Abstract
Background and Aims
Endoscopic ultrasound (EUS)-guided fine-needle aspiration (FNA) or biopsy is widely practiced. Optimal sonographic visualization of the needle is critical for image guided interventions. There are several commercially available needles but no bench-top testing and direct comparison of these needles to reveal their inherent echogenicity. The aims are to provide bench-top data that can be used to guide clinical applications and to promote future device research and development.
Methods
Descriptive bench-top testing and comparison. Bench-top testing of 8 commonly used EUS-FNA needles (all of 22 gauge in size): SonoTip Pro Control (Medi-Globe); Expect Slimline (Boston Scientific); EchoTip, EchoTip Ultra, EchoTip ProCore High Definition, (Cook Medical); ClearView (Conmed); EZ Shot2 (Olympus); BNX (Beacon Endoscopic); and 2 new prototype needles that are coated by echogenic polymers by Medi-Globe. Blinded evaluation of standardized and unedited videos by 43 EUS endoscopists and 17 radiologists specialized in gastrointestinal ultrasound examination that is unfamiliar with EUS needle devices.
Results
There was no significant difference in the ratings and rankings of these needles between endosonographers and radiologists. Overall, one prototype needle was rated as the best, ranking 10% to 40% higher than all other needles (p<0.01). Among the commercially available needles, the EchoTip Ultra needle and the ClearView needle were top choices. The EZ Shot 2 needle was ranked statistically lower than other needles (30%–75% worse, p<0.001).
Conclusions
All FNA needles have their inherent and different echogenicity, and these differences are similarly recognized by EUS endoscopists and radiologists. Needles with polymeric coating from the entire shaft to the needle tip may offer better echogenicity.
Keywords: Endoscopic ultrasound, needle, bench-top testing, echogenicity, tip echo-definition, shaft echo-definition, high angles better echogenicity
Introduction
The first reported endoscopic ultrasound (EUS)-guided fine-needle aspiration (FNA) in the GI tract was in 1992 by Vilmann et al. The first needle assembly for EUS-guided biopsy, of which all other commercially available needles have striking similarities, was invented by the same group [1–3]. EUS-FNA ushered in an era of therapeutic or interventional EUS [4–6]. All EUS-FNA needles have 4 main components: a metal needle, a stylet, a sheath, and a handle for controlling the sheath and needle lengths. The sheath size varies from 4 French to 6 French in diameter and the needle size ranges from 25 gauge to 19 gauge. The needle length is up to 8 to 9 cm. The most commonly used needle size is 22 gauge. Some endoscopists prefer inserting the needles with the stylet fully inserted and other prefer stylet withdrawal about 1 to 2 cm before targeted needle puncture.
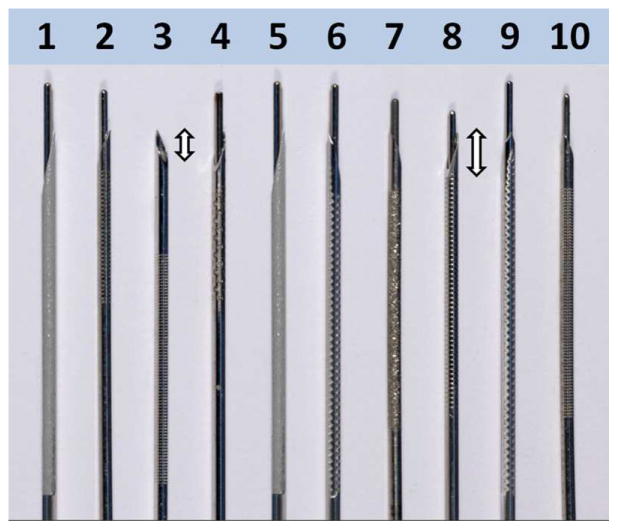
For any sonographically-guided needle interventions, needle visualization and related echogenicity is of paramount importance for the safety, success, and ease of performing interventional EUS. The needle tip optimal visibility is critical in situations in which a small lesion is targeted for tissue sampling. Based on the radiology literature, needle size, needle angle position to the transducer, and needle movement affect needle visualization [7–12]. Water or air priming of the needle, insulation, and the insertion of a stylet do not seem to influence needle visibility [9]. Recently, various echogenic features have been implemented to enhance the needle visibility, such as polymeric coating and laser treatment of the needle shaft [4, 10]. Most of the currently available disposable needles are modified by laser etching, mechanical dimpling, or sandblasting of the leading tip to enhance their echogenicity [4] (Figure 1).
Figure 1.
Image of all 10 study needles showing the needle tips (double-headed arrows), shafts, and treated mid shafts to enhance their echogenicity.
Although different device companies and EUS experts have claimed better echogenicity associated with certain needles, no bench-top testing has been reported and direct comparisons among the EUS-FNA needles. We designed the Needle Identification Comparison and Evaluation (NICE) study to assess their inherent echogenicity, needle tip echo-definition (TED) and shaft echo-definition (SED). The aims are to provide bench-top data that can be used to guide clinical applications and to promote future device research and development. The authors hypothesized that needles with longer and certain types of echogenic treatment were better than others. The authors also wanted to address whether radiologists rate and rank these needles differently from endoscopists.
Materials & Methods
This study was conducted at the GastroUnit, Division of Endoscopy, Copenhagen University Hospital Herlev, Denmark. This study was exempt from institutional research board review. A standard linear array EUS endoscope (Pentax EG 3270 UK) connected to a single EUS machine (Hitachi Ascendus, Tokyo, Japan) was used for all needle testing with a scanning frequency of 7.5 MHz and a compound scanning mode. The following settings were used in the system: frame rate of 35; mode dTHI-W-R (resolution)/P (penetration); dynamic range 70; and black and white gain of 13, B-gray map 3, BG (B-gain) 13, echo enhance 4, focus 1, 5cm depth, HIREZ 8, acoustic energy 100%. The manual gain controls were set in the middle and the gain was fixed for all needles’ testing.
We tested and compared 10 types of EUS-FNA needles (Supplemental Figure 1 and Table 1), 22 gauge in size. Two needles were prototype needles coated with echogenic polymers from their shafts to needle tips. The other 8 needles were commercially available with echogenic shaft treatment of variable lengths as depicted in Figure 1. We tested all needles in a single day thus eliminating potential machine or setup variations as confounding factors or bias. Only one investigator performed each step of the testing setup in order to minimize potential differences caused by individual skills. The order of testing was generated randomly and blinded to the biostatisticians and to a group of expert endosonographers (NICE-Endosonographers, or NICE-E) and another group of expert interventional ultrasound radiologists (NICE-Radiologists, or NICE-R). The NICE-R study group members were invited to avoid potential bias such as personal preference and conflict of interest because many of these needles potentially may be identified by experienced endosonographers based on their intrinsic echo patterns generated by the needles.
Table 1.
Features of ten tested endoscopic ultrasound fine-needle aspiration needles.
| Needle brand name (Manufacturer) | Needle gauge | Ranking of the echogenic treatment in terms of length* | |
|---|---|---|---|
| 1 | A new prototype needle (Medi-Globe) | 22 | Entire needle (1) |
| 2 | EchoTip (Cook Medical) | 22 | 5 |
| 3 | EchoTip®ProCore HD (Cook Medical) | 22 | 4 |
| 4 | EZ Shot 2 (Olympus) | 22 | 6 |
| 5 | A new prototype needle (Medi-Globe) | 22 | Entire needle (1) |
| 6 | ClearView Conmed | 22 | 2 |
| 7 | BNX (Beacon Endoscopic) | 22 | 3 |
| 8 | Expect Slimline (Boston Scientific) | 22 | 3 |
| 9 | SonoTip Pro (Medi-Globe) | 22 | 2 |
| 10 | EchoTip Ultra (Cook Medical) | 22 | 3 |
Needles 7, 8 and 10 have the same treatment lengths and are ranked the 3rd in terms of treatment length. HD: high definition. Needles 6 and 9 have the same treatment lengths and are ranked the 2nd. Needles 1 and 5 are coated with echogenic polymers and are ranked the 1st and in their instances the entire needles were visible.
Medi-Globe GmbH, Rosenheim, Germany; Boston Scientific, Natick, Mass, USA; Cook Medical, Winston-Salem, NC, USA; Conmed, Billercia, Mass, USA; Olympus America, Center Valley, Pa, USA; Beacon Endoscopic, Newton, Mass, USA. BNX needle and new 22 G SharkCore needle are made by the Beacon Endoscopic and they have an identical needle shaft, and differ only in tip from the SharkCore.
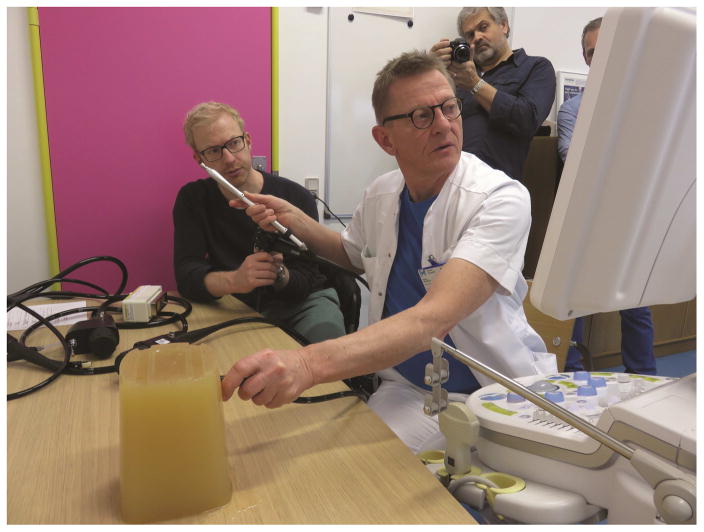
One investigator (PV) experienced in sonographically guided FNA, obtained all in-plane needle endosonograms. Because many endoscopists do not withdraw the stylet before needle insertion and in order to prevent air entrapment at the needle tips, all needles were studied with their stylets in place. The tested FNA needle was inserted through the endoscopic channel of a linear EUS endoscope. In clinical applications, the needle trajectory is generally between 30° and 50° respecting the axis of the echoendoscope. For each needle, test parameters were evaluated both with the needle in neutral position and with the needle maximally bent by means of the elevator. A custom-made bench-top tissue equivalent phantom block was used for this study (Figure 2) [13–14]. The phantom material was made from agar, flea seeds were added to create tissue-mimicking echogenicity. A single built-in video recorder was used to record endosonographic video footages in Audio Video Interleave (AVI) format (raw video footage) (Videos 1 and 2 includes all study videos in a much lower resolution format due to size limits). NICE-E and NICE-R study group members used these recorded raw video footages for evaluation. A two-minutes video was dedicated to each single needle testing and consisted of about 10 seconds of initial needle tip visualization and the index puncturing with the elevator fully open producing an insertion angle of about 30°: 15 seconds of needle in-plane motionless view (Figure 3) were shown followed by about 15 seconds with the needle moving to and fro 7 times mimicking the standard aspiration maneuver. Then the needle was withdrawn and reinserted with the elevator maximally activated producing an insertion angle of about 50°. Finally, 15 seconds of needle in-plane motionless view (Figure 4) were followed by about 15 seconds showing the needle moving to and fro 7 times.
Figure 2.
Image showing the bench-top testing setup: a tissue equivalent phantom block and the performing endosonographer (PV).
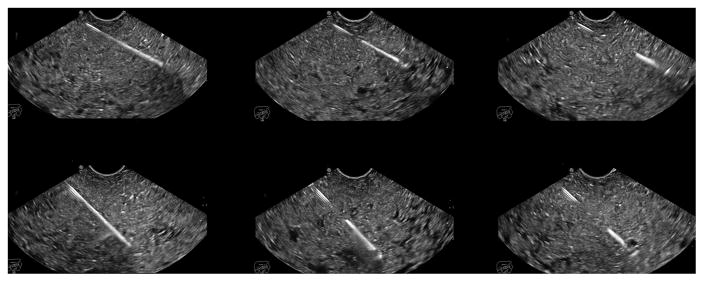
Figure 3.
Endoscopic ultrasonographic images showing different echogenicity of three studied needles at about 30° and 50° needle insertion angles: one prototype needle (5), Medi-Globe; EchoTip Ultra (10), Cook Medical; EZ Shot2 (4), Olympus.
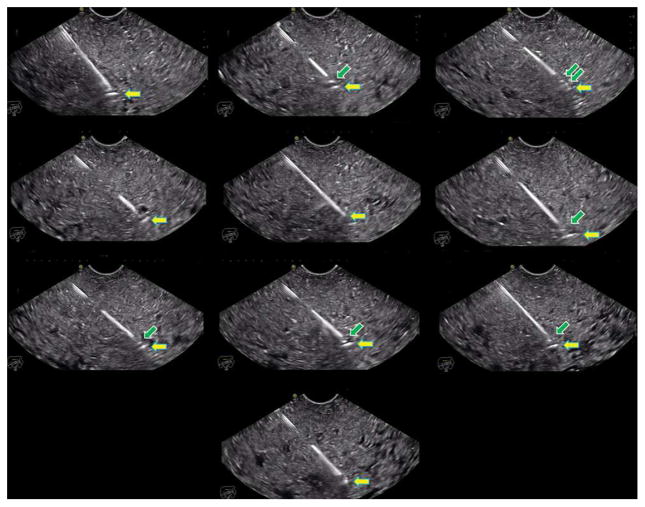
Figure 4.
Endoscopic ultrasonographic images showing different echogenicity of all 10 studied needles at about 50° insertion angles.
The NICE-E study group consisted of experienced endosonographers and the NICE-R study group consisted of ultrasound radiologists who were unfamiliar with EUS FNA needles but had at least 5 years of post-graduate clinical experience in percutaneous ultrasound guided needle aspiration. All members practice in major academic and/or tertiary referral centers. Each study group member independently evaluated the video footages generated from this study. They were blinded to brands and types of these needles and scored the needle echogenicity, SED and TED on a scale of 1 to 10. Needle tip and shaft echo-definition was defined as the sharpness of needle tip outline from the surrounding tissue phantom. A score of 5 meant acceptable echogenicity, tip and shaft echo-definition, a score of 1 meant un-acceptable echo signals, and a score of 10 meant optimal needle echogenicity for clinical interventions. Finally, each study group member was asked to rank all 10 tested needles and select the top, intermediate 4 and bottom 3 needles. The NICE study aimed to answer these questions: (1) Whether the endosonographers’ current needle choices and their personal belief can influence their rating and ranking of the needles; (2) Whether operators with more than 15 years’ experience rate needles differently than more junior endoscopists; (3) Whether radiologists rate and rank these needles differently from endoscopists.
Statistical Analysis
Survey participants’ characteristics were reported as frequency (percentage) for categorical variables and mean (standard deviation) for numerical variables. Multilevel mixed-effects generalized linear models with Poisson distributions and random intercepts were used to calculate needles’ average ranks and incidence-rate ratios (IRR) based on pooled data containing overall, TED and SED scores while accounting for clustering by participants and missing data. Needle comparisons on separate overall, TED and SED scores were additionally examined using negative binomial models fit with generalized estimating equations (GEE). Models were adjusted for participant location (North America, European and other areas). We additionally examined the effects of current needle usage and personal needle preferences on needle ratings using likelihood ratio tests.
Results
The NICE-E study group consisted of 43 endosonographers primarily based in Europe (18) and in the United States (19) (Supplemental Table 1). Twenty (47%) of study group members had more than 15 years of EUS experience. They listed currently using needles made by Cook Medical (36, 84%), Boston Scientific (22, 51%), Olympus (6, 14%), Medi-Globe (6, 14%), and Beacon Endoscopic (5, 12%). In a pre-study evaluation the participants were asked whether they considered different needles to be different in terms of echogenicity, 13 (30%) thought all needles are the same. Among operators with a preferred choice, this was Cook Medical needles (17, 40%), Boston Scientific needles (13, 30%), Medi-Globe (3, 7%), Beacon Endoscopic (2, 5%), and Olympus needles (1, 2%). The NICE-R study group consisted of 17 ultrasound radiologists, all based either in Europe (12) or in the United States (5). Nine of them did not use percutaneous needles made by any of these companies listed in our study.
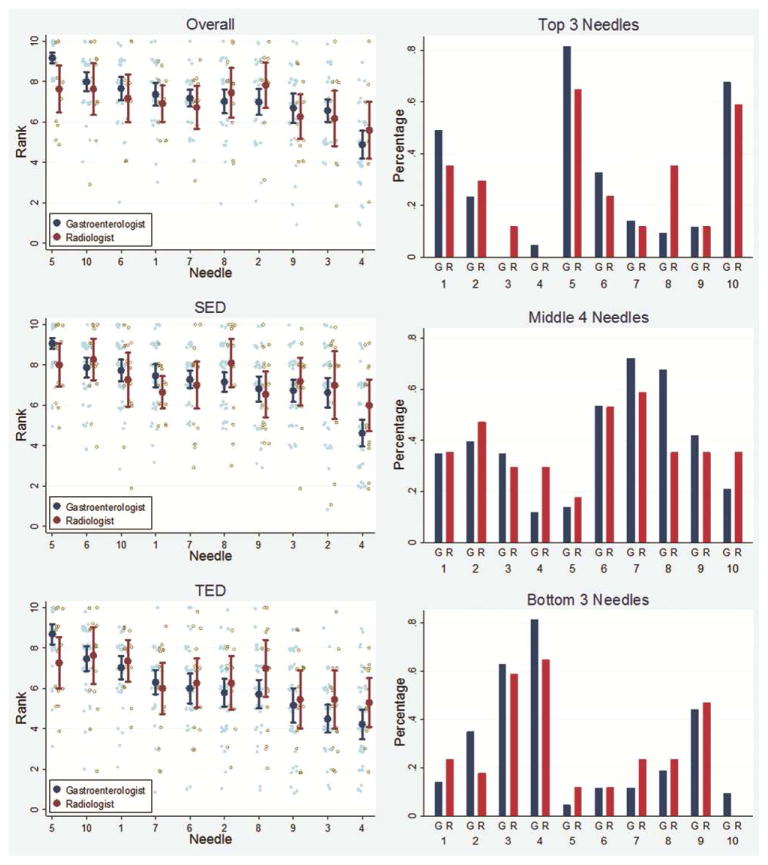
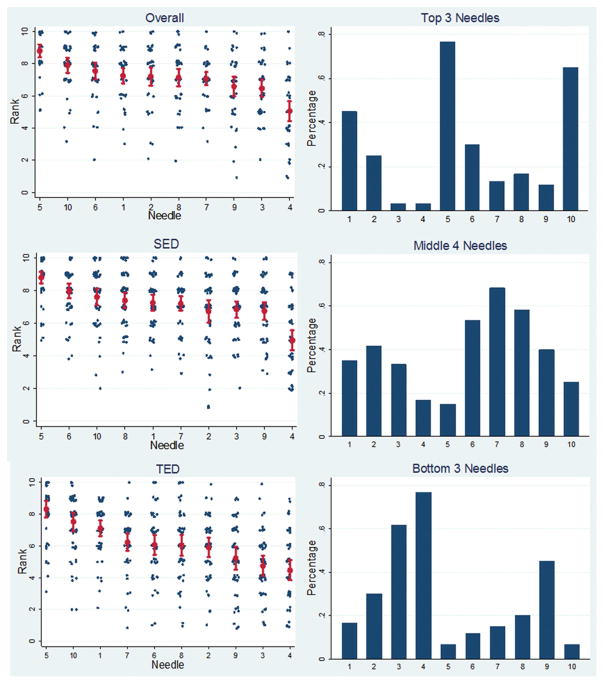
There were no significant differences in the ratings and rankings of these needles between endosonographers and radiologists (Figure 5). Overall, needle number 5, a prototype needle made by Medi-Globe, was rated as the best needle, ranking 10% to 40% higher than all other needles (p<0.01 for all comparisons). (Figure 6) (Table 2). The EchoTip Ultra needle was the second best needle and was rated the best commercially available needle. The EchoTip Ultra needle was ranked significantly higher than all other commercially available needles except for the ClearView needle, which was ranked slightly lower, but not statistically supported. The EZ Shot 2 needle was ranked statistically lower than all other needles (30%–75% worse, p<0.001). Two prototype needles (1 and 5) and the EchoTip Ultra needle (10) were selected by participants as consistently being among the top 3 needles. EZ Shot 2 (4), EchoTip ProCore High Definition (3), and SonoTip Pro (9) were selected as the bottom 3 needles.
Figure 5.
Comparisons of rankings between NICE-E and NICE-R study groups. All ratings and rankings are similar without statistical differences. TED: tip echo-definition. SED: shaft echo-definition.
Figure 6.
Overall rankings of 10 studied needles by a combined NICE-E and NICE-R study group. TED: tip echo-definition. SED: shaft echo-definition.
Table 2.
Studied needles’ average rank and incidence-rate ratios (IRRs)
| Pooled | Overall | TED | SED | |||||
|---|---|---|---|---|---|---|---|---|
| Needle | Avg. Rank (95% CI) | IRR | Avg. Rank (95% CI) | IRR | Avg. Rank (95% CI) | IRR | Avg. Rank (95% CI) | IRR |
| 5 | 8.63 (8.07, 9.19) | -ref- | 8.82 (8.39–9.24) | -ref- | 8.40 (7.94–8.86) | -ref- | 8.72 (8.36, 9.07) | -ref- |
| 10 | 7.72 (7.20, 8.24) | 0.89 p=0.004 (0.83, 0.97) | 7.92 (7.44–8.41) | 0.90 p=0.001 (0.84, 0.96) | 7.51 (7.02–7.99) | 0.89 p=0.007 (0.82, 0.97) | 7.76 (7.31, 8.20) | 0.89 p<0.001 (0.84, 0.94) |
| 1 | 7.27 (6.77, 7.77) | 0.84 p<0.001 (0.78, 0.91) | 7.27 (6.79–7.74) | 0.82 p<0.001 (0.76, 0.89) | 7.17 (6.76–7.58) | 0.85 p<0.001 (0.79, 0.92) | 7.34 (6.93, 7.76) | 0.84 p<0.001 (0.80, 0.89) |
| 6 | 7.23 (6.73, 7.72) | 0.84 p<0.001 (0.77, 0.90) | 7.53 (7.03–8.03) | 0.85 p<0.001 (0.80, 0.92) | 6.16 (5.67–6.66) | 0.73 p<0.001 (0.67, 0.80) | 8.00 (7.63, 8.38) | 0.92 p=0.001 (0.87, 0.97) |
| 8 | 6.92 (6.44, 7.40) | 0.80 p<0.001 (0.74, 0.87) | 7.13 (6.62–7.64) | 0.81 p<0.001 (0.74, 0.88) | 6.09 (5.57–6.60) | 0.72 p<0.001 (0.66, 0.80) | 7.53 (7.11, 7.95) | 0.86 p<0.001 (0.81, 0.92) |
| 7 | 6.91 (6.42, 7.39) | 0.80 p<0.001 (0.74, 0.87) | 7.07 (6.68–7.47) | 0.80 p<0.001 (0.76, 0.85) | 6.30 (5.86–6.74) | 0.75 p<0.001 (0.69, 0.81) | 7.33 (6.96, 7.71) | 0.84 p<0.001 (0.80, 0.89) |
| 2 | 6.69 (6.21, 7.16) | 0.77 p<0.001 (0.72, 0.84) | 7.21 (6.65–7.77) | 0.82 p<0.001 (0.75, 0.90) | 5.94 (5.48–6.41) | 0.71 p<0.001 (0.64, 0.78) | 6.98 (6.44, 7.52) | 0.80 p<0.001 (0.73, 0.87) |
| 9 | 6.29 (5.83, 6.74) | 0.73 p<0.001 (0.67, 0.79) | 6.57 (5.98–7.17) | 0.75 p<0.001 (0.67, 0.82) | 5.35 (4.81–5.89) | 0.64 p<0.001 (0.57, 0.71) | 6.93 (6.48, 7.38) | 0.80 p<0.001 (0.75, 0.85) |
| 3 | 6.04 (5.60, 6.48) | 0.70 p<0.001 (0.64, 0.76) | 6.47 (5.94–7.00) | 0.73 p<0.001 (0.67, 0.80) | 4.74 (4.27–5.21) | 0.56 p<0.001 (0.50, 0.63) | 6.96 (6.57, 7.36) | 0.80 p<0.001 (0.75, 0.85) |
| 4 | 5.05 (4.65, 5.45) | 0.58 p<0.001 (0.54, 0.64) | 5.02 (4.42–5.63) | 0.57 p<0.001 (0.50, 0.65) | 4.72 (4.22–5.21) | 0.56 p<0.001 (0.50, 0.64) | 5.31 (4.77, 5.85) | 0.61 p<0.001 (0.55, 0.68) |
Needles were sorted in descending order based on the pooled average rank.
TED: tip echo-definition, SED: shaft echo-definition, Avg.: average.
The NICE study also answered these relevant questions. The first was whether the endosonographers’ current needle choice and their personal believe could influence their rating and ranking of the needles. The answer was no. For example, although many endosonographers use Cook needles and believe they are better needles, there was no support that the use of specific needles modifies physicians’ rankings, with a likelihood ratio test p=0.996. Also, there was no support indicated that people who believe Cook or Boston Scientific needles are better were more likely to rate those needles higher, likelihood ratio test p=0.989. The second question was whether operators with more than 15 years’ experience rated needles differently from more junior endoscopists. We found no support that the years of experienced influenced rankings, IRR=1.06 p=0.332.
Additional and post survey video image analysis by the authors revealed following findings: (1) The needle echogenicity was increased when the needles were inserted at a higher angle (about 50°) than at a lower angle (about 30°) (Figure 3); (2) Needles (1 and 5) coated with polymers were echogenic from the entire shaft to the tip, whereas laser etched or sandblasted current commercial needles were visible only within the treated mid or distal shaft segment. The tips of some non-polymer coated needles were only visible as a single dot (Figure 4, Supplemental Figures 2 and 3). (3) The needle echogenicity and its ratings by endosonographers was depending on the lengths and types of echogenic treatment to the needle shaft. In general, the longer the treated shaft, the better the ratings, provided that the type of treatment was the same. (4) Of all the studied needles, only needle 3 (EchoTip ProCore H) has a cut-off on the distal shaft. This cut-off probably explains the third white dot observed on EUS images.
Discussion
The NICE study demonstrated that all FNA needles had their inherent and different echogenicity, and endosonographers and radiologists shared these differences similarly. The rating and ranking of these needles were the same between senior and junior endosonographers, and were independent of their current practice habits (ie, current needle selections) and their perceived most echogenic needles. Needle echogenicity relies on its echogenic technology, length and location of treatment and possible additional needle tip echogenic features. Polymer coating of the entire shaft and needle tip were preferred by endoscopists and radiologists (needles 5 and 1 were among top 3 preferred needles), likely due to maximal treatment length without skip areas. For non-polymer coated needles, certain echogenicity enhancing patterns or structures were better than others. Needles 2 and 10 were both made by Cook Medical and the echogenicity-enhancing pattern appeared similar. However, needle 10 was ranked much higher than needle 2 (ranked 5th) in this study. This difference is probably explained by the more than 70% longer echogenicity-enhancing pattern in needle 10. On the flip side, needles 2 and 4 had a similar length of echogenicity-enhancing pattern, but the length was the shortest of all studied needles, yet needle 4 was ranked the lowest in this study. The difference may be related to different methods of structuring the needle surface by the manufacturers. Needle 4, the EZ Shot 2 needle’s echogenic structure includes many holes on the shaft aiming to trap air bubbles to increase its visibility. Apparently, this feature is less effective than that used in other EUS needles. It seems that both the method for creating echo-enhancing structure as well as the length of the structure influences EUS needle visibility. As for needle 3, probably because of its cut-off on the distal shaft and the longer length of untreated distal needle shaft, it was rated second worse and ranked among one of the bottom 3 needles. On the other hand, needles 10 and 2, made by the same manufacturer, ranked high. All 3 needles have used the same echogenicity-enhancing pattern. It is noted that needle 6 (ClearView) and needle 9 (SonoTip Pro) are made with the same echogenicity-enhancing structure, length, and location on the shaft. On overall ranking, needle 6 was ranked the 3rd and needle 9 the 8th; on SED ranking, needle 6 was ranked the 2nd and needle 9 the 9th. If needle 6 is used as reference, IRR for needle 9 is 0.87 (0.80, 0.95), p=0.002 on overall rankings. This reveals the limitations of subjective evaluation in any study and recall bias. In order to minimize intra-observer variation, the authors asked each study group member to rate the needles individually and again to group all needles into top, middle, and bottom categories. Another way is to retest the same needle (either the same video again; or better yet a different video shot with the same needle).
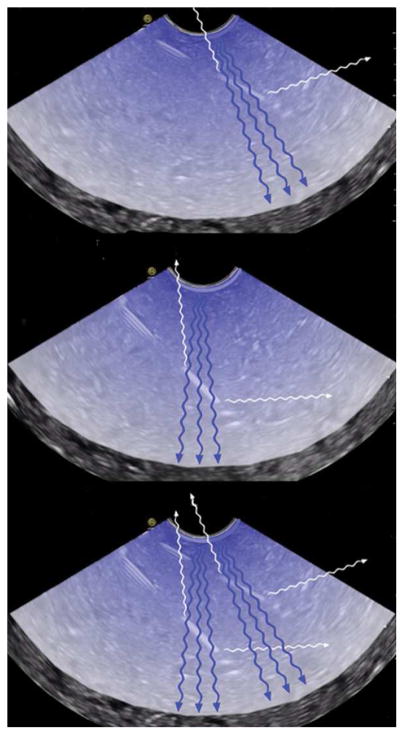
It is interesting to note that the needle echogenicity is greater when the needles are inserted at a higher angle (about 50°) than at a lower angle (30°): the higher angle the better the echogenicity (HABE) phenomenon. This finding collaborates study results published in radiology literature showing that needle size, needle angle respect to the transducer, and needle movement affect needle visualization [7–12]. The authors hypothesize that this phenomenon is related to echo reflection and its associated echo reception density (Figure 7). At higher insertion angles, the more direct and dense echo reflections are brought back to the sensor. The HABE phenomenon can potentially direct endosonographers where to place the lesion on the opposite part of the screen from needle entrance and insert the needles at a higher angle if the echogenicity of the needles is suboptimal. Advanced 2-dimensional ultrasound imaging techniques including compound imaging and tissue harmonic imaging may also play an important role for this phenomenon and has been studied in the radiology literature [15–16]. In our study, compound imaging and tissue harmonic imaging were always on for all tested needles. Additionally, ultrasound beam steering and transducer shape were also important factors for needle enhancement visualization [17]. Future experiments with EUS needles with echogenicity-enhancing surfaces and these new US techniques may help elucidate and/or improve needle enhancement and visualization.
Figure 7.
Endoscopic ultrasonographic images demonstrating the authors’ hypothesis of the higher angle the better echogenicity (HABE) phenomenon. The ultrasound fan hits the treated area of the needle and is reflected in a spread manner. At a lower angle of 30° (top image), the ultrasound emitter/receiver recollects less spread-out reflection. At a higher angle of 50° (middle image), the ultrasound emitter/receiver recollects a higher level of spread out reflection. The bottom is a composite image showing and comparing both scenarios.
Published in radiology literature, water or air priming of the needle, insulation, and the insertion of a stylet do not seem to influence needle visibility [9]. Because many endoscopists do not withdraw the stylet before needle insertion mainly to avoid sampling the gut wall and tissue outside the lesion, many needles have stylet tips within the needle bevels when fully inserted, and in order to prevent air entrapment at the needle tips, the authors studied all needles with their stylets inserted. There are pros and cons of studying needle tip’s visibility using this approach. As the authors have mentioned previously, the stylet tip may accentuate the needle tip’s visibility as a white dot or may even cause the most distal white dot on EUS video images. The authors believe that this interference is very small and insignificant. The authors suspect the white dot represent the needle tip when the stylet tip is not sticking outside the needle tips, or the stylet tip when the stylet tip are outside the needle tip. Occasionally, a second white dot can be seen above the needle tip in the non-polymer coated needles. We suspect this proximal second white dot represents the needle bevel-shaft junction in some cases and the needle tip when the stylet tip is outside the needle tip in others. The visibility may depend on the direction of the needle bevel: more visible when the bevel is facing toward or away from the echo fan.
Needle visibility may be influenced by physical properties of the tissue which is passed and targeted by the needle (reflection, scattering, absorption, diffraction). In the NICE study a tissue equivalent, but very homogeneous phantom was used. In more inhomogeneous biological tissues differences of visibility between needles may be more pronounced than in the homogeneous phantom tissue. However, using a phantom was the only way to have “same conditions” for all needles. Another limitation of this study is that the authors did not address needle flexibility or elasticity, which is important for EUS-FNA. The NICE study only focused on needle visibility. These needle-related mechanical features, such as internal friction, retention or loss of shape (nitinol versus stainless steel), etc. also influence needle’s functionality and utilization. These attributes are important because a bent-out-of-shape needle in a torqued endoscope may go off plane and be difficult to visualize; and echogenicity may become even more important.
In conclusion, all FNA needles have their inherent and different echogenicity, and these differences are shared similarly by endosonographers and radiologists. Needles with longer and certain echogenic features are ranked better than others. Needles with polymeric coating may offer the best echogenicity.
Supplementary Material
A composition of all 10 NICE study videos in a much lower resolution format due to publication limitations.
Image of the 10 tested endoscopic ultrasound FNA needles: Prototype needle number one (1), Medi-Globe; EchoTip (2), Cook Medical; EchoTip ProCore High Definition (3), Cook Medical; EZ Shot2 (4), Olympus; Prototype needle number two (5), Medi-Globe; ClearView (6), Conmed; BNX (7), Beacon Endoscopic; Expect Slimline (8), Boston Scientific; SonoTip Pro (9), Medi-Globe; EchoTip Ultra (10), Cook Medical.
Endoscopic ultrasonographic images showing different echogenicity, shaft echo-definition, and tip echo-definition of the top ranked (one prototype needle) and the second best (EchoTip Ultra) needles at about 50° angles. The polymer-coated shaft and tip (single headed arrow) of needle 5 are visible uniformly and throughout its entirety. The non-polymer coated needle (10) is visible at its mid shaft (double-headed arrow) due to echogenic treatment and its tip as a white dot (single-headed arrow).
Supplemental Figure 3. Endoscopic ultrasonographic images showing different echogenicity, shaft echo-definition, and tip echo-definition of the second best (EchoTip Ultra) and 5th ranked (EchoTip) needles at about 50° insertion angles. These non-polymer coated needles are visible at their mid shafts (double-headed arrow) due to echogenic treatment and their needle or stylet tips as a white dot (single-headed and yellow arrow). Another white dot above the tip (green arrow) in needle 2 is possibly the needle bevel shaft junction or needle bevel.
Supplemental Table 1: Survey participants’ characteristics (N=60)
Acronyms
- EUS
Endoscopic ultrasound
- FNA
final needle aspiration
- GI
gastrointestinal
- R&D
research and development
- SD
standard deviation
- HD
high-definition
- NICE
Needle Identification Comparison and Evaluation Study
- TED
tip echo-definition
- SED
shaft echo-definition
- HABE
high angles better echogenicity
- IRR
incidence-rate ratios
- GEE
generalized estimating equation
Footnotes
Author Contributions:
Study concept and design: Shou-jiang Tang, Andreas S. Vilmann, Adrian Saftoiu, Peter P. Fink, Peter Vilmann
Acquisition of Data: All authors on the list
Drafting of the article: Shou-jiang Tang, Andreas Vilmann, Peter Vilmann
Critical revision for important intellectual content: All authors on the list
Final approval of the article: All authors on the list
Disclosure Statement: Drs. Shou-jiang Tang, Andreas Vilmann, Costin Streba, Wanmei Wang, Michael Griswold, and Ruonan Wu have no conflict of interest or financial to disclose related to this study. Both Drs. Peter Vilmann and Adrian Saftoiu are members of a Medi-Globe Global Advisory Board. Peter Fink is an employee of Medi-Globe. The rest of the co-authors are study participants which were blinded to the used needle line up in this study. The study is investigator-initiated and not funded by industry or Medi-Globe.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vilmann P, Jacobsen GK, Henriksen FW, Hancke S. Endoscopic ultrasonography with guided fine needle aspiration biopsy in pancreatic disease. Gastrointest Endosc. 1992;38(2):172–3. doi: 10.1016/s0016-5107(92)70385-x. [DOI] [PubMed] [Google Scholar]
- 2.Vilmann P, Hancke S, Henriksen FW, Jacobsen GK. Endosonographically-guided fine needle aspiration biopsy of malignant lesions in the upper gastrointestinal tract. Endoscopy. 1993;25(8):523–7. doi: 10.1055/s-2007-1010389. [DOI] [PubMed] [Google Scholar]
- 3.Vilmann P, Hancke S. A new biopsy handle instrument for endoscopic ultrasound–guided fine-needle aspiration biopsy. Gastrointest Endosc. 1996;43:238–242. doi: 10.1016/s0016-5107(96)70324-3. [DOI] [PubMed] [Google Scholar]
- 4.Adler DG, Conway JD, Coffie JM, Disario JA, Mishkin DS, Shah RJ, Somogyi L, Tierney WM, Wong Kee Song LM, Petersen BT ASGE Technology Committee. EUS accessories. Gastrointest Endosc. 2007;66(6):1076–81. doi: 10.1016/j.gie.2007.07.035. [DOI] [PubMed] [Google Scholar]
- 5.Wani S, Muthusamy VR, Komanduri S. EUS-guided tissue acquisition: an evidence-based approach (with videos) Gastrointest Endosc. 2014 Dec;80(6):939–59.e7. doi: 10.1016/j.gie.2014.07.066. [DOI] [PubMed] [Google Scholar]
- 6.ASGE Technology Committee. Kaul V, Adler DG, Conway JD, Farraye FA, Kantsevoy SV, Kethu SR, Kwon RS, Mamula P, Pedrosa MC, Rodriguez SA, Tierney WM. Interventional EUS. Gastrointest Endosc. 2010;72(1):1–4. doi: 10.1016/j.gie.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 7.Hopkins RE, Bradley M. In-vitro visualization of biopsy needles with ultrasound: a comparative study of standard and echogenic needles using an ultrasound phantom. Clin Radiol. 2001;56(6):499–502. doi: 10.1053/crad.2000.0707. [DOI] [PubMed] [Google Scholar]
- 8.Sviggum HP, Ahn K, Dilger JA, Smith HM. Needle echogenicity in sonographically guided regional anesthesia: blinded comparison of 4 enhanced needles and validation of visual criteria for evaluation. J Ultrasound Med. 2013;32(1):143–8. doi: 10.7863/jum.2013.32.1.143. [DOI] [PubMed] [Google Scholar]
- 9.Schafhalter-Zoppoth I, McCulloch CE, Gray AT. Ultrasound visibility of needles used for regional nerve block: an in vitro study. Reg Anesth Pain Med. 2004;29(5):480–8. [PubMed] [Google Scholar]
- 10.Culp WC, McCowan TC, Goertzen TC, Habbe TG, Hummel MM, LeVeen RF, Anderson JC. Relative ultrasonographic echogenicity of standard, dimpled, and polymeric-coated needles. J Vasc Interv Radiol. 2000;11(3):351–8. doi: 10.1016/s1051-0443(07)61429-8. [DOI] [PubMed] [Google Scholar]
- 11.Nichols K, Wright LB, Spencer T, Culp WC. Changes in ultrasonographic echogenicity and visibility of needles with changes in angles of insonation. J Vasc Interv Radiol. 2003;14(12):1553–7. doi: 10.1097/01.rvi.0000099527.29957.a6. [DOI] [PubMed] [Google Scholar]
- 12.Wiesmann T, Bornträger A, Zoremba M, Neff M, Wulf H, Steinfeldt T. Compound imaging technology and echogenic needle design: effects on needle visibility and tissue imaging. Reg Anesth Pain Med. 2013;38(5):452–5. doi: 10.1097/AAP.0b013e31829730d5. [DOI] [PubMed] [Google Scholar]
- 13.Hocking G, Hebard S, Mitchell CH. A review of the benefits and pitfalls of phantoms in ultrasound-guided regional anesthesia. Reg Anesth Pain Med. 2011;36(2):162–70. doi: 10.1097/aap.0b013e31820d4207. [DOI] [PubMed] [Google Scholar]
- 14.Culjat MO, Goldenberg D, Tewari P, Singh RS. A review of tissue substitutes for ultrasound imaging. Ultrasound Med Biol. 2010;36(6):861–73. doi: 10.1016/j.ultrasmedbio.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 15.Mesurolle B, Helou T, El-Khoury M, Edwardes M, Sutton EJ, Kao E. Tissue harmonic imaging, frequency compound imaging, and conventional imaging: use and benefit in breast sonography. J Ultrasound Med. 2007;26(8):1041–51. doi: 10.7863/jum.2007.26.8.1041. [DOI] [PubMed] [Google Scholar]
- 16.Mesurolle B, Bining HJS, El Khoury M, Barhdadi A, Kao E. Contribution of tissue harmonic imaging and frequency compound imaging in interventional breast sonography. J Ultrasound Med. 2006;25(7):845–55. doi: 10.7863/jum.2006.25.7.845. [DOI] [PubMed] [Google Scholar]
- 17.Reusz G, Sarkany P, Gal J, Csomos A. Needle-related ultrasound artifacts and their importance in anaesthetic practice. Br J Anaesth. 2014;112(5):794–802. doi: 10.1093/bja/aet585. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A composition of all 10 NICE study videos in a much lower resolution format due to publication limitations.
Image of the 10 tested endoscopic ultrasound FNA needles: Prototype needle number one (1), Medi-Globe; EchoTip (2), Cook Medical; EchoTip ProCore High Definition (3), Cook Medical; EZ Shot2 (4), Olympus; Prototype needle number two (5), Medi-Globe; ClearView (6), Conmed; BNX (7), Beacon Endoscopic; Expect Slimline (8), Boston Scientific; SonoTip Pro (9), Medi-Globe; EchoTip Ultra (10), Cook Medical.
Endoscopic ultrasonographic images showing different echogenicity, shaft echo-definition, and tip echo-definition of the top ranked (one prototype needle) and the second best (EchoTip Ultra) needles at about 50° angles. The polymer-coated shaft and tip (single headed arrow) of needle 5 are visible uniformly and throughout its entirety. The non-polymer coated needle (10) is visible at its mid shaft (double-headed arrow) due to echogenic treatment and its tip as a white dot (single-headed arrow).
Supplemental Figure 3. Endoscopic ultrasonographic images showing different echogenicity, shaft echo-definition, and tip echo-definition of the second best (EchoTip Ultra) and 5th ranked (EchoTip) needles at about 50° insertion angles. These non-polymer coated needles are visible at their mid shafts (double-headed arrow) due to echogenic treatment and their needle or stylet tips as a white dot (single-headed and yellow arrow). Another white dot above the tip (green arrow) in needle 2 is possibly the needle bevel shaft junction or needle bevel.
Supplemental Table 1: Survey participants’ characteristics (N=60)