Abstract
Sex hormones promote immunoregulatory effects on multiple sclerosis. In the current study we evaluated the composition of the gut microbiota and the mucosal-associated regulatory cells in estrogen or sham treated female mice before and after autoimmune encephalomyelitis (EAE) induction. Treatment with pregnancy levels of estrogen induces changes in the composition and diversity of gut microbiota. Additionally, estrogen prevents EAE-associated changes in the gut microbiota and might promote the enrichment of bacteria that are associated with immune regulation. Our results point to a possible cross-talk between the sex hormones and the gut microbiota which could promote neuroprotection.
Keywords: Estrogen, EAE, regulatory B cell, M2-like macrophage/microglia, microbiota
Graphical abstract
1. Introduction
Multiple sclerosis (MS) is a chronic, immune–mediated demyelinating disease of the central nervous system (CNS) (Sospedra and Martin, 2005, Steinman, 2001). MS exhibits a distinct female predominance similar to a majority of human autoimmune diseases. However, women with MS often experience clinical improvement during pregnancy, indicating that sex hormones might have a therapeutic effect in MS. Although sex hormones are involved in regulating the immune response, the relevant mechanisms are poorly understood.
Previously, we demonstrated that 17β-estradiol (E2) mediated protection against EAE was dependent on the presence of IL-10 and programed death ligand 1 (PDL1) expressing regulatory B cells (Bregs), which were increased with E2 treatment in spleen, spinal cord and brain (Bodhankar et al., 2013, Bodhankar and Offner, 2011, Bodhankar et al., 2012, Bodhankar et al., 2011, Zhang et al., 2015a, Zhang et al., 2015b). We further showed that E2 treatment induced regulatory and anti-inflammatory macrophages and microglia.
A growing number of studies have demonstrated that the gut microbiota plays a critical role in shaping the immune system (Palm et al., 2015). For instance, commensal microbes can stimulate T cells responses that are important for host defense against enteric pathogens, yet may also drive local or systemic tissue damage (Haghikia et al., 2015, Ivanov et al., 2008, Maeda et al., 2016, Tan et al., 2016). On the other hand, the microbiota can elicit immune regulatory responses that promote immune homeostasis. The balance of these interactions has been shown to affect the pathogenesis of several auto-immune diseases, including multiple sclerosis (Berer et al., 2011, Erny et al., 2015, Lee et al., 2011, Mielcarz and Kasper, 2015, Ochoa-Reparaz and Kasper, 2014, Wang et al., 2014a).
Several factors, including diet, are thought to shape the gut microbiota-immune axis. It was reported that fatty acids that are metabolized by gut microbiota could affect the course of EAE; long-chain fatty acids were shown to enhance Th1 and Th17 cell responses and exacerbate EAE, whereas short-chain fatty acids were shown to promote regulatory T cell responses and ameliorate EAE (Haghikia, Jorg, 2015).
Recent studies also demonstrated that sex hormones either impact the gut microbiota or could be affected by it. In addition to the differences in gut microbiota composition between males and females, different levels of these hormones within each sex could affect the microbiota and thus the immune response (Gomez et al., 2015). Herein, we evaluated the effect of estrogen treatment before and during EAE on the composition of gut microbiota and further determined this effect on regulatory cells in the mesenteric lymph nodes (MLN) of these mice.
2. Material and methods
2.1 Animals
Eleven-week old female C57BL/6 wild-type mice were purchased from the Jackson Laboratory. IL-10 transcriptional reporter mice were obtained from Dr. Christopher Karp, Division of Molecular Immunology, University of Cincinnati College of Medicine, Cincinnati, Ohio. The generation and characterization of these mice has been described (Madan et al., 2009). Briefly, the IL10-GFP reporter mice have a floxed neomycin-IRES eGFP cassette (Mohrs et al., 2001) inserted between the endogenous stop site and the poly (A) site of Il10 to help track IL-10 producing cells in vivo. These mice (designated as Vert-X) are homozygous, develop normally and are viable and fertile without any obvious abnormal phenotype. The mice were housed in the Animal Resource Facility at the VA Portland Health Care System (VAHPCS) in accordance with institutional guidelines. This study was conducted in accordance with National Institutes of Health guidelines for the use of experimental animals and protocols were approved by the VAPHCS Animal Care and Use Committee.
2.2 Hormone treatment and induction of EAE
Female mice were implanted subcutaneously with 2.5mg/60-day release 17β-estradiol pellets (Innovative Research of America, Sarasota, FL) or were sham-treated (control) one week prior to subcutaneous immunization at four sites on the flanks with 200μg mouse (m)MOG-35-55 peptide (PolyPeptide Laboratories, San Diego, CA) in 400μg Complete Freund’s adjuvant comprised of Incomplete Freund’s adjuvant (IFA, Sigma-Aldrich, St. Louis, MO) containing heat-killed Mycobacterium tuberculosis (Mtb, Difco, Detroit, MI). Mice received intraperitoneal injections of pertussis toxin (Ptx, List Biologicals, Campbell, CA) on the day of immunization (75 ng) and 2 days later (200 ng).
All mice were monitored daily for clinical signs of EAE and scored using the following scale: 0=normal; 1=limp tail or mild hind limb weakness; 2=moderate hind limb weakness or mild ataxia; 3=moderately severe hind limb weakness; 4=severe hind limb weakness or mild forelimb weakness or moderate ataxia; 5=paraplegia with no more than moderate forelimb weakness; and 6=paraplegia with severe forelimb weakness or severe ataxia or moribund condition. Mice were scored daily and were evaluated for incidence, day of onset, day of maximal clinical signs (peak) and for total disease score over the course of the experiment (Cumulative Disease Index, CDI). Mean ± SD were calculated for these parameters for each experimental group.
2.3 Fecal samples collections and 16S rRNA gene sequencing
Feces were freshly harvested from each individual mouse before and after EAE induction: day 0 (before EAE induction) and days 3, 7, 10, 14 and 21 (post-immunization) and kept at −20C until analysis. Microbiota composition and diversity was evaluated by 16S rRNA gene sequencing. Fecal DNA from 12 mice from each group was purified using the PowerSoil DNA isolation kit and the 16S rRNA gene amplified using validated primers that target the 515F/806R 16S region (Caporaso et al., 2012). These primers allowed multiplexing and simultaneous sequencing of up to 600 stool samples with the Illumina MiSeq platform (Caporaso, Lauber, 2012).
2.4 16s rRNA Sequence processing and taxonomic assignment
Primers and sequence adapters were removed with the Illumina MiSeq Reporter (version 2.5). The sequences were further processed using scripts implemented through the workflow package Quantitative Insights into Microbial Ecology (QIIME) version 1.9.0 (Caporaso et al., 2010). Individual sequence reads were joined using FASTQ-join (eautils, version 1.1.2-537; (Aronesty, 2013)), with a maximum number of 3 mismatches and minimum overlap of 6. Operational taxonomic units (OTUs) were identified with an open-reference approach against the greengenes reference database(Caporaso, Kuczynski, 2010, Navas-Molina et al., 2013) using uclust (version 1.2.22q (Edgar, 2010)). Chimeric sequences were removed with the blast fragments approach implemented in identify_chimeric_seqs.py. Taxonomy was assigned to individual OTUs using the RDP Classifier (version 2.2; (Wang et al., 2007)) with a minimum confidence of 0.80. The resulting OTU table was imported into R for filtering and statistical analysis (described below).
2.5 Isolation of leukocytes from mesenteric LN and spinal cord
LNs were removed from euthanized animals under sterile conditions and single cell suspensions of leukocytes were prepared by disaggregation of the tissue through a 100μm nylon mesh (BD Falcon, Bedford, MA). Cells were washed once with RPMI 1640 supplemented with 10% heat-inactivated FBS (hiFBS; Thermo Scientific, Waltham, MA). Cells were enumerated at a 1:1 dilution with 0.2% trypan blue stain (Life Technologies, Grand Island, NY) using a Cellometer Auto T4 Cell Viability Counter (Nexcelom, Lawrence, MA), washed, and resuspended at 107 cells/mL in stimulation medium (RPMI 1640 containing 10% FBS [Thermo Scientific, Waltham, MA], 1% sodium pyruvate [Life Technologies, Grand Island, NY], 1% L-glutamine [Thermo Scientific, Waltham, MA], and 0.4% β-ME [Sigma-Aldrich, St. Louis, MO]).
Spinal cords were passed through 100μm mesh screens and washed as above. Cells were resuspended in 80% Percoll (GE Healthcare, Pittsburgh, PA) then overlaid with 40% Percoll to establish a density gradient and centrifuged at 300 g for 30min following a previously described method(Campanella et al., 2002). Leukocytes were collected from the resultant interface, counted, and resuspended in stimulation medium for assay.
2.6 Flow cytometry
Antibodies
Leukocytes were labeled with a combination of the following antibodies obtained from BD Bioscience (San Jose, CA): APC CD19 (1D3), PE CD1d (1B1), PerCP CD5 (53–7.3), PerCP-Cy5.5 CD11b (M1/70), PE CD45 (30-F11), PE PDL1 (MIH5), from Ebioscience (San Diego, CA), APC CD206 (C068C2), (CXNFT) from Biolegend (San Diego, CA) and PE CD9 (MZ3) and PE ARG1 from R&D systems (Minneapolis, MN).
Extracellular staining
Single cell suspensions were washed and resuspended in staining buffer (1X PBS, 0.5% BSA [Sigma-Aldrich], 0.1% sodium azide [Sigma-Aldrich]). Fc receptors were blocked with anti-CD16/32 antibody (2.4G2, BD Biosciences) and cells were incubated with monoclonal antibodies (mAbs) listed above. Unbound mAbs were washed with staining buffer prior to four-color fluorescence flow cytometry analysis using a BD Accuri C6 flow cytometer (BD Biosciences).
Intracellular staining
1×106 cells were incubated in 1mL of stimulation medium (as above). Then, Fc receptors were blocked with anti-CD16/32 monoclonal antibodies (mAb) (2.3G2; BD Biosciences) before cell surface staining. Cells were fixed and permeabilized with Fixation/Permeabilization buffer (BD Biosciences) according to the manufacturer’s instructions. Permeabilized cells were washed with 1X Perm/Wash Buffer (BD Biosciences) and were stained with anti-ARG1 and anti-CD206 (BD Biosciences, San Jose, CA, USA). Isotype-matched mAb served as negative controls to demonstrate specificity and to establish a background for the levels of, ARG1 or CD206 expression. All data were acquired and analyzed using the Accuri C6 (BD Biosciences) software included with the instrument.
2.7 Statistical analysis
Data were reported using GraphPad Prism (v5.0, San Diego, CA) and expressed as the mean ± SD. Statistical significance for the disease course was calculated using the Mann-Whitney U test. Statistical significance for flow cytometry data was calculated using Student’s t-test or one way ANOVA with Tukey correction. P-values ≤0.05 were considered to be significant (*p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001).
2.8 Microbial community analysis
Beta diversity was evaluated on the rarefied OTU table (rarefied to 48,000 reads) using principal coordinate analysis on the weighted UniFrac distances in Qiime for each time point. Significance of clustering between E2-treated mice and sham in the PCoA was assessed by PERMANOVA. Alpha diversity was evaluated with the Chao1 estimator, Shannon Index, and inverse Simpson index using phyloseq (McMurdie and Holmes, 2013) in R. Differentially abundant OTUs were identified at time 0 and time 21 using DeSEQ2 (Love et al., 2014). DeSEQ2 uses a negative binomial generalized linear model to identify individual taxa that are differentially abundant between groups. In order to minimize the amount of comparisons made, we filtered taxa that were not present in at least 20% of the samples. P-values for this analysis were adjusted for multiple comparisons using the Benjamini-Hochberg method. Significantly different taxa were defined as taxa that had a log fold change between groups of at least 1 and adjusted p-value of 0.10.
2.9 Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
3. Results
3.1 E2-mediated prevention of EAE modulates the intestinal microbiota
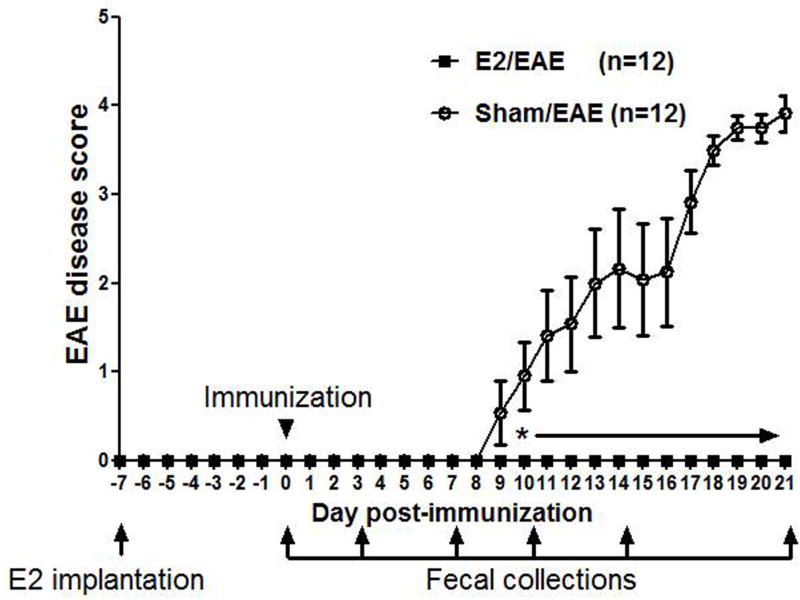
In order to evaluate the effect of E2 on gut microbiota and immune response, 11 week old female C57BL/6 WT mice that were housed for 3 weeks in the same conditions were either sham treated or implanted with E2 pellets 7 days prior to immunization with mMOG-35-55 peptide/CFA/Ptx. As demonstrated before, E2 treated mice were completely protected against EAE compared to sham treated mice. Fecal samples were collected from these mice at different time points: Day 0 –just prior to immunization (after 7 days of sham or estrogen treatment), and days 3, 7, 10, 14 and 21 post-immunization (p.i.). This experimental scheme is shown in Figure 1.
Figure 1. E2 treatment protects female C57BL/6 mice from EAE.
Female C57BL/6 mice were implanted with sham or E2 pellets one week prior to immunization with mouse (m)MOG-35–55 peptide/CFA/Ptx. Fecal samples were collected from these mice at different time points: Day 0 –just prior to immunization (after 7 days of sham or estrogen treatment), and days 3, 7, 10, 14 and 21 post-immunization (p.i.). Daily mean scores were analyzed by Mann Whitney U test. * p<0.05.
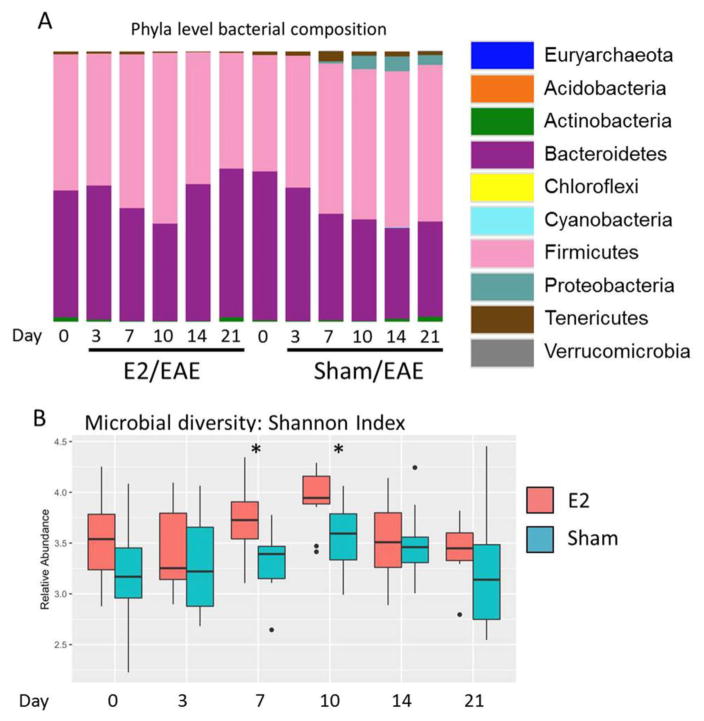
Fecal microbiota diversity and composition were then identified by 16S rRNA gene sequencing. Comparison at the phylum level of all time points showed that Firmicutes and Bacteroidetes persisted as the overwhelmingly dominant phyla in E2-treated animals, whereas in sham-treated animals that succumbed to EAE a marked expansion of more minor phyla (Proteobacteria and Tenericutes) was observed concomitant with disease onset (day 10) (Fig 2A). Analysis of microbial diversity within each group also revealed E2-treated animals exhibited significantly higher diversity than sham-treated controls at multiple time points (Fig 2B, Supplementary Fig 1). Interestingly, the increased diversity in E2-treated animals was a relatively early event, observed at d7 and d10 post EAE induction, time points of disease onset in sham-treated animals.
Figure 2. Bacterial composition and diversity in E2 or sham treated mice.
A) Microbiota community composition (phylum-level) is shown in either sham (S)- or E2-treated mice (E) during the course of EAE progression. Day 0 – just prior to immunization (after 7 days of sham or estrogen treatment), and days 3, 7, 10, 14 and 21 post-immunization (p.i.). are shown (D0–D21). B). The alpha (within-sample) diversity for each time point is shown for E2 (red bars) or sham (blue bars) treated animals as represented by Shannon Index values. Median and interquartile range are denoted by horizontal bars. Circles represent sample outliers. For A&B, n = 11–12 mice per group and are representative of two pooled experiments. * = p ≤ 0.05 by Wilcoxon Rank Sum test at the indicated time points between E2- and sham- treated animals, with Benjamini-Hochberg correction for multiple comparisons.
Our results suggest that E2 limits the loss of microbial diversity and outgrowth of specific phyla, for instance Proteobacteria that is observed during the course of EAE in sham-treated animals.
3.2 E2 treatment affects the microbiota composition before EAE induction
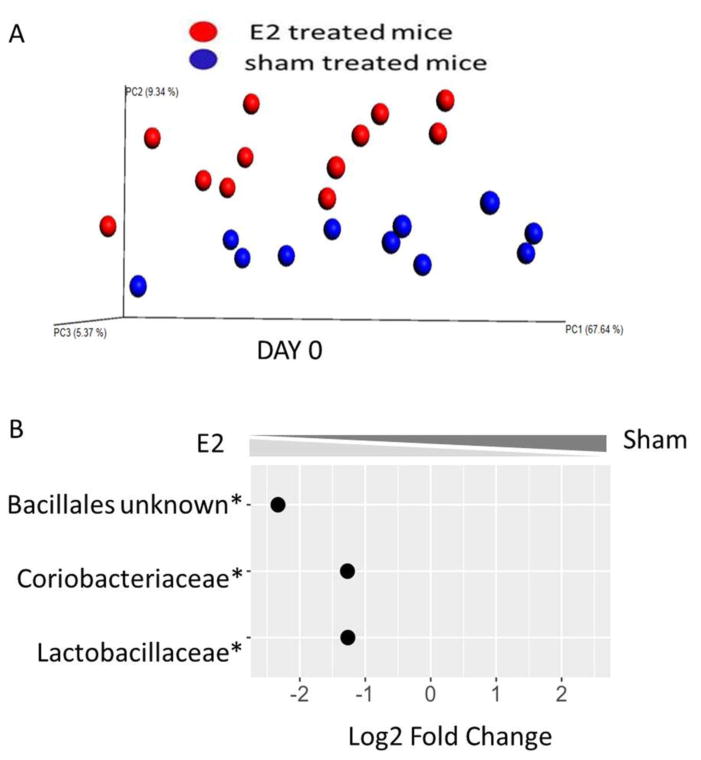
In order to further evaluate E2 effects on the gut microbiota, independent from the effects of EAE, we compared mice 7 days post- E2 or sham treatment prior to EAE induction. As shown by principal coordinate analysis (PCoA) of mice on day 0 (Fig. 3A), E2 treated mice and sham treated mice grouped into two significantly distinct clusters (PERMANOVA p=0.05). Further to these observations, we were able to identify three bacterial orders/families that were significantly different between sham and E2 treated mice on day 0: Lactobacillaceae, Coriobacteriaceae and unidentified family from the Bacillales order were significantly enriched after E2 treatment compared to sham (p<0.001). These data demonstrate that high levels of estrogen affect the composition of the gut microbiota of female mice.
Figure 3. E2 treatment induces changes in composition of gut microbiota.
A) Principal coordinate analysis (PCoA) plot of unweighted distances between E2-pretreated (red dots) and sham (blue dots) mice, before EAE induction (day 0). Mice were implanted with E2 or sham for seven days. Sham (n = 11) vs E2 (n = 12) groups were compared by PERMAVOVA. Each point represents an individual animal. B) Fold change (log2) of microbial families that exhibited a significant difference in relative abundance in the feces of E2 and sham treated mice at the same time point (day 0). A negative binomial generalized linear model implemented in DeSEQ2 was used to identify differentially abundant microbial families between groups, with a significance threshold of p ≤ 0.05 following Benjamini-Hochberg correction for multiple comparisons.
3.3 E2 treatment affects the microbiota composition during EAE
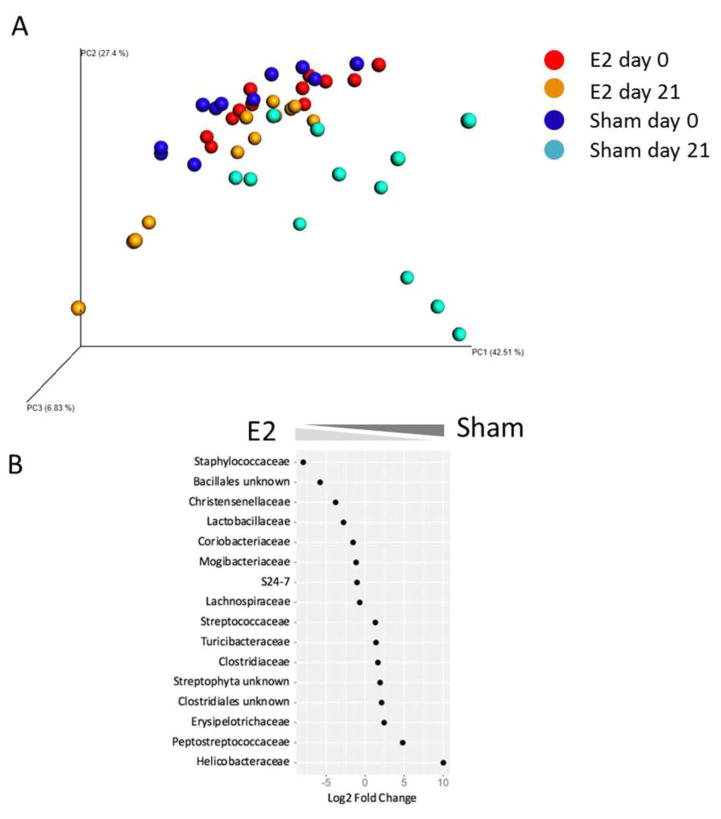
In order to evaluate the effect of EAE on the microbiota of E2 or sham treated mice, we performed a PCoA analysis which combined data from mice on day 0 and day 21 p.i. As demonstrated in Figure 4A, while E2 day 0 mice and E2 day 21 mice clustered together, sham day 0 mice significantly differed from sham day 21 mice (PERMANOVA p=0.001). We further identify eight bacterial orders/families that were significantly increased in E2/EAE mice and eight bacterial orders/families that were significantly increased in sham/EAE mice on day 21 p.i. (Fig 4B). Of particular interest are the Lactobacillaceae that were significantly elevated in the E2 treated mice at both time points (day 0 and day 21 p.i.) compared to sham treated mice, and were shown to induce anti-inflammatory effects (Lavasani et al., 2010). The relative abundances throughout the different time points of this bacterial family are presented in Supplementary Figure 2. Taken together, these observations suggest that estrogen treatment impacts the gut microbiota by preventing dysbiotic or disease-associated changes to the intestinal microbiota and promoting bacteria that are involved in immune regulation.
Figure 4. E2 treatment reduces gut dysbiosis during EAE.
A) Principal coordinate analysis (PCoA) plot of unweighted distances between E2-pretreated (red dots) vs. sham (blue dots) mice, before EAE induction (day 0), E2/EAE day 21 p.i. (orange) and sham/EAE day 21 p.i. (cyan). B) Relative abundances of significantly changed bacterial families in the gut of E2 and sham treated mice, day 21 p.i. A negative binomial generalized linear model implemented in DeSEQ2 was used to identify differentially abundant microbial families between groups, with a significance threshold of p ≤ 0.05 following Benjamini-Hochberg correction for multiple comparisons.
3.4 E2 protection against EAE induces a regulatory phenotype in the mesenteric lymph nodes and spinal cord
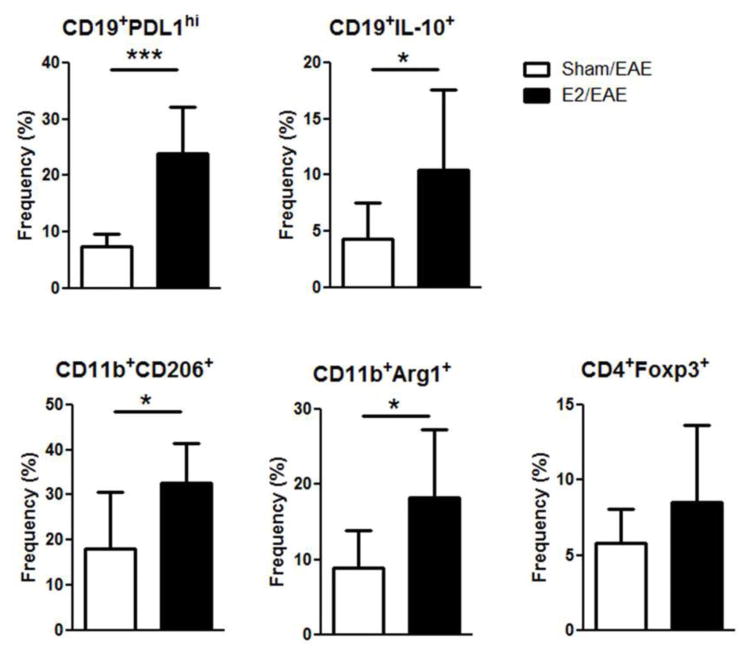
We previously reported that E2 treatment can induce Bregs that are involved in ameliorating clinical signs of EAE through PDL1 and IL-10 dependent mechanisms (Bodhankar, Wang, 2011, Offner and Polanczyk, 2006). However, in light of the clear impact of E2 administration on the gut microbiota, we hypothesized that E2 may promote the local expansion of intestinal Bregs or other regulatory immune cell populations. We therefore examined whether E2 treatment affects immune cells in the gut-draining mesenteric lymph nodes (MLN) on day 21 p.i. As demonstrated in figure 5, the frequencies of PDL1 on CD19 B cells and the M2 markers (Arg1 and CD206) on CD11b+ cells were significantly increased in E2 treated mice compared to sham treated mice (p<0.001 and p<0.05, respectively). Interestingly, we did not observe a higher frequency of CD4+FOXP3+ T cells in the MLN of E2 treated mice. To determine the frequency of IL-10 producing B cells in the MLN, IL-10 GFP mice were with implanted with E2 pellets 7 days prior to immunization with mMOG-35-55 peptide/CFA/Ptx. E2 treated mice had a significantly higher frequency of IL-10 producing CD5+CD1dhi B cells in the mesenteric LN compared to sham treated mice (p<0.05) (Fig 5). Our results demonstrate that E2 treatment impacts the immune response in lymphatic organs that are involved with gut immunity.
Figure 5. E2 treatment protects against EAE by inducing regulatory B cells and anti-inflammatory macrophage in MLN.
Frequency of CD19+PDL1hi, CD11b+CD206+, CD11b+Arg1+ and CD4+Foxp3+ cells in E2 (n=6) or sham (n=6) treated C57BL/6 mice on day 21 p.i. or CD19+IL-10+ cells in E2 (n=6) or sham (n=6) treated IL10/GFP mice on day 21 p.i. *p<0.05, ***p<0.001 Student’s t-test.
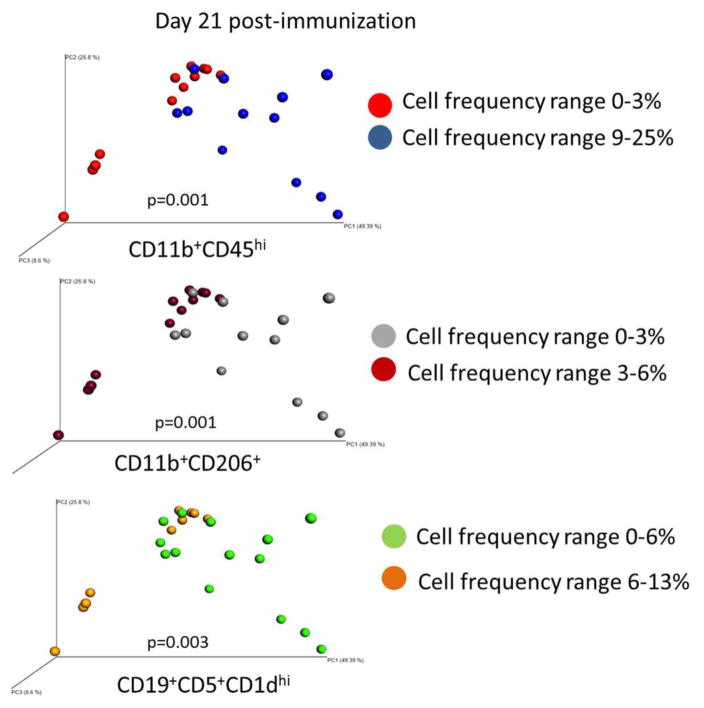
We next examined how gut microbiota composition relates to the frequency of activated CD11b+CD45hi cells, regulatory B cells and M2-like CD11b+ cells in the CNS. Principal coordinate analysis (PCoA) of EAE mice on day 21 p.i. showed that microbial composition of the gut was related to the frequencies of CD11b+CD45hi (activated macrophage/microglia), CD11b+CD206+ (M2-like) and CD19+CD5+CD1dhi (Breg) cells in the spinal cord, which were divided mainly into two distinct clusters (PERMANOVA p=0.001, p=0.001 and p=0.003, respectively) corresponding with the treatment groups (E2 vs. sham, Figure 6). Together these findings indicate changes in the microbiota induced by estrogen treatment in the EAE model are also associated with a significant segregation of immune phenotype concomitant with disease state.
Figure 6. Estrogen-induced changes in microbiota correlate with activated and regulatory cells in the spinal cord.
PCoA analysis of EAE mice based on CD11b+CD45hi cell, CD11b+CD206+ (M2-like) cell or CD19+CD5+CD1dhi (Breg) cell frequency in spinal cord. P values represent PERMANOVA values for each comparison.
4. Discussion
During pregnancy, women with MS often show clinical improvement during the third trimester, usually followed by temporary post-partum exacerbations (Confavreux et al., 1998, Debouverie et al., 2008, Orton et al., 2006, Whitacre, 2001). These observations pointed towards the involvement of sex hormones, such as estrogen and estriol in disease regulation (Baker et al., 2004, Bebo et al., 2001, Drew et al., 2003, Itoh et al., 2016, Offner and Polanczyk, 2006). It was further demonstrated that the composition of gut microbiota changes during pregnancy (Koren et al., 2012). Herein, we demonstrate for the first time that treatment of mice with pregnancy levels of estrogen induces changes in the composition and diversity of gut microbiota in mice. Additionally, estrogen prevents EAE-associated changes in the gut microbiota and might promote the enrichment of bacteria that are associated with immune regulation.
The impact of cross talk between sex hormones and the microbiota on the immune system is far from being completely understood. We demonstrated that E2 treatment significantly increases the abundance of Lactobacillaceae. This family was associated with induction of regulatory cells and was also shown to be affected by estrogen (Shen et al., 2016, Stanisavljevic et al., 2016). It was reported that different strains of lactobacilli might attenuate EAE by inducing IL-10 producing cells (Lavasani, Dzhambazov, 2010). Interestingly, the abundance of Lactobacillaceae family was increased in the E2 treated mice compared to the sham treated mice throughout the experiment, until mice were euthanized on day 21 p.i. It was demonstrated in various studies that different strains of this family are reduced in MS patients (Mirza and Mao-Draayer, 2017).
Our data suggest that while EAE might induce gut dysbiosis, pretreatment with E2 could protect mice from these disease-associated changes. It is important to note that the changes in the gut microbiota of sham treated mice might also be effected by the eating and drinking habits of the mice during EAE. However, our data suggest the E2 treatment might, on one hand, promote the abundance of some anti-inflammatory related bacteria and on the other hand, prevent the severe dysbiosis that is induced by EAE. We showed that after disease induction the relative abundance of Lachnospiraceae was increased in E2 mice relative to sham treated mice that developed disease, whereas taxa such as Erysipelotrichaceae spp., which have been associated with induction of inflammatory cytokines such as TNFα (Dinh et al., 2015), were under-represented in E2-treated animals. Recently, Chen et al. reported the nod-like receptor (NLR) p12 is crucial in attenuation of colon inflammation by promoting protective bacterial growth. The authors demonstrated that Nlrp12 deficient mice are susceptible to dysbiosis and colitis and exhibit similar changes to those observed in EAE induction, with a loss of Lachnospiraceae family members and increases in the abundance of the Erysipelotrichaceae spp (Chen et al., 2017). Strikingly, Nlrp12 deficiency was also shown to exacerbate EAE (Gharagozloo et al., 2015, Lukens et al., 2015). It is possible that E2 treatment may therefore limit disease by similar mechanisms to Nlrp12, or indeed directly promote Nlrp12 expression.
Previously we demonstrated that E2 protection against EAE is mediated by regulatory B cells and M2 macrophages (Benedek et al., 2016, Benedek, 2017, Bodhankar, Vandenbark, 2012, Bodhankar, Wang, 2011, Subramanian et al., 2011, Zhang, Lapato, 2015b). These cells were found to be increased in the spleen and CNS of E2 treated mice during EAE. In the current study we show that the frequencies of these regulatory cells were also increased in the gut mucosal immune compartment (MLN) of E2 treated mice compared to sham mice. Furthermore we demonstrate that frequencies of activated CD11b+CD45hi cells, M2-like cells and regulatory B cells in the CNS correlated with the clustering of gut microbiota composition of the treated mice (Fig. 6). Dysbiosis of gut microbes by antibiotic (ABX) treatment leads to reduced EAE severity and is associated with increased levels of IL-10 and IL-13 and increased frequencies of regulatory T and B cells in the mesenteric and cervical lymph nodes (LN) (Ochoa-Reparaz et al., 2010, Ochoa-Reparaz et al., 2008). It was further reported that polysaccharides from Bacteroides fragilis could expand intestinal regulatory T cells and protect against EAE (Wang, Begum-Haque, 2014a, Wang et al., 2014b). Interestingly, Rosser et al. reported that ABX treatment of mice led to reduction in the frequency of Bregs (Rosser et al., 2014). While these are two contradictory results that could be due to differences in housing conditions, diet and type of ABX cocktail, it is clear that the gut microbiota could induce either an enhanced pro-inflammatory response or an anti-inflammatory response by promoting diverse populations of regulatory cells.
It is possible that an estrogen-modified microbiota would have a protective effect in EAE by inducing regulatory cells. On the other hand, it is possible that estrogen-affected cells induce changes in the gut microbiota. Yerkovetskiy et al. demonstrated that microbes are able to regulate sex hormone levels and that sex hormones could change the microbial composition of the gut (Yurkovetskiy et al., 2013). It was also suggested that estrogen-like compounds may be metabolized by gut microbes to produce estrogenic metabolites that could affect the immune system (Chen and Madak-Erdogan, 2016). High fat diets were shown to increase the lipopolysaccharide (LPS) producing Gram-negative bacteria, whereas estrogen was reported to reduce LPS-induced inflammation and decrease the concentration of these bacteria (Blasco-Baque et al., 2012). While further experiments are needed to determine whether the microbiota composition, which was observed in estrogen-treated mice is sufficient to induce protection against EAE, these findings point to a feedback loop between sex hormones and gut microbiota that affects the immune system.
5. Conclusions
In summary, our present study demonstrates that estrogen treatment induces changes in the gut microbiota composition which are associated with protection against EAE. Furthermore, while EAE lead to dysbiosis, estrogen pretreatment reduced these changes and increased the frequency of regulatory B cells and anti-inflammatory macrophages in the MLN and spinal cord of mice. Further study is needed to better understand the cross talk between sex hormones and the gut microbiota, which could open new avenues of research to identify particular bacteria that could promote immune regulation.
Supplementary Material
Highlights.
Estrogen treatment induces changes in the gut microbiota composition.
While EAE lead to gut dysbiosis, estrogen pretreatment reduced these changes.
A possible crosstalk between sex hormones and microbiota affects the immune system.
Acknowledgments
This work was supported by NIH/NINDS grant RO1 NS080890 (H.O). This material is the result of work supported with resources and the use of facilities at the VA Portland Health Care System, Portland, OR. M.A is supported by the Spondylitis Association of America. The contents do not represent the views of the Department of Veterans Affairs or the US government.
Footnotes
Authors’ contributions:
HO directed the research. GB and MA designed the experiments. GB wrote the manuscript. JZ, HN, GK, SD, HS and PS carried out the experiments, LK performed data and statistical analysis. AAV critically reviewed the manuscript. All authors critically reviewed data and edited the manuscript.
Competing interests
The authors declare that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aronesty E. Comparison of Sequencing Utility Programs. The Open Bioinformatics Journal. 2013;7:8. [Google Scholar]
- Baker AE, Brautigam VM, Watters JJ. Estrogen modulates microglial inflammatory mediator production via interactions with estrogen receptor beta. Endocrinology. 2004;145:5021–32. doi: 10.1210/en.2004-0619. [DOI] [PubMed] [Google Scholar]
- Bebo BF, Jr, Fyfe-Johnson A, Adlard K, Beam AG, Vandenbark AA, Offner H. Low-dose estrogen therapy ameliorates experimental autoimmune encephalomyelitis in two different inbred mouse strains. Journal of immunology. 2001;166:2080–9. doi: 10.4049/jimmunol.166.3.2080. [DOI] [PubMed] [Google Scholar]
- Benedek G, Zhang J, Bodhankar S, Nguyen H, Kent G, Jordan K, et al. Estrogen induces multiple regulatory B cell subtypes and promotes M2 microglia and neuroprotection during experimental autoimmune encephalomyelitis. Journal of neuroimmunology. 2016;293:45–53. doi: 10.1016/j.jneuroim.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedek GZ, Nguyena J, Kent H, Seiferta G, Vandenbarka H, AA, Offner H. Novel feedback loop between M2 macrophages/microglia and regulatory B cells in estrogen-protected EAE mice. Journal of neuroimmunology. 2017;305:59–67. doi: 10.1016/j.jneuroim.2016.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, Johner C, et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479:538–41. doi: 10.1038/nature10554. [DOI] [PubMed] [Google Scholar]
- Blasco-Baque V, Serino M, Vergnes JN, Riant E, Loubieres P, Arnal JF, et al. High-fat diet induces periodontitis in mice through lipopolysaccharides (LPS) receptor signaling: protective action of estrogens. PloS one. 2012;7:e48220. doi: 10.1371/journal.pone.0048220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodhankar S, Galipeau D, Vandenbark AA, Offner H. PD-1 Interaction with PD-L1 but not PD-L2 on B-cells Mediates Protective Effects of Estrogen against EAE. Journal of clinical & cellular immunology. 2013;4:143. doi: 10.4172/2155-9899.1000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodhankar S, Offner H. Gpr30 Forms an Integral Part of E2-Protective Pathway in Experimental Autoimmune Encephalomyelitis. Immunology, endocrine & metabolic agents in medicinal chemistry. 2011;11:262–74. doi: 10.2174/1871522211108040262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodhankar S, Vandenbark AA, Offner H. Oestrogen treatment of experimental autoimmune encephalomyelitis requires 17beta-oestradiol-receptor-positive B cells that up-regulate PD-1 on CD4+ Foxp3+ regulatory T cells. Immunology. 2012;137:282–93. doi: 10.1111/imm.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodhankar S, Wang C, Vandenbark AA, Offner H. Estrogen-induced protection against experimental autoimmune encephalomyelitis is abrogated in the absence of B cells. European journal of immunology. 2011;41:1165–75. doi: 10.1002/eji.201040992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanella M, Sciorati C, Tarozzo G, Beltramo M. Flow cytometric analysis of inflammatory cells in ischemic rat brain. Stroke. 2002;33:586–92. doi: 10.1161/hs0202.103399. [DOI] [PubMed] [Google Scholar]
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nature methods. 2010;7:335–6. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. The ISME journal. 2012;6:1621–4. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KL, Madak-Erdogan Z. Estrogen and Microbiota Crosstalk: Should We Pay Attention? Trends in endocrinology and metabolism: TEM. 2016;27:752–5. doi: 10.1016/j.tem.2016.08.001. [DOI] [PubMed] [Google Scholar]
- Chen L, Wilson JE, Koenigsknecht MJ, Chou WC, Montgomery SA, Truax AD, et al. NLRP12 attenuates colon inflammation by maintaining colonic microbial diversity and promoting protective commensal bacterial growth. Nature immunology. 2017;18:541–51. doi: 10.1038/ni.3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. The New England journal of medicine. 1998;339:285–91. doi: 10.1056/NEJM199807303390501. [DOI] [PubMed] [Google Scholar]
- Debouverie M, Pittion-Vouyovitch S, Louis S, Guillemin F, Group L. Natural history of multiple sclerosis in a population-based cohort. European journal of neurology: the official journal of the European Federation of Neurological Societies. 2008;15:916–21. doi: 10.1111/j.1468-1331.2008.02241.x. [DOI] [PubMed] [Google Scholar]
- Dinh DM, Volpe GE, Duffalo C, Bhalchandra S, Tai AK, Kane AV, et al. Intestinal microbiota, microbial translocation, and systemic inflammation in chronic HIV infection. J Infect Dis. 2015;211:19–27. doi: 10.1093/infdis/jiu409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew PD, Chavis JA, Bhatt R. Sex steroid regulation of microglial cell activation: relevance to multiple sclerosis. Annals of the New York Academy of Sciences. 2003;1007:329–34. doi: 10.1196/annals.1286.031. [DOI] [PubMed] [Google Scholar]
- Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–1. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- Erny D, Hrabe de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18:965–77. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharagozloo M, Mahvelati TM, Imbeault E, Gris P, Zerif E, Bobbala D, et al. The nod-like receptor, Nlrp12, plays an anti-inflammatory role in experimental autoimmune encephalomyelitis. Journal of neuroinflammation. 2015;12:198. doi: 10.1186/s12974-015-0414-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez A, Luckey D, Taneja V. The gut microbiome in autoimmunity: Sex matters. Clinical immunology. 2015;159:154–62. doi: 10.1016/j.clim.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghikia A, Jorg S, Duscha A, Berg J, Manzel A, Waschbisch A, et al. Dietary Fatty Acids Directly Impact Central Nervous System Autoimmunity via the Small Intestine. Immunity. 2015;43:817–29. doi: 10.1016/j.immuni.2015.09.007. [DOI] [PubMed] [Google Scholar]
- Itoh N, Kim R, Peng M, DiFilippo E, Johnsonbaugh H, MacKenzie-Graham A, et al. Bedside to bench to bedside research: Estrogen receptor beta ligand as a candidate neuroprotective treatment for multiple sclerosis. Journal of neuroimmunology. 2016 doi: 10.1016/j.jneuroim.2016.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, de Frutos RL, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell host & microbe. 2008;4:337–49. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren O, Goodrich JK, Cullender TC, Spor A, Laitinen K, Backhed HK, et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell. 2012;150:470–80. doi: 10.1016/j.cell.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavasani S, Dzhambazov B, Nouri M, Fak F, Buske S, Molin G, et al. A novel probiotic mixture exerts a therapeutic effect on experimental autoimmune encephalomyelitis mediated by IL-10 producing regulatory T cells. PloS one. 2010;5:e9009. doi: 10.1371/journal.pone.0009009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(Suppl 1):4615–22. doi: 10.1073/pnas.1000082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome biology. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukens JR, Gurung P, Shaw PJ, Barr MJ, Zaki MH, Brown SA, et al. The NLRP12 Sensor Negatively Regulates Autoinflammatory Disease by Modulating Interleukin-4 Production in T Cells. Immunity. 2015;42:654–64. doi: 10.1016/j.immuni.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan R, Demircik F, Surianarayanan S, Allen JL, Divanovic S, Trompette A, et al. Nonredundant roles for B cell-derived IL-10 in immune counter-regulation. Journal of immunology. 2009;183:2312–20. doi: 10.4049/jimmunol.0900185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda Y, Kurakawa T, Umemoto E, Motooka D, Ito Y, Gotoh K, et al. Dysbiosis Contributes to Arthritis Development via Activation of Autoreactive T Cells in the Intestine. Arthritis & rheumatology. 2016;68:2646–61. doi: 10.1002/art.39783. [DOI] [PubMed] [Google Scholar]
- McMurdie PJ, Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PloS one. 2013;8:e61217. doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielcarz DW, Kasper LH. The gut microbiome in multiple sclerosis. Current treatment options in neurology. 2015;17:344. doi: 10.1007/s11940-015-0344-7. [DOI] [PubMed] [Google Scholar]
- Mirza A, Mao-Draayer Y. The gut microbiome and microbial translocation in multiple sclerosis. Clinical immunology. 2017 doi: 10.1016/j.clim.2017.03.001. [DOI] [PubMed] [Google Scholar]
- Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15:303–11. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- Navas-Molina JA, Peralta-Sanchez JM, Gonzalez A, McMurdie PJ, Vazquez-Baeza Y, Xu Z, et al. Advancing our understanding of the human microbiome using QIIME. Methods in enzymology. 2013;531:371–444. doi: 10.1016/B978-0-12-407863-5.00019-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa-Reparaz J, Kasper LH. Gut microbiome and the risk factors in central nervous system autoimmunity. FEBS letters. 2014;588:4214–22. doi: 10.1016/j.febslet.2014.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa-Reparaz J, Mielcarz DW, Haque-Begum S, Kasper LH. Induction of a regulatory B cell population in experimental allergic encephalomyelitis by alteration of the gut commensal microflora. Gut microbes. 2010;1:103–8. doi: 10.4161/gmic.1.2.11515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa-Reparaz J, Rynda A, Ascon MA, Yang X, Kochetkova I, Riccardi C, et al. IL-13 production by regulatory T cells protects against experimental autoimmune encephalomyelitis independently of autoantigen. Journal of immunology. 2008;181:954–68. doi: 10.4049/jimmunol.181.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offner H, Polanczyk M. A potential role for estrogen in experimental autoimmune encephalomyelitis and multiple sclerosis. Annals of the New York Academy of Sciences. 2006;1089:343–72. doi: 10.1196/annals.1386.021. [DOI] [PubMed] [Google Scholar]
- Orton SM, Herrera BM, Yee IM, Valdar W, Ramagopalan SV, Sadovnick AD, et al. Sex ratio of multiple sclerosis in Canada: a longitudinal study. The Lancet Neurology. 2006;5:932–6. doi: 10.1016/S1474-4422(06)70581-6. [DOI] [PubMed] [Google Scholar]
- Palm NW, de Zoete MR, Flavell RA. Immune-microbiota interactions in health and disease. Clinical immunology. 2015;159:122–7. doi: 10.1016/j.clim.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosser EC, Oleinika K, Tonon S, Doyle R, Bosma A, Carter NA, et al. Regulatory B cells are induced by gut microbiota-driven interleukin-1beta and interleukin-6 production. Nature medicine. 2014;20:1334–9. doi: 10.1038/nm.3680. [DOI] [PubMed] [Google Scholar]
- Shen J, Song N, Williams CJ, Brown CJ, Yan Z, Xu C, et al. Effects of low dose estrogen therapy on the vaginal microbiomes of women with atrophic vaginitis. Scientific reports. 2016;6:24380. doi: 10.1038/srep24380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- Stanisavljevic S, Lukic J, Sokovic S, Mihajlovic S, Mostarica Stojkovic M, Miljkovic D, et al. Correlation of Gut Microbiota Composition with Resistance to Experimental Autoimmune Encephalomyelitis in Rats. Frontiers in microbiology. 2016;7:2005. doi: 10.3389/fmicb.2016.02005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman L. Multiple sclerosis: a two-stage disease. Nature immunology. 2001;2:762–4. doi: 10.1038/ni0901-762. [DOI] [PubMed] [Google Scholar]
- Subramanian S, Yates M, Vandenbark AA, Offner H. Oestrogen-mediated protection of experimental autoimmune encephalomyelitis in the absence of Foxp3+ regulatory T cells implicates compensatory pathways including regulatory B cells. Immunology. 2011;132:340–7. doi: 10.1111/j.1365-2567.2010.03380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan TG, Sefik E, Geva-Zatorsky N, Kua L, Naskar D, Teng F, et al. Identifying species of symbiont bacteria from the human gut that, alone, can induce intestinal Th17 cells in mice. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:E8141–E50. doi: 10.1073/pnas.1617460113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Applied and environmental microbiology. 2007;73:5261–7. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Begum-Haque S, Telesford KM, Ochoa-Reparaz J, Christy M, Kasper EJ, et al. A commensal bacterial product elicits and modulates migratory capacity of CD39(+) CD4 T regulatory subsets in the suppression of neuroinflammation. Gut microbes. 2014a;5:552–61. doi: 10.4161/gmic.29797. [DOI] [PubMed] [Google Scholar]
- Wang Y, Telesford KM, Ochoa-Reparaz J, Haque-Begum S, Christy M, Kasper EJ, et al. An intestinal commensal symbiosis factor controls neuroinflammation via TLR2-mediated CD39 signalling. Nature communications. 2014b;5:4432. doi: 10.1038/ncomms5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitacre CC. Sex differences in autoimmune disease. Nature immunology. 2001;2:777–80. doi: 10.1038/ni0901-777. [DOI] [PubMed] [Google Scholar]
- Yurkovetskiy L, Burrows M, Khan AA, Graham L, Volchkov P, Becker L, et al. Gender bias in autoimmunity is influenced by microbiota. Immunity. 2013;39:400–12. doi: 10.1016/j.immuni.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Benedek G, Bodhankar S, Lapato A, Vandenbark AA, Offner H. IL-10 producing B cells partially restore E2-mediated protection against EAE in PD-L1 deficient mice. Journal of neuroimmunology. 2015a;285:129–36. doi: 10.1016/j.jneuroim.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Lapato A, Bodhankar S, Vandenbark AA, Offner H. Treatment with IL-10 producing B cells in combination with E2 ameliorates EAE severity and decreases CNS inflammation in B cell-deficient mice. Metabolic brain disease. 2015b;30:1117–27. doi: 10.1007/s11011-015-9661-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.