Abstract
Introduction
Biologics are generally macromolecules, large in size with poor stability in biological environments. Delivery of biologics to tissues at the back of the eye remains a challenge. To overcome these challenges and treat posterior ocular diseases, several novel approaches have been developed. Nanotechnology-based delivery systems, like drug encapsulation technology, macromolecule implants and gene delivery are under investigation. We provide an overview of emerging technologies for biologics delivery to back of the eye tissues. Moreover, new biologic drugs currently in clinical trials for ocular neovascular diseases have been discussed.
Areas Covered
Anatomy of the eye, posterior segment disease and diagnosis, barriers to biologic delivery, ocular pharmacokinetic, novel biologic delivery system
Expert Opinion
Anti-VEGF therapy represents a significant advance in developing biologics for the treatment of ocular neovascular diseases. Various strategies for biologic delivery to posterior ocular tissues are under development with some in early or late stages of clinical trials. Despite significant progress in the delivery of biologics, there is unmet need to develop sustained delivery of biologics with nearly zero-order release kinetics to the back of the eye tissues. In addition, elevated intraocular pressure associated with frequent intravitreal injections of macromolecules is another concern that needs to be addressed.
Keywords: barriers, biologics delivery, back of the eye, implants, nanomicelles, nanoparticles, ocular delivery, peptide, protein, pharmacokinetics
1. INTRODUCTION
Back-of-the-eye diseases are commonly diagnosed in ageing population. Statistics indicate that 76 million people may suffer from blindness by 2020 [1]. Posterior segment of the eye presents unique anatomical, physiological and biochemical barriers that protect the eye from various toxic insults [2], [3]. Back-of-the-eye tissues comprise sclera, choroid, choriocapillaries, Bruch’s membrane, retinal pigmented epithelium (RPE), retina and inner retinal membrane. Such highly protected back-of-the-eye tissues are affected with vision threatening diseases such as age related macular degeneration (AMD), diabetic macular edema (DME), cystoid macular edema (CME), diabetic retinopathy (DR), posterior vitreoretinopathy and retinal vein occlusion (RVO) [4], [5], [6]. If treatment is not initiated at early stages, it may result in impaired vision leading to complete vision loss [7], [4], [8]. Treatment of posterior ocular diseases is obstructed by ocular barriers (static and dynamic) as well as physicochemical properties of therapeutic agents. Biologics are gaining significant attention due to their high efficacy and minimal side effects.
Biologics such as macromolecules (protein, peptides, aptamers, SiRNA and antibody) are discussed in this article. In pharmaceutical industry, biologics have received enormous medical attention as therapeutic agents in the past decennium. Biologics are currently recommended for the treatment of various ocular diseases. Macromolecules such as rituximab and infliximab are indicated for inflammatory eye diseases and uveitis [9,10], so is aflibercept for AMD and DME [11,12], while ranibizumab, and bevacizumab are indicated for AMD, DR, DME and RVO [13–18]. Despite promising results such as improved visual acuity, inhibiting macular edema and vision loss (Table 1), however major challenge still remains i.e. delivery of biologics to the back-of-the-eye tissues (retina-choroid) [3], [19]. Biologics display numerous delivery related limitations such as absorption/permeability across biological membranes, poor bioavailability and in vivo stability [20,21]. Short in vivo half-life of biologics demands frequent intravitreal administrations which lowers patient compliance. Significant research has been conducted to impart macromolecular stability, overcome ocular barriers, sustain release, and lowering administration frequency. Innovative technologies have been applied for macromolecular delivery to anterior and posterior ocular tissues.
Table 1.
List of commercially available macromolecules/biologics for treatment of posterior ocular diseases
| Trade Name | Generic Name | Type of Molecule | Biological Target | Disease | Reference |
|---|---|---|---|---|---|
| Lucentis® | Ranibizumab | Humanized monoclonal antibody fragment | VEGF-A isoforms | AMD, DME, DR, RVO | [125–128] |
| Eylea® | Aflibercept | Fusion protein | VEGF-A, VEGF-B isoforms and PLGF | AMD, DME, DR | [129–131] |
| Avastin® (off label) | Bevacizumab | Humanized full length monoclonal antibody | VEGF-A isoforms | AMD | [125,132] |
| Macugen® | Pegaptanib | RNA aptamer | VEGF-A165 only | AMD | [133] |
PLGF = Placental growth factor, VEGF = Vascular endothelial growth factor, RNA = Ribonucleic acid, RVO = Retinal vein occlusion, AMD = Age related Macular Degeneration; CNV = Choroidal Neovascularization, DME = Diabetic Macular Edema
Biologics are promising therapeutics for neovascular ocular diseases. Several anti-VEGF biologics were developed in last decade and are currently in the market. These anti-VEGF drugs have shown tremendous improvement in visual acuity, edema reduction and improvement in neovascularization of cornea/retina. Several retinal delivery systems are in different stages of clinical trials and others are being developed. Despite success in the treatment of back-of-the-eye diseases with biologics, it is very difficult to deliver proteins/peptides to the back-of-the-eye tissues. Delivery of macromolecules to the back-of-the-eye tissues has a number of concerns which have been discussed in this article. Several concerns have been raised about the long term use of intravitreal injections of anti-VEGF for the treatment of posterior diseases. One of those questions is the potential elevation of intraocular pressure (IOP) caused by frequent intraocular injections. Several studies have been conducted to investigate if there is any relationship between the elevation of IOP after intravitreal injections of anti-VEGF agents [22,23]. Results demonstrated that patients receiving bevacizumab and/or ranibizumab had sustained IOP elevation [22]. Moreover, patients with pre-existing glaucoma experienced higher rates of elevated IOP compared with patients with no prior history of IOP related effects [22]. Elevated levels of IOP was observed in another study performed on AMD patients but did not investigate in preexisting glaucoma [23]. Both studies indicated significant elevation of IOP with intravitreal injection and is more pronounced in patients with a history of glaucoma. These studies clearly draw a correlation between anti-VEGF treatment and IOP elevation. However, another study was performed in AMD patients to evaluate elevation of IOP after multiple intravitreal injections of ranibizumab [24]. This study included AMD patients receiving ≥ 15 injections (as frequent injection group) and ≤ 5 injections (fewer control group). Results indicated no significance change in elevation of IOP between the two groups [24]. These studies demonstrate lack of uniformity in findings regarding elevation in IOP and frequency intravitreal injections. This leaves a room for more research to understand and explain the elevation of IOP. However, there is no report of elevated IOP for topically administered biologics. Whether IOP is mainly caused by frequent intraocular injections or not, remains to be determined. This should be a wake-up call for pharmaceutical scientists developing protein or peptide drugs for back-of-the-eye delivery that require intraocular injections. Therefore, novel drug delivery strategies that will minimize or eradicate concerns associated with frequent intraocular injections are pivotal. Elevation of IOP is not the only side effect caused by frequent intravitreal injections, possibility of retinal detachment, retinal hemorrhage, and endophthalmitis have been reported [25].
Topical administration is the most patient compliance route for ocular drugs. There are some reports of topical administration for back-of-the-eye delivery of small molecule therapeutics mainly peptides such as calpain [26], KAL821 [27] and cyclosporine [28]. A few reports have contradicted feasibility of delivering macromolecules to the back-of-the-eye through topical route. [29–31]. PK of bevacizumab after topical administration in human eyes revealed that no bevacizumab was detectable in aqueous or vitreous fluids in topically treated eyes [29]. These results indicate that bevacizumab is a large molecule to cross cornea to reach anterior and posterior segments. On the other hand, in vivo studies on rabbit model detected ranibizumab after 7 and 14 days following topical administration after 2 hours [31]. Davis et al utilized annex A5 associated liposomes to deliver bevacizumab to the posterior segment of the eye through topical administration. The study was performed on rat and rabbit models demonstrating significant levels of bevacizumab to the posterior segment of the eye (127ng/g on rats and 18ng/g on rabbit retina) [30]. Therefore, these results suggest that it is feasible to deliver biologics to posterior segment of the eye through topical administration. A proper delivery system has to be selected that can cross anterior segment barriers in order for the biologics to reach posterior chamber at therapeutic levels.
The use of anti-VEGF may cause inhibition of VEGF A which in turn may result into toxicity on ciliary body hindering properly functioning [32]. Anti-VEGF biologics have shown remarkable results for the treatment of posterior ocular diseases, but frequent intravitreal injections, possible effects on IOP elevation and long term toxicities ban be major concerns. Ciliary body is involved in production of aqueous humor and anchors the lens. If these functions are impaired, more side effects may occur. For that reason, it is important for clinicians to be cautious when administering anti-VEGF therapeutics. It is essential to perform a test that will ensure normal functioning of ciliary body of patients undergoing anti-VEGF therapy. Bevasiranib is a siRNA that targets VEGF-A which has been associated with ciliary body damage [32]. Hence it is necessary not only for the scientists and clinicians to perform studies not only on how to treat AMD with bevasiranib, but also to investigate the side effects that may occur with dysfunction of ciliary body.
In this review, we have described the recent perspectives on the delivery of biologics to ocular tissues with special emphasis to back-of-the-eye tissues (retina-choroid). Moreover, we have also discussed barriers to ocular delivery of macromolecules and strategies to overcome such barriers. Inventive approaches like colloidal systems (nanoparticles, liposomes, neosomes, micelles, hydrogels, and dendrimers), microneedles and implants are described. Additionally, ocular pharmacokinetics and routes of macromolecule drug elimination have been discussed.
2. OCULAR ANATOMY AND PHYSIOLOGY
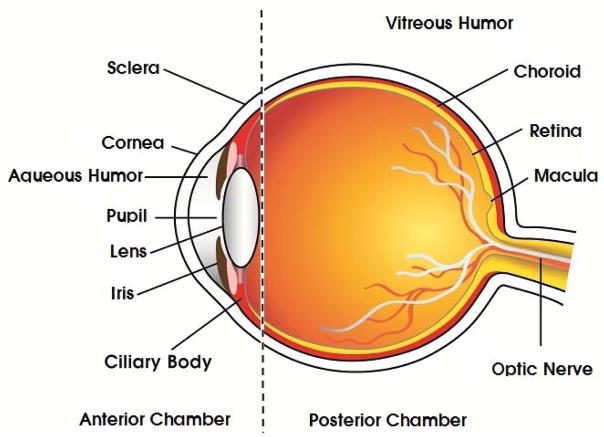
Human eye is a sensitive and complex organ with unique anatomy and physiology. For easy understanding, the ocular structure can be broadly divided into two segments namely, anterior and posterior compartments (Fig. 1). Anterior segment covers one-third of the globe including aqueous humor and the remainder is occupied by posterior segment including vitreous fluid. Anterior ocular compartment comprises of cornea, conjunctiva, aqueous humor, lens, iris and ciliary body. On the other hand, posterior compartment is composed of sclera, choroid, choriocapillaries, Bruch’s membrane, RPE retina, inner limiting membrane and vitreous body.
Fig. 1.
Structure of Human eye
3. POSTERIOR OCULAR DISEASES AND DIAGNOSIS
3.1 Diabetic and Cystoid Macular Edema
Diabetic Macular Edema (DME) originates from venous occlusions resulting in retinal microvascular damage with macular leakage, capillary dropout, upregulation of angiogenic growth factors and subsequent neovascularization [33]. Diabetes affects the blood vessels by weakening the inner lining so that the vessels become porous and leaky [34–36]. Leakage from the retinal blood vessels cause retinal swelling, a condition called diabetic macular edema. This swelling usually occurs in the macula which is responsible for central vision. Consequently macular edema results in vision loss of varying degree. Cystoid macular edema (CME) typically occurs 1 to 4 months after cataract extraction, reducing visual acuity in 20/30 to 20/80 range. It is a condition in which the macula becomes swollen with fluid-filled cysts. CME can occur in diabetes, retinal vein occlusion, or uveitis [36]. Previous main treatment modalities for diabetic related disease conditions of the eye, including DME and diabetic retinopathy consisted of laser photocoagulation and vitrectomy, respectively. Currently, much research has focused on the use of anti-vascular endothelium growth factors (Anti-VEGF) agents. Over the past ten years several anti-VEGF agents have evolved. Pegaptanib, which works by binding to VEGF 165 isoform protein, ranibizumab (Fab fragment) and bevacizumab inhibit VEGF A isoforms, VEGF trap fusion protein such as aflibercept that inhibits VEGF A, VEGF B and PIGF. Conbercept, a recombinant fusion protein, inhibits PIGF and VEGF isoforms A, B and C. These anti-VEGF agents have changed treatment decisions and outcomes for patients over the years. [37]. Although, they have their own limitations such as absorption/permeability across biological membranes, poor bioavailability, in vivo stability and short in vivo half-life.
3.2 Diabetic Retinopathy
DR is a progressive process where in the retinal blood vessels continue to become weak, narrow and close [38]. As the blood vessels close, these lose their oxygen carrying potential to the retinal tissues, resulting in a condition such as Retinal Ischemia. It is characterized by retina being deprived of sufficient oxygen and nutrients to maintain normal health and functioning of the eye. Typically, the retina responds to ischemia by attempting to compensate for the reduced circulation by growing new, but abnormal blood vessels, a process of neovascularization. These new blood vessels are extremely fragile and tend to break easily leading to hemorrhage. If this condition left untreated, hemorrhage leads to scarring and ultimately retinal detachment with profound vision loss. Diabetic retinopathy follows a similar pathophysiological progression as DME, therefore treatment modalities can be similar. However, treatment options for DR are minimal with potential side effects. Surgical interventions such as laser photocoagulation can help to maintain central vision, although with the loss of peripheral vision. Anti-VEGF treatment has provided an effective alternative, requiring multiple treatments due to biological limitations [39,40]. Compromised neuronal and vascular survival can be seen as potential side effects. New research for the possible prevention/treatment of DR is currently being investigated utilizing Niaspan, a prolonged release form of niacin [41]. Niacin is currently the most clinically proficient medication for increasing high-density lipoproteins (HDL). High levels of HDL have been associated with a decrease in DR prevalence. MicroRNA-126 (miR-126) has potentiality improved in the pathogenesis of DR by regulating VEGF, angiopoietin-1(Ang-1) and vascular cell adhesion molecule-1(VCAM-1) [41].
3.3 Age-related Macular Degeneration
AMD is a vision threatening ocular disease affecting macula region, retinal pigment epithelium, Bruch’s membrane and choriocapillaris [42]. AMD is a major cause of central vision loss particularly in individuals of 65 years or older. The disease is characteristically manifested in two forms - “dry” and “wet” [43]. The majority of patients with vision loss suffer from the “wet” form of AMD due to choroidal neovascularization (CNV) and related pathologies such as subretinal hemorrhage, fibrovascular scarring and RPE detachment. CNV formation involves various pathophysiological steps such as senile RPE degeneration, deposition of drusen, disruption of Bruch’s membrane, CNV formation and cicatrisation of the CNV [44,45]. AMD can be divided into three stages: early, intermediate and late AMD. Late AMD can be subdivided into geographic atrophy or (Dry AMD) and neovascular AMD or (Wet AMD). The progression of AMD are dependent on the amount and size of drusen deposits below the retina. Treatment options for AMD vary based on the progressive stage. At this point of time there are no treatment options for early stage AMD. Once the AMD is reached to intermediate stage, the treatment is focused on daily intake of vitamins and minerals in hopes to slow the progressive nature of the disease. The vitamin and mineral regiment is based on Age-Related Eye Disease Studies (AREDS and AREDS2) which appear to protect AMD. Late stage neovascular “wet” AMD can be treated with the use of multiple treatment modalities. Injections of anti-VEGF agents, currently the main form of therapy, is aimed at blocking the increased levels of VEGF released from the eye. A less common procedure is use of verteporfin, a photodynamic therapy (PDT). In this procedure verteporfin medication is injected into the venous blood supply and is up-taken by newly forming blood vessels. The photodynamic laser is directed into the AMD affected eye, which activates the medication, leading to production of free oxygen radicals, causing destruction of newly formed vessels, while saving normal vessel architecture. The anticipated outcome of this treatment is to slow the progression of neovascular “wet” AMD vision loss. The third treatment option for AMD utilizes the previously mentioned method of laser photocoagulation according to NIH National Eye Institute [46].
3.4 Proliferative Vitreoretinopathy
Proliferative Vitreoretinopathy (PVR) may develop along with some retinal detachment (RD). It is the most common cause of surgical failure in the rhegmatogenous treatment [47]. PVR can also be defined as the growth and contraction of cellular membranes within the vitreous cavity and retinal cells. In many cases a fibrotic process may occur in the retina itself [48]. Treatment of PVR has mainly been surgically based. Although, clinical success can differ based on the severity of PVR present. With complex PVR, the success maybe 40%. Due to the invasive nature of surgery and reduced clinical success, new treatment strategies are being developed utilizing pharmacological agents. Due to the pathophysiology of PVR disease process, three groups of pharmacological agents are being investigated. (1). Anti-inflammatories such as corticosteroids, (2). Antineoplastic/antiproliferative agents, examples include 5-fluorouracil (5-FU), taxol, daunorubicin, colchicine, retinoic acid, vincristine, cisplatin, ribozymes, adriamycin, mitomycin, dactomycin and many others, (3). Antigrowth factor/growth factor pathway inhibitors eg. Hypericin, platelet-derived growth factor (PDGFR) kinase inhibitor AG1295. Continued research on the pathogenesis of PVR is required to tailor non-surgical treatment strategies and improve overall success. [49].
3.5 Diagnosis of Posterior Ocular Diseases
Visual acuity of 20/20 is a pivotal parameter in the progression of DME, DR, AMD and PVR. Posterior ocular diseases may be primarily diagnosed with dilated eye exam. If primary diagnosis shows any of the following: abnormal blood vessels, swelling, blood or fatty deposits in retina or scar tissue, retinal detachment, abnormalities in optic nerve, then it probably indicates posterior ocular disease. Further confirmation is necessary with funduscopic examination (color stereo funds photographs), fluorescein angiography and optical coherence tomography. However, PVR is difficult to diagnose when media opacity occurs due to corneal, lenticular and vitreous opacities. With such conditions, “ultra-sonographic characteristics showing funnel-shaped retinal detachment with opposition of the posterior retina or the presence of an anterior membrane bridging the mouth of the funnel 28 may provide evidence for a definitive diagnosis” [50].
4. CHALLENGES FOR OCULAR DELIVERY OF MACROMOLECULES
Macromolecule drug delivery to the eye is challenging due to size, stability, surface charge, non-specificity and toxicity. Topically administered drug solutions/products encounter the transparent collagenous cornea and conjunctiva as the primary barriers for drug penetration [51]. Cornea is composed of layers, from anterior to posterior, namely corneal epithelium, Bowman’s layer, corneal stroma, Dua’s layer, Descemet’s membrane and endothelium. Hydrophilic molecules encounter barriers from corneal permeation due to tight junctions and lipophilicity of corneal epithelium [52,53] whereas hydrophobic molecules may permeate epithelium easily. However, hydrophilic stroma contains charged collagen fibers that can act as a barrier to lipid soluble drugs and may impede permeation. Moderately charged small molecules are shown to permeate cornea [52,54]. Stromal collagen fibers are highly organized and intertwined with narrow pore size which may prevent macromolecular permeation. Moreover, tears and tear turn-over rate act as a barrier to macromolecule bioavailability [55]. Corneal layers along with tear production result in <5% bioavailability of topical doses including biologics [56]. Similar to cornea, conjunctival epithelium also possess tight junctions. However, conjunctival intercellular spaces are wider relative to cornea and allow passage of macromolecules [56]. Molecules that traverse conjunctiva are drained into subconjunctival blood and lymphatics [57,58]. Additionally, blood-aqueous barrier (BAB) comprising endothelial cells in the uvea and non-pigmented layer of the epithelium of ciliary body possess incomplete barrier functionality. BAB regulates active and paracellular molecule permeation. Small molecules may be rapidly drained into circulation relative to macromolecules. For example, fluorescently labeled dextrans (~ 150 kDa) demonstrated permeation across BAB[59].
Sclera may act as a barrier and inhibit drug translocation into deeper ocular tissues. Studies indicated that high molecular size molecules have reduced rate and extend penetration [60–63]. Ex vivo studies with human sclera demonstrated that ~150 kDa molecules (dextran and bevacizumab) easily translocate sclera [63]. Molecules that traverse choroid may be drained by choriocapillaries. Studies demonstrated choriocapillaries permeability of 40 kDa horseradish peroxidase in rats [64]. However, high molecular weights molecules such as hemoglobin (68 kDa) and lactoperoxidase (84 kDa) and ferritin (480 kDa) exhibit poor penetration [65]. Retinal pigment epithelium (RPE) makes up high tight junctions and poses a significant barrier to macromolecules. An exponential decrease in permeability was noticed for 0.376 kDa to 77 kDa fluorescently tagged molecules across excised bovine eyes [66]. Human retina limits the diffusion of compounds with molecular weight larger than 76 kDa [67]. Highest resistance to diffusion is offered by inner and outer plexiform layers in the retina [67,68]. Moreover, inner limiting membrane restricts the permeation of macromolecules larger than 150 kDa [67], [68], [69]. Discovery of novel delivery approaches that combine high specificity and therapeutic payload with minimal/no toxicity remains a challenge. However, several promising systems have emerged recently which are discussed below.
5. OCULAR PHARMACOKINETICS
Animal models have been developed to study the distribution and elimination of ocular drugs with different routes of administration [70]. Human and rabbit eyes share common characteristics. Hence, rabbits are commonly selected as the pharmacokinetic animal model. Human eyes have high retinal vasculature, larger vitreous cavity, small lens and large serum compartment relative to rabbits [71]. These factors may contribute to different pharmacokinetic profile for biologics/macromolecules in human and rabbit. However, rabbit model is established to study pharmacokinetics for convenience. In ocular drug development, the pharmacokinetic parameters in rabbit have shown predictable correlation to human. Moreover, such models aid to determine drug elimination pathways. [72,73]. Other animal models evaluated include monkey, rat, pig, bovine, and horse. Table 2 summarizes pharmacokinetic results of macromolecules administered through various routes in animal models.
Table 2.
Pharmacokinetics results on various species treated with biologics.
| Species | Macromolecule/Biologic | Route of Administration | AUC (μg/ml* day | Cmax (μg/ml) | Tmax (Days) | Half-life (Days) | Remarks/Conclusions | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| New Zealand Rabbits | VEGF-Trap (Aflibercept) | Intravitreal administration | Aqueous Humor | - | - | - | - | VEGF production in the eye will be inhibited for more than 6 weeks after intravitreal administration of 500 μg/ml of aflibercept in the eye. | 2 |
| Vitreous Humor | - | 500 | 0.25 | 4.5 | |||||
| Serum | - | 1.6 | 10 | - | |||||
| Dutch-belted rabbits | Bevacizumab (Avastin) | Intravitreal administration | Aqueous Humor | 295 | 400 | 3 | 4.88 | In this study small amounts of bevacizumab was observed in untreated eye after treating one eye with 1.25mg of bevacizumab. | [134] |
| Vitreous Humor | 3300 | 37.7 | 1 | 4.32 | |||||
| Serum | 54 | 3.3 | 8 | 6.86 | |||||
| Dutch-belted rabbits | Ranibizumab (Lucentis) | Intravitreal administration | Aqueous Humor | - | 17.9 | 3 | 2.84 | No ranibizumab was detected in the serum and in untreated eye after 0.5 mg treatment. | [71] |
| Vitreous Humor | - | 162 | 1 | 2.88 | |||||
| Serum | - | - | - | - | |||||
| Human Beings | Ranibizumab (Lucentis) | Intravitreal administration (Single/Multiple dose) | Aqueous Humor | - | - | - | Steady state concentration of ranibizumab in vitreous humor attained with monthly intravitreal dosing. | [75]* | |
| Vitreous Humor | 1,900,000 d* ng/ml | 140,000 ng/ml | - | 9 | |||||
| Serum | 21 d* ng/ml | 1.5 ng/ml | - | 2 | |||||
| Monkeys | Ranibizumab (Lucentis) | Intravitreal administration (500 or 2000 μg/eye) | Aqueous Humor | 231/1500 | 116/478 | 0.25/1 | 2.54/2.63 | Results provide dose for the treatment of AMD and other neovascular ocular diseases | [76] |
| Vitreous Humor | 689/3110 | 169/612 | 0.25/1 | 2.63/3.95 | |||||
| Serum | 315/1270 d* ng/ml | 150/616 | 0.25/0.25 | 3.59/3.47 | |||||
| New Zealand Rabbits | Bevacizumab (Avastin) | Intravitreal administration | Aqueous Humor | 44.8 | 5.83 | 1 | 6.51 | A half-life of 6.61 days in vitreous humor reached after 1.25 mg/0.05mL of bevacizumab was used. Low concentrations of bevacizumab observed in untreated eye. | [135] |
| Vitreous Humor | 1455.1 | 406.25 | 1 | 6.61 | |||||
| Serum | 5.3 | 0.413 | 8 | 5.87 | |||||
| New Zealand Rabbits | VEGF-Trap | Intravitreal administration | Aqueous Humor | 3945.1 (μg/ml* h) | 86.2 | 1 | 87.1 h | Half-life of VEGF-Trap shorter compared bevacizumab, but longer than ranibizumab. | [136] |
| Vitreous Humor | 10009.8 (μg/ml* h) | 49.5 | 1 | 36.8 h | |||||
| Serum | - | - | - | - | |||||
| Cynomologus Macaques | Bevacizumab (Avastin) | Intravitreal administration | Aqueous Humor | 5680±2336 (μg/ml* h) | 49500±10900 ng/mL | 1 | 2.8±0.6 | Reduction in VEGF in treated eyes and negligible or no effect on untreated eyes. | [137] |
| Vitreous Humor | - | - | - | - | |||||
| Serum | 526.2±17.1 (μg/ml* h) | 1430±186 ng/mL | 7 | 12.3±2.6 |
This was a single or multiple dose at various concentrations study. Data reported in the table was for 0.5 mg/eye monthly for 12 dose
In vivo models play an important role in pharmacokinetics study. Poor pharmacokinetic design may generate erroneous results [74]. Bakri et al. have compared the pharmacokinetics of intravitreal bevacizumab and ranibizumab using twenty-eight Dutch-belted rabbits [71]. In this study, 0.5 mg of ranibizumab and 1.25 mg of bevacizumab were intravitreally injected into rabbits and drug concentrations in different tissues i.e. aqueous humor, vitreous and serum were measured and pharmacokinetic data was noted. The half-lives of ranibizumab and bevacizumab were 2.88 and 4.32 days respectively. Maximum concentrations for ranibizumab and bevacizumab in vitreous cavity were 162μg/ml and 400μg/ml respectively at day-1. Because ranibizumab has smaller molecular weight (48 kDa) relative to bevacizumab (149 kDa); it could have higher retinal penetration as well as more rapid elimination. Another study performed by Xu L. et al., reported that the pharmacokinetics of ranibizumab in 674 patients with age-related macular degeneration [75]. One compartment model is the best fit model for ranibizumab systemic concentration and first order elimination from systemic circulation. The elimination half-life in vitreous was 9 days where as intrinsic systemic elimination half-life was 2 hours.
Monkeys are employed as a model to study pharmacokinetic profiles. Reports suggest variable pharmacokinetics profiles for ranibizumab in monkey relative to rabbit. Ranibizumab (0.5 mg) plasma half-lives are calculated as 2.6 days in monkeys and 2.9 days in rabbits. Vitreous humor had a maximum concentration of 169 μg/ml at 6 hours in monkeys relative to 162 μg/ml at 24 hours in rabbit [71,76]. Lampalizumab elimination following an intravitreal injection in cynomolgus monkeys indicated slower ocular elimination with a T 1/2 approximately 3 days compared to systemic elimination with T 1/2 of 0.8 hours [77]. Drolet DW et al. have studied the pharmacokinetic and safety profile of an anti-vascular endothelial growth factor aptamer (NX1838) in rhesus monkeys [78]. The concentrations of NX1838 in plasma and vitreous humor were dose dependent. A large fraction of NX1838 was cleared intact from the vitreous into the plasma with a half-life of approximately 94 hours. The remaining NX1838 in the vitreous humor was active even after 28 days of administration without any toxicological effects [78]. Pegaptanib sodium (Macugen; Eyetech Pharmaceuticals/Pfizer) is an RNA aptamer developed against VEGF. It was effective in clinical trials in treating choroidal neovascularization associated with age-related macular degeneration [79].
6. NOVEL COLLOIDAL DELIVERY SYSTEMS
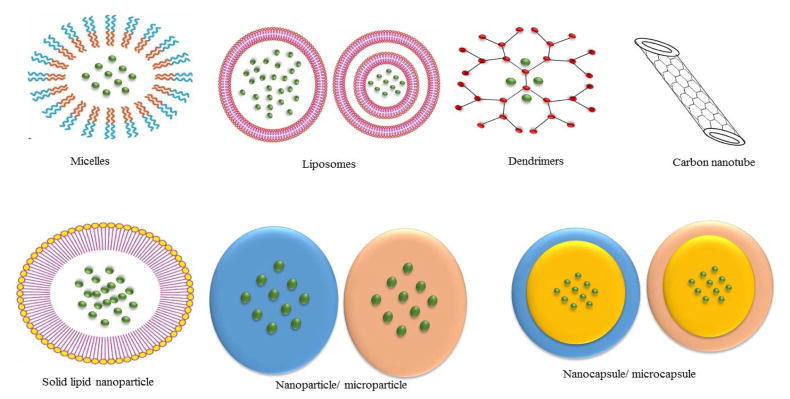
In recent years, several delivery systems have been developed for biologics for back-of-the-eye tissue delivery. Systems such as particulates, vesicular and controlled release systems have been discovered (Fig 2). Advancement in biotechnology allowed upsurge in drug pipeline production of therapeutic biologics such as peptides, proteins, aptamers, siRNA, oligonucleotides and others. All have attempted to improve mode of delivery. These delivery systems have advantages and disadvantages (Table 3), hence the selection of a particular delivery system is crucial. In the following sections we have discussed different novel drug delivery systems that have currently been developed.
Fig. 2.
Different delivery systems that may be utilized to deliver macromolecules to back of the eye.
 Drugs
Drugs
Nanoparticle size (10–1000 nm)
Micronanoparticles (> 1 μm)
Table 3.
Advantages and disadvantages of different delivery systems for back-of-the-eye delivery of biologics
| Delivery System | Advantages | Disadvantages | Ref |
|---|---|---|---|
| Conventional | Patient compliance. Easily administered |
Rapid pre-corneal elimination Blurred vision Unable to cross ocular barriers to the back of the eye Poor bioavailability |
[3,138] |
| Vesicular | Biocompatible, biodegradable, safe materials can be used. Can encapsulate hydrophilic and hydrophobic drugs. Can sustain release of encapsulated drug |
Oxidative degradation of phospholipids. Instability e.g. liposomes. Large size e.g. discosomes Non patient compliance |
[3,139] [19] |
| Particulate | Biocompatible, biodegradable, non-toxic materials. Control and prolonged release of therapeutic molecules. High bioavailability to the target site. Different routes of administration. Large drug payload e.g. lipid nanoparticles |
Patient non-compliance Associated with IOP, retina hemorrhage, retinal detachment, and endophthalmitis. Initial burst release of drugs Degradation of specific polymers may results to lower pH, hence irritation. Poor stability of macromolecules Low encapsulation efficiency |
[25,140–143] [144,145] |
| Control Release | Control and sustained release of therapeutic molecules. Various routes of administration can be applied. High bioavailability of therapeutic molecules to the target site Control of IOP Improved patient compliance e.g. implants |
Replacement of the unit in case of contamination or depletion of the active agent. Might require a medical personnel and surgery to insert and remove the device e.g. implants. Education to handle and clean e.g. contact lenses Active agent might not able to cross ocular barrier to the posterior segment in case of contact lenses. Costly |
[146–150] |
| Advanced | No toxicity, safe Stable Enhanced bioavailability Rapid drug delivery Tissue targeting Possibility of treating vascular and neuronal ocular diseases |
Immune responses Random integration Vehicle selection can be challenging Poor cell permeability and degradation of nucleases Intracellular and extracellular barriers |
[141,151–154] |
6.1 Vesicular Systems
6.1.1 Nanomicelle
Nanomicelles are composed of amphiphilic molecules capable of self-assembling colloidal systems with size range between 10–100 nm [80]. Nanomicelles are normally formed above critical micellar concentration, where the amphiphilic molecules assemble to form a hydrophobic core with a hydrophilic corona in aqueous environment. Iriyama et al applied Nanomicellar carriers for gene delivery to impede choroidal neovascularization. Nanomicelles composed of polyion complex (PIC) can encapsulate plasmid DNA (Yellow Fluorescent Protein - YFP) or soluble Fms-like tyrosine kinase-1 (psFIt-1) was effective in delivering DNA in CNV area. A significant reduction (~65%) in CNV was observed [81]. In another study, Ideta et al evaluated FITC-P(Lys) loaded PIC nanomicelles for the treatment of CNV in rats. Long systemic circulation and accumulation of PIC nanomicelles at CNV lesion site were witnessed [82]. Such results indicate that PIC nanomicelles systems may be employed to deliver therapeutic biologics to back-of-the-eye.
Antagonistic peptide for VEGFR1 (anti-FIt1) conjugated to hyaluronate was investigated in rats for retinal neovascularization and diabetic retinopathy [83]. Anti-FIt1-hyaluronate conjugate self-assembled in aqueous solution and generated nanomicelles. In vitro studies indicated that anti-FIt1-hyaluronate conjugate inhibited retinal neovascularization in laser induced CNV rats [83]. Moreover, retinal vascular permeability and deformation of retinal structure were significantly reduced in diabetic retinopathy rats [83]. Such system may be exploited to encapsulate a therapeutic macromolecule (a small molecule was encapsulated by the same group) [84] which may produce an additive effect, from anti-FIt1 and the encapsulated macromolecules.
6.1.2 Liposomes
Liposomes are biodegradable and biocompatible lipid vesicles comprising one or more layers of phospholipid in addition to other components such as cholesterol and polymers [85]. Liposomes ubiquitously are known to encapsulate and deliver both hydrophilic and lipophilic drugs. The fact that liposomes possess cell membrane like structure make them an impeccable colloidal delivery system [86]. Liposomes have been intensively explored for small molecule drug delivery to the eye. With recent advancements in biologics, investigators evaluated liposomes, encapsulating proteins or peptides, for back-of-the-eye delivery. Downregulation of endotoxin induced uveitis with intravitreal injection of liposomes encapsulated with vasoactive intestinal peptide (VIP) was evaluated [87]. Following intravitreal injection of VIP liposome in rats, high peptide concentrations was observed in vitreous, sclera, retina, conjunctiva and ciliary body [87]. Moreover, inflammatory cytokines and chemokine mRNA expression were found to be significantly reduced [87,88]. In a different approach, the same group developed rhodamine conjugated VIP liposomes incorporated in hyaluronic acid gel [88]. Researchers revealed significant improvement with gel formulation. Similarly Abrishami et al have evaluated nanoliposome encapsulating bevacizumab by intravitreal administration in rats. These studies indicated higher concentration (5X) of bevacizumab even on day 42 in nanoliposomal delivery compared to free drug alone [89]. Such results suggest that biologics may be encapsulated in liposome to achieve higher concentrations in the ocular compartments.
6.2 Particulates
6.2.1 Nanoparticles
Nanoparticles and microparticles are colloidal delivery systems being explored for macromolecule drug delivery. These particles are composed of biocompatible and biodegradable polymers. Nanoparticle delivery system has several advantages such as higher corneal penetration, extended release and large dissolution area which improve bioavailability of active ingredient compared to conventional delivery systems [90]. Patel et al, developed novel biodegradable pentablock copolymer based nanoparticle system for sustained release of proteins. Various pentablock copolymers were utilized to encapsulate biologics such as IgG, bevacizumab and FITC-BSA [25,91]. Pentablock copolymers examined in several ocular cell lines, did not demonstrate any cytotoxicity or inflammatory mediator release with in vitro studies. [25,92]. Moreover, Ig-G, bevacizumab and FITC-BSA were released over prolonged period of time. In addition, released Ig-G was stable as native Ig-G for prolonged time [25,91]. Therefore, such formulations may be applied to anti-VEGF therapies in order to be delivered to the back-of-the-eye tissues for treatment of posterior diseases. In addition Yandrapu et al investigated sustained release of bevacizumab from the core of porous microparticles. PLA nanoparticles encapsulating bevacizumab were first prepared before mixing with PLGA microparticles [93]. In vitro study indicated sustained release of bevacizumab for 4 months with no change in conformation and activity [93]. Therefore, this formulation may be employed for delivery of biologics to the back-of-the-eye for the treatment of posterior ocular diseases. However, PLGA may generate lactic acid which can cause irritation. Microparticles/nanoparticles if injected intravitreally, can cause blurred vision due to particle floatation. In contrast, bevacizumab encapsulated in pentablock polymer nanoparticles and suspended in gel have shown sustained release for extended periods (2–4 months) and can deliver therapeutics for prolonged period of time without frequent injections [25,91,92]. Recombination technology RNA has been extensively used as therapeutic agent for the treatment of a wide-range of ocular diseases. RNA can undergo several modifications, engineering, and/or assembly. The altered RNA can then encapsulated into a nanoscale delivery system. RNA, such as packaging RNA (pRNA), which may contain up to 117 nucleotides and small interfering RNA (siRNA), has been broadly explored in cancer, viral infection and genetic diseases. Recently, Feng et al investigated the distribution and clearance of pRNA and dsRNA nanoparticles to the cornea and retina after subconjunctival injection. In vivo study was performed in mice and nanoparticles containing pRNA or dsRNA were labeled using Alexa647 for easy quantification and imaging [94]. Results indicated that pRNA and dsRNA were observed in sclera, corneal and conjunctiva cells, but only pRNA-X was detected in retinal cells [94]. These results suggest that RNA therapy can be useful and delivered to the back-of-the-eye tissues for the treatment of posterior segment neovascular diseases.
Inherited retinal diseases are complicated. At present there are no feasible treatment options for patients with inherited retinal diseases [95]. Consequently, gene therapy for the treatment of inherited and acquired ocular diseases is currently progressing. Mitra et al studied non-viral nanoparticles containing glycol chitosan and plasmid DNA for their stability against DNases, aggregation, plasmid stability and expression [96]. This study was conducted in vivo in albino mice and results point out that the particles were stable and no aggregation was observed [96]. Further, significant amount of plasmid expression was observed in the retinal pigmented epithelium on day 14 after sub-retinal injection [96]. Recent studies have shown multiple blindness is associated with mutations of the RPE65 gene [97]. Koirala et al have investigated persistence of a non-viral vector mediated RPE65 in terms of viability as a gene transfer therapy for diseases related with RPE. Nanoparticles containing plasmid DNA (VMD2-hRPE65-S/MAR) and naked DNA were sub-retinally injected in mice with RPE65gene mutation. Following injections, the animals were monitored for 15 months [97]. Results demonstrated that expression of RPE65 was positive improvement 32% for nanoparticles mediated delivery compared to 44 % for naked DNA [97]. Further, fundus and toxic reduction were observed in the eyes injected with nanoparticles and naked DNA compared to untreated eyes [97]. Results indicate that compacted DNA nanoparticles may be exploited as gene therapies for long term improvement of RPE degenerative diseases.
Recent studies have shown that mutations in gene RPE65 are associated with multiple blindness diseases [97]. Koirala et al have investigated persistence of a non-viral vector mediated RPE65 in terms of viability as a gene transfer therapy for diseases related with RPE. Nanoparticles containing plasmid DNA (VMD2-hRPE65-S/MAR) and naked DNA were subretinally injected in mice with RPE65gene mutation and animals were monitored for 15 months [97]. Results demonstrated that expression of RPE65 was 32% for nanoparticles mediated delivery compared to 44 % for naked DNA [97]. Further, funds and toxic reduction were observed in the eyes injected with nanoparticles and naked DNA compared to untreated eyes [97]. Results indicate that compacted DNA nanoparticles may be exploited as gene therapies for long term improvement of RPE degenerative diseases.
6.2.2 Microparticles
Acylation of peptides has been identified as a major challenge for sustained release of peptides from delivery systems [20,98,99]. Reversible hydrophobic ion pairing (HIP) complex strategy was used to minimize octreotide acylation during long time delivery from PLGA microparticles by Vaishya et al. Sodium dodecyl sulfate and dextran sulfate with different molecular weights were used as ion pairing agents to prepare HIP complex with octreotide [21]. Microparticles were prepared with HIP complexed with octreotide and PLGA polymer. Results showed higher encapsulation efficiency. Octreotide was released for extended time period. A large percentage of released octreotide was in native form and only less than 7% was acylated [21]. In addition, Shirangi et al inhibited acylation by temporarily and reversibly block the amine groups in the peptide chain with a protective group. Results indicated that octreotide with these modifications encapsulated in PLGA microsphere had 5% acylation while unprotected octreotide generated 52.5 % acylation after 50 days of incubation [99]. These studies clearly suggest that such modification on peptides can be utilized to deliver peptide to the back-of-the-eye tissues with lower acylated products.
Two different chitosan microparticle (CMP) formulations were prepared encapsulating either BSA or, green fluorescent protein fused to the activator of transcription peptide (tat-EGFP). In vitro release kinetics presented high release of BSA compared to tat-EGFP [100]. In vivo studies showed presence of CMPs in the photoreceptor layer of retina and remained there for at least 8 weeks after subretinal injection. While higher concentrations resulted into toxicity, lower concentrations were well tolerated [100].
6.3 Controlled Release Delivery Systems
6.3.1 Nanotubes
Nanotubes have also been exploited for back-of-the-eye therapeutic delivery. Panda et al studied self-assembly dipeptide phenylalanine-α, and β-hydrophenylalanine nanotubes. These delivery systems sustained intravitreal delivery of targeted tyrosine kinase inhibitor (pazopanib) [101]. Nanotubes were able to deliver pazopanib with 25% efficiency and no toxicity observed in retinal cells. Pazopanib was found in vitreous humor, retina and choroid RPE at higher concentrations for 15 days relative to pazopanib solution alone in vivo [101]. These results suggest that nanotubes can be applied as a delivery system which may sustain higher drug concentrations in ocular tissues.
6.3.3 Implants
Implants are a popular mode of ocular delivery. Commercially available implants include Vitrasert, Retisert, Ozurdex and Iluvien [102]. Most of these implants are confined to small molecule drugs. At present, no implants carrying biologics are available in the market, but some are in the pipeline. NT-501(Renexus, Neurotech) is an implantable encapsulated cell therapy device containing genetically modified human retinal pigmented epithelium cells that can secrete therapeutic doses of ciliary neurotrophic factor (CNTF) into the back-of-the-eye for the treatment of retinal degenerative diseases [103]. In addition to NT-501, NT-503 uses engineered RPE cells to produce soluble VEGF receptor protein (Fc-Fusion protein). The construct can be delivered with similar implants [103]. Port Delivery System (Genentech) is a non-biodegradable implant that is applicable to deliver ranibizumab into vitreous cavity over extended time period. The main important feature of this implant is its ability to refill via trans-conjunctival injection [104]. Implants presently on the market or undergoing clinical trials comprise nonbiodegradable polymers, encompassing drugs i.e. ganciclovir, fluocinolone acetonide, triamcinolone acetonide, and ranibizumab. Moreover, biodegradable polymers, containing dexamethasone, triamcinolone acetonide, and ranibizumab have also been developed [102].
6.3.4 Dendrimers
Dendrimers have been exploited as nanocarrier system in drug delivery. It is defined as a branched structure on the outside and a center core in encapsulating and targeting a variety of molecules. Dendrimers can entrap numerous molecular weights of either hydrophilic or hydrophobic molecules [105]. When designing a dendrimer it is vital to consider molecular weight, size, surface charge, molecular geometry and functional group as these parameters play key role in drug delivery [86]. Marano et al utilized amino acid dendrimers to deliver anti-VEGF oligonucleotide into the eye for the treatment of laser induced CNV. In vivo studies showed inhibition of CNV and oligonucleotide was observed in retinal cell layers [106]. Despite many advantages, dendrimer uses in ocular drug delivery have been limited due to high toxicity profile [107]. The interaction between positive charge of dendrimers and negative charge of biological membrane cause membrane disruption and erosion [108]. Many attempts have been invested to modify the surface charge of dendrimers to reduce the toxicity, such as acetylation [109], carbonhydrate conjugation [110], peptide conjugation [111] or PEGylation [112].
7. Future perspectives
Anti-VEGF, VEGF trap and aptamer therapies are recently approved for the treatment of back-of-the-eye diseases. These therapies represent an innovation to counteract neovascularization, edema, arresting and/or diminishing vision loss. Despite all the success with VEGF inhibitor therapies, several limitations exist for complete vision recovery. Therefore, there is a need to identify novel approaches that will reduce frequent intravitreal administration, increase patient compliance and complete vision restoration. Table 4 describes biologics that are currently in clinical trials.
Table 4.
List of biologics currently in different phases of clinical trials
| Drug | Type of Molecule | Biological Target | Clinical Phase | Disease |
|---|---|---|---|---|
| E10030/Fovista | DNA aptamer | PDGF-BB | III | AMD |
| ARC1905 | RNA aptamer | C5 complement | I | AMD |
| POT-4 | Peptide | C3 complement | II | AMD |
| Infliximab | Monoclonal antibody | TNF-α | II | AMD |
| Bevasiranib | siRNA | VEGF-A | III | AMD |
| siRNA-027 | siRNA | VEGFR-1 | I | AMD, CNV |
| PF-04523655 | siRNA | DDIT4 | II | AMD, DME, CNV |
| ALN-VSP02 | Dual siRNA | VEGF-A and KSP | I | |
| Ad-PEDF | Adenovirus/gene | PEDF | I | AMD, CNV |
| AAV-SFLT01 | Adeno-associated virus/gene | VEGF | I | AMD |
| JSM6427 | Monoclonal antibody | α5β1 integrin | I | AMD, CNV |
| Retinostat | Lentiviral vector/gene | Endostatin, angiostatin | I | AMD |
| Volociximab | Monoclonal antibody | α5β1 integrin | I | AMD |
| HI-con1 | Fusion Protein | Pathologic blood vessels | II | AMD, CNV |
| NT-503 | Human RPE cells/implant | VEGF | II | AMD, CNV |
| KH902 | Fusion Protein | VEGF | III | AMD, CNV, DME |
| Abicipar pegol/DARPins | Non antibody protein scaffold | VEGF-A | II/III | AMD, DME |
Source: [155]
AMD = Age related Macular Degeneration; CNV = Choroidal Neovascularization; DME=Diabetic Macular Edema
7.1 Aptamers
Aptamers are single stranded DNA or RNA (ssDNA or ssRNA) oligonucleotide ligands that bind to molecular targets with high affinity and specificity. RNA aptamer (Pegaptanib) has been indicated for the treatment of posterior ocular diseases and have shown significant improvement in the treatment of back-of-the-eye diseases [17]. Another aptamer E10030 which is a DNA aptamer, has been indicated against platelets-derived growth factor (PDGF) which stimulates angiogenesis. It is currently in Phase III clinical trial, in combination with anti-VEGF agents for the treatment of AMD [113–115].
Studies reported that inhibition of PDGF escalates sensitivity of endothelial cells to anti-VEGF agents [114,116]. Therefore, combination therapy of anti-VEGF and other growth factors may be beneficial to improve vision. Another aptamer ARC1905 has also been utilized in combination with ranibizumab for the treatment of subfoveal CNV secondary to AMD [17,113].
7.2 Small Interference RNAs
Small interfering RNA (siRNA) sometimes referred to as short interfering or silencing RNA is a double stranded RNA molecule with 20–25 base pairs. Such siRNA provides an opportunity to induce gene silencing in cells. Usually the gene silencing takes place after transcription stage. The application of siRNA for various posterior segment ocular diseases may be considered as a promising approach. An exploitation of siRNA directed against VEGF for the treatment of neovascularization has been demonstrated. Bevasiranib is a dsRNA with 21 nucleotide base pairs targeting VEGF-A. Biodistribution and pharmacokinetics studies of bevasiranib after single intravitreal injection in rabbit eyes show that radiolabeled dsRNA was detected in vitreous, retina, RPE, choroid and sclera with highest concentrations found in the vitreous [117]. Another siRNA-027(AGN211745) has been designed to target VEGFR-1 [118,119]. PF-04523655 (RTP-801) which targets DNA damage inducible transcript 4 (DDIT4) [120,121] is currently in clinical trial. siRNA therapy is well tolerated in patients with neovascular AMD and may improve in visual acuity [17,119,121]. Thus siRNA therapy represents an important new class of therapy against uncontrolled angiogenesis.
7.3 Stem Cells
Stem cell therapy has been investigated mostly for the treatment of cancer diseases. Emerging stem cell therapies for restoration of sight should be focused on two areas such as cornea and retina [122]. Limbal stem cell deficiency (LSCD) can lead to corneal vascularization which may result in visual impairment or blindness [123]. Limbal stem cells have been transplanted successfully from an exogenous source other than the patient with successful renewal of corneal epithelium [123,124]. Limbal cells may be collected from donors, or cells grown in culture. Stem cell therapy has been confirmed as a treatment for anterior segment diseases [124]; and may be used for posterior segment diseases too (Please see 6.3.3 Implants).
Besides aptamers, siRNA and stem cells, antisense oligonucleotides may also be considered as treatment regimen in vision impairment. Oligonucleotides function by blocking synthesis of proteins interfering at the transcription or translation levels. Therefore, an oligonucleotide can be developed to block proteins synthesis such as VEGF. It can be delivered to the back-of-the-eye from topical administration. This may constitute a significant improvement in treatment of retinal pathologies.
8. CONCLUSIONS
The delivery of therapeutic biologics to back-of-the-eye tissues is a formidable task at this present time. While the mechanism by which biologics are effective are yet to be fully understood, an ideal delivery system with effective drug levels at given time over an extended period with minimizing systemic drug exposure requires more standardization. Moreover, the delivery system should emphasize patient compliance with the ability to be self-administered. Recent anti-VEGF therapies have proved to be an example of a more efficient treatment modality. However, it is important to explore other biologics that may provide a superior treatment outcome. Patient compliance will continue to be emphasized in designing future retinal drug delivery systems. A reasonable strategy to circumvent drawbacks of an individual technology is to combine the benefits from several varying technologies.
9. EXPERT OPINION
Treatment of posterior ocular diseases is a challenge. At present, anti-VEGFs are the main strain and first line of treatment. Moreover, anti-VEGF may be considered as gold standard. However, other therapeutics such as siRNA, oligonucleotides, aptamers and gene therapy are still under investigation for posterior ocular disease treatment. On the other hand, stem cell implants and fusion protein technologies for biologics have demonstrated remarkable results in clinical trials. Despite all these therapeutics, attention should be emphasized to improve delivery of biologics to back-of-the eye with non-invasive technology. Molecular weight, size, charge and shape and biological barriers definitely add hindrance to posterior ocular delivery. At present, most of the biologics are delivered with an invasive technique i.e, as intravitreal injection. Such administration of biologics lack specificity, sustained drug delivery and may be eliminated from vitreous cavity resulting in requirement of frequent drug administration to maintain therapeutic levels. Transcleral or conjunctival-scleral pathway and uveal pathway may be explored as an alternative route of drug delivery to back-of-the eye. A delivery system encapsulating macromolecules with high drug loading and unchanged confirmation upon release providing sustained release is highly desirable. Moreover, such delivery system should be patient compliance and affordable. Such a drug delivery system may utilize either of the two pathways (transcleral or and uveal pathway) to reach back of the eye is highly desirable. Example of such system include nanomicelles. Nanomicelles with their small size and hydrophilic surface corona have been demonstrated to deliver cyclosporine to back-of-the-eye tissues following topical drop administration on to cornea [28]. Such a polymeric delivery systems with small size and hydrophilic surface may be further explored to non-invasively delivery macromolecules to posterior ocular tissues. Moreover, sustain drug release may be achieved with hydrogels. Encapsulating macromolecules/biologics in polymeric nanomicelles and their incorporation into hydrogels sounds potential for sustained drug delivery. Such an incorporation may not only sustain macromolecule drug release but also maintains therapeutic drug levels and reduces frequency of drug administration. Moreover, delivery of drugs to back-of-the-eye may be achieved following topical drop administration into cul-de-sac or precorneal pocket. In near future, such a technology may replace current invasive methods of drug administration techniques such as intravitreal and/or periocular injection resulting in an economical and patient compliance method of posterior ocular disease treatment.
Article highlights box.
Back of the eye diseases such as AMD, DME and DR are vision threatening diseases, normally diagnosed in elderly population.
Treatment of posterior ocular diseases is challenging due to ocular barriers as well as physicochemical properties of therapeutic agents.
Biologics also known as macromolecules have recently gained attention as therapeutic agents used to treat back of the eye diseases. These molecules have shown remarkable results relative to small molecule drugs and other treatments
Small number of biologics have been approved and several are in the pipeline for treatment of back of the eye diseases.
With increase in number of biologics in pipeline; an ideal delivery system, one that will ensure stability of the molecule, sustained release, patient compliance and affordable needs to be developed.
Acknowledgments
Funding
The authors were supported by grants from the U.S. Department of Health and Human Services, National Institutes of Health, National Eye Institute (R01-EY-09171, R01-EY-10569).
ABBREVIATIONS
- AMD
Age Macular Degeneration
- BAB
Blood Aqueous Barrier
- BSA
Bovine Serum Albumin
- CME
Cystoid Macular Edema
- CNV
Choroidal Neovascularization
- DME
Diabetic Macular Edema
- DNA
Deoxyribonucleic Acid
- DR
Diabetic Retinopathy
- HIP
Hydrophobic Ion Pairing
- IOP
Intraocular Pressure
- LSCD
Limbal Stem Cell Deficiency
- PIC
Polyion Complex
- PDGF
Platelets Derived Growth Factor
- PLA
Poly Lactic Acid
- PLGA
Poly (Lactic-co-Glycolic) Acid
- PVR
Proliferative Vitreoretinopathy
- VEGF
Vascular Endothelial Growth Factor
- RNA
Ribonucleic Acid
- RPE
Retinal Pigmented Epithelium
- RVO
Retinal Vein Occlusion
- siRNA
Small Interference Ribonucleic Acid
Footnotes
Declaration of interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.The global economic cost of visual impairment. 2014 http://www.icoph.org/resources/146/The-Global-Economic-Cost-of-Visual-Impairment.html.
- 2.Boddu SH, Gupta H, Patel S. Drug delivery to the back of the eye following topical administration: An update on research and patenting activity. Recent patents on drug delivery & formulation. 2014;8(1):27–36. doi: 10.2174/1872211308666140130093301. [DOI] [PubMed] [Google Scholar]
- 3.Gaudana R, Ananthula HK, Parenky A, Mitra AK. Ocular drug delivery. The AAPS journal. 2010;12(3):348–360. doi: 10.1208/s12248-010-9183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zajac-Pytrus HM, Pilecka A, Turno-Krecicka A, Adamiec-Mroczek J, Misiuk-Hojlo M. The dry form of age-related macular degeneration (amd): The current concepts of pathogenesis and prospects for treatment. Advances in clinical and experimental medicine: official organ Wroclaw Medical University. 2015;24(6):1099–1104. doi: 10.17219/acem/27093. [DOI] [PubMed] [Google Scholar]
- 5.Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye and vision. 2015;2(17) doi: 10.1186/s40662-015-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiersbitzky S, Ballke EH, Burghardt R, Spangenberg U, Joswig T, Baufeld W, Ordt HA, Paul W. long-term study of various immunologic functions in children with chronic nonspecific lung diseases. Zeitschrift fur Erkrankungen der Atmungsorgane. 1985;164(3):241–253. [PubMed] [Google Scholar]
- 7.Mohamed R, El-Remessy AB. Imbalance of the nerve growth factor and its precursor: Implication in diabetic retinopathy. Journal of clinical & experimental ophthalmology. 2015;6(5) doi: 10.4172/2155-9570.1000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8•.Fogli S, Mogavero S, Egan CG, Del Re M, Danesi R. Pathophysiology and pharmacological targets of vegf in diabetic macular edema. Pharmacological research. 2016;103:149–157. doi: 10.1016/j.phrs.2015.11.003. This is a detail overview and updated diabetic macular edema treatment. [DOI] [PubMed] [Google Scholar]
- 9.Takeuchi M. A systematic review of biologics for the treatment of noninfectious uveitis. Immunotherapy. 2013;5(1):91–102. doi: 10.2217/imt.12.134. [DOI] [PubMed] [Google Scholar]
- 10••.Posarelli C, Arapi I, Figus M, Neri P. Biologic agents in inflammatory eye disease. Journal of ophthalmic & vision research. 2011;6(4):309–316. The different class of macromolecules have been indicated for the inflammatory and the detail pathways of those macomolecules have been discuued) [PMC free article] [PubMed] [Google Scholar]
- 11.McKibbin M, Devonport H, Gale R, Gavin M, Lotery A, Mahmood S, Patel PJ, Ross A, Sivaprasad S, Talks J, Walters G. Aflibercept in wet amd beyond the first year of treatment: Recommendations by an expert roundtable panel. Eye. 2015;29(Suppl 1):S1–S11. doi: 10.1038/eye.2015.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heier JS, Bressler NM, Avery RL, Bakri SJ, Boyer DS, Brown DM, Dugel PU, Freund KB, Glassman AR, Kim JE, Martin DF, et al. Comparison of aflibercept, bevacizumab, and ranibizumab for treatment of diabetic macular edema: Extrapolation of data to clinical practice. JAMA ophthalmology. 2016;134(1):95–99. doi: 10.1001/jamaophthalmol.2015.4110. [DOI] [PubMed] [Google Scholar]
- 13.Bressler NM, Varma R, Mitchell P, Suner IJ, Dolan C, Ward J, Ferreira A, Ehrlich JS, Turpcu A. Effect of ranibizumab on the decision to drive and vision function relevant to driving in patients with diabetic macular edema: Report from restore, ride, and rise trials. JAMA ophthalmology. 2016;134(2):160–166. doi: 10.1001/jamaophthalmol.2015.4636. [DOI] [PubMed] [Google Scholar]
- 14.Gibson JM, McGinnigle S. Diabetes: Intravitreous ranibizumab for proliferative diabetic retinopathy. Nature reviews Endocrinology. 2016;12(3):130–131. doi: 10.1038/nrendo.2016.1. [DOI] [PubMed] [Google Scholar]
- 15.Rush RB, Rush SW. Ranibizumab versus bevacizumab for neovascular age-related macular degeneration with an incomplete posterior vitreous detachment. Asia-Pacific journal of ophthalmology. 2016;5(3):171–175. doi: 10.1097/APO.0000000000000157. [DOI] [PubMed] [Google Scholar]
- 16.Chin-Yee D, Eck T, Fowler S, Hardi A, Apte RS. A systematic review of as needed versus treat and extend ranibizumab or bevacizumab treatment regimens for neovascular age-related macular degeneration. The British journal of ophthalmology. 2015 doi: 10.1136/bjophthalmol-2015-306987. [DOI] [PubMed] [Google Scholar]
- 17••.Amadio M, Govoni S, Pascale A. Targeting vegf in eye neovascularization: What’s new?: A comprehensive review on current therapies and oligonucleotide-based interventions under development. Pharmacological research. 2016;103:253–269. doi: 10.1016/j.phrs.2015.11.027. This review has summerized all the anti-VEGF drugs along with their pharmacological mechanisms and target and activities. [DOI] [PubMed] [Google Scholar]
- 18.Ho M, Liu DT, Lam DS, Jonas JB. Retinal vein occlusions, from basics to the latest treatment. Retina. 2016;36(3):432–448. doi: 10.1097/IAE.0000000000000843. [DOI] [PubMed] [Google Scholar]
- 19.Cholkar K, Patel A, Vadlapudi AD, Mitra AK. Novel nanomicellar formulation approaches for anterior and posterior segment ocular drug delivery. Recent patents on nanomedicine. 2012;2(2):82–95. doi: 10.2174/1877912311202020082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaishya RD, Mandal A, Patel S, Mitra AK. Extended release microparticle-in-gel formulation of octreotide: Effect of polymer type on acylation of peptide during in vitro release. International journal of pharmaceutics. 2015;496(2):676–688. doi: 10.1016/j.ijpharm.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaishya RD, Mandal A, Gokulgandhi M, Patel S, Mitra AK. Reversible hydrophobic ion-paring complex strategy to minimize acylation of octreotide during long-term delivery from plga microparticles. International journal of pharmaceutics. 2015;489(1–2):237–245. doi: 10.1016/j.ijpharm.2015.04.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Good TJ, Kimura AE, Mandava N, Kahook MY. Sustained elevation of intraocular pressure after intravitreal injections of anti-vegf agents. The British journal of ophthalmology. 2011;95(8):1111–1114. doi: 10.1136/bjo.2010.180729. [DOI] [PubMed] [Google Scholar]
- 23.Hoang QV, Tsuang AJ, Gelman R, Mendonca LS, Della Torre KE, Jung JJ, Freund KB. Clinical predictors of sustained intraocular pressure elevation due to intravitreal anti-vascular endothelial growth factor therapy. Retina. 2013;33(1):179–187. doi: 10.1097/IAE.0b013e318261a6f7. [DOI] [PubMed] [Google Scholar]
- 24.Yu AL, Seidensticker F, Schaumberger M, Welge-Lussen U, Wolf A. Evaluation of intraocular pressure elevation after multiple injections of intravitreal ranibizumab. Clinical ophthalmology. 2014;8:743–747. doi: 10.2147/OPTH.S58410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel SP, Vaishya R, Pal D, Mitra AK. Novel pentablock copolymer-based nanoparticulate systems for sustained protein delivery. AAPS PharmSciTech. 2015;16(2):327–343. doi: 10.1208/s12249-014-0196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozaki T, Nakazawa M, Yamashita T, Ishiguro S. Delivery of topically applied calpain inhibitory peptide to the posterior segment of the rat eye. PloS one. 2015;10(6):e0130986. doi: 10.1371/journal.pone.0130986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schopf LR, Popov AM, Enlow EM, Bourassa JL, Ong WZ, Nowak P, Chen H. Topical ocular drug delivery to the back of the eye by mucus-penetrating particles. Translational vision science & technology. 2015;4(3):11. doi: 10.1167/tvst.4.3.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28•.Cholkar K, Gilger BC, Mitra AK. Topical, aqueous, clear cyclosporine formulation design for anterior and posterior ocular delivery. Translational vision science & technology. 2015;4(3):1. doi: 10.1167/tvst.4.3.1. This is the first article that deals with clear, micellar topical formulations of cyclosporine for ocular delivery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moisseiev E, Waisbourd M, Ben-Artsi E, Levinger E, Barak A, Daniels T, Csaky K, Loewenstein A, Barequet IS. Pharmacokinetics of bevacizumab after topical and intravitreal administration in human eyes. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2014;252(2):331–337. doi: 10.1007/s00417-013-2495-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis BM, Normando EM, Guo L, Turner LA, Nizari S, O’Shea P, Moss SE, Somavarapu S, Cordeiro MF. Topical delivery of avastin to the posterior segment of the eye in vivo using annexin a5-associated liposomes. Small. 2014;10(8):1575–1584. doi: 10.1002/smll.201303433. [DOI] [PubMed] [Google Scholar]
- 31.Chen JJ, Ebmeier SE, Sutherland WM, Ghazi NG. Potential penetration of topical ranibizumab (lucentis) in the rabbit eye. Eye. 2011;25(11):1504–1511. doi: 10.1038/eye.2011.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ford KM, Saint-Geniez M, Walshe TE, D’Amore PA. Expression and role of vegf--a in the ciliary body. Investigative ophthalmology & visual science. 2012;53(12):7520–7527. doi: 10.1167/iovs.12-10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ciulla TA, Amador AG, Zinman B. Diabetic retinopathy and diabetic macular edema: Pathophysiology, screening, and novel therapies. Diabetes care. 2003;26(9):2653–2664. doi: 10.2337/diacare.26.9.2653. [DOI] [PubMed] [Google Scholar]
- 34.Shah CA. Diabetic retinopathy: A comprehensive review. Indian J Med Sci. 2008;62(12):500–519. [PubMed] [Google Scholar]
- 35.Simo R, Carrasco E, Garcia-Ramirez M, Hernandez C. Angiogenic and antiangiogenic factors in proliferative diabetic retinopathy. Current diabetes reviews. 2006;2(1):71–98. doi: 10.2174/157339906775473671. [DOI] [PubMed] [Google Scholar]
- 36.Rotsos TG, Moschos MM. Cystoid macular edema. Clinical ophthalmology. 2008;2(4):919–930. doi: 10.2147/opth.s4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marashi A. Using anti-vegf in diabetic retinopathy. Adv Ophthalmol Vis Syst. 2016;4(4) [Google Scholar]
- 38.Crawford TN, Alfaro DV, 3rd, Kerrison JB, Jablon EP. Diabetic retinopathy and angiogenesis. Current diabetes reviews. 2009;5(1):8–13. doi: 10.2174/157339909787314149. [DOI] [PubMed] [Google Scholar]
- 39.Gupta N, Mansoor S, Sharma A, Sapkal A, Sheth J, Falatoonzadeh P, Kuppermann B, Kenney M. Diabetic retinopathy and vegf. Open Ophthalmol J. 2013;7:4–10. doi: 10.2174/1874364101307010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim JH, Kim JH, Yu YS, Cho CS, Kim KW. Blockade of angiotensin ii attenuates vegf-mediated blood-retinal barrier breakdown in diabetic retinopathy. J Cereb Blood Flow Metab. 2009;29(3):621–628. doi: 10.1038/jcbfm.2008.154. [DOI] [PubMed] [Google Scholar]
- 41•.Wang Y, Yan H. Microrna-126 contributes to niaspan treatment induced vascular restoration after diabetic retinopathy. Scientific reports. 2016;6:26909. doi: 10.1038/srep26909. While the microrna - 126 reponse for angiogenesis with several therapeutic targets. Niaspan treatment significantly improved clinical and histopathological outcomes of DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nowak JZ. Age-related macular degeneration (amd): Pathogenesis and therapy. Pharmacol Rep. 2006;58(3):353–363. [PubMed] [Google Scholar]
- 43.Chappelow AV, Kaiser PK. Neovascular age-related macular degeneration: Potential therapies. Drugs. 2008;68(8):1029–1036. doi: 10.2165/00003495-200868080-00002. [DOI] [PubMed] [Google Scholar]
- 44.Green WR, McDonnell PJ, Yeo JH. Pathologic features of senile macular degeneration. 1985. Retina. 2005;25(5 Suppl):615–627. doi: 10.1097/00006982-200507001-00011. [DOI] [PubMed] [Google Scholar]
- 45.Bressler SB, Silva JC, Bressler NM, Alexander J, Green WR. Clinicopathologic correlation of occult choroidal neovascularization in age-related macular degeneration 1992. Retina. 2005;25(5 Suppl):827–832. doi: 10.1097/00006982-200507001-00014. [DOI] [PubMed] [Google Scholar]
- 46••.Agarwal A, Rhoades WR, Hanout M, Soliman MK, Sarwar S, Sadiq MA, Sepah YJ, Do DV, Nguyen QD. Management of neovascular age-related macular degeneration: Current state-of-the-art care for optimizing visual outcomes and therapies in development. Clinical ophthalmology. 2015;9:1001–1015. doi: 10.2147/OPTH.S74959. This review has summarized all the available or potential therapies for AMD and discussed their advantages and disadvantages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pastor JC. Proliferative vitreoretinopathy: An overview. Survey of ophthalmology. 1998;43(1):3–18. doi: 10.1016/s0039-6257(98)00023-x. [DOI] [PubMed] [Google Scholar]
- 48.Kon CH, Asaria RH, Occleston NL, Khaw PT, Aylward GW. Risk factors for proliferative vitreoretinopathy after primary vitrectomy: A prospective study. The British journal of ophthalmology. 2000;84(5):506–511. doi: 10.1136/bjo.84.5.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sadaka A, Giuliari GP. Proliferative vitreoretinopathy: Current and emerging treatments. Clinical ophthalmology. 2012;6:1325–1333. doi: 10.2147/OPTH.S27896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oh Woong Kwon MIR, Song Ji Hun. Retinal detachment and proliferative vitreoretinopathy. Retinal diseases amenable to pharmacotherapy. :147–151. [Google Scholar]
- 51.Doughty MJ, Zaman ML. Human corneal thickness and its impact on intraocular pressure measures: A review and meta-analysis approach. Survey of ophthalmology. 2000;44(5):367–408. doi: 10.1016/s0039-6257(00)00110-7. [DOI] [PubMed] [Google Scholar]
- 52.Yi X, Wang Y, Yu FS. Corneal epithelial tight junctions and their response to lipopolysaccharide challenge. Investigative ophthalmology & visual science. 2000;41(13):4093–4100. [PubMed] [Google Scholar]
- 53.Mitic LL, Van Itallie CM, Anderson JM. Molecular physiology and pathophysiology of tight junctions i. Tight junction structure and function: Lessons from mutant animals and proteins. American journal of physiology Gastrointestinal and liver physiology. 2000;279(2):G250–254. doi: 10.1152/ajpgi.2000.279.2.G250. [DOI] [PubMed] [Google Scholar]
- 54.Edward A, Prausnitz MR. Predicted permeability of the cornea to topical drugs. Pharmaceutical research. 2001;18(11):1497–1508. doi: 10.1023/a:1013061926851. [DOI] [PubMed] [Google Scholar]
- 55.van Haeringen NJ, Glasius E. Lysosomal hydrolases in tears and the lacrimal gland: Effect of acetylsalicylic acid on the release from the lacrimal gland. Investigative ophthalmology & visual science. 1980;19(7):826–829. [PubMed] [Google Scholar]
- 56.Huang AJ, Tseng SC, Kenyon KR. Paracellular permeability of corneal and conjunctival epithelia. Investigative ophthalmology & visual science. 1989;30(4):684–689. [PubMed] [Google Scholar]
- 57.Kim SH, Galban CJ, Lutz RJ, Dedrick RL, Csaky KG, Lizak MJ, Wang NS, Tansey G, Robinson MR. Assessment of subconjunctival and intrascleral drug delivery to the posterior segment using dynamic contrast-enhanced magnetic resonance imaging. Investigative ophthalmology & visual science. 2007;48(2):808–814. doi: 10.1167/iovs.06-0670. [DOI] [PubMed] [Google Scholar]
- 58.Nakao S, Hafezi-Moghadam A, Ishibashi T. Lymphatics and lymphangiogenesis in the eye. Journal of ophthalmology. 2012;2012:783163. doi: 10.1155/2012/783163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bellhorn RW. Permeability of blood-ocular barriers of neonatal and adult cats to fluorescein-labeled dextrans of selected molecular sizes. Investigative ophthalmology & visual science. 1981;21(2):282–290. [PubMed] [Google Scholar]
- 60.Prausnitz MR, Noonan JS. Permeability of cornea, sclera, and conjunctiva: A literature analysis for drug delivery to the eye. Journal of pharmaceutical sciences. 1998;87(12):1479–1488. doi: 10.1021/js9802594. [DOI] [PubMed] [Google Scholar]
- 61.Olsen TW, Edelhauser HF, Lim JI, Geroski DH. Human scleral permeability. Effects of age, cryotherapy, transscleral diode laser, and surgical thinning. Investigative ophthalmology & visual science. 1995;36(9):1893–1903. [PubMed] [Google Scholar]
- 62.Maurice DM, Polgar J. Diffusion across the sclera. Experimental eye research. 1977;25(6):577–582. doi: 10.1016/0014-4835(77)90136-1. [DOI] [PubMed] [Google Scholar]
- 63.Wen H, Hao J, Li SK. Characterization of human sclera barrier properties for transscleral delivery of bevacizumab and ranibizumab. Journal of pharmaceutical sciences. 2013;102(3):892–903. doi: 10.1002/jps.23387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pino RM, Essner E. Permeability of rat choriocapillaris to hemeproteins. Restriction of tracers by a fenestrated endothelium. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society. 1981;29(2):281–290. doi: 10.1177/29.2.7252121. [DOI] [PubMed] [Google Scholar]
- 65.Essner E, Gordon SR. Observations on the permeability of the choriocapillaris of the eye. Cell and tissue research. 1983;231(3):571–577. doi: 10.1007/BF00218115. [DOI] [PubMed] [Google Scholar]
- 66.Pitkanen L, Ranta VP, Moilanen H, Urtti A. Permeability of retinal pigment epithelium: Effects of permeant molecular weight and lipophilicity. Investigative ophthalmology & visual science. 2005;46(2):641–646. doi: 10.1167/iovs.04-1051. [DOI] [PubMed] [Google Scholar]
- 67.Jackson TL, Antcliff RJ, Hillenkamp J, Marshall J. Human retinal molecular weight exclusion limit and estimate of species variation. Investigative ophthalmology & visual science. 2003;44(5):2141–2146. doi: 10.1167/iovs.02-1027. [DOI] [PubMed] [Google Scholar]
- 68.Tao Y, Li XX, Jiang YR, Bai XB, Wu BD, Dong JQ. Diffusion of macromolecule through retina after experimental branch retinal vein occlusion and estimate of intraretinal barrier. Current drug metabolism. 2007;8(2):151–156. doi: 10.2174/138920007779815968. [DOI] [PubMed] [Google Scholar]
- 69.Mordenti J, Cuthbertson RA, Ferrara N, Thomsen K, Berleau L, Licko V, Allen PC, Valverde CR, Meng YG, Fei DT, Fourre KM, et al. Comparisons of the intraocular tissue distribution, pharmacokinetics, and safety of 125i-labeled full-length and fab antibodies in rhesus monkeys following intravitreal administration. Toxicologic pathology. 1999;27(5):536–544. doi: 10.1177/019262339902700507. [DOI] [PubMed] [Google Scholar]
- 70.Tojo K. A pharmacokinetic model for ocular drug delivery. Chemical & pharmaceutical bulletin. 2004;52(11):1290–1294. doi: 10.1248/cpb.52.1290. [DOI] [PubMed] [Google Scholar]
- 71.Bakri SJ, Snyder MR, Reid JM, Pulido JS, Ezzat MK, Singh RJ. Pharmacokinetics of intravitreal ranibizumab (lucentis) Ophthalmology. 2007;114(12):2179–2182. doi: 10.1016/j.ophtha.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 72.Fauser S, Kalbacher H, Alteheld N, Koizumi K, Krohne TU, Joussen AM. Pharmacokinetics and safety of intravitreally delivered etanercept. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2004;242(7):582–586. doi: 10.1007/s00417-004-0895-x. [DOI] [PubMed] [Google Scholar]
- 73.Iyer MN, He F, Wensel TG, Mieler WF, Benz MS, Holz ER. Intravitreal clearance of moxifloxacin. Transactions of the American Ophthalmological Society. 2005;103:76–81. discussion 81–73. [PMC free article] [PubMed] [Google Scholar]
- 74.Del Amo EM, Urtti A. Rabbit as an animal model for intravitreal pharmacokinetics: Clinical predictability and quality of the published data. Experimental eye research. 2015;137:111–124. doi: 10.1016/j.exer.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 75.Xu L, Lu T, Tuomi L, Jumbe N, Lu J, Eppler S, Kuebler P, Damico-Beyer LA, Joshi A. Pharmacokinetics of ranibizumab in patients with neovascular age-related macular degeneration: A population approach. Investigative ophthalmology & visual science. 2013;54(3):1616–1624. doi: 10.1167/iovs.12-10260. [DOI] [PubMed] [Google Scholar]
- 76.Gaudreault J, Fei D, Rusit J, Suboc P, Shiu V. Preclinical pharmacokinetics of ranibizumab (rhufabv2) after a single intravitreal administration. Investigative ophthalmology & visual science. 2005;46(2):726–733. doi: 10.1167/iovs.04-0601. [DOI] [PubMed] [Google Scholar]
- 77.Le KN, Gibiansky L, Good J, Davancaze T, van Lookeren Campagne M, Loyet KM, Morimoto A, Jin J, Damico-Beyer LA, Hanley WD. A mechanistic pharmacokinetic/pharmacodynamic model of factor d inhibition in cynomolgus monkeys by lampalizumab for the treatment of geographic atrophy. The Journal of pharmacology and experimental therapeutics. 2015;355(2):288–296. doi: 10.1124/jpet.115.227223. [DOI] [PubMed] [Google Scholar]
- 78.Drolet DW, Nelson J, Tucker CE, Zack PM, Nixon K, Bolin R, Judkins MB, Farmer JA, Wolf JL, Gill SC, Bendele RA. Pharmacokinetics and safety of an anti-vascular endothelial growth factor aptamer (nx1838) following injection into the vitreous humor of rhesus monkeys. Pharmaceutical research. 2000;17(12):1503–1510. doi: 10.1023/a:1007657109012. [DOI] [PubMed] [Google Scholar]
- 79.Ng EW, Shima DT, Calias P, Cunningham ET, Jr, Guyer DR, Adamis AP. Pegaptanib, a targeted anti-vegf aptamer for ocular vascular disease. Nature reviews Drug discovery. 2006;5(2):123–132. doi: 10.1038/nrd1955. [DOI] [PubMed] [Google Scholar]
- 80.Vadlapudi AD, Mitra AK. Nanomicelles: An emerging platform for drug delivery to the eye. Therapeutic delivery. 2013;4(1):1–3. doi: 10.4155/tde.12.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Iriyama A, Oba M, Ishii T, Nishiyama N, Kataoka K, Tamaki Y, Yanagi Y. Gene transfer using micellar nanovectors inhibits choroidal neovascularization in vivo. PloS one. 2011;6(12):e28560. doi: 10.1371/journal.pone.0028560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ideta R, Yanagi Y, Tamaki Y, Tasaka F, Harada A, Kataoka K. Effective accumulation of polyion complex micelle to experimental choroidal neovascularization in rats. FEBS letters. 2004;557(1–3):21–25. doi: 10.1016/s0014-5793(03)01315-2. [DOI] [PubMed] [Google Scholar]
- 83.Oh EJ, Choi JS, Kim H, Joo CK, Hahn SK. Anti-flt1 peptide - hyaluronate conjugate for the treatment of retinal neovascularization and diabetic retinopathy. Biomaterials. 2011;32(11):3115–3123. doi: 10.1016/j.biomaterials.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 84.Kim H, Choi JS, Kim KS, Yang JA, Joo CK, Hahn SK. Flt1 peptide-hyaluronate conjugate micelle-like nanoparticles encapsulating genistein for the treatment of ocular neovascularization. Acta biomaterialia. 2012;8(11):3932–3940. doi: 10.1016/j.actbio.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 85.Ing TS, Yu AW, Thompson KD, Ansari AU, McShane AP, Gandhi VC, Filkins JP. Peritoneal dialysis using conventional, lactate--containing solution sterilized by ultrafiltration. The International journal of artificial organs. 1992;15(11):658–660. [PubMed] [Google Scholar]
- 86••.Patel A, Cholkar K, Agrahari V, Mitra AK. Ocular drug delivery systems: An overview. World journal of pharmacology. 2013;2(2):47–64. doi: 10.5497/wjp.v2.i2.47. This review has provided an overview of major delivery systems for ocular therapeutics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lajavardi L, Bochot A, Camelo S, Goldenberg B, Naud MC, Behar-Cohen F, Fattal E, de Kozak Y. Downregulation of endotoxin-induced uveitis by intravitreal injection of vasoactive intestinal peptide encapsulated in liposomes. Investigative ophthalmology & visual science. 2007;48(7):3230–3238. doi: 10.1167/iovs.06-1305. [DOI] [PubMed] [Google Scholar]
- 88.Lajavardi L, Camelo S, Agnely F, Luo W, Goldenberg B, Naud MC, Behar-Cohen F, de Kozak Y, Bochot A. New formulation of vasoactive intestinal peptide using liposomes in hyaluronic acid gel for uveitis. Journal of controlled release: official journal of the Controlled Release Society. 2009;139(1):22–30. doi: 10.1016/j.jconrel.2009.05.033. [DOI] [PubMed] [Google Scholar]
- 89.Abrishami M, Zarei-Ghanavati S, Soroush D, Rouhbakhsh M, Jaafari MR, Malaekeh-Nikouei B. Preparation, characterization, and in vivo evaluation of nanoliposomes-encapsulated bevacizumab (avastin) for intravitreal administration. Retina. 2009;29(5):699–703. doi: 10.1097/IAE.0b013e3181a2f42a. [DOI] [PubMed] [Google Scholar]
- 90.Baranowski P, Karolewicz B, Gajda M, Pluta J. Ophthalmic drug dosage forms: Characterisation and research methods. TheScientificWorldJournal. 2014;2014:861904. doi: 10.1155/2014/861904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Patel SP, Vaishya R, Mishra GP, Tamboli V, Pal D, Mitra AK. Tailor-made pentablock copolymer based formulation for sustained ocular delivery of protein therapeutics. Journal of drug delivery. 2014;2014:401747. doi: 10.1155/2014/401747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Patel SP, Vaishya R, Yang X, Pal D, Mitra AK. Novel thermosensitive pentablock copolymers for sustained delivery of proteins in the treatment of posterior segment diseases. Protein and peptide letters. 2014;21(11):1185–1200. doi: 10.2174/092986652111141001122054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93•.Yandrapu SK, Upadhyay AK, Petrash JM, Kompella UB. Nanoparticles in porous microparticles prepared by supercritical infusion and pressure quench technology for sustained delivery of bevacizumab. Molecular pharmaceutics. 2013;10(12):4676–4686. doi: 10.1021/mp400487f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Feng L, Li SK, Liu H, Liu CY, LaSance K, Haque F, Shu D, Guo P. Ocular delivery of prna nanoparticles: Distribution and clearance after subconjunctival injection. Pharmaceutical research. 2014;31(4):1046–1058. doi: 10.1007/s11095-013-1226-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zulliger R, Conley SM, Naash MI. Non-viral therapeutic approaches to ocular diseases: An overview and future directions. Journal of controlled release: official journal of the Controlled Release Society. 2015;219:471–487. doi: 10.1016/j.jconrel.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mitra RN, Han Z, Merwin M, Al Taai M, Conley SM, Naash MI. Synthesis and characterization of glycol chitosan DNA nanoparticles for retinal gene delivery. ChemMedChem. 2014;9(1):189–196. doi: 10.1002/cmdc.201300371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Koirala A, Conley SM, Makkia R, Liu Z, Cooper MJ, Sparrow JR, Naash MI. Persistence of non-viral vector mediated rpe65 expression: Case for viability as a gene transfer therapy for rpe-based diseases. Journal of controlled release: official journal of the Controlled Release Society. 2013;172(3):745–752. doi: 10.1016/j.jconrel.2013.08.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98•.Ghassemi AH, van Steenbergen MJ, Barendregt A, Talsma H, Kok RJ, van Nostrum CF, Crommelin DJ, Hennink WE. Controlled release of octreotide and assessment of peptide acylation from poly(d,l-lactide-co-hydroxymethyl glycolide) compared to plga microspheres. Pharmaceutical research. 2012;29(1):110–120. doi: 10.1007/s11095-011-0517-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shirangi M, Najafi M, Rijkers DT, Kok RJ, Hennink WE, van Nostrum CF. Inhibition of octreotide acylation inside plga microspheres by derivatization of the amines of the peptide with a self-immolative protecting group. Bioconjugate chemistry. 2016;27(3):576–585. doi: 10.1021/acs.bioconjchem.5b00598. [DOI] [PubMed] [Google Scholar]
- 100.Wassmer S, Rafat M, Fong WG, Baker AN, Tsilfidis C. Chitosan microparticles for delivery of proteins to the retina. Acta biomaterialia. 2013;9(8):7855–7864. doi: 10.1016/j.actbio.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 101.Panda JJ, Yandrapu S, Kadam RS, Chauhan VS, Kompella UB. Self-assembled phenylalanine-alpha,beta-dehydrophenylalanine nanotubes for sustained intravitreal delivery of a multi-targeted tyrosine kinase inhibitor. Journal of controlled release: official journal of the Controlled Release Society. 2013;172(3):1151–1160. doi: 10.1016/j.jconrel.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 102.Wang J, Jiang A, Joshi M, Christoforidis J. Drug delivery implants in the treatment of vitreous inflammation. Mediators of inflammation. 2013;2013:780634. doi: 10.1155/2013/780634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sotoodehnejadnematalahi F, Burke B. Human activated macrophages and hypoxia: A comprehensive review of the literature. Iranian journal of basic medical sciences. 2014;17(11):820–830. [PMC free article] [PubMed] [Google Scholar]
- 104.Drug delivery to the posterior segment. http://www.neurotechusa.com/
- 105.Madaan K, Kumar S, Poonia N, Lather V, Pandita D. Dendrimers in drug delivery and targeting: Drug-dendrimer interactions and toxicity issues. Journal of pharmacy & bioallied sciences. 2014;6(3):139–150. doi: 10.4103/0975-7406.130965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Marano RJ, Toth I, Wimmer N, Brankov M, Rakoczy PE. Dendrimer delivery of an anti-vegf oligonucleotide into the eye: A long-term study into inhibition of laser-induced cnv, distribution, uptake and toxicity. Gene therapy. 2005;12(21):1544–1550. doi: 10.1038/sj.gt.3302579. [DOI] [PubMed] [Google Scholar]
- 107.Yavuz B, Pehlivan SB, Unlu N. Dendrimeric systems and their applications in ocular drug delivery. ScientificWorldJournal. 2013;2013:732340. doi: 10.1155/2013/732340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jain K, Kesharwani P, Gupta U, Jain NK. Dendrimer toxicity: Let’s meet the challenge. International journal of pharmaceutics. 2010;394(1–2):122–142. doi: 10.1016/j.ijpharm.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 109.Waite CL, Sparks SM, Uhrich KE, Roth CM. Acetylation of pamam dendrimers for cellular delivery of sirna. BMC Biotechnol. 2009;9:38. doi: 10.1186/1472-6750-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Agrawal P, Gupta U, Jain NK. Glycoconjugated peptide dendrimers-based nanoparticulate system for the delivery of chloroquine phosphate. Biomaterials. 2007;28(22):3349–3359. doi: 10.1016/j.biomaterials.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 111.Agashe HB, Dutta T, Garg M, Jain NK. Investigations on the toxicological profile of functionalized fifth-generation poly (propylene imine) dendrimer. J Pharm Pharmacol. 2006;58(11):1491–1498. doi: 10.1211/jpp.58.11.0010. [DOI] [PubMed] [Google Scholar]
- 112.Stasko NA, Johnson CB, Schoenfisch MH, Johnson TA, Holmuhamedov EL. Cytotoxicity of polypropylenimine dendrimer conjugates on cultured endothelial cells. Biomacromolecules. 2007;8(12):3853–3859. doi: 10.1021/bm7008203. [DOI] [PubMed] [Google Scholar]
- 113.2016 https://clinicaltrials.gov/ct2/show/NCT01940900.
- 114.Ishikawa M, Jin D, Sawada Y, Abe S, Yoshitomi T. Future therapies of wet age-related macular degeneration. Journal of ophthalmology. 2015;2015:138070. doi: 10.1155/2015/138070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.2016 http://www.ophthotech.com/wp-content/uploads/Fovista_Fact_Sheet.pdf.
- 116.Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. The Journal of clinical investigation. 2003;111(9):1287–1295. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dejneka NS, Wan S, Bond OS, Kornbrust DJ, Reich SJ. Ocular biodistribution of bevasiranib following a single intravitreal injection to rabbit eyes. Molecular vision. 2008;14:997–1005. [PMC free article] [PubMed] [Google Scholar]
- 118.Shen J, Samul R, Silva RL, Akiyama H, Liu H, Saishin Y, Hackett SF, Zinnen S, Kossen K, Fosnaugh K, Vargeese C, et al. Suppression of ocular neovascularization with sirna targeting vegf receptor 1. Gene therapy. 2006;13(3):225–234. doi: 10.1038/sj.gt.3302641. [DOI] [PubMed] [Google Scholar]
- 119.Kaiser PK, Symons RC, Shah SM, Quinlan EJ, Tabandeh H, Do DV, Reisen G, Lockridge JA, Short B, Guerciolini R, Nguyen QD, et al. Rnai-based treatment for neovascular age-related macular degeneration by sirna-027. American journal of ophthalmology. 2010;150(1):33–39. e32. doi: 10.1016/j.ajo.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 120.Rittenhouse KD, Johnson TR, Vicini P, Hirakawa B, Kalabat D, Yang AH, Huang W, Basile AS. Rtp801 gene expression is differentially upregulated in retinopathy and is silenced by pf-04523655, a 19-mer sirna directed against rtp801. Investigative ophthalmology & visual science. 2014;55(3):1232–1240. doi: 10.1167/iovs.13-13449. [DOI] [PubMed] [Google Scholar]
- 121.Nguyen QD, Schachar RA, Nduaka CI, Sperling M, Basile AS, Klamerus KJ, Chi-Burris K, Yan E, Paggiarino DA, Rosenblatt I, Khan A, et al. Phase 1 dose-escalation study of a sirna targeting the rtp801 gene in age-related macular degeneration patients. Eye. 2012;26(8):1099–1105. doi: 10.1038/eye.2012.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Atram Sandeep C, NNB, Wankhade Vikrant P, Dr, Pande1 Shrikant D, Dr, Tapar Kiran K. Current trends towards an ocular drug delivery system. International Journal of Pharmacy and Pharmaceutical Science Research. 2013;3(1):28–34. [Google Scholar]
- 123.Meller D, Thomasen H, Steuhl KP. ocular surface reconstruction in limbal stem cell deficiency: Transplantation of cultivated limbal epithelium. Der Ophthalmologe: Zeitschrift der Deutschen Ophthalmologischen Gesellschaft. 2012;109(9):863–868. doi: 10.1007/s00347-011-2510-y. [DOI] [PubMed] [Google Scholar]
- 124.Basu S, Mohamed A, Chaurasia S, Sejpal K, Vemuganti GK, Sangwan VS. Clinical outcomes of penetrating keratoplasty after autologous cultivated limbal epithelial transplantation for ocular surface burns. American journal of ophthalmology. 2011;152(6):917–924. e911. doi: 10.1016/j.ajo.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 125••.Comparison of Age-related Macular Degeneration Treatments Trials Research G. Maguire MG, Martin DF, Ying GS, Jaffe GJ, Daniel E, Grunwald JE, Toth CA, Ferris FL, 3rd, Fine SL. Five-year outcomes with anti-vascular endothelial growth factor treatment of neovascular age-related macular degeneration: The comparison of age-related macular degeneration treatments trials. Ophthalmology. 2016 doi: 10.1016/j.ophtha.2016.03.045. This clinical report has evaluated for the effect of anti-VEGF treatment in visual acuity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L, Gibson A, Sy J, Rundle AC, Hopkins JJ, Rubio RG, et al. Ranibizumab for diabetic macular edema: Results from 2 phase iii randomized trials: Rise and ride. Ophthalmology. 2012;119(4):789–801. doi: 10.1016/j.ophtha.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 127.Jackson TL, Desai R, Simpson A, Neffendorf JE, Petrarca R, Smith K, Wittes J, Lewis C, Membrey L, Haynes R, Costen M, et al. Epimacular brachytherapy for previously treated neovascular age-related macular degeneration (merlot): A phase 3 randomized controlled trial. Ophthalmology. 2016 doi: 10.1016/j.ophtha.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 128.Tadayoni R, Waldstein SM, Boscia F, Gerding H, Pearce I, Priglinger S, Wenzel A, Barnes E, Gekkieva M, Pilz S, Mones J, et al. Individualized stabilization criteria-driven ranibizumab versus laser in branch retinal vein occlusion: Six-month results of brighter. Ophthalmology. 2016 doi: 10.1016/j.ophtha.2016.02.030. [DOI] [PubMed] [Google Scholar]
- 129.Pfau M, Fassnacht-Riederle HM, Freiberg FJ, Wons JB, Wirth M, Becker MD, Michels S. switching therapy from ranibizumab and/or bevacizumab to aflibercept in neovascular age-related macular degeneration (amd): One-year results. Klinische Monatsblatter fur Augenheilkunde. 2016 doi: 10.1055/s-0042-101348. [DOI] [PubMed] [Google Scholar]
- 130.Korobelnik JF, Do DV, Schmidt-Erfurth U, Boyer DS, Holz FG, Heier JS, Midena E, Kaiser PK, Terasaki H, Marcus DM, Nguyen QD, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121(11):2247–2254. doi: 10.1016/j.ophtha.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 131.Sivaprasad S, Prevost AT, Bainbridge J, Edwards RT, Hopkins D, Kelly J, Luthert P, Murphy C, Ramu J, Sarafraz-Shekary N, Vasconcelos J, et al. Clinical efficacy and mechanistic evaluation of aflibercept for proliferative diabetic retinopathy (acronym clarity): A multicentre phase iib randomised active-controlled clinical trial. BMJ open. 2015;5(9):e008405. doi: 10.1136/bmjopen-2015-008405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Comparison of Age-related Macular Degeneration Treatments Trials Research G. Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE, Toth C, Redford M, Ferris FL., 3rd Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: Two-year results. Ophthalmology. 2012;119(7):1388–1398. [Google Scholar]
- 133.Shiragami C, Ono A, Kobayashi M, Manabe S, Yamashita A, Shiraga F. Effect of switching therapy to pegaptanib in eyes with the persistent cases of exudative age-related macular degeneration. Medicine. 2014;93(18):e116. doi: 10.1097/MD.0000000000000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bakri SJ, Snyder MR, Reid JM, Pulido JS, Singh RJ. Pharmacokinetics of intravitreal bevacizumab (avastin) Ophthalmology. 2007;114(5):855–859. doi: 10.1016/j.ophtha.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 135.Sinapis CI, Routsias JG, Sinapis AI, Sinapis DI, Agrogiannis GD, Pantopoulou A, Theocharis SE, Baltatzis S, Patsouris E, Perrea D. Pharmacokinetics of intravitreal bevacizumab (avastin(r)) in rabbits. Clinical ophthalmology. 2011;5:697–704. doi: 10.2147/OPTH.S19555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Park SJ, Oh J, Kim YK, Park JH, Park JY, Hong HK, Park KH, Lee JE, Kim HM, Chung JY, Woo SJ. Intraocular pharmacokinetics of intravitreal vascular endothelial growth factor-trap in a rabbit model. Eye. 2015;29(4):561–568. doi: 10.1038/eye.2014.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Miyake T, Sawada O, Kakinoki M, Sawada T, Kawamura H, Ogasawara K, Ohji M. Pharmacokinetics of bevacizumab and its effect on vascular endothelial growth factor after intravitreal injection of bevacizumab in macaque eyes. Investigative ophthalmology & visual science. 2010;51(3):1606–1608. doi: 10.1167/iovs.09-4140. [DOI] [PubMed] [Google Scholar]
- 138•.Abdelkader H, Alany RG. Controlled and continuous release ocular drug delivery systems: Pros and cons. Current drug delivery. 2012;9(4):421–430. doi: 10.2174/156720112801323125. The advantages and disadvantages of the of different drug delivery system have been summerized and discussed in details. [DOI] [PubMed] [Google Scholar]
- 139.Mishra GP, Bagui M, Tamboli V, Mitra AK. Recent applications of liposomes in ophthalmic drug delivery. Journal of drug delivery. 2011;2011:863734. doi: 10.1155/2011/863734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Puglia C, Offerta A, Carbone C, Bonina F, Pignatello R, Puglisi G. Lipid nanocarriers (lnc) and their applications in ocular drug delivery. Current medicinal chemistry. 2015;22(13):1589–1602. doi: 10.2174/0929867322666150209152259. [DOI] [PubMed] [Google Scholar]
- 141.Yellepeddi VK, Palakurthi S. Recent advances in topical ocular drug delivery. Journal of ocular pharmacology and therapeutics: the official journal of the Association for Ocular Pharmacology and Therapeutics. 2016;32(2):67–82. doi: 10.1089/jop.2015.0047. [DOI] [PubMed] [Google Scholar]
- 142.Hennig R, Goepferich A. Nanoparticles for the treatment of ocular neovascularizations. European journal of pharmaceutics and biopharmaceutics: official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2015;95(Pt B):294–306. doi: 10.1016/j.ejpb.2015.02.027. [DOI] [PubMed] [Google Scholar]
- 143.Shah SS, Denham LV, Elison JR, Bhattacharjee PS, Clement C, Huq T, Hill JM. Drug delivery to the posterior segment of the eye for pharmacologic therapy. Expert review of ophthalmology. 2010;5(1):75–93. doi: 10.1586/eop.09.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pignatello R, Carbone C, Puglia C, Offerta A, Bonina FP, Puglisi G. Ophthalmic applications of lipid-based drug nanocarriers: An update of research and patenting activity. Ther Deliv. 2015;6(11):1297–1318. doi: 10.4155/tde.15.73. [DOI] [PubMed] [Google Scholar]
- 145.Musumeci T, Bucolo C, Carbone C, Pignatello R, Drago F, Puglisi G. Polymeric nanoparticles augment the ocular hypotensive effect of melatonin in rabbits. International journal of pharmaceutics. 2013;440(2):135–140. doi: 10.1016/j.ijpharm.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 146.MacDonald IM, Sauve Y, Sieving PA. Preventing blindness in retinal disease: Ciliary neurotrophic factor intraocular implants. Canadian journal of ophthalmology Journal canadien d’ophtalmologie. 2007;42(3):399–402. [PubMed] [Google Scholar]
- 147.Myles ME, Neumann DM, Hill JM. Recent progress in ocular drug delivery for posterior segment disease: Emphasis on transscleral iontophoresis. Advanced drug delivery reviews. 2005;57(14):2063–2079. doi: 10.1016/j.addr.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 148.Hughes PM, Olejnik O, Chang-Lin JE, Wilson CG. Topical and systemic drug delivery to the posterior segments. Advanced drug delivery reviews. 2005;57(14):2010–2032. doi: 10.1016/j.addr.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 149.Marano RJ, Wimmer N, Kearns PS, Thomas BG, Toth I, Brankov M, Rakoczy PE. Inhibition of in vitro vegf expression and choroidal neovascularization by synthetic dendrimer peptide mediated delivery of a sense oligonucleotide. Experimental eye research. 2004;79(4):525–535. doi: 10.1016/j.exer.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 150.Yasin MN, Svirskis D, Seyfoddin A, Rupenthal ID. Implants for drug delivery to the posterior segment of the eye: A focus on stimuli-responsive and tunable release systems. Journal of controlled release: official journal of the Controlled Release Society. 2014;196:208–221. doi: 10.1016/j.jconrel.2014.09.030. [DOI] [PubMed] [Google Scholar]
- 151••.Rowe-Rendleman CL, Durazo SA, Kompella UB, Rittenhouse KD, Di Polo A, Weiner AL, Grossniklaus HE, Naash MI, Lewin AS, Horsager A, Edelhauser HF. Drug and gene delivery to the back of the eye: From bench to bedside. Investigative ophthalmology & visual science. 2014;55(4):2714–2730. doi: 10.1167/iovs.13-13707. The strategies and process of developing a drug from preclinial, animal and how to overcome the ocular barriers have been described. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tucker BA, Mullins RF, Stone EM. Stem cells for investigation and treatment of inherited retinal disease. Human molecular genetics. 2014;23(R1):R9–R16. doi: 10.1093/hmg/ddu124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wan C, Li F, Li H. Gene therapy for ocular diseases meditated by ultrasound and microbubbles (review) Molecular medicine reports. 2015;12(4):4803–4814. doi: 10.3892/mmr.2015.4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Marchetti V, Krohne TU, Friedlander DF, Friedlander M. Stemming vision loss with stem cells. The Journal of clinical investigation. 2010;120(9):3012–3021. doi: 10.1172/JCI42951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.https://clinicaltrials.gov