedsn/edsn and Itga5tm1Hyn/+; Nischedsn/edsn mice compared to wild-type (one-way ANOVA: p = 0.017 and p <0.001 respectively). Moreover, there was a significant enhancement of NF-κB labelling in the double mutant compared to Nischedsn/edsn (one-way ANOVA: p = 0.026) (Fig 6F). In agreement with IHC, we found significantly higher levels of NF-κB p65 by western blot in both mutants compared to wild-type (t-test: p = 0.039 and p = 0.041) (Fig 7F). We also examined levels of activated NF-κB p65 by western blot employing an antibody that recognises phosphorylated-NF-κB [Ser 276] p65 (p-NF-κB p65) and detected significantly higher levels of the activated protein in both mutants compared to wild-type (t-test: p = 0.030 and p = 0.035) (Fig 7G).
In addition, we investigated the expression of two pathways that are implicated in the development of chronic OM (see Introduction), or are regulated by NISCH interacting partners. First, we evaluated levels of focal adhesion kinase (FAK) and SRC. Cross-talk between integrins and VEGF is a critical factor in the regulation of angiogenesis, vascularisation and vascular permeability [25]. Integrins regulate VE-cadherin via the activation of SRC, and FAK catalytic activity is required when α5β1 integrin stimulates SRC activation through FAK phosphorylation. FAK inhibition prevents VEGF-stimulated vascular permeability underlining the importance of FAK activity in the regulation of adherens junctions [32]. Tyr 397 in human FAK becomes phosphorylated upon integrin engagement and creates a binding site for SRC. This results in release of the inactive conformation of SRC (Tyr 527) and leads to autophosphorylation of SRC on Tyr 416. Activated SRC further phosphorylates FAK on additional residues, one of which is Tyr 576. The activated FAK-SRC complex then initiates multiple downstream signalling pathways [33,34]. IHC of ME epithelial cells showed there was increased expression of total FAK in both Nischedsn/edsn and Itga5tm1Hyn/+; Nischedsn/edsn compared to wild-type mice (one-way ANOVA: p = 0.015 and p < 0.001 respectively). A significant difference was also observed between Nischedsn/edsn and Itga5tm1Hyn/+; Nischedsn/edsn ears (one-way ANOVA: p = 0.005) (Fig 6G).
To study protein levels of FAK we used an antibody raised against the last 50 amino acids at the C-terminal of the human protein. There are nine known mouse isoforms of FAK (http://www.uniprot.org/uniprot/P34152) produced by alternative promoter usage and alternative splicing and the antibody is potentially able to detect six of them. We detected three main bands in ME epithelial cell lysates at 124, 115 and 100kDa. The full-length canonical isoform is 124kDa. We detected significantly increased levels of the full length 124kDa FAK1 protein in Nischedsn/edsn mutants compared to wild-type (t-test: p = 0.014). The level of the 115kDa form was significantly raised in Nischedsn/edsn (t-test: p = 0.014) and Itga5tm1Hyn/+; Nischedsn/edsn (t-test: p = 0.001) mice compared to wild-type, while the 100kDa form was the main isoform detected in wild-type samples. A significant difference was detected in the levels of the 100kDa between wild-type and Itga5tm1Hyn/+; Nischedsn/edsn (t-test: p = 0.0005) (Fig 7H). Furthermore we used phosphorylated-FAK (p-FAK) [Y576] and phosphorylated-SRC (p-SRC) [Y527] antibodies to test the activity of the FAK-SRC complex in middle ear epithelia. Using a p-FAK [Y576] antibody, which recognises activated FAK, we detected an increase in the activated FAK in the Nischedsn/edsn tissue compared to the wild-type ME epithelial cell lysate (t-test: p = 0.007) (Fig 7I). Not surprisingly, in the double mutant Itga5tm1Hyn/+; Nischedsn/edsn, levels of activated FAK were not significantly different to wild-type. Using a p-SRC [Y527] antibody, which recognises inactive SRC, we detected complementary results to that seen with activated FAK. We found reduced levels of protein in Nischedsn/edsn ME lysates compared to the wild types (t-test: p = 0.002) but no differences between wild-type and Itga5tm1Hyn/+; Nischedsn/edsn (Fig 7J).
Finally, we proceeded to evaluate activation of the TGF-β pathway during chronic ME disease by assessment of phosphorylated-SMAD2 (p-SMAD2). IHC analysis of p-SMAD2 revealed significantly raised levels in Itga5tm1Hyn/+; Nischedsn/edsn mice compared to wild-type (one-way ANOVA: p < 0.001). However, there was no significant difference between Nischedsn/edsn and wild-type mice (one-way ANOVA: p = 0.680) (Fig 6H). In addition, protein levels of p-SMAD2 in ME epithelial cells were significantly higher in Itga5tm1Hyn/+; Nischedsn/edsn mice compared to wild-type (t-test: p = 0.029) or Nischedsn/edsn (t-test: p = 0.024) mice. Again no difference was detected between wild-type and Nischedsn/edsn mice (t-test: p = 0.864) (Fig 7K).
IHC analysis of this suite of proteins in airway epithelia revealed many similarities to that seen in ME epithelia (S5 Fig). Levels of NISCH appeared to be raised in both Nischedsn/edsn and Itga5tm1Hyn/+; Nischedsn/edsn mice compared to wild-type. Furthermore, increased epithelia expression of p-LIMK1/2 in both Nischedsn/edsn and Itga5tm1Hyn/+; Nischedsn/edsn mice mirrored the findings in ME epithelia. Similarly, RAC1 epithelial levels were significantly raised in Itga5tm1Hyn/+; Nischedsn/edsn mice, while NF-κB and FAK expression was raised in both Nischedsn/edsn and Itga5tm1Hyn/+; Nischedsn/edsn mice. However, we observed no significant changes in p-SMAD2 levels in mutant airways. In contrast, in lung tissue we observed very few significant changes in protein levels by western blot analysis (S5 Fig). These results may reflect the complexity of tissues and cell types isolated from dissected material and the visible mesenchymal expression for many of these proteins in airway tissue.
Discussion
In a large-scale ENU mutagenesis screen we recovered a new recessive mouse model of chronic OM, edison. The edison mutant carries a Leu972Pro change in the Nischarin gene. Nischedsn/edsn homozygotes display a progressive middle ear disease with 56% of mice displaying bilateral OM by 20 weeks and elevated ABR thresholds of 20–30 dB SPL indicative of a conductive hearing loss. We derived an additional ENU allele (NischV33A) in the Nischarin gene which also presents with progressive chronic OM, but where ABR thresholds were only very moderately increased and at 12 weeks only 10% of the mice had bilateral OM. It appears that the NischV33A allele is severely hypomorphic. Compound heterozygotes of the edison and NischV33A alleles showed an intermediate non-complementing OM phenotype. We did not identify any sensorineural element to the hearing loss in the Nischedsn/edsn mutant.
The chronic OM in Nischedsn/edsn mice is exemplified by exudate within the ME cavity and a thickened mucoperiosteum and polypoid exophytic growths, sometimes associated with an inflamed tympanic membrane. Serous or granulocyte-rich effusions were observed, but as the mice aged a thick effusion was predominately observed rich in macrophages and PMNs. Examination of ME exudates for upregulation of both inflammatory genes and hypoxia genes found that both Il-1b and Tnfa were both upregulated, as were Hif1a and the HIF responsive gene Vegfa. This is similar to the findings reported for other mouse models of chronic OM, such as the Jeff [8], Junbo [9] and Tgif1 [35] mutants. Both the middle ear and the lungs have substantial similarities in structure and function [36] and, intriguingly, we identified a lung defect in Nischedsn/edsn mice. During embryonic development, we found a significant reduction in airspace width, while in the adults, we observed an emphysema-like phenotype with enlarged airspace width and a reduced number of airspaces. In utero, the network of airways is generated first followed by formation of the gas-exchanging units (alveoli) that develop from the distal ends of the small airways. As a consequence disruption to lung development often results in narrower airspaces in embryonic lungs but enlarged airspaces post-natally because of insufficient generation of alveoli. These lung abnormalities likely account for the deficit of Nischedsn/edsn mice recovered from the various crosses that we report.
The discovery of the involvement of Nischarin in the development of inflammatory middle ear disease identifies a novel gene and associated pathways that are involved in OM. This led us to explore the intersection with known pathways of OM [11,13,14] and the downstream signalling mechanisms that lead to the OM phenotype. Nischarin is a highly conserved protein across mammalian species, consisting of an N-terminal phox homology (PX) domain, 6 putative leucine-rich repeats, a coiled-coil domain, an alanine/proline-rich region and a long C-terminal region. NISCH has a multitude of interacting partners, including ITGA5 [37], PAK1 [26], Rac1 [27,38], LIMK1 [28], Rab14, PI3P [38], and LKB1 [39]. Association of NISCH with these interacting partners underlines its broad impact on the regulation of cell motility, cell invasion, vesicle maturation, as well as its role as a tumour suppressor [28,37,38,40]. Most notably, the binding of NISCH to ITGA5 [37] is thought to mediate the translocation of ITGA5 from the cell membrane to endosomes [24] thus regulating ITGA5 levels. As we discuss above, integrins have been shown to play a critical role in modulating VEGF-induced angiogenesis and vascularization [25]. These pathways thus intersect with the hypoxia-response pathways mediated by HIF-1a which have been demonstrated to be involved with the development of chronic OM in the Junbo and Jeff models [14]. Hypoxia leads to upregulation of VEGFA and downstream pathway genes resulting in VEGF-induced angiogenesis and vascular leak. VEGFR inhibitors moderated angiogenesis and lymphangiogenesis in the Junbo mouse. For these reasons we sought to explore the role of ITGA5 in the edison mutant, and also to characterise the responses of downstream pathways which may illuminate the mechanisms of OM development in edison.
We found a strong genetic interaction between edison and Itga5 mutants. Itga5tm1Hyn/+; Nischedsn/edsn double mutants compared to Nischedsn/edsn mice showed significantly elevated ABR thresholds as well as a very significant raised frequency of bilateral OM in mice from 4 weeks onwards. In summary, the OM was more highly penetrant from an earlier age, and commensurately less progressive. Given the interactions of ITGA5 and NISCH, along with the interactions of NISCH with diverse molecules involved in LIMK1 and NF-κB signalling, we sought to interpret this genetic interaction in the context of known signalling pathways and interactions downstream of NISCH. Moreover, in developing a mechanistic model, we took into account the reports that interaction of ITGA5 with NISCH affects some of the downstream interactions of the NISCH molecule itself.
RAC1 signalling regulates disparate cellular functions mediated through a variety of effector proteins [30,31]. PAK1 is a key downstream effector of RAC1 [41,42], with binding of RAC1 leading to activation of PAK1 [43]. NISCH represses this pathway, and NISCH has been shown to block RAC1 induced cell migration through binding to PAK1; interaction with NISCH strongly inhibits the kinase activity of PAK1 [23]. RAC1 activation of PAK1 enhances the interaction between NISCH and PAK1, while notably expression of ITGA5 also increases the association between NISCH and PAK1 [23]. LIMK1 is a downstream effector of PAK1 [44] and has been shown to be involved in vascular permeability [45]. NISCH is also an interacting partner with LIMK1, regulating cell invasion through repression of the LIMK1-cofilin pathway [28]. NISCH also regulates PAK1-independent RAC1 signalling through direct interaction with RAC1 [27]. RAC1 stimulates the phosphorylation and degradation of IkB and up-regulates NF-κB [46,47]. Overexpression of NISCH has been shown to suppress the ability of RAC1 to stimulate NF-κB activation [27]. The Junbo OM mutant carries a mutation in the Evi1 gene, and it is noteworthy that EVI1 is a negative-feedback regulator of NF-κB [13]. The mutation in Junbo leads to activation of NF-κB and inappropriate regulation of the inflammatory response [13].
We have assessed the expression of the critical genes within these pathways in the ME epithelia of wild-type, Nischedsn/edsn and Itga5tm1Hyn/+; Nischedsn/edsn mice. First, we observed that levels of ITGA5 were raised in Nischedsn/edsn mice though not significantly, reflecting the role of NISCH in regulating ITGA5 levels. Not surprisingly, ITGA5 was significantly reduced compared to edison mice in the double mutant, Itga5tm1Hyn/+; Nischedsn/edsn. We found significantly raised levels of activated p-PAK1 in both mutant lines compared to wild-type, which was mirrored by downstream raised levels of p-LIMK1/2 in both Nischedsn/edsn and Itga5tm1Hyn/+; Nischedsn/edsn mice. Raised levels of p-LIMK1/2 were also reflected in the IHC assessment. LIMK1 levels were also raised in Nischedsn/edsn Itga5tm1Hyn/+ mice. We were unable to assess directly levels of p-LIMK1 due to the ineffectiveness of available antibodies. While RAC1 protein levels were not affected in Nischedsn/edsn mice, they were significantly raised in the double mutant, Itga5tm1Hyn/+; Nischedsn/edsn mice which was mirrored in the IHC assessment. We also found by both IHC and western analysis that NF-κB levels were raised in both Nischedsn/edsn and Itga5tm1Hyn/+; Nischedsn/edsn mice compared to wild-type. Moreover, there is evidence from IHC that the effect of the two mutations are additive and that the double mutant shows a significantly higher level of NF-κB expression compared to Nischedsn/edsn mice. While the protein analysis shows a similar trend the differences between Nischedsn/edsn and Itga5tm1Hyn/+; Nischedsn/edsn mice are not significant.
Overall, our analysis indicates that the edison mutation leads to activation of PAK1 and PAK1-independent RAC1 pathways with increased levels of NF-κB and p-LIMK1/2 each of which may lead to inflammatory and vascular permeability effects. In addition, a combination of mutations in NISCH and ITGA5 can lead to an exacerbation of raised protein levels which may underlie the more severe phenotype seen in the double mutant, Itga5tm1Hyn/+; Nischedsn/edsn. This may reflect the role of ITGA5 in enhancing binding of NISCH to PAK1, and provides us with a model of the mechanism underlying the Nischedsn/edsn and Itga5tm1Hyn/+; Nischedsn/edsn phenotypes (Fig 8). We surmise that impairing function of NISCH leads to derepression of RAC1 pathways, while reducing levels of ITGA5 in combination with impaired NISCH leads to further derepression and activation of downstream pathways manifested in the more severe phenotype. However, we did not observe significantly raised levels of RAC1 in the single mutant. Nevertheless, we found raised levels of NF-κB that may reflect activation of PAK1 independent pathways.
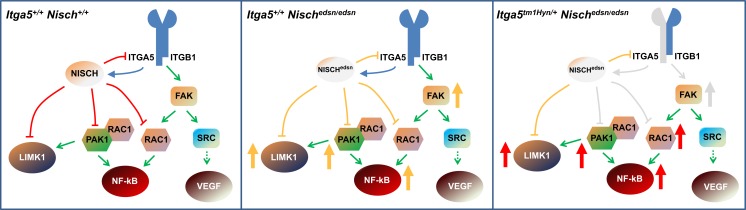
Fig 8. Proposed mechanism.
Model of the mechanism of action in wild-type mice (Itga5+/+; Nisch+/+), during the onset OM in edison mice (Itga5+/+; Nischedsn/edsn) and in double mutants (Itga5tm1Hyn/+; Nischedsn/edsn). Nisch, Nischarin; Nischedsn, Nischarin with the edison mutation; ITGA5, integrin α5; ITGB1, integrin β1; FAK, Focal adhesion kinase; PAK1, p21-activated kinase 1; LIMK1, LIM domain kinase 1; Rac1, Ras-related C3 botulinum toxin substrate 1; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; SRC, Proto-oncogene tyrosine-protein kinase; VEGF, Vascular endothelial growth factor. Red capped lines represent inhibition, yellow capped lines represent reduced inhibition and grey capped lines represent low inhibition. The blue arrow indicates the role of ITGA5 in enhancing binding of NISCH to PAK1. Green arrows indicate direct activation of downstream members of the pathway and dashed green arrows indicate indirect activation. Grey arrows indicate slightly raised levels of proteins, while yellow indicates raised levels and red indicates highly raised levels.
ITGA5 is known to be involved with the activation of SRC protein tyrosine kinase, as well as FAK, both of which are involved with mediating VEGF induced vascular leak [32]. ITGA5 can mediate its effects by phosphorylation of FAK and binding of activated FAK to SRC leading to conformational SRC activation [33]. Alternatively, for example in the context of neuroblastoma cell motility, FAK is required for integrin α5β1-mediated SRC phosphorylation [34]. We thus investigated FAK and SRC levels in the mutant mice, focusing on activated FAK and inactive SRC. We found total levels of the full length isoform of the FAK protein raised in Nischedsn/edsn mice. However, in Itga5tm1Hyn/+; Nischedsn/edsn mice, full length FAK protein was not significantly different to wild-type. Importantly, we detected raised levels of activated FAK along with reduced levels of inactive SRC in Nischedsn/edsn mice. These changes may contribute to angiogenesis and vascular permeability through upregulation of VEGF signalling pathways.
The genes mutated in previously characterised OM models (Junbo, Jeff and Tgif1), have been reported to regulate the TGF-β signalling pathway [11,12,35]. There is considerable cross-talk between TGF-β signalling and hypoxia pathways and mutations that perturb the TGF-β pathway might be expected to perturb hypoxia responses with downstream consequences on VEGFA and VEGF signalling, as is observed in the mutants studied [14]. We discuss above the raised levels of HIF-1a and VEGFA found in the edison mutant. We thus assessed TGF-β signalling (in edison), and found that p-SMAD2 levels are significantly raised in Itga5tm1Hyn/+; Nischedsn/edsn mice, but not in Nischedsn/edsn. The lack of raised p-SMAD2 levels in the Nischedsn/edsn mouse suggests that activation of TGF-β signalling is not a primary event underlying the development of chronic OM in the edison mouse. Rather, the upregulation of p-SMAD2 in the double mutant may reflect the very severe inflammatory state of the middle ear leading to activation of TGF-β pathways.
In summary (see Fig 8), we conclude that mutations in Nischarin impact upon PAK-dependent and PAK-independent RAC pathways with downstream signalling effects on LIMK1 and NF-κB. Moreover, the interplay between Nischarin and ITGA5 likely underlies impacts on FAK and SRC signalling which may lead to VEGF mediated vascular leak. The combined effects on LIMK1, NF-κB and FAK signalling can account for the observed inflammatory changes in the edison middle ear, which along with vascular leak and middle ear exudate, produce a chronic OM. Together, the pathways changes we describe provide a mechanism underlying the observed phenotypic changes in edison. Moreover, these studies further enhance our knowledge of the relevant genetic pathways that contribute to middle ear inflammatory disease, as well as a panel of new genes that are candidates for genetic susceptibility to chronic OM in the human population.
Materials and methods
Ethics statement
Mice were bred and maintained by Mary Lyon Centre, MRC Harwell and were housed in specific-pathogen free conditions. All animal experimentation was approved by the Animal Welfare and Ethical Review Body at MRC Harwell (License Numbers: 30/3015 and 30/3280). The humane care and use of mice in this study was under the authority of the appropriate UK Home Office Project License.
Phenotype-driven ENU-mutagenesis screen
The founder mouse carrying the edison mutation was generated in a large-scale phenotype-driven ENU mutagenesis program at MRC Harwell [20]. Briefly, Male C57BL/6J mice were mutagenized and mated to C3H.Pde6b+ females (a C3H stock that does not carry the retinal degeneration allele Pde6brd). G3 offspring were screened for a variety of abnormalities, including deafness and vestibular dysfunction. The edison founder was identified due to lack of a Preyer reflex when presented with a calibrated 20 kHz, 90 dB SPL tone burst via a click-box test.
Mapping
The founder edison mouse was maintained by repeated outcrossing to C3H/HeH and intercrossing to produce homozygous mutant progeny, identified by the lack of a Preyer reflex. For linkage analysis genomic DNA from 13 affected mice were screened with 63 strain specific SNP markers spaced equidistantly across the genome using the Pyrosequencing SNP genotyping system (QIAGEN). Additional SNP markers were used within linked regions to further fine map the causal mutation. Markers and primer sequences are available on request from the authors.
Whole-genome sequencing
Genomic DNA from a single affected edison mouse was sent for next-generation sequencing (High-Throughput Genomics, WTCHG). Identification, analysis and dissemination of sequence variant information identified through whole-genome sequencing were achieved with the tools provided by the MRC Harwell Biocomputing custom sequence analysis pipeline. Sequence reads were mapped to the NCBI37/mm9 assembly of the reference mouse genome. Within the critical interval, the mean read depth was 8.17 and the read coverage was 99.64%. Sequence variants were identified and were subsequently categorised into those which occurred within exons and splice donor/acceptor sites, and were not known or strain-specific variants. This led to the identification of a T to C base substitution within exon 14 of Nisch, which is predicted to cause a Leu972Pro missense change in NISCH protein. This sequence variant was validated using Sanger sequencing (Source BioScience, UK). Affected and unaffected mice were genotyped using the LightScanner SNP genotyping system (Idaho Technology Inc., USA) to confirm the association of genotype to phenotype. In all cases the genotype correlated with the phenotype.
Gene-driven identification of an additional Nisch allele
DNA from the MRC Harwell ENU-DNA sperm archive (http://www.har.mrc.ac.uk/services/archiving-distribution/enu-dna-archive) was screened with the LightScanner platform (Idaho Technology Inc., USA). Briefly, male C57BL/6J mice were treated with ENU and crossed to C3H/HeH females. F1 progeny (C3H/HeH.C57BL/6J) were rederived and male F1 animals had sperm and DNA samples taken for archiving. Ten exons of Nisch were screened in DNA from ~10,000 F1 ENU mutagenised animals and potential mutations confirmed with Sanger sequencing (Source BioScience, UK).
Genetic background
All edison mice used for phenotyping were congenic on a C3H/HeH background. They were backcrossed for at least ten generations. The NischV33A strain was rederived by in vitro fertilisation of C57BL/6J oocytes with F1 sperm from the MRC Harwell ENU-DNA sperm archive and maintained on a mixed C3H/HeH and C57BL/6J genetic background. NischV33A/+ mice were intercrossed for phenotypic analysis and crossed to congenic Nischedsn/+ mice for complementation testing. Cryopreserved Itga5tm1Hyn mutant sperm was imported from the Jackson Laboratory (Stock No. 002274) [48] and the colony was rederived using in vitro fertilisation of C57BL/6J oocytes. Itga5tm1Hyn mice had been backcrossed 4–5 generations onto a C3H/HeH background, before crossing to congenic Nischedsn mice for phenotypic analysis.
Genotyping
Genotyping for edison mice was performed using an allelic discrimination assay with primers 5’-GGC AGC ACA AAG ATG GCG GTA AC-3’ and 5’-AAC TGC CGC AAC CGC AAC A-3’ and labelled probes 5’-[6-FAM]_AGC AGC TCG AGC ACA T-3’ (edsn) and 5’-[TET]_CAG CTC GGG CAC ATG-3’ (Wild-type). The Applied Biosystems 7900HT Fast System (Applied Biosystems, USA) was used for amplification and analysis. To genotype NischV33A mice, PCR amplification was performed with primers 5’-GAC TGA GTA CCT TGC AGC TA-3’ and 5’-CTG TAA CGG TGT TTG ATC GTC-3’ and an unlabelled probe 5’-CCC TTT AGG CTT ATG TCA TCC AGG TTA C_[SpC3]-3’. The LightScanner System (Idaho Technology Inc., USA) was used for subsequent unlabelled probe genotyping analysis. Itga5tm1Hyn mice were genotyped with mutant and control specific primers, as described on the Jackson Laboratory Mice Database (http://jaxmice.jax.org/strain/002274).
Skull morphology and radiography
Analysis of skulls from 20 wk mice was performed using a Faxitron Mx-20 DC-4 specimen X-ray System. ImageJ software was used to measure the skull length, nasal bone length, frontal bone length, parietal bone length and skull width. Allometric comparisons were performed against skull length with at least 12 mice of each genotype.
Auditory brainstem response
Mice were anesthetized and hearing thresholds determined using ABR, as previously described [7]. The ABR threshold was measured for each ear. Click-evoked hearing assessments for edison mice were conducted at 3, 4, 6, 8, 12, 16 and 20 wk with cohorts containing at least 14 mice of each genotype at each time point. Frequency-specific (8, 16, and 32 kHz) analysis of auditory function for edison mice was conducted over a longitudinal time course at 4, 6, 8, 12 and 20 wk with 5 mice of each genotype. At least 10 NischV33A mice were used for click-evoked ABR analysis across a longitudinal time course at 4, 6, 8 and 12 wk. Finally, click-evoked analysis of Itga5tm1Hyn Nischedsn hearing thresholds were measured across a longitudinal time course at 4, 6, 8, 12, 16 and 20 wk with at least 8 mice of each genotype.
Histology
Mouse 3, 4, 6, 8, 12, 16 and 20 wk heads from Nisch+/+, Nischedsn/+ and Nischedsn/edsn mice were fixed for 48 hours in 10% neutral buffered formaldehyde, decalcified in D.F.B decalcifying agent (Kristensen; Pioneer Research Chemicals) for 72 hours and embedded in paraffin following routine procedures. Perinatal heads were processed in the same manner without the decalcification steps. Four-micrometre-thick sections were obtained, de-paraffinized in xylene substitute and rehydrated via a graded ethanol. For morphological observations, sections were stained with haemotoxylin and eosin (H&E). The histological sections were used to investigate the middle ear inflammation of the mice. Evaluation of mean mucosal thickness was by blinded assessment of a standard 1000 μm length of ME mucosa (avoiding the cochlea and the region close to the Eustachian tube), the mucosal thickness was averaged from five measurements.
To study the lung morphology of edison mice, H&E stained sections from adult and perinatal lungs were viewed using a Zeiss Axiostar Plus bright-field microscope and analysed using cellB imaging software (Olympus). The data was analysed as previously described [49].
Scanning electron microscopy
To study the ultra-structure of the organ of Corti we dissected the inner ears from five 20 wk Nisch+/+ and Nischedsn/edsn mice and prepared the samples as previously described [50]. Inner ears were imaged using a JEOL 6010 LV scanning electron microscope under high vacuum conditions.
Blood and bulla fluid collection
Blood and bulla fluids were collected, as previously described [14], for analysis using Real-time quantitative PCR.
Real-time quantitative PCR (RT-qPCR)
Real-time quantitative PCR was performed as previously described [14]. For ear fluid analysis, each sample pool comprised the fluid from both ears of four individual samples. For blood analysis each sample pool comprised four individual samples. Murine TaqMan gene expression assays used for analysis were Hif1a (Mm01283756_m1), Il1b (Mm01336189_m1), Tnfa (Mm00443258_m1), Vegfa (Mm00437304_m1), Src (Mm00436785_m1), Evi1 (Mm00491303_m1) and Fbxo11 (Mm01227499_m1). Ppia (Mm02342429_g1) was used as the endogenous control.
Immunohistochemistry
For immunohistochemical analysis, the avidin–biotin complex (ABC) method was used to look for the localization of NISCH, ITGA5, p-PAK, p-LIMK1/2, RAC1, NF-κB p65, FAK and p-SMAD2 in wild-type and mutant mouse ME and lungs. The sections through the ears of mice were de-parafinized, and endogenous peroxidase activity was quenched with 3% hydrogen peroxide in isopropanol for 30 min. Vectastain Elite ABC kit (Vector Laboratories, PK 6101) was used to perform the immunohistochemistry. The antibodies were as follows: rabbit polyclonal anti-NISCH (sc-98980, Santa Cruz Biotechnology), rabbit polyclonal anti-ITGA5 (sc-10729, Santa Cruz Biotechnology), rabbit polyclonal anti-p-αPAK (Thr212) (sc-101772, Santa Cruz Biotechnology), rabbit polyclonal anti-p-LIMK1/2 (Thr508/505) (sc-28409-R, Santa Cruz Biotechnology), rabbit polyclonal anti-RAC1 (sc-95, Santa Cruz Biotechnology), rabbit polyclonal anti-p-SMAD2 (Ser465/467) (AB3849, Chemicon International), rabbit polyclonal anti-FAK (sc-558, Santa Cruz Biotechnology) rabbit polyclonal anti-NF-κB p65 (ab131485, Abcam). The sections were incubated with the antibodies overnight at the following dilutions: p-PAK, 1:50; NISCH, ITGA5, p-LIMK1/2, NF-κB p65, FAK and p-SMAD2, 1:200; Rac1, 1:400. For F4/80 visualisation, sections were treated with 0.05% trypsin in calcium chloride for 20 min at 37°C, blocked with 10% rabbit serum (X0902, DAKO), incubated with rat anti mouse F4/80 (MCA497GA, Serotec) antibody overnight at 1:100 dilution and the next day after the washes were incubated with biotinylated rabbit anti-rat secondary antibody at 1:400 dilution (E0468, DAKO). The serum and the secondary antibody for all the other antibodies were from the Vectastain Elite ABC kit and they were used according to the manufacturer's instructions. DAB+ chromogen system (DAKO K3468) was used to develop the specific signals. The slides were counterstained with haematoxylin.
Western blot
Total protein extracted from the ME epithelial cells and lungs of two-months-old wild-type, Nischedsn/edsn and Itga5tm1Hyn/+; Nischedsn/edsn mice were used for the western blot analysis. Each middle ear sample consisted of combined epithelial cells scooped out of both ears of one mouse. Each lung sample consisted of whole lung tissue from one mouse. Either three or four biological replicates were performed for each antibody. The tissues were homogenised in CelLytic MT Cell Lysis Reagent (Sigma C3228), protease inhibitors, phosphatase inhibitors and vanadate and centrifuged at 4°C. Protein concentration was determined using the DC Protein Assay kit (Bio-Rad). Samples (30 μg from the lung samples and 10 μg from the middle ear samples) were loaded into 12% NuPAGE Bis-Tris gel, 7% NuPAGE Tris Acetate gel or 3–8% NuPAGE Tris acetate gels (Invitrogen), blotted onto nitrocellulose membrane (Invitrogen) and immunostained. 5% non-fat milk in TBST was used as blocking solution and antibody diluent. The antibodies and the dilutions they were used at for the western blot analysis were as follows: rabbit polyclonal anti-ITGA5 (sc-10729, Santa Cruz Biotechnology) 1:500, rabbit polyclonal anti-PAK1 (2602, Cell Signaling) 1:1000, rabbit polyclonal anti-p-LIMK1/2 (Thr508/505) (sc-28409-R, Santa Cruz Biotechnology) 1:500, goat polyclonal anti LIMK1 (sc-8387, Santa Cruz Biotechnology) 1:500, rabbit polyclonal anti-RAC1 (sc-95, Santa Cruz Biotechnology) 1:500, rabbit polyclonal anti-FAK (sc-558, Santa Cruz Biotechnology) 1:500, rabbit polyclonal anti-NF-κB p65 (ab131485, Abcam) 1:1000, rabbit polyclonal p-NFκB p65 (Ser 276) (sc-101749, Santa Cruz Biotechnology) 1:500, rabbit polyclonal anti-phospho-FAK (Tyr576) (44-652G, Invitrogen), rabbit polyclonal anti-phospho-SRC (Tyr527) (2105, Cell Signaling), rabbit polyclonal anti-phospho-SMAD2 (Ser465 /467) (3101, Cell Signaling) 1:500 and actin (A 2066, Sigma). Goat anti-rabbit IgG (H+L)-HRP conjugate (1706515, Bio-Rad), 1:3000, was used as a secondary antibody for all the primary antibodies except for LIMK1 for which Rabbit anti-goat IgG (H+L) secondary antibody, HRP, 1:5000 (81–1620, Invitrogen) was used. ECL or ECL 2 (GE Healthcare) were used as detection system.
Data analysis
All data are given as unadjusted mean +/- SEM (standard error of the mean) unless stated otherwise. Data were analysed to establish normal distribution. Where data was normally distributed an ANOVA or Student’s t-test were conducted. If data was not normally distributed the non-parametric equivalents of these tests were used (Kruskal-Wallis One Way Analysis of Variance on Ranks or Mann-Whitney Rank Sum Test) to establish if data were significant. The Holm-Sidak method (ANOVA) or Dunn’s method (Ranks) was used for multiple comparisons versus a control group. Results with values of P < 0.05 were considered statistically significant. SigmaPlot 11.0 software was used to perform all statistical analysis.
Supporting information
(A) Characteristic image of a male Nischedsn/edsn mutant and a wild-type littermate. (B) Weight data for male edison mice over a 20 wk longitudinal time course shows Nischedsn/edsn mice are significantly smaller than both wild-type and Nischedsn/+ littermates throughout the time course. In addition, Nischedsn/+ mice are also smaller than those wild-type for the allele. * P < 0.05; ** P < 0.01; *** P < 0.001. Nisch+/+, n = 9; Nischedsn/+, n = 20; Nischedsn/edsn, n = 18 (C) Dorsoventral view of a 20 wk wild-type mouse skull showing the measurements used to analyse skull morphology in this study. SL, skull length; SW, skull width; NB, nasal bone; FB, frontal bone; PB, parietal bone. (D) Allometric comparisons against skull length show abnormal growth in Nischedsn/edsn skulls at the nasal bone and frontal bone. ns P > 0.05; * P < 0.05; *** P < 0.001. Nisch+/+, n = 13; Nischedsn/+, n = 18; Nischedsn/edsn, n = 12. (E) Dissected and stained skulls of Nisch+/+, Nischedsn/+ and Nischedsn/edsn 20 wk mice. Eustachian tube (ET) angle measurements are indicated by dashed lines. R, right ear; L, left ear. (F) Mean angle between the midline of the skull and the bony part of the left and the right ET. ns P > 0.05. Nisch+/+, n = 6; Nischedsn/+, n = 6; Nischedsn/edsn, n = 6. Error bars indicate standard error of mean. Statistics were conducted using one-way ANOVA’s and Holm-Sidak’s multiple comparison tests for post-hoc analysis.
(TIF)
(A) Frequency-specific ABR thresholds (8 kHz, 16 kHz, 32 kHz and click-evoked) of Nischedsn/edsn mice across a longitudinal time course displayed parallel shifts in audiometric profiles across frequencies, consistent with a conductive hearing loss. n = 5. (B) H&E mid-modiolar sections of the cochlea showed comparable structure of inner ears in 20 wk Nisch+/+ and Nischedsn/edsn mice. Nisch+/+ n = 5; Nischedsn/edsn n = 5. Scale bar = 1 mm. OC, organ of Corti; SG, spiral ganglion; SL, spiral ligament; RM, Reissner’s membrane. (C) H&E sections of the organ of Corti from the mid cochlear turn displayed no differences in the morphology between Nisch+/+ and Nischedsn/edsn mice at 20 wk. Nisch+/+ n = 5; Nischedsn/edsn n = 5. Scale bar = 100 μm. OHC, outer hair cell; IHC, inner hair cell. (D) Scanning electron microscopy (SEM) images showed normal hair cell morphology in 20 wk Nisch+/+ and Nischedsn/edsn mice. Nisch+/+ n = 5; Nischedsn/edsn n = 5. Scale bar = 10 μm.
(TIF)
Relative Quantification (RQ) of gene expression using TaqMan RT-qPCR for Nischedsn/edsn mice at 20 wk. Blood, n = 3 pools; Ear fluid, n = 3 pools. ns P > 0.05; ** P < 0.01; *** P < 0.001. Error bars indicate 95% confidence interval. A Student's t-test of the replicate 2(−ΔCt) values was performed to analyse the data.
(TIF)
(A, B) Visual inspection of the tympanic membrane was used as a semi-quantitative measure for the prevalence of OM. (A) Itga5tm1Hyn/+; Nisch+/+ mice show a small incidence of unilateral OM at 4 wk, whereas in (B) Itga5tm1Hyn/+; Nischedsn/+ animals prevalence of OM increases with age compared to littermates with onset at 12 wk. (C-F) H&E stained transverse sections of the MEC and mucoperiosteum, in 20 wk (C, E) Itga5tm1Hyn/+; Nisch+/+ and (D, F) Itga5tm1Hyn/+; Nischedsn/+ animals. Itga5tm1Hyn/+; Nischedsn/+ mice develop OM with a diffuse mucosal inflammation of mild severity, with the presence of a cellular middle ear effusion. C, cochlea; ET, Eustachian tube; E, exudate; MEC, middle ear cavity; MP, mucoperiosteum (arrowheads); TB, temporal bone; TM, tympanic membrane. C, D scale bar = 2 mm; E, F scale bar = 200 μm.
(TIF)
Immunohistochemistry of lung sections of Nisch+/+, Nischedsn/edsn and Itga5tm1Hyn/+; Nischedsn/edsn mice at 3 wk and total lung extracts at 8 wk, with (A) NISCH, (B) ITGA5, (C) PAK1, (D) p-LIMK1/2, (E) RAC1, (F) NF-κB p65, (G) p-SMAD2 and (H) FAK antibodies. To quantify the results from the staining, airway epithelial cells in wild-types and mutants were counted in three different regions from four lungs for each genotype. The results presented from the western blots are from four independent experiments for all the antibodies except for p-LIMK1/2, where three independent experiments were used to present the data. Scale bar = 100 μm. * P < 0.05. Error bars indicate standard error of mean. Immunohistochemistry data was analysed by one-way ANOVAs and Holm-Sidak’s multiple comparison procedures for post-hoc testing. For western blot analysis a Student’s t-test was performed.
(TIF)
Acknowledgments
We thank the staff in the Mary Lyon Centre, specifically Andy Hinton, Lisa Ireson and Lucie Vizor for the husbandry and humane care of these mice; the pathology and histology teams; the genotyping core; and imaging team at MRC Harwell. Additionally, we thank the High-Throughput Genomics Group at the Wellcome Trust Centre for Human Genetics for the generation of the sequencing data.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by an award from the Medical Research Council (http://www.mrc.ac.uk) to SDMB (MC_U142684175). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Monasta L, Ronfani L, Marchetti F, Montico M, Vecchi Brumatti L, et al. (2012) Burden of disease caused by otitis media: systematic review and global estimates. PLoS One 7: e36226 doi: 10.1371/journal.pone.0036226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casselbrant ML, Mandel EM, Fall PA, Rockette HE, Kurs-Lasky M, et al. (1999) The heritability of otitis media: a twin and triplet study. JAMA 282: 2125–2130. [DOI] [PubMed] [Google Scholar]
- 3.Rovers M, Haggard M, Gannon M, Koeppen-Schomerus G, Plomin R (2002) Heritability of symptom domains in otitis media: a longitudinal study of 1,373 twin pairs. Am J Epidemiol 155: 958–964. [DOI] [PubMed] [Google Scholar]
- 4.Rye MS, Bhutta MF, Cheeseman MT, Burgner D, Blackwell JM, et al. (2011) Unraveling the genetics of otitis media: from mouse to human and back again. Mamm Genome 22: 66–82. doi: 10.1007/s00335-010-9295-1 [DOI] [PubMed] [Google Scholar]
- 5.Tyrer HE, Crompton M, Bhutta MF (2013) What have we learned from murine models of otitis media? Curr Allergy Asthma Rep 13: 501–511. doi: 10.1007/s11882-013-0360-1 [DOI] [PubMed] [Google Scholar]
- 6.Nolan PM, Peters J, Strivens M, Rogers D, Hagan J, et al. (2000) A systematic, genome-wide, phenotype-driven mutagenesis programme for gene function studies in the mouse. Nat Genet 25: 440–443. doi: 10.1038/78140 [DOI] [PubMed] [Google Scholar]
- 7.Hardisty-Hughes RE, Parker A, Brown SD (2010) A hearing and vestibular phenotyping pipeline to identify mouse mutants with hearing impairment. Nat Protoc 5: 177–190. doi: 10.1038/nprot.2009.204 [DOI] [PubMed] [Google Scholar]
- 8.Hardisty RE, Erven A, Logan K, Morse S, Guionaud S, et al. (2003) The deaf mouse mutant Jeff (Jf) is a single gene model of otitis media. J Assoc Res Otolaryngol 4: 130–138. doi: 10.1007/s10162-002-3015-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parkinson N, Hardisty-Hughes RE, Tateossian H, Tsai HT, Brooker D, et al. (2006) Mutation at the Evi1 locus in Junbo mice causes susceptibility to otitis media. PLoS Genet 2: e149 doi: 10.1371/journal.pgen.0020149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hardisty-Hughes RE, Tateossian H, Morse SA, Romero MR, Middleton A, et al. (2006) A mutation in the F-box gene, Fbxo11, causes otitis media in the Jeff mouse. Hum Mol Genet 15: 3273–3279. doi: 10.1093/hmg/ddl403 [DOI] [PubMed] [Google Scholar]
- 11.Tateossian H, Hardisty-Hughes RE, Morse S, Romero MR, Hilton H, et al. (2009) Regulation of TGF-beta signalling by Fbxo11, the gene mutated in the Jeff otitis media mouse mutant. Pathogenetics 2: 5 doi: 10.1186/1755-8417-2-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurokawa M, Mitani K, Irie K, Matsuyama T, Takahashi T, et al. (1998) The oncoprotein Evi-1 represses TGF-beta signalling by inhibiting Smad3. Nature 394: 92–96. doi: 10.1038/27945 [DOI] [PubMed] [Google Scholar]
- 13.Xu X, Woo CH, Steere RR, Lee BC, Huang Y, et al. (2012) EVI1 acts as an inducible negative-feedback regulator of NF-kappaB by inhibiting p65 acetylation. J Immunol 188: 6371–6380. doi: 10.4049/jimmunol.1103527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheeseman MT, Tyrer HE, Williams D, Hough TA, Pathak P, et al. (2011) HIF-VEGF pathways are critical for chronic otitis media in Junbo and Jeff mouse mutants. PLoS Genet 7: e1002336 doi: 10.1371/journal.pgen.1002336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Segade F, Daly KA, Allred D, Hicks PJ, Cox M, et al. (2006) Association of the FBXO11 gene with chronic otitis media with effusion and recurrent otitis media: the Minnesota COME/ROM Family Study. Arch Otolaryngol Head Neck Surg 132: 729–733. doi: 10.1001/archotol.132.7.729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rye MS, Wiertsema SP, Scaman ES, Oommen J, Sun W, et al. (2011) FBXO11, a regulator of the TGFbeta pathway, is associated with severe otitis media in Western Australian children. Genes Immun 12: 352–359. doi: 10.1038/gene.2011.2 [DOI] [PubMed] [Google Scholar]
- 17.Jotic A, Jesic S, Zivkovic M, Tomanovic N, Kuveljic J, et al. (2015) Polymorphisms in Toll-like receptors 2 and 4 genes and their expression in chronic suppurative otitis media. Auris Nasus Larynx 42: 431–437. doi: 10.1016/j.anl.2015.04.010 [DOI] [PubMed] [Google Scholar]
- 18.Emonts M, Veenhoven RH, Wiertsema SP, Houwing-Duistermaat JJ, Walraven V, et al. (2007) Genetic polymorphisms in immunoresponse genes TNFA, IL6, IL10, and TLR4 are associated with recurrent acute otitis media. Pediatrics 120: 814–823. doi: 10.1542/peds.2007-0524 [DOI] [PubMed] [Google Scholar]
- 19.Sale MM, Chen WM, Weeks DE, Mychaleckyj JC, Hou X, et al. (2011) Evaluation of 15 functional candidate genes for association with chronic otitis media with effusion and/or recurrent otitis media (COME/ROM). PLoS One 6: e22297 doi: 10.1371/journal.pone.0022297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thaung C, West K, Clark BJ, McKie L, Morgan JE, et al. (2002) Novel ENU-induced eye mutations in the mouse: models for human eye disease. Hum Mol Genet 11: 755–767. [DOI] [PubMed] [Google Scholar]
- 21.Quwailid MM, Hugill A, Dear N, Vizor L, Wells S, et al. (2004) A gene-driven ENU-based approach to generating an allelic series in any gene. Mamm Genome 15: 585–591. doi: 10.1007/s00335-004-2379-z [DOI] [PubMed] [Google Scholar]
- 22.Poobalasingam T, Yates LL, Walker SA, Pereira M, Gross NY, et al. (2017) Heterozygous Vangl2Looptail mice reveal novel roles for the planar cell polarity pathway in adult lung homeostasis and repair. Dis Model Mech. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alahari SK (2003) Nischarin inhibits Rac induced migration and invasion of epithelial cells by affecting signaling cascades involving PAK. Exp Cell Res 288: 415–424. [DOI] [PubMed] [Google Scholar]
- 24.Lim KP, Hong W (2004) Human Nischarin/imidazoline receptor antisera-selected protein is targeted to the endosomes by a combined action of a PX domain and a coiled-coil region. J Biol Chem 279: 54770–54782. doi: 10.1074/jbc.M411315200 [DOI] [PubMed] [Google Scholar]
- 25.De S, Razorenova O, McCabe NP, O'Toole T, Qin J, et al. (2005) VEGF-integrin interplay controls tumor growth and vascularization. Proc Natl Acad Sci U S A 102: 7589–7594. doi: 10.1073/pnas.0502935102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alahari SK, Reddig PJ, Juliano RL (2004) The integrin-binding protein Nischarin regulates cell migration by inhibiting PAK. EMBO J 23: 2777–2788. doi: 10.1038/sj.emboj.7600291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reddig PJ, Xu D, Juliano RL (2005) Regulation of p21-activated kinase-independent Rac1 signal transduction by nischarin. J Biol Chem 280: 30994–31002. doi: 10.1074/jbc.M502546200 [DOI] [PubMed] [Google Scholar]
- 28.Ding Y, Milosavljevic T, Alahari SK (2008) Nischarin inhibits LIM kinase to regulate cofilin phosphorylation and cell invasion. Mol Cell Biol 28: 3742–3756. doi: 10.1128/MCB.01832-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orr AW, Hahn C, Blackman BR, Schwartz MA (2008) p21-activated kinase signaling regulates oxidant-dependent NF-kappa B activation by flow. Circ Res 103: 671–679. doi: 10.1161/CIRCRESAHA.108.182097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gastonguay A, Berg T, Hauser AD, Schuld N, Lorimer E, et al. (2012) The role of Rac1 in the regulation of NF-kappaB activity, cell proliferation, and cell migration in non-small cell lung carcinoma. Cancer Biol Ther 13: 647–656. doi: 10.4161/cbt.20082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bosco EE, Mulloy JC, Zheng Y (2009) Rac1 GTPase: a "Rac" of all trades. Cell Mol Life Sci 66: 370–374. doi: 10.1007/s00018-008-8552-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen XL, Nam JO, Jean C, Lawson C, Walsh CT, et al. (2012) VEGF-induced vascular permeability is mediated by FAK. Dev Cell 22: 146–157. doi: 10.1016/j.devcel.2011.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao X, Guan JL (2011) Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv Drug Deliv Rev 63: 610–615. doi: 10.1016/j.addr.2010.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu L, Bernard-Trifilo JA, Lim Y, Lim ST, Mitra SK, et al. (2008) Distinct FAK-Src activation events promote alpha5beta1 and alpha4beta1 integrin-stimulated neuroblastoma cell motility. Oncogene 27: 1439–1448. doi: 10.1038/sj.onc.1210770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tateossian H, Morse S, Parker A, Mburu P, Warr N, et al. (2013) Otitis media in the Tgif knockout mouse implicates TGFbeta signalling in chronic middle ear inflammatory disease. Hum Mol Genet 22: 2553–2565. doi: 10.1093/hmg/ddt103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takahashi H (2001) The middle ear: the role of ventilation in disease and surgery Tokyo; New York: Springer; viii, 105 p. p. [Google Scholar]
- 37.Alahari SK, Lee JW, Juliano RL (2000) Nischarin, a novel protein that interacts with the integrin alpha5 subunit and inhibits cell migration. J Cell Biol 151: 1141–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuijl C, Pilli M, Alahari SK, Janssen H, Khoo PS, et al. (2013) Rac and Rab GTPases dual effector Nischarin regulates vesicle maturation to facilitate survival of intracellular bacteria. EMBO J 32: 713–727. doi: 10.1038/emboj.2013.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jain P, Baranwal S, Dong S, Struckhoff AP, Worthylake RA, et al. (2013) Integrin-binding protein nischarin interacts with tumor suppressor liver kinase B1 (LKB1) to regulate cell migration of breast epithelial cells. J Biol Chem 288: 15495–15509. doi: 10.1074/jbc.M112.418103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baranwal S, Wang Y, Rathinam R, Lee J, Jin L, et al. (2011) Molecular characterization of the tumor-suppressive function of nischarin in breast cancer. J Natl Cancer Inst 103: 1513–1528. doi: 10.1093/jnci/djr350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar R, Vadlamudi RK (2002) Emerging functions of p21-activated kinases in human cancer cells. J Cell Physiol 193: 133–144. doi: 10.1002/jcp.10167 [DOI] [PubMed] [Google Scholar]
- 42.Bokoch GM (2003) Biology of the p21-activated kinases. Annu Rev Biochem 72: 743–781. doi: 10.1146/annurev.biochem.72.121801.161742 [DOI] [PubMed] [Google Scholar]
- 43.Buchwald G, Hostinova E, Rudolph MG, Kraemer A, Sickmann A, et al. (2001) Conformational switch and role of phosphorylation in PAK activation. Mol Cell Biol 21: 5179–5189. doi: 10.1128/MCB.21.15.5179-5189.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edwards DC, Sanders LC, Bokoch GM, Gill GN (1999) Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat Cell Biol 1: 253–259. doi: 10.1038/12963 [DOI] [PubMed] [Google Scholar]
- 45.Gorovoy M, Han J, Pan H, Welch E, Neamu R, et al. (2009) LIM kinase 1 promotes endothelial barrier disruption and neutrophil infiltration in mouse lungs. Circ Res 105: 549–556. doi: 10.1161/CIRCRESAHA.109.195883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perona R, Montaner S, Saniger L, Sanchez-Perez I, Bravo R, et al. (1997) Activation of the nuclear factor-kappaB by Rho, CDC42, and Rac-1 proteins. Genes Dev 11: 463–475. [DOI] [PubMed] [Google Scholar]
- 47.Bonizzi G, Karin M (2004) The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol 25: 280–288. doi: 10.1016/j.it.2004.03.008 [DOI] [PubMed] [Google Scholar]
- 48.Yang JT, Rayburn H, Hynes RO (1993) Embryonic mesodermal defects in alpha 5 integrin-deficient mice. Development 119: 1093–1105. [DOI] [PubMed] [Google Scholar]
- 49.Yates LL, Schnatwinkel C, Hazelwood L, Chessum L, Paudyal A, et al. (2013) Scribble is required for normal epithelial cell-cell contacts and lumen morphogenesis in the mammalian lung. Dev Biol 373: 267–280. doi: 10.1016/j.ydbio.2012.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mburu P, Romero MR, Hilton H, Parker A, Townsend S, et al. (2010) Gelsolin plays a role in the actin polymerization complex of hair cell stereocilia. PLoS One 5: e11627 doi: 10.1371/journal.pone.0011627 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Characteristic image of a male Nischedsn/edsn mutant and a wild-type littermate. (B) Weight data for male edison mice over a 20 wk longitudinal time course shows Nischedsn/edsn mice are significantly smaller than both wild-type and Nischedsn/+ littermates throughout the time course. In addition, Nischedsn/+ mice are also smaller than those wild-type for the allele. * P < 0.05; ** P < 0.01; *** P < 0.001. Nisch+/+, n = 9; Nischedsn/+, n = 20; Nischedsn/edsn, n = 18 (C) Dorsoventral view of a 20 wk wild-type mouse skull showing the measurements used to analyse skull morphology in this study. SL, skull length; SW, skull width; NB, nasal bone; FB, frontal bone; PB, parietal bone. (D) Allometric comparisons against skull length show abnormal growth in Nischedsn/edsn skulls at the nasal bone and frontal bone. ns P > 0.05; * P < 0.05; *** P < 0.001. Nisch+/+, n = 13; Nischedsn/+, n = 18; Nischedsn/edsn, n = 12. (E) Dissected and stained skulls of Nisch+/+, Nischedsn/+ and Nischedsn/edsn 20 wk mice. Eustachian tube (ET) angle measurements are indicated by dashed lines. R, right ear; L, left ear. (F) Mean angle between the midline of the skull and the bony part of the left and the right ET. ns P > 0.05. Nisch+/+, n = 6; Nischedsn/+, n = 6; Nischedsn/edsn, n = 6. Error bars indicate standard error of mean. Statistics were conducted using one-way ANOVA’s and Holm-Sidak’s multiple comparison tests for post-hoc analysis.
(TIF)
(A) Frequency-specific ABR thresholds (8 kHz, 16 kHz, 32 kHz and click-evoked) of Nischedsn/edsn mice across a longitudinal time course displayed parallel shifts in audiometric profiles across frequencies, consistent with a conductive hearing loss. n = 5. (B) H&E mid-modiolar sections of the cochlea showed comparable structure of inner ears in 20 wk Nisch+/+ and Nischedsn/edsn mice. Nisch+/+ n = 5; Nischedsn/edsn n = 5. Scale bar = 1 mm. OC, organ of Corti; SG, spiral ganglion; SL, spiral ligament; RM, Reissner’s membrane. (C) H&E sections of the organ of Corti from the mid cochlear turn displayed no differences in the morphology between Nisch+/+ and Nischedsn/edsn mice at 20 wk. Nisch+/+ n = 5; Nischedsn/edsn n = 5. Scale bar = 100 μm. OHC, outer hair cell; IHC, inner hair cell. (D) Scanning electron microscopy (SEM) images showed normal hair cell morphology in 20 wk Nisch+/+ and Nischedsn/edsn mice. Nisch+/+ n = 5; Nischedsn/edsn n = 5. Scale bar = 10 μm.
(TIF)
Relative Quantification (RQ) of gene expression using TaqMan RT-qPCR for Nischedsn/edsn mice at 20 wk. Blood, n = 3 pools; Ear fluid, n = 3 pools. ns P > 0.05; ** P < 0.01; *** P < 0.001. Error bars indicate 95% confidence interval. A Student's t-test of the replicate 2(−ΔCt) values was performed to analyse the data.
(TIF)
(A, B) Visual inspection of the tympanic membrane was used as a semi-quantitative measure for the prevalence of OM. (A) Itga5tm1Hyn/+; Nisch+/+ mice show a small incidence of unilateral OM at 4 wk, whereas in (B) Itga5tm1Hyn/+; Nischedsn/+ animals prevalence of OM increases with age compared to littermates with onset at 12 wk. (C-F) H&E stained transverse sections of the MEC and mucoperiosteum, in 20 wk (C, E) Itga5tm1Hyn/+; Nisch+/+ and (D, F) Itga5tm1Hyn/+; Nischedsn/+ animals. Itga5tm1Hyn/+; Nischedsn/+ mice develop OM with a diffuse mucosal inflammation of mild severity, with the presence of a cellular middle ear effusion. C, cochlea; ET, Eustachian tube; E, exudate; MEC, middle ear cavity; MP, mucoperiosteum (arrowheads); TB, temporal bone; TM, tympanic membrane. C, D scale bar = 2 mm; E, F scale bar = 200 μm.
(TIF)
Immunohistochemistry of lung sections of Nisch+/+, Nischedsn/edsn and Itga5tm1Hyn/+; Nischedsn/edsn mice at 3 wk and total lung extracts at 8 wk, with (A) NISCH, (B) ITGA5, (C) PAK1, (D) p-LIMK1/2, (E) RAC1, (F) NF-κB p65, (G) p-SMAD2 and (H) FAK antibodies. To quantify the results from the staining, airway epithelial cells in wild-types and mutants were counted in three different regions from four lungs for each genotype. The results presented from the western blots are from four independent experiments for all the antibodies except for p-LIMK1/2, where three independent experiments were used to present the data. Scale bar = 100 μm. * P < 0.05. Error bars indicate standard error of mean. Immunohistochemistry data was analysed by one-way ANOVAs and Holm-Sidak’s multiple comparison procedures for post-hoc testing. For western blot analysis a Student’s t-test was performed.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.