Abstract
Objective
To assess whether the addition of oncolytic reovirus (Reolysin®) to weekly paclitaxel prolonged progression-free survival (PFS) in the treatment of women with recurrent or persistent ovarian, tubal or primary peritoneal cancer.
Patients and Methods
Patients with recurrent or persistent epithelial ovarian, tubal, or peritoneal carcinoma, measurable or detectable disease, and three or fewer prior regimens were randomly assigned to paclitaxel (80 mg/m2 intravenously days 1, 8, and 15 every 4 weeks) or the combination of paclitaxel (80 mg/m2 intravenously days 1, 8, and 15) plus reovirus 3×1010 TCID50/day intravenously on days 1-5, both every 4 weeks until disease progression or toxicity. The primary end point was PFS. The study was designed with 80% power for a one-sided alternative at a 10% level of significance to detect a reduction in the hazard by 37.5%.
Results
The study accrued 108 patients, 100 of whom were evaluable for toxicity. Median PFS was 4.3 months for paclitaxel and 4.4 months for paclitaxel plus reovirus (hazard ratio, 1.11; 90% two-sided CI, 0.78 to 1.59; one-sided P = 0.687). The proportion responding (overall response rate) to paclitaxel was 20% among 45 patients with measurable disease receiving paclitaxel alone, and 17.4% among the 46 patients treated with the combination. The asymptotic relative probability of responding was 0.87 (90% CI, 0.42 to 1.79). Severe adverse events were more common in the combination regimen than in paclitaxel arm for severe neutropenia (grade ≥ 4, 12% versus 0%), and severe respiratory adverse events (grade ≥ 3, 25% versus 2%). No deaths were considered treatment related.
Conclusion
The addition of reovirus to weekly paclitaxel in the treatment of women with recurrent or persistent ovarian, tubal or peritoneal cancer did not sufficiently reduce the hazard of progression or death to warrant further investigation.
Keywords: Oncolytic virus, recurrent ovarian cancer, paclitaxel
Introduction
Few FDA approved options exist for the treatment of recurrent ovarian cancer. In patients with recurrent disease, re-treatment with paclitaxel using a weekly schedule has demonstrated activity, possibly through anti-angiogenic as well as direct cytotoxic mechanisms [1]. Gynecologic Oncology Group (GOG)-0126N demonstrated a 21% objective response rate (and a 46% rate of stable disease) in this population [2].
Reovirus Serotype 3 – Dearing Strain (Reolysin®) is a naturally occurring, ubiquitous, non-enveloped human reovirus with a genome that consists of 10 segments of double-stranded RNA. While community-acquired reovirus infection in humans is generally mild and limited to the upper respiratory and gastrointestinal tract, reovirus has been shown to replicate specifically in, and be cytopathic to, transformed cells possessing an activated Ras signaling pathway. The specificity of the reovirus for Ras-transformed cells, coupled with its relatively nonpathogenic nature in humans, makes it an attractive anticancer therapy candidate. In transformed cells with mutations of the Ras proto-oncogene (approximately 30-40% of all human tumors), reovirus has been shown to possess cytopathic activity [3]. Activated Ras is present in greater than 20% of ovarian cancers, and appears to be dependent on histology [4]. Importantly,activating Ras mutations are not requisite for reovirus efficacy, since activation or over-expression of regulatory elements in Ras signaling pathways can also lead to antitumor effects from reovirus [3]. In ovarian cancer, it has been shown that increased Ras signaling contributes to pathogenesis seen with reovirus [4].
Given the susceptibility of ovarian cancer cells to reovirus and the safety of IV reovirus in patients with advanced malignancies, reovirus has been investigated using IV and intraperitoneal (IP) administration in patients with recurrent ovarian cancer [5], demonstrating viral replication in peritoneal tumors when reovirus is delivered systemically [6].
Recent preclinical data suggests that reovirus has a synergistic effect when administered with taxanes [7]. In an in vitro model, exposure of cells to reovirus in combination with docetaxel or paclitaxel demonstrated enhanced apoptotic cell death when compared to either agent alone. Furthermore, in a murine model, reovirus monotherapy slowed tumor growth and prolonged median overall survival time compared to control treatment, whereas docetaxel alone had no effect. When administered in conjunction with reovirus, the combined therapy significantly suppressed tumor growth and replicating virus was identified within tumors [8]. Thus, we set out to assess whether weekly paclitaxel, when combined with intravenous reovirus, reduces the risk of disease progression when compared with paclitaxel alone.
Methods
This was an open-label prospective randomized phase IIB trial of single-agent weekly paclitaxel compared with weekly paclitaxel plus reovirus (GOG-186-H; ClinicalTrials.gov. Identifier: NCT01166542). Eligible patients included women with measurable (per RECIST 1.1) or detectable persistent or recurrent epithelial ovarian, fallopian tube, or primary peritoneal carcinoma with documented disease progression. Detectable disease required at least one of the following conditions: cancer antigen (CA)-125 at least 2× upper limit of normal (ULN), ascites and/or pleural effusion attributed to tumor, or solid and/or cystic abnormalities on radiographic imaging that did not meet RECIST 1.1 definitions for target lesions. Patients must have had one prior platinum-based chemotherapeutic regimen for management of primary disease containing carboplatin, cisplatin, or another organoplatinum compound. This initial treatment may have included intraperitoneal therapy, consolidation, non-cytotoxic agents or extended therapy administered after surgical or non-surgical assessment. If patients were treated with paclitaxel for their primary disease, this could have been given weekly or every 3 weeks. Patients were allowed to have received two additional cytotoxic regimens for management of recurrent or persistent cancer, with no more than one non-platinum, non-taxane regimen. Treatment with weekly paclitaxel for recurrent or persistent disease was not allowed. Patients were also allowed to have received non-cytotoxic (biologic and/or targeted agents such as bevacizumab) therapy as part of their primary treatment regimen but were not allowed to have received any non-cytotoxic therapy for management of recurrent or persistent disease. Patients with either platinum-sensitive (platinum-free interval [PFI] >182 days) or platinum-resistant (PFI <=182 days) disease were eligible. Importantly, patients who had received only one prior cytotoxic regimen (platinum-based regimen for management of primary disease), must have had a PFI of less than 12 months, or had progressed during platinum-based therapy, or had persistent disease after a platinum-based therapy.
Patients aged 18 years or older with a GOG performance status of 0 or 1 were eligible. Patients with a performance status of 2 were eligible if they had received only one prior regimen.
Patients must have been able to avoid direct contact with pregnant or nursing women, infants and immune-compromised individuals while on study and for ≥3 weeks following the last dose of reovirus administration. Additionally, patients with known HIV or hepatitis B or C or those receiving immunosuppressive therapy including chronic oral steroids (at an equivalent dose of greater than prednisone 5 mg daily) were excluded due to risk of viral infectivity of reovirus. Patients with a pre-existent infection were not eligible.
Drug Administration and Supportive Care
Paclitaxel was administered at 80 mg/m2 as a continuous intravenous (IV) infusion days 1, 8, 15 every 4 weeks. Hypersensitivity reactions were prevented with premedication with corticosteroids, diphenhydramine, and H2 antagonists prior to paclitaxel administration and the paclitaxel was administered over 60 minutes. Among those randomly assigned to reovirus, 3×1010 TCID50/day was administered IV over 60 minutes on days 1-5 of each cycle after paclitaxel. Treatment with acetaminophen was precluded while receiving reovirus due to preclinical reports of elevated ALT with this combination. Thus, non-acetaminophen containing antipyretics were recommended if needed. Treatment was continued every 28 days (one cycle) until disease progression or until adverse events (AEs) prohibited further therapy.
Reovirus was handled according to Biosafety Level (BSL) 2 guidelines, and in accordance with institutional biosafety policies and procedures, which generally included decontamination of all equipment and work surfaces with an appropriate disinfectant (minimum of 2% bleach solution).
Dose Modifications
Growth factors were not allowed, and subsequent cycles of therapy were administered if the absolute neutrophil count was >= 1,500/microL and the platelet count was >= 100,000/microL. Patients who failed to recover adequate counts within a 2-week delay were removed from the study. Dose reduction of both paclitaxel (to 60 mg/m2) and reovirus (to 1×1010 TCID50) was required after an initial episode of febrile neutropenia or grade 4 neutropenia persisting for at least 7 days. Similar dose reductions were instituted for grade 4 thrombocytopenia. Recurrent hematologic toxicity and neutropenic complications led to an additional dose reduction of both paclitaxel (to 40 mg/m2) and reovirus (to 3×109 TCID50). Dose reductions for grade 2 peripheral neuropathy required a reduction in the paclitaxel dose, and grade 2 or greater renal toxicity and grade 3 or greater elevations in liver associated enzymes and bilirubin required a dose reduction in both paclitaxel and reovirus. Patients with persistent grade 3 or greater nausea, emesis, diarrhea or constipation despite appropriate medical management required a dose reduction in both paclitaxel and reovirus. Patients requiring greater than two dose reductions for any cause were removed from the study. Dose escalations or re-escalations were not allowed.
Study End Points
Tumor measurements using computed tomography or magnetic resonance imaging were made once during every other cycle according to RECIST 1.1 for the first 6 months and then every 3 months thereafter until disease progression. Patient response was reported as the best response during therapy. Progression (for those with measurable disease) was defined as at least a 20% increase in the sum of the diameters of target lesions, taking as a reference the smallest sum on study. Other criteria sufficient for declaring progression included new lesions or unequivocal progression of existing non target lesions. Patients who progressed within 6 weeks were deemed to have progressive disease (PD). Partial response (PR) was defined as at least a 30% decrease in the sum of the diameters of target lesions, taking as a reference the baseline sum. Complete response (CR) was defined as the disappearance of all target and non-target lesions and no evidence of new lesions with normal CA-125 levels. Stable disease was declared for patients who neither progressed nor had CR/PR for at least 6 weeks. Progression-free survival (PFS) was defined as the time from study entry until disease progression or death. OS was defined as the time on study until death. Censored cases were observed until the date of last contact.
For those with detectable but non-measurable disease, assessment was based on CA-125, effusions (ie, ascites), and/or evaluation of indeterminate solid or cystic abnormalities. The date of progression by CA-125 level was determined by values greater than 2× maximum (ULN, nadir) that was confirmed at least 8 days later.
Statistical Design
The study used the intent to treat principle for evaluating efficacy. The primary objective of this study was to assess the activity of the combination regimen relative to the reference (paclitaxel) through a stratified Cox hazard ratio (HR) of the PFS endpoint. Patients were stratified according to their platinum-free interval PFI (those with a PFI ≤ 182 days versus those with PFI > 182 days) and measurable disease status (measurable versus non-measurable or “detectable” disease). The allocation ratio was 1:1 using stratified blocks. Dynamic allocation was not used. The study had 3 kinds of outcomes: (1) further study recommended, (2) a non-definitive negative result, and (3) no further study recommended [9], and it was powered to detect a reduction in the hazard rate by 37.5% (HR 0.625) with 80% power at the approximate 10% level of significance. The study had about a 10% chance of declaring a non-definitive negative result under the null and alternative hypotheses (Ho: HR=1 and Ha: HR=0.625). In this case, investigators could consider other literature or sources before making a final recommendation. The target enrollment was 110 patients (55 per arm) with a final analysis triggered after 88 PFS events. The study recommended further study, a non-definitive negative, or a negative result if the observed Cox HR was less than 0.757, between 0.757 and 0.857, and greater than 0.857, respectively. An interim futility analysis was conducted after 44 PFS events using a Lan-Demets beta spending function with a 47% chance of stopping under Ho [10] and [9]. Secondary objectives included assessments of tumor response, OS, and toxicity (among all treated patients).
Results
From December 2010 to September 2014, GOG member institutions randomized 108 patients (54 to the reference; 54 to the combination). Two patients were deemed ineligible, and a total of 8 patients were never treated. One patient in Figure 1 on the experimental arm was ineligible and never treated. Because she was never treated, she was excluded from the toxicity comparison. Thus, 100 treated patients (48 in the paclitaxel arm and 52 patients in the combination arm) were evaluable for toxicity (Figure 1). Patient characteristics are presented in Table 1. Only 3.5% of the overall population with serous ovarian cancer had low-grade (grade 1) disease, with the remaining proportion having high-grade (grade 2 or 3) disease. Approximately 30% of patients received only one prior chemotherapy regimen, and the same proportion received three prior regimens. Prior bevacizumab exposure was reported in 42% of patients overall. Measurable disease was present in 84% of patients, and approximately 67% were platinum-resistant. Treatment arms were well balanced by platinum sensitivity, measurable disease, and prior bevacizumab use. A median of four cycles were administered in both arms of the study (overall range, 1-16).
Figure 1.
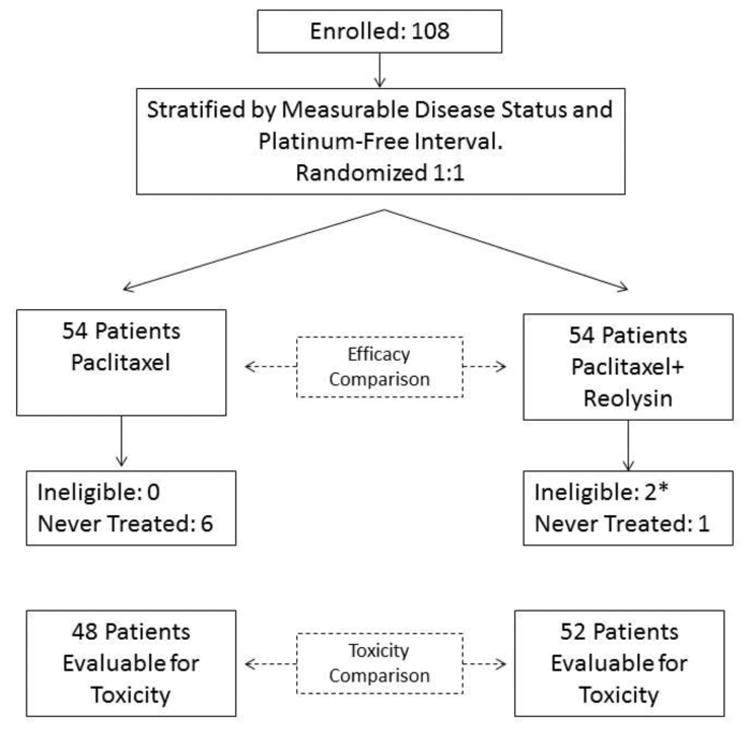
a) CONSORT diagram and b) schema.
* One ineligible patient was also never treated.
* One patient on the experimental arm was ineligible and never treated. This patient is tabulated in the figure as “ineligible” but is excluded from the toxicity comparison.
Table 1. Patient Characteristics of All Enrolled Patients.
| Characteristic | Regimen | |||||
|---|---|---|---|---|---|---|
| Paclitaxel | Paclitaxel+Reovirus | Total | ||||
| N | % | N | % | N | % | |
| Age Group | ||||||
| 30-39 | 1 | 1.9 | 0 | 0 | 1 | 0.9 |
| 40-49 | 4 | 7.4 | 4 | 7.4 | 8 | 7.4 |
| 50-59 | 19 | 35.2 | 11 | 20.4 | 30 | 27.8 |
| 60-69 | 20 | 37.0 | 26 | 48.1 | 46 | 42.6 |
| 70-79 | 9 | 16.7 | 11 | 20.4 | 20 | 18.5 |
| >=80 | 1 | 1.9 | 2 | 3.7 | 3 | 2.8 |
| Ethnicity | ||||||
| Hispanic | 3 | 5.6 | 0 | 0 | 3 | 2.8 |
| Non-Hispanic | 51 | 94.4 | 50 | 92.6 | 101 | 93.5 |
| Unknown/Unsp. | 0 | 0 | 4 | 7.4 | 4 | 3.7 |
| Race | ||||||
| Asian | 1 | 1.9 | 1 | 1.9 | 2 | 1.9 |
| Black/African American | 1 | 1.9 | 3 | 5.6 | 4 | 3.7 |
| American Indian | 1 | 1.9 | 1 | 1.9 | 2 | 1.9 |
| White | 50 | 92.6 | 48 | 88.9 | 98 | 90.7 |
| Unknown/Unsp. | 1 | 1.9 | 1 | 1.9 | 2 | 1.9 |
| Performance Status | ||||||
| 0 | 33 | 61.1 | 32 | 59.3 | 65 | 60.2 |
| 1 | 20 | 37.0 | 22 | 40.7 | 42 | 38.9 |
| 2 | 1 | 1.9 | 0 | 0 | 1 | 0.9 |
| Cell Type/Grade | ||||||
| Endometrioid, grade 1 | 2 | 3.7 | 0 | 0 | 2 | 1.9 |
| Endometrioid, grade 2 | 0 | 0 | 1 | 1.9 | 1 | 0.9 |
| Endometrioid, grade 3 | 2 | 3.7 | 0 | 0 | 2 | 1.9 |
| Serous | 40 | 74.1 | 45 | 83.3 | 85 | 78.7 |
| Serous, grade 1 | 1 | 2.5 | 2 | 4.4 | 3 | 3.5 |
| Serous, grade 2 | 5 | 12.5 | 3 | 6.7 | 8 | 9.4 |
| Serous, grade 3 | 33 | 82.5 | 40 | 88.9 | 73 | 85.9 |
| Serous, grade N/A | 1 | 2.5 | 0 | 0.0 | 1 | 1.2 |
| Clear Cell | 2 | 3.7 | 0 | 0 | 2 | 1.9 |
| Mixed Epithelial | 1 | 1.9 | 3 | 5.6 | 4 | 3.7 |
| Undifferentiated | 1 | 1.9 | 0 | 0 | 1 | 0.9 |
| Adenocarcinoma, NOS | 5 | 9.3 | 4 | 7.4 | 9 | 8.3 |
| Mucinous | 1 | 1.9 | 0 | 0 | 1 | 0.9 |
| Other | 0 | 0 | 1 | 1.9 | 1 | 0.9 |
| Number of Prior Regimens | ||||||
| 1 Prior Regimen | 13 | 24.1 | 19 | 35.2 | 32 | 29.6 |
| 2 Prior Regimens | 24 | 44.4 | 21 | 38.9 | 45 | 41.7 |
| 3 Prior Regimens | 17 | 31.5 | 14 | 25.9 | 31 | 28.7 |
| Prior Immunotherapy | ||||||
| No | 50 | 92.6 | 50 | 92.6 | 100 | 92.6 |
| Yes | 4 | 7.4 | 4 | 7.4 | 8 | 7.4 |
| Prior Surgery | ||||||
| No | 3 | 5.6 | 5 | 9.3 | 8 | 7.4 |
| Yes | 51 | 94.4 | 49 | 90.7 | 100 | 92.6 |
| Prior Bevacizumab | ||||||
| No | 31 | 57.4 | 32 | 59.7 | 63 | 58.3 |
| Yes | 23 | 42.6 | 22 | 40.7 | 45 | 41.7 |
| Measurable Disease | ||||||
| No | 9 | 16.7 | 8 | 14.8 | 17 | 15.7 |
| Yes | 45 | 83.3 | 46 | 85.2 | 91 | 84.3 |
| Platinum Sensitive | ||||||
| Platinum Resistant | 36 | 66.7 | 36 | 66.7 | 72 | 66.7 |
| Platinum Sensitive 6-12 M | 14 | 25.9 | 12 | 22.2 | 26 | 24.1 |
| Platinum Sensitive >12 M | 4 | 7.4 | 6 | 11.1 | 10 | 9.3 |
| Total | 54 | 50.0 | 54 | 50.0 | 108 | 100.0 |
Adverse Events (AEs)
Treatment emergent AEs are listed in Table 2. Severe neutropenia (grade ≥ 4) appeared to be associated with reovirus administration (11.5% versus 0.0%). Additionally, severe respiratory/thoracic/mediastinal adverse events (grade ≥ 3) appeared to be associated with reovirus administration (25.0% versus 2.1%, risk ratio was 12.0 [95% CI 1.63 ∼ 88.3]). When looking at the more detailed list of AEs, it is noted that some toxicities that could be attributed to a viral illness were higher on the combination arm (e.g. fever, myalgia, and headache) though the risk of false positive outcomes is higher. There were no significant differences observed in AEs that could be attributed to a viral illness when examining fatigue, sepsis, or cough. There were no deaths attributed to treatment.
Table 2.
All Adverse Events Regardless of Attribution Using CTCAE v. 4.0 Tabulated by Max Grade among Treated Patients Who Submitted Toxicity Data.
| AE Category | Treatment Group | 0 | 1 | 2 | 3 | 4 | 5 | Total |
|---|---|---|---|---|---|---|---|---|
| Leukopenia | Paclitaxel | 13 | 18 | 14 | 3 | 0 | 0 | 48 |
| Paclitaxel+Reovirus | 10 | 9 | 16 | 14 | 3 | 0 | 52 | |
| Thrombocytopenia | Paclitaxel | 37 | 11 | 0 | 0 | 0 | 0 | 48 |
| Paclitaxel+Reovirus | 22 | 24 | 5 | 1 | 0 | 0 | 52 | |
|
| ||||||||
| Neutropenia1 | Paclitaxel | 23 | 6 | 14 | 5 | 0 | 0 | 48 |
| Paclitaxel+Reovirus | 13 | 11 | 13 | 9 | 6 | 0 | 52 | |
|
| ||||||||
| Anemia | Paclitaxel | 5 | 19 | 17 | 7 | 0 | 0 | 48 |
| Paclitaxel+Reovirus | 4 | 18 | 20 | 7 | 3 | 0 | 52 | |
| Other Investigations | Paclitaxel | 18 | 17 | 9 | 3 | 1 | 0 | 48 |
| Paclitaxel+Reovirus | 27 | 12 | 9 | 2 | 2 | 0 | 52 | |
| Other Blood/Lymphatics | Paclitaxel | 47 | 0 | 0 | 1 | 0 | 0 | 48 |
| Paclitaxel+Reovirus | 51 | 0 | 0 | 1 | 0 | 0 | 52 | |
| Cardiac | Paclitaxel | 41 | 5 | 2 | 0 | 0 | 0 | 48 |
| Paclitaxel+Reovirus | 39 | 5 | 4 | 3 | 1 | 0 | 52 | |
| Ear and labyrinth | Paclitaxel | 38 | 9 | 0 | 1 | 0 | 0 | 48 |
| Paclitaxel+Reovirus | 48 | 4 | 0 | 0 | 0 | 0 | 52 | |
| Endocrine | Paclitaxel | 48 | 0 | 0 | 0 | 0 | 0 | 48 |
| Paclitaxel+Reovirus | 51 | 0 | 1 | 0 | 0 | 0 | 52 | |
| Eye | Paclitaxel | 36 | 10 | 1 | 1 | 0 | 0 | 48 |
| Paclitaxel+Reovirus | 45 | 6 | 1 | 0 | 0 | 0 | 52 | |
| Nausea | Paclitaxel | 13 | 20 | 11 | 4 | 0 | 0 | 48 |
| Paclitaxel+Reovirus | 8 | 28 | 13 | 3 | 0 | 0 | 52 | |
| Vomiting | Paclitaxel | 27 | 11 | 6 | 4 | 0 | 0 | 48 |
| Paclitaxel+Reovirus | 29 | 10 | 12 | 1 | 0 | 0 | 52 | |
| Other Gastrointestinal | Paclitaxel | 6 | 11 | 13 | 17 | 0 | 1* | 48 |
| Paclitaxel+Reovirus | 5 | 13 | 18 | 14 | 1 | 1* | 52 | |
| General and administration site | Paclitaxel | 8 | 10 | 25 | 5 | 0 | 0 | 48 |
| Paclitaxel+Reovirus | 0 | 14 | 25 | 13 | 0 | 0 | 52 | |
| Hepatobiliary | Paclitaxel | 48 | 0 | 0 | 0 | 0 | 0 | 48 |
| Paclitaxel+Reovirus | 49 | 1 | 0 | 2 | 0 | 0 | 52 | |
| Immune System | Paclitaxel | 46 | 2 | 0 | 0 | 0 | 0 | 48 |
| Paclitaxel+Reovirus | 50 | 1 | 1 | 0 | 0 | 0 | 52 | |
| Infections/infestations | Paclitaxel | 25 | 1 | 17 | 3 | 2 | 0 | 48 |
| Paclitaxel+Reovirus | 26 | 1 | 18 | 5 | 2 | 0 | 52 | |
| Injury/poisoning | Paclitaxel | 42 | 5 | 1 | 0 | 0 | 0 | 48 |
| Paclitaxel+Reovirus | 48 | 3 | 0 | 1 | 0 | 0 | 52 | |
| Metabolism/nutrition | Paclitaxel | 7 | 19 | 11 | 9 | 2 | 0 | 48 |
| Paclitaxel+Reovirus | 10 | 13 | 18 | 10 | 1 | 0 | 52 | |
| Musculoskeletal/connective tissue | Paclitaxel | 17 | 20 | 9 | 2 | 0 | 0 | 48 |
| Paclitaxel+Reovirus | 17 | 25 | 9 | 1 | 0 | 0 | 52 | |
| Neoplasms benign/malignant | Paclitaxel | 47 | 0 | 0 | 0 | 0 | 1* | 48 |
| Paclitaxel+Reovirus | 47 | 0 | 0 | 0 | 0 | 5* | 52 | |
| Peripheral sensory neuropathy | Paclitaxel | 19 | 25 | 3 | 1 | 0 | 0 | 48 |
| Paclitaxel+Reovirus | 20 | 27 | 5 | 0 | 0 | 0 | 52 | |
| Nervous system | Paclitaxel | 25 | 16 | 5 | 2 | 0 | 0 | 48 |
| Paclitaxel+Reovirus | 21 | 21 | 4 | 5 | 1 | 0 | 52 | |
| Psychiatric | Paclitaxel | 25 | 18 | 5 | 0 | 0 | 0 | 48 |
| Paclitaxel+Reovirus | 28 | 13 | 11 | 0 | 0 | 0 | 52 | |
| Renal/urinary | Paclitaxel | 29 | 13 | 4 | 1 | 0 | 1* | 48 |
| Paclitaxel+Reovirus | 33 | 13 | 4 | 1 | 1 | 0 | 52 | |
| Reproductive/breast | Paclitaxel | 44 | 4 | 0 | 0 | 0 | 0 | 48 |
| Paclitaxel+Reovirus | 49 | 2 | 0 | 1 | 0 | 0 | 52 | |
|
| ||||||||
| Respiratory/thoracic/mediastinal2 | Paclitaxel | 15 | 19 | 13 | 1 | 0 | 0 | 48 |
| Paclitaxel+Reovirus | 9 | 21 | 9 | 12 | 1 | 0 | 52 | |
| Skin/subcutaneous3 | Paclitaxel | 8 | 11 | 29 | 0 | 0 | 0 | 48 |
| Paclitaxel+Reovirus | 12 | 20 | 20 | 0 | 0 | 0 | 52 | |
|
| ||||||||
| Vascular disorders | Paclitaxel | 22 | 6 | 9 | 11 | 0 | 0 | 48 |
| Paclitaxel+Reovirus | 23 | 9 | 5 | 15 | 0 | 0 | 52 | |
The estimate of the odds ratio is undefined (i.e. is infinitely large). The exact 95% CI for the odds ratio of a severe adverse event (grade 4 or worse) for experimental treatment to control is [1.51, ].
The estimate of the odds ratio is 15.7. The exact 95% CI for the odds ratio of a severe adverse event (grade 3 or worse) for experimental treatment to control is [2.13, 679.6].
The estimate of the odds ratio is 0.410. The exact 95% CI for the odds ratio of a moderate adverse event (grade 2 or worse) for experimental treatment to control is [0.17, 0.98].
Activity (Table 3)
Table 3. Response to Therapy and Patient Outcomes among All Enrolled Patients.
| Paclitaxel | Paclitaxel +Reovirus | |||||
|---|---|---|---|---|---|---|
| Characteristic | Category | n | % | n | % | Total |
| Off Study | No | 4 | 7.4 | 3 | 5.6 | 7 |
| Yes | 50 | 92.6 | 51 | 94.4 | 101 | |
| Reason Off Therapy | On Study/Unspecified | 4 | 7.4 | 3 | 5.6 | 7 |
| Disease Progression | 28 | 51.9 | 30 | 55.6 | 58 | |
| Refusal | 9 | 16.7 | 4 | 7.4 | 13 | |
| Adverse Events | 6 | 11.1 | 13 | 24.1 | 19 | |
| Death | 1 | 1.9 | 1 | 1.9 | 2 | |
| Other Disease | 1 | 1.9 | 0 | 0.0 | 1 | |
| Other Reason | 5 | 9.3 | 3 | 5.6 | 8 | |
| Response | Complete response | 3 | 5.6 | 1 | 1.9 | 4 |
| Partial response | 6 | 11.1 | 7 | 13.0 | 13 | |
| Stable disease | 16 | 29.6 | 16 | 29.6 | 32 | |
| Increasing disease | 12 | 22.2 | 17 | 31.5 | 29 | |
| Indeterminate | 8 | 14.8 | 5 | 9.3 | 13 | |
| Non-measurable | 9 | 16.7 | 8 | 14.8 | 17 | |
| Cycles of Treatment1 | 0 | 6 | 11.1 | 2 | 3.7 | 8 |
| 1 | 2 | 3.7 | 5 | 9.3 | 7 | |
| 2 | 16 | 29.6 | 16 | 29.6 | 32 | |
| 3 | 2 | 3.7 | 3 | 5.6 | 5 | |
| 4 | 7 | 13.0 | 8 | 14.8 | 15 | |
| 5 | 3 | 5.6 | 4 | 7.4 | 7 | |
| 6 | 3 | 5.6 | 4 | 7.4 | 7 | |
| 7 | 0 | 0.0 | 1 | 1.9 | 1 | |
| 8 | 3 | 5.6 | 0 | 0.0 | 3 | |
| 9+ | 12 | 22.2 | 11 | 20.4 | 23 | |
| Alive/Cause of Death1 | Alive | 22 | 40.7 | 22 | 40.7 | 44 |
| Dead - Disease-related | 30 | 55.6 | 29 | 53.7 | 59 | |
| Dead - Rx & Disease | 0 | 0.0 | 1 | 1.9 | 1 | |
| Dead - Neither Rx/Dis | 1 | 1.9 | 2 | 3.7 | 3 | |
| Dead - Reason Pending | 1 | 1.9 | 0 | 0.0 | 1 | |
Analysis obtained at the time of primary endpoint maturation.
There were similar median PFS in both arms (4.3 months with paclitaxel versus 4.4 months with paclitaxel and reovirus, hazard ratio 1.11, 90% CI 0.78-1.59). A similar proportion of patients with measurable disease who were treated with weekly paclitaxel plus reovirus responded to treatment (20% for paclitaxel alone versus 17.4% for the combination, odds ratio 0.84 (90% Exact CI 0.30-2.33)). The asymptotic estimate of the relative probability of response (experimental to reference) was 0.87 (90% CI 0.42-1.79). The probability of responding by CA-125 among evaluable patients was similar between the combination arm (12/39, 30.8%) compared to the reference arm (10/29, 34.5%). An analysis of OS was conducted at the time of the primary analysis and again at a later point when the data were more mature. The following results were obtained upon the second OS analysis: OS with paclitaxel (13.1 months) was not significantly different from that with paclitaxel and reovirus (12.6 months), hazard ratio 1.006 (90% CI 0.690-1.468). There remained no survival difference even when stratified by measurable disease and PFI (HR 0.912, 90% CI 0.623-1.334).
Discussion
Weekly paclitaxel has substantial activity in the treatment of recurrent or persistent ovarian, tubal and peritoneal cancer in both the platinum-sensitive and platinum-resistant settings. However, the addition of intravenous reovirus to weekly paclitaxel did not improve PFS or OS or objective response, regardless of whether the assessment was in the population with measurable or with CA-125 evaluable disease.
Oncolytic reovirus has demonstrated activity against many cancers, including colorectal, pancreatic, lung and head and neck cancers as well as multiple myeloma [11]. Initial interest in reovirus against ovarian cancer was established after the observation that reovirus infected multiple ovarian cancer cell lines but not a normal ovarian cell line [4]. Subsequent clinical investigation of reovirus in ovarian cancer has included the demonstration of intravenous reovirus replicating within peritoneal carcinomatosis from ovarian cancer [6], with sparing of replication in normal tissues [12]. Following the establishment of the proof of principle that systemic delivery of a replicative virus could infect distant disease, the feasibility of combining reovirus with taxanes was established [8], ultimately leading to the development of GOG 186-H. Despite the background work which led to enthusiasm for the concept of patients with recurrent ovarian cancer being treated with reovirus and weekly paclitaxel, this study was not able to demonstrate improved outcomes compared with patients treated with weekly paclitaxel alone.
There are a number of factors that could contribute to the lack of additional activity afforded by the addition of reovirus to weekly paclitaxel. While reovirus does not require activated RAS for replication, its function ultimately acts on the RAS signaling pathway, and the reovirus progeny from RAS transformed cells are more infectious leading to an increase in the release of viral particles [13]. Given that only 20% of ovarian cancers are expected to harbor activating RAS mutations, reovirus efficacy could be limited in a population unselected for RAS mutation. Additionally, limited data exist demonstrating that the systemic administration of reovirus leads to replication in peritoneal tumors from ovarian cancer [6] and [8]. Given that there were no translational research objectives in this study, no archival specimens exist to assess whether selection for RAS activation would lead to alternative conclusions regarding the activity of reovirus. It is conceivable that other factors that prevent active reovirus replication could play a role in the lack of synergistic activity of reovirus with weekly paclitaxel. Since reovirus has been shown to replicate and demonstrate cytotoxic activity in hypoxic conditions [14], the use of weekly paclitaxel, which was shown to target proliferating endothelial cells [15], may contribute to the reversal of a hypoxic phenotype thereby attenuating the replication of reovirus in ovarian cancers. Continued investigation of populations of patients with ovarian cancer in which reovirus may be more effective (such as those with low-grade serous carcinoma, commonly with activation of the MEK-ERK-RAS-RAF pathway) is critical to the development of reovirus in ovarian cancer. Given that only 3.5% of the overall population with serous ovarian cancer had low-grade disease, no meaningful conclusions can be drawn from the patients treated with reovirus with weekly paclitaxel.
An interesting finding in this study overall is the consistent response to weekly paclitaxel in patients with ovarian cancer (Table 4). Summarizing the data from patients treated with weekly paclitaxel on 5 prospective NCI-funded clinical trials, an overall response was seen in 53/230 (23%), with response rates in the individual studies ranging from 20 to 28%. These data, reporting consistent responses to weekly paclitaxel, argue for subsequent studies in this population being designed as single-arm combinations of investigational agents with weekly paclitaxel, thereby maximizing the opportunity to study new agents in patients with recurrent ovarian cancer.
Table 4. Response to weekly taxanes across completed GOG clinical trials (On-line only).
| GOG Study | Taxane | N | Response rate (%) |
|---|---|---|---|
| 126-N2 | Weekly paclitaxel | 48 | 10/48 (21%) |
| 126-R16 | Weekly Nab-paclitaxel | 47 | 11/47 (23%) |
| 186-H3 | Weekly paclitaxel | 45 | 9/45 (20%) |
| 186-J17 | Weekly paclitaxel | 44 | 10/44 (23%) |
| 186-K18 | Weekly paclitaxel | 46 | 13/46 (28%) |
In summary, the addition of reovirus to weekly paclitaxel in women with recurrent ovarian cancer led to no improvement in PFS or other measures of patient outcome. Results from this study do not support further investigation of this combination in this patient population at these doses and schedule.
Figure 2. Progression-free survival.
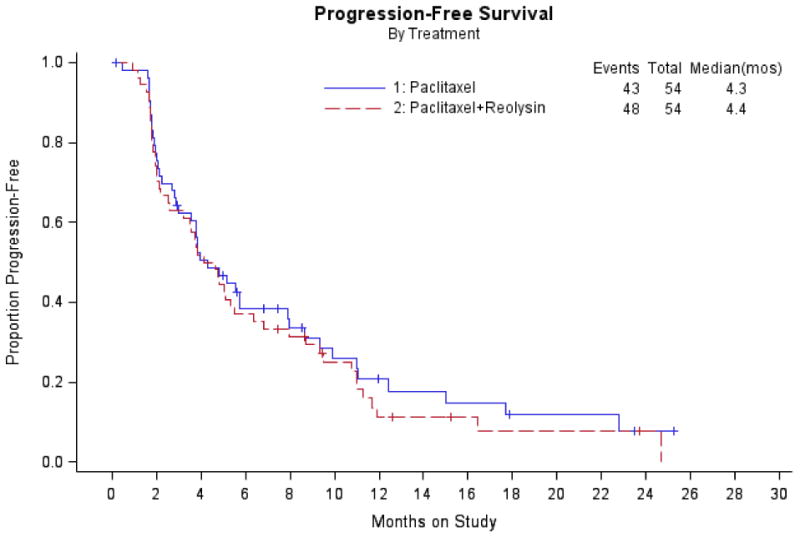
Figure 3. Overall survival.
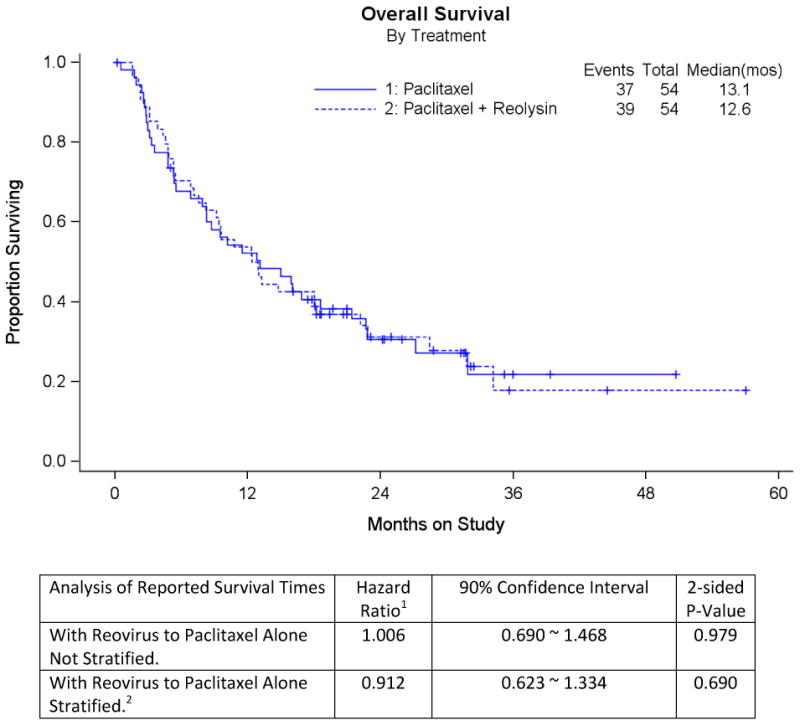
1 Hazard ratios are reported for rates on Paclitaxel+Reovirus to Paclitaxel Alone. This analysis occurred after the primary analysis was conducted.
2 Analysis stratified by measurable disease (Yes/No) and platinum-free interval (≤ 182 days versus > 182 days).
Highlights.
Reovirus when added to paclitaxel is not active in unselected patients with recurrent ovarian cancer
Severe neutropenia and respiratory toxicity is more common with reovirus exposure
The activity of weekly paclitaxel in recurrent ovarian cancer is confirmed
Acknowledgments
This study was supported by National Cancer Institute grants to the Gynecologic Oncology Group (GOG) Administrative Office (CA 27469), the Gynecologic Oncology Group Statistical Office (CA 37517), NRG Oncology (1 U10 CA180822) and NRG Operations (U10CA180868). The following Gynecologic Oncology institutions participated in this study: Ohio State University Comprehensive Cancer Center, University of Oklahoma Health Sciences Center, University of New Mexico, Case Western Reserve University, Carilion Clinic Gynecological Oncology, Froedtert and the Medical College of Wisconsin, University of New Mexico, University of Texas Southwestern Medical Center, Indiana University Hospital/Melvin and Bren Simon Cancer Center, University of Mississippi Medical Center, Wake Forest University Health Sciences, Women's Cancer Center of Nevada and Mayo Clinic.
Dr. Joan Walker received grant funding from NCI and GOG/NRG. She also received support for travel to meetings for the study or other purposes from Mateon.
Dr. David O'Malley was on the Advisory Board for Clovis, Tesaro, Novocure, Janssen, AstraZeneca, Genentech/Roche and Eisai. He served as a consultant for Clovis and Tesaro and was on the Steering Committee for Amgen.
Dr. Debra Richardson received grant funding from NRG. She also served on the Advisory Board for Genentech and AstraZeneca.
Dr. Carol Aghajanian received personal fees from Oxigene for Steering Committee Meetings, Cerulean Advisory Board, Bayer Advisory Board, VentiRx Advisory Board, AstraZeneca Advisory Board and Travel, ImmunoGen Advisory Board, Oxigene Advisory Board, Abbvie for travel for investigator meetings ×2, and Clovis Advisory Board.
Footnotes
CONFLICTS OF INTEREST: All other co-authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cohn DE, Valmadre S, Resnick KE, Eaton LA, Copeland LJ, Fowler JM. Bevacizumab and weekly taxane chemotherapy demonstrates activity in refractory ovarian cancer. Gynecol Oncol. 2006;102(2):134–9. doi: 10.1016/j.ygyno.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 2.Gynecologic Oncology Group. Markman M, Blessing J, Rubin SC, Connor J, Hanjani P, et al. Phase II trial of weekly paclitaxel (80 mg/m2) in platinum and paclitaxel-resistant ovarian and primary peritoneal cancers: a Gynecologic Oncology Group study. Gynecol Oncol. 2006;101(3):436–40. doi: 10.1016/j.ygyno.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 3.Gemignani ML, Schlaerth AC, Bogomolniy F, Barakat RR, Lin O, Soslow R, et al. Role of KRAS and BRAF gene mutations in mucinous ovarian carcinoma. Gynecol Oncol. 2003;90(2):378–81. doi: 10.1016/s0090-8258(03)00264-6. [DOI] [PubMed] [Google Scholar]
- 4.Hirasawa K, Nishikawa SG, Norman KL, Alain T, Kossakowska A, Lee PW. Oncolytic reovirus against ovarian and colon cancer. Cancer Res. 2002;62(6):1696–701. [PubMed] [Google Scholar]
- 5.Cohn DE, Nuovo G, Coffey MC, O'Malley D, Villalona-Calero MA, Grever MR, Deam D, Zwiebel JA, Phelps MA. Phase I/II trial of reovirus serotype 3-Dearing strain in patients with recurrent ovarian cancer. J Clin Oncol. 2010;28:TPS253. [Google Scholar]
- 6.Phelps M, Cohn DE, O'Malley DM, Wei L, Wilkins D, Campbell A, Schaaf LJ, Coffey MC, Villalona-Calero MA, Grever MR, Nuovo GJ, Zwiebel JA. Reovirus replication in ovarian and peritoneal tumors after intravenous administration. Cancer Res. 2010;70:2594. doi: 10.1158/1538-7445.AM10-2594. [DOI] [Google Scholar]
- 7.Heinemann L, Simpson GR, Boxall A, Kottke T, Relph KL, Vile R, et al. Synergistic effects of oncolytic reovirus and docetaxel chemotherapy in prostate cancer. BMC Cancer. 2011;11:221. doi: 10.1186/1471-2407-11-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Comins C, Spicer J, Protheroe A, Roulstone V, Twigger K, White CM, et al. REO-10: a phase I study of intravenous reovirus and docetaxel in patients with advanced cancer. Clin Cancer Res. 2010;16(22):5564–72. doi: 10.1158/1078-0432.CCR-10-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sargent DJ, Chan V, Goldberg RM. A three-outcome design for phase II clinical trials. Control Clin Trials. 2001;22(2):117–25. doi: 10.1016/s0197-2456(00)00115-x. [DOI] [PubMed] [Google Scholar]
- 10.Lan KKG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70:659–663. [Google Scholar]
- 11.Gong J, Sachdev E, Mita AC, Mita MM. Clinical development of reovirus for cancer therapy: An oncolytic virus with immune-mediated antitumor activity. World J Methodol. 2016;6(1):25–42. doi: 10.5662/wjm.v6.i1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nuovo GJ, Garofalo M, Valeri N, Roulstone V, Volinia S, Cohn DE, et al. Reovirus-associated reduction of microRNA-let-7d is related to the increased apoptotic death of cancer cells in clinical samples. Mod Pathol. 2012;25(10):1333–44. doi: 10.1038/modpathol.2012.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marcato P, Shmulevitz M, Pan D, Stoltz D, Lee PW. Ras transformation mediates reovirus oncolysis by enhancing virus uncoating, particle infectivity, and apoptosis-dependent release. Mol Ther. 2007;15(8):1522–30. doi: 10.1038/sj.mt.6300179. [DOI] [PubMed] [Google Scholar]
- 14.Gupta-Saraf P, Miller CL. HIF-1alpha downregulation and apoptosis in hypoxic prostate tumor cells infected with oncolytic mammalian orthoreovirus. Oncotarget. 2014;5(2):561–74. doi: 10.18632/oncotarget.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Vinals F, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15(3):220–31. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coleman RL, Brady WE, McMeekin DS, Rose PG, Soper JT, Lentz SS, et al. A phase II evaluation of nanoparticle, albumin-bound (nab) paclitaxel in the treatment of recurrent or persistent platinum-resistant ovarian, fallopian tube, or primary peritoneal cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 2011;122(1):111–5. doi: 10.1016/j.ygyno.2011.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richardson DL, Sill MW, Cho JK, et al. A randomized placebo controlled Phase IIb trial of weekly paclitaxel plus/minus pazopanib in persistent or recurrent ovarian cancer. 15th Biennial Meeting of the International Gynecologic Cancer Society; 8–10 Nov, 2014; Melbourne, Australia. 2014. IGCSM-1335. [Google Scholar]
- 18.Matulonis UA, Sill M, Thaker PH, et al. NRG/GOG 186K: A randomized phase II study of NCI-supplied cabozantinib versus weekly paclitaxel in the treatment of persistent or recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer—final results. Gynecol Oncol; Presented at: 2016 SGO Annual Meeting; March 19-22, 2016; San Diego, CA. 2016. p. 207. Late-breaking abstract. (#4) [Google Scholar]


