Abstract
Background
Evolving animal studies and limited epidemiological data show that prenatal air pollution exposure is associated with childhood obesity. Timing of exposure and child sex may play an important role in these associations. We applied an innovative method to examine sex-specific sensitive prenatal windows of exposure to PM2.5 on anthropometric measures in preschool-aged children.
Methods
Analyses included 239 children born ≥37 weeks gestation in an ethnically-mixed lower-income urban birth cohort. Prenatal daily PM2.5 exposure was estimated using a validated satellite-based spatio-temporal model. Body mass index z-score (BMI-z), fat mass, % body fat, subscapular and triceps skinfold thickness, waist and hip circumferences and waist-to-hip ratio (WHR) were assessed at age 4.0±0.7 years. Using Bayesian distributed lag interaction models (BDLIMs), we examined sex differences in sensitive windows of weekly averaged PM2.5 levels on these measures, adjusting for child age, maternal age, education, race/ethnicity, and pre-pregnancy BMI.
Results
Mothers were primarily Hispanic (55%) or Black (26%), had ≤12 years of education (66%) and never smoked (80%). Increased PM2.5 exposure from 8–17 and 15–22 weeks gestation was significantly associated with increased BMI z-scores and fat mass in boys, but not in girls. Higher PM2.5 exposure from 10–29 weeks gestation was significantly associated with increased WHR in girls, but not in boys. Prenatal PM2.5 was not significantly associated with other measures of body composition. Estimated cumulative effects across pregnancy, accounting for sensitive windows and within-window effects, were 0.21 (95%CI=0.01–0.37) for BMI-z and 0.36 (95%CI=0.12–0.68) for fat mass (kg) in boys, and 0.02 (95%CI=0.01–0.03) for WHR in girls, all per μg/m3 increase in PM2.5.
Conclusions
Increased prenatal PM2.5 exposure was more strongly associated with indices of increased whole body size in boys and with an indicator of body shape in girls. Methods to better characterize vulnerable windows may provide insight into underlying mechanisms contributing to sex-specific associations.
Keywords: prenatal exposure, particulate matter, child obesity, anthropometry, sensitive windows, sex difference
1. INTRODUCTION
Nearly one third of children in the United States (U.S.) are overweight or obese, a proportion that has more than doubled since 1980 (Ogden et al., 2016). While some data suggest that U.S. obesity rates are stabilizing, rates remain high in preschoolers (CDC 2013) and continue to show disparities for lower-socioeconomic status (SES) groups (Yanovski and Yanovski 2011). Moreover, correlated phenotypes in infants and toddlers, such as faster weight gain and higher body fat, predict later life obesity-related trajectories (Barker 2012; Taveras et al., 2009). For example, children who are obese are five times as likely as those who are not to be obese adults (CDC 2013). Moreover, obesity increases the risk of a number of physical and mental health disorders over the life course (Bogers et al., 2007; CDC 2013; Strazzullo et al., 2010). Such trends result in U.S. spending approaching nearly $190 billion annually on obesity related healthcare expenses (Cawley and Meyerhoefer 2012). Identifying potentially modifiable risk factors is a research priority.
While current standards mandate prevention to start soon after birth (2010), research increasingly shows that programming of obesity begins prenatally (Sutton et al., 2016). Moreover, while attempts to curb childhood obesity have largely focused on physical activity and diet, childhood obesity is likely influenced by a range of environmental factors beyond nutrition and exercise (Birch et al., 2011). Recent evidence points to a role for chemical environmental exposures in programming obesity (Vrijheid et al., 2016).
Emerging evidence specifically supports a role for particulate air pollution, a pro-oxidant environmental exposure, in obesity programming. Particulate air pollution has been increasingly linked to obesity and related phenotypes in animal models (Bolton et al., 2014). Oxidative stress (OS) plays a role in the generation and maintenance of an obesity phenotype in both isolated adipocytes and animals (Aroor and DeMarco 2014; De Marchi et al., 2013; Imhoff and Hansen 2010; Ye et al., 2015). Notably, obesity involves excess accumulation of adipose tissue, as well as dysregulation of glucose and lipid metabolism. Reactive oxygen species (ROS) promote adipogenic signaling pathways and disrupted adipogenesis (Atashi et al., 2015; Iyer et al., 2010). Moreover, recent prospective epidemiological data link early postnatal ambient pollution to child obesity (McConnell et al., 2015), and also link ambient pollution exposure, even below air quality guidelines, to glucose tolerance during pregnancy and food reward hormone dysregulation (Calderon-Garciduenas et al., 2015; Fleisch et al., 2014), both of which are identified intermediate pathways to offspring/child obesity (Hillier et al., 2007; Jastreboff et al., 2014; Kubo et al., 2014; Poston 2010).
Ambient air pollution effects likely begin in utero. In pregnant women, inhaled particles translocate from the lungs via the blood to other organs including the placenta (MohanKumar et al., 2008). Particulate matter can also invoke a chronic inflammatory process in the mother’s lung resulting in systemic inflammation and consequent placental OS (Liu et al., 2016). Our group and others have linked in utero air pollution exposure with low birth weight, a potential predecessor of overweight/obesity in later life (Bell et al., 2007; Kloog et al., 2012; Lakshmanan et al., 2015; Padula et al., 2012).
Prospective human studies examining the association between prenatal traffic-related air pollution exposure and childhood obesity remain sparse. Rundle et al. linked prenatal exposure to polyaromatic hydrocarbons (PAHs) to obesity in children assessed at ages 5 and 7 years (Rundle et al., 2012). Fleisch et al. showed that prenatal exposure to fine particulate matter was associated with more rapid postnatal weight gain in 6-month-old infants (Fleisch et al., 2015). This group also demonstrated a link between proximity to major roadways at birth (<50 meters) and fat mass at both early- and mid-childhood (median 3.3 and 7.7 years of age) (Fleisch et al., 2016). Lavigne et al. showed that increased exposure to air pollution during pregnancy was associated with higher levels of umbilical cord blood adinopectin, which regulates glucose and fatty acid breakdown in the developing fetus, and may contribute to obesity in later childhood (Lavigne et al., 2016).
Notably, existing studies of prenatal air pollution effects on childhood obesity have considered subjective assignment of exposure timing, such as air pollution exposure in a certain trimester or averaged over the entire pregnancy or over a certain length of time before pregnancy. This makes it difficult to compare the results across studies as well as to better delineate the critical windows affecting fetal programming. Clinically defined trimesters do not necessarily correspond to relevant vulnerable periods of body growth. Measuring exposure in an arbitrarily defined susceptibility window that does not overlap with periods of physiological vulnerability may lead to underestimated or even missed associations (Wilson et al., 2017a). In addition, animal data demonstrate sex-specific vulnerability to prenatal oxidant injury (Minghetti et al., 2013), which has been linked to both air pollution and infant growth (Sun et al., 2009). Recent analyses from our group combined advanced statistical methods with highly temporally resolved exposure data to more objectively identify susceptibility windows and enhance the power to detect associations and identify vulnerable groups (i.e., effect modification) (Chiu et al., 2016; Hsu et al., 2015). These analyses demonstrated sex-specific and time-varying associations of prenatal air pollution exposure on asthma and neurodevelopmental outcomes in children, but to our knowledge, this has not yet been examined for early childhood growth indicators.
We leveraged data on daily exposure to particulate matter with a diameter ≤2.5 μm (PM2.5) measured over gestation and applied advanced statistical methods to more precisely identify the sensitive windows of time-varying prenatal PM2.5 exposure effects on anthropometric measurements in preschool-aged children from an ethnically mixed lower-SES inner city population. We also examined effect modification by child sex.
2. MATERIALS AND METHODS
Participants were from the Asthma Coalition on Community, Environment and Social Stress (ACCESS) project, a pregnancy cohort originally funded to recruit 500 mother-child pairs to examine independent and interactive effects of early life stress and physical toxins on childhood respiratory health (Wright et al., 2008). Between August 2002 and January 2007, English- or Spanish-speaking pregnant women (≥18 years old) receiving care at Brigham & Women’s Hospital (BWH), Boston Medical Center (BMC), and affiliated community health centers were enrolled (at 28.4 ± 7.9 weeks gestation). Seventy-eight percent (78%) of women receiving prenatal care, who were approached by research staff on select clinic days, were eligible and agreed to enroll. There were no significant differences on race/ethnicity, education, and income between women enrolled and those who declined. A total of 455 women gave birth to a live born infant and continued follow-up. Supplemental funding was obtained to assess anthropometry in children aged 3 to 5 years; of n=358 children age-eligible for this initiative, n=277 completed anthropometry assessments at age 4.0±0.7 years of age. Among these children, n=30 were born <37 weeks, and n=8 did not have prenatal PM2.5 data (i.e., did not have accurate addresses during pregnancy), resulting in n=239 available for analysis. Those included in analyses did not differ significantly from those not included in analyses based on key covariates including race/ethnicity, maternal age at enrollment, maternal educational status, maternal pre-pregnancy weight, prenatal smoking, child’s birth weight or gestational age. Procedures were approved by the human studies committees at BWH and BMC. Mothers provided written consent in their preferred language.
2.1 Prenatal PM2.5 Exposure
As described previously (Chiu et al., 2016), we used a validated hybrid satellite based spatio-temporal prediction model to estimate each woman’s prenatal exposure to PM2.5, an index of ambient pollution from traffic and other sources, based on residence over pregnancy (i.e., at enrollment and updated if they moved). In brief, the model combines the aerosol optical depth (AOD) data derived by Moderate Resolution Imaging Spectroradiometer (MODIS) at a 10 km spatial resolution with traditional land-use regression (LUR) predictors to yield residence-specific estimates of daily PM2.5, as detailed elsewhere (Kloog et al., 2011). The model was run using day-specific AOD data were calibrated against ground monitor-based PM2.5 measurements derived from 78 monitoring stations covering New England and incorporated traditional LUR terms (traffic density, point sources, etc) and meteorological variables (temperature, wind speed, visibility, elevation, distance to major roads, percent of open space, point emissions and area emissions). The relationship between AOD and PM2.5 was calibrated daily using data from grid cells with both AOD values and monitor data using mixed models with random slopes for day, nested within regions. If the AOD data was not available for certain locations due to metrological conditions such as cloud coverage or snow, the predictions at these locations were imputed by fitting the model with a thin plate spline of latitude and longitude and a random intercept for each cell. The R2 of “out of sample” ten-fold cross validation for daily values were 0.83 for days with AOD and 0.81 for days without AOD data. We then calculated weekly averaged PM2.5 levels for each week over pregnancy for each participant to reduce potential noise caused by day-to-day PM2.5 variation and autocorrelations.
2.2 Anthropometric Measurements
2.2.1 Body mass index (BMI)
Children’s height while standing was measured without shoes (to nearest 0.1 cm) using a portable stadiometer (Shorr Productions, Olney, MD) and weight was measured (to nearest 0.1 kg) in light clothing using a calibrated portable electronic scale (Seca model 881; Seca Corporation, Hanover, MD). BMI (kg/m2) was calculated as weight divided by square of height. BMI z-scores were calculated based on sex- and age- specific normative U.S. data using the Centers for Disease Control (CDC) 2000 growth chart (CDC 2000).
2.2.2 Bioimpedance
Bipolar bioelectrical impedance was used to estimate fat mass (kg), fat free mass (kg), and percent body fat using the BIM4 bio-impedance analyzer (Impedimed, Queensland, Australia) which has been validated in young children (Rush et al., 2013).
2.2.3 Skinfold thickness
Subscapular (SS) and triceps skinfold (TS) thicknesses (mm) were measured using Harpenden skinfold calipers and standard techniques (Oken et al., 2005); the sum (SS + TS) and ratio (SS:TS) were also calculated.
2.2.4 Waist and hip circumferences
Waist circumference was measured midway between the lowest rib and the top of the iliac crest at end expiration and hip circumference (to nearest 0.1 cm) over the great trochanters using a measuring tape (Hoechstmass Balzer GmbH, Sulzbach, Germany). Waist-to-hip ratio (WHR) was calculated by dividing waist circumference by hip circumference.
2.3 Covariates
Maternal age, race/ethnicity, and educational status were ascertained at enrollment; information about child’s sex, date of birth, gestational age at birth, and birth weight were obtained by medical record review. Women’s pre-pregnancy BMI (kg/m2) was calculated from height and pre-pregnancy weight reported at enrollment. An internal validation study comparing self-report and measured height and weight available in 121 women assessed early in pregnancy (<10 weeks gestation) showed good agreement across all levels of height and weight (Wright et al., 2013). We calculated length of gestation using last menstrual period, and sex-specific birth weight for gestational age z-scores were calculated based on the U.S. national reference (Lakshmanan et al., 2015; Oken et al., 2003).
2.4 Statistical Analysis
Analyses included 239 mothers and their singleton children born at ≥37 weeks gestation. In order to identify sensitive windows for the effects of prenatal PM2.5 in relation to anthropometric outcomes, we applied a distributed lag model (DLM) (Gasparrini et al., 2010; Zanobetti et al., 2000). Using a constrained DLM, we estimate the time-varying association for each participant’s weekly exposures throughout the gestational period and anthropometric outcomes. Significant sensitive exposure windows were identified as weeks during pregnancy with a statistically significant association as previously described (Chiu et al., 2016; Hsu et al., 2015).
To examine effect modification by child sex, we estimated the interaction of prenatal PM2.5 × sex using Bayesian distributed lag interaction models (BDLIMs) as recently detailed elsewhere (Wilson et al., 2017b). In brief, BDLIM extends the traditional constrained DLM framework for critical windows to more fully account for effect modification - in our case, by child sex. The constrained DLM assumes that boys and girls either have the same sensitive window and within-window effect or that they have both different windows and different within-window effects. In contrast, BDLIM partitions the DLM into shape and scale components, which allows the additional possibilities of boys and girls having the same sensitive window but different within-window effect or the same within-window effect but different sensitive windows. The BDLIM for child i (i=1, …, n) who is sex j (j=1 for female and j=0 for male) is , where aj is a fixed sex-specific intercept, βj, is the regression coefficient characterizing the sex-specific association between weighted PM2.5 exposure and anthropometric outcome, is the weighted exposure, and is the covariate regression term. BDLIM partitions the time-varying association between exposure and outcome into two components: 1) the weights, wjt, that identify critical windows of susceptibility and 2) the coefficients, βj, that identify the magnitude of the within-window effects. When the weights are constant over time in both groups, BDLIM is equivalent to a model with mean exposure over pregnancy interacted with child sex. When the weights vary by time, the model identifies time periods with greater weight (i.e., potential sensitive windows) that will graphically appear as a bump during which exposure is significantly associated with the respective anthropometric indicators. This data-driven approach automatically determines whether the wjt and βj are the same or different for each group based on the likelihood of each pattern of heterogeneity. In other words, the BDLIM is able to identify the best fit pattern of effect modification by sex among the four patterns: 1) boys and girls have the same weights (shape of distributed lag function) and the same coefficients (within window effect or scale); 2) boys and girls have the same weight (windows) function but different coefficients; 3) boys and girls have different weights but the same coefficients; 4) and boys and girls have both different weights and coefficients. The association between exposure and outcome was then estimated under the effect modification pattern that is best supported by the data. Deviance information criterion (DIC) was used to determine the best fitting model with optimal parameters (e.g., number of knots and degrees of freedom used) and whether the weights and effects were different across sex (Wilson et al., 2017b).
The BDLIM incorporates the data from all exposure time points simultaneously and assumes that the association between the outcome and exposure at a given time point varies smoothly as a function of time while controlling for exposure at all other time points. It also yields an estimate of a cumulative effect across all time points (in our case, across the entire pregnancy) accounting for both the sensitive windows and the strength of within-window associations for each sex group, by calculating the sum of βj × wjt for each time point.
Standard controls (maternal age, child’s sex, child’s age at anthropometric assessment) as well as potential confounders (maternal race/ethnicity, education, pre-pregnancy BMI) were included in analyses. We also conducted sensitivity analyses additionally adjusting for postnatal daily PM2.5 levels predicted by the spatio-temporal model averaged over the first 2 years of life and perinatal smoking, as well as birth weight for gestational age z-score, a potential pathway variable. All analyses were implemented in R statistical software (v3.3.1, Vienna, Austria).
3. RESULTS
3.1 Participant characteristics
Most mothers were ethnic minority (55% Hispanic, 26% African American), had ≤ 12 years of education (66%), and never smoked (80%) (Table 1). Table 2 summarizes the distribution of child anthropometry measurements and prenatal PM2.5 exposure by sex. Prenatal PM2.5 levels were similar for boys and girls and there were no significant sex differences in terms of maternal age at enrollment, race/ethnicity, education, pre-pregnancy BMI, and smoking status or child’s birth weight for gestational age z-score or age at anthropometry assessment (Tables 1–2).
Table 1.
ACCESS participant characteristics
| All children (n=239) | Girls (n=109) | Boys (n=130) | ||||
|---|---|---|---|---|---|---|
| Race/Ethnicity (n, %) | ||||||
| Black | 61 | 25.5 | 30 | 27.5 | 31 | 23.9 |
| Hispanic | 132 | 55.2 | 61 | 56.0 | 71 | 54.6 |
| White/Other | 46 | 19.3 | 18 | 16.5 | 28 | 21.5 |
| Maternal education (n, %) | ||||||
| >12 yrs | 81 | 33.9 | 40 | 36.7 | 41 | 31.5 |
| ≤12 yrs | 158 | 66.1 | 69 | 63.3 | 89 | 68.5 |
| Maternal smoking status (n, %) | ||||||
| Never smoked | 192 | 80.3 | 87 | 79.8 | 105 | 80.8 |
| Smoked prenatally, but not postnatally | 11 | 4.6 | 4 | 3.7 | 7 | 5.4 |
| Did not smoke prenatally, but smoked postnatally | 15 | 6.3 | 9 | 8.3 | 6 | 4.6 |
| Smoked both pre- and postnatally | 21 | 8.8 | 9 | 8.3 | 12 | 9.2 |
| Maternal age at enrollment (yr; mean, SD) | 28.1 | 5.8 | 28.7 | 6.1 | 27.6 | 5.5 |
| Maternal pre-pregnancy BMI (kg/m2; median, IQR) | 28.0 | 25.9–31.2 | 28.0 | 25.7–31.4 | 28.0 | 26.2–30.7 |
| Child age at anthropometry measure (yr; mean, SD) | 4 | 0.7 | 4 | 0.7 | 4 | 0.7 |
| Birth weight for gestational age z-score (mean, SD) | −0.10 | 1.12 | −0.20 | 1.16 | −0.01 | 1.07 |
Table 2.
Anthropometry measurements and prenatal PM2.5 exposure levels by child sex
| Variables | All children | Girls | Boys | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Median | IQR | Median | IQR | Median | IQR | |
| Prenatal PM2.5 level (μg/m3)a | 10.7 | 9.9–11.4 | 10.8 | 10.0–11.5 | 10.6 | 9.8–11.4 |
| Body Mass Index (BMI) | ||||||
| BMI z-score | 0.50 | −0.27–1.52 | 0.61 | −0.30–1.48 | 0.44 | −0.26–1.53 |
| Bioimpedance | ||||||
| Fat mass (kg) | 3.0 | 2.1–3.9 | 2.1 | 1.1–3.2 | 3.2 | 2.8–4.4 |
| Fat free mass (kg) | 14 | 13–16 | 15 | 14–16 | 13 | 12–15 |
| % body fat (%) | 17 | 13–21 | 11 | 6.5–16 | 19 | 17–22 |
| Skin fold | ||||||
| Tricep skinfold (mm) | 9.1 | 7.8–11.0 | 9.8 | 8.4–12.0 | 8.4 | 7.4–10.0 |
| Subscapular skinfold (mm) | 6.2 | 5.2–7.8 | 6.8 | 5.6–8.6 | 5.8 | 5.0–7.0 |
| Waist and Hip Circumferences | ||||||
| Waist circumference (cm) | 52 | 50–56 | 53 | 50–57 | 52 | 49–55 |
| Hip circumference (cm) | 56 | 52–59 | 56 | 52–60 | 55 | 52–59 |
| Waist-to-hip ratio | 0.94 | 0.90–0.98 | 0.94 | 0.90–0.98 | 0.94 | 0.91–0.98 |
Averaged over pregnancy
3.2 Identification of sensitive windows and sex-specific associations
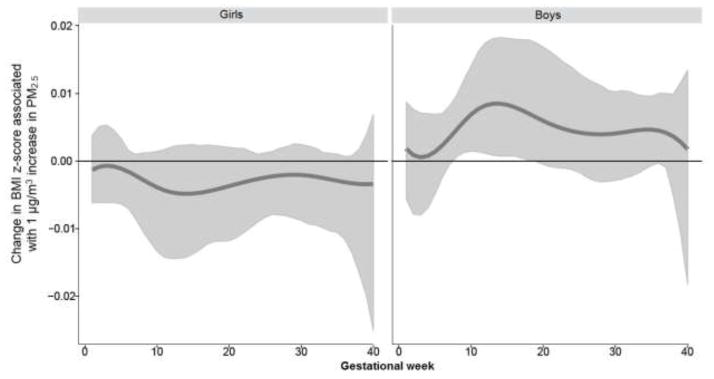
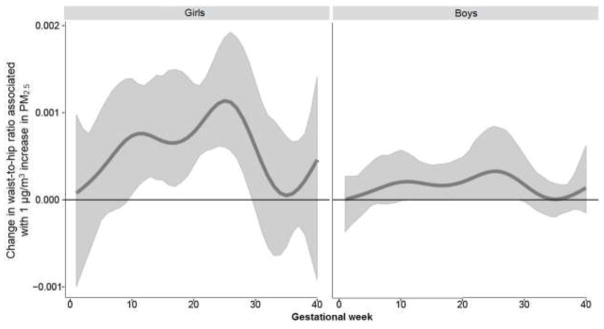
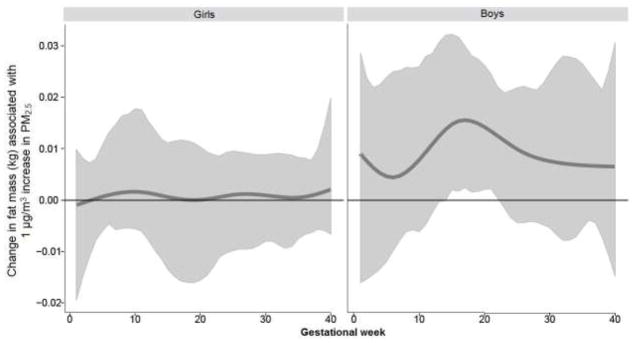
When examining the association between prenatal PM2.5 exposure over gestation and anthropometry using DLMs in the sample as a whole, only the calculated index, WHR, was significantly associated with exposure at 2–22 weeks gestation (Supplemental Materials, Figure S1). However, examining the interaction between prenatal PM2.5 and child’s sex in relation to anthropometric outcomes using BDLIMs revealed significant sexually-dimorphic associations in several indices (Figures 1–3). When interpreting the figures, “sensitive windows” graphically appear as a bump during which exposure is significantly associated with the respective outcome. Analyses revealed a significant association between higher PM2.5 exposure in early-to-mid pregnancy (8–17 weeks of gestation) and higher BMI z-scores in boys, but not in girls (Figure 1). Higher PM2.5 levels in mid-pregnancy (15–22 weeks of gestation) were significantly associated with increased fat mass among boys, but not in girls (Figure 2). On the other hand, a significant association between higher PM2.5 levels and increased WHR among girls with a sensitive window spanning 10–29 weeks gestation was seen, whereas the association was only suggestive in boys (Figure 3). We did not find statistically significant sensitive prenatal windows for the remaining anthropometric measurements. For the respective outcome measurements with identified significant sex-specific sensitive exposure windows, the BDLIM analysis found that effect modification by sex was attributable to difference in the magnitude of the within-window association (i.e., βj) between boys and girls, while the window (i.e., wjt) was not different between the two sexes (the normalized posterior density was 0.56, 0.98, and 0.97 for BMI-z, fat mass, and WHR models, respectively, which can be interpreted as a probability that the model with a common window but different magnitudes was the best fitting pattern of effect modification).
Figure 1. Associations between weekly PM2.5 levels over gestation and BMI-z: interaction by sex.
This figure demonstrates the association between PM2.5 exposure over pregnancy and BMI z-scores using BDLIM assuming week-specific effects. Models were adjusted for maternal age, race/ethnicity, education, pre-pregnancy BMI, and child’s age at anthropometric measurement. The y-axis represents the change in BMI z-scores corresponding to per μg/m3 increase in PM2.5; the x-axis is gestational age in weeks. Solid lines show the predicted change in BMI z-score. Gray areas indicate 95% confidence intervals (CIs). A sensitive window is identified for the weeks where the estimated pointwise 95% CI (shaded area) does not include zero.
Figure 3. Associations between weekly PM2.5 levels over gestation and waist-to-hip ratio: interaction by sex.
This figure demonstrates the association between PM2.5 exposure over pregnancy and waist-to-hip ratio using BDLIM assuming week-specific effects. Models were adjusted for maternal age, race/ethnicity, education, pre-pregnancy BMI, and child’s age at anthropometric measurement. The y-axis represents the change in waist-to-hip ratio corresponding to per μg/m3 increase in PM2.5; the x-axis is gestational age in weeks. Solid lines show the predicted change in waist-to-hip ratio. Gray areas indicate 95% confidence intervals (CIs). A sensitive window is identified for the weeks where the estimated pointwise 95% CI (shaded area) does not include zero.
Figure 2. Associations between weekly PM2.5 levels over gestation and fat mass: interaction by sex.
This figure demonstrates the association between PM2.5 exposure over pregnancy and fat mass (kg) using BDLIM assuming week-specific effects. Models were adjusted for maternal age, race/ethnicity, education, pre-pregnancy BMI, and child’s age at anthropometry measurement. The y-axis represents the change in fat mass corresponding to per μg/m3 increase in PM2.5; the x-axis is gestational age in weeks. Solid lines show the predicted change in fat mass. Gray areas indicate 95% confidence intervals (CIs). A sensitive window is identified for the weeks where the estimated pointwise 95% CI (shaded area) does not include zero.
3.3 Cumulative effects across pregnancy accounting for sensitive windows
In order to further assess the associations over the entire pregnancy while accounting for time-varying associations, we also estimated the cumulative effects accounting for sensitive windows and within-window associations identified by BDLIMs (Table 3). The estimated cumulative effects of prenatal PM2.5 exposure (per μg/m3 increase in PM2.5) were significant for BMI-z (0.21, 95%CI=0.003–0.37) and fat mass (0.36, 95%CI=0.12–0.68) in boys, but not in girls. The estimated cumulative effect of prenatal PM2.5 was significant for WHR among girls (0.02, 95%CI=0.01–0.03) and only suggestive in boys (0.006, 95%CI= −0.0001–0.014).
Table 3.
Estimated cumulative effects of prenatal PM2.5 exposure over gestation: accounting for time-varying interaction by sex, sensitive windows, and within-window effects identified by BDLIMa
| Anthropometry indices | Girls | Boys | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Cumulative Effect | 95% CI | Cumulative Effect | 95% CI | |||
| BMI z-score | −0.12 | −0.37 | 0.03 | 0.21 | 0.003 | 0.37 |
| Fat mass (kg) | 0.03 | −0.29 | 0.28 | 0.36 | 0.12 | 0.68 |
| Waist-to-hip ratio | 0.023 | 0.01 | 0.03 | 0.006 | −0.0001 | 0.01 |
Models were adjusted for maternal age, race/ethnicity, education, pre-pregnancy BMI, and child’s age at anthropometric measurement; corresponding to per μg/m3 increase in PM2.5.
3.4 Sensitivity analyses
Finally, sensitivity analyses additionally including postnatal PM2.5 levels, perinatal smoking, and birth weight for gestational age z-scores in the models showed similar sex-specific patterns for significant associations as in our main analyses (Supplemental Materials, Figures S2–S4 and Table S1).
4. DISCUSSION
These data add to a sparse literature linking in utero traffic-related air pollution exposure and early childhood body composition in two significant ways. First, this is the first study to leverage weekly address-specific PM2.5 exposure estimates and Bayesian distributed lag interaction models to examine time-varying effects in a data driven fashion rather than assigning a priori exposure time periods arbitrarily (e.g., averaged over a certain length of time before birth or using clinically defined trimesters) in order to more definitively identify sensitive windows for effects on child growth. In addition, the use of BDLIM allowed us to directly examine the time-varying interaction by sex and estimation of cumulative effects across the pregnancy accounting for both sensitive windows and within-window effects. Our data suggested that increased exposure to PM2.5 in early to mid-pregnancy was associated with increased fat mass and higher BMI z-scores among boys. Among girls, higher exposure to PM2.5 from early-to-mid pregnancy was associated with increased WHR.
More refined estimation of the sensitive time windows in which air pollution has the greatest impact may enhance our insight into underlying mechanisms as well as the etiology of sex-specific effects on child growth. Oxidative stress is one of the leading hypothesized mechanisms that may be operating between prenatal particulate air pollution and early growth outcomes. Particulate air pollution may result in increased placental or fetal oxidative stress and related DNA damage or inflammation, which may inhibit nutrient transfer from mother to fetus and disrupt programming of the fetal metabolic system, thereby disrupting fetal growth (Fleisch et al., 2015; Kannan et al., 2006). Excess fetal oxidative stress or inflammation resulting from air pollution exposure could also induce fetal or infant adipose tissue inflammation and hypertrophy, which may be related to excessive weight gain (Sun et al., 2009). Increased oxidative stress could also induce neuroinflammation and central nervous system effects, which may be linked to disrupted satiety signals (Bolton et al., 2014).
Further, these analyses also found that the sensitive windows for prenatal exposure to PM2.5 may be sex specific. While prior human data are not available, animal studies have demonstrated sex-specific associations between prenatal air pollutants and offspring growth outcomes. In a rodent study, Bolton et al. (Bolton et al., 2012) found that adult male offspring born to pregnant mice exposed to diesel exhaust weighed 12% more compared to those born to non-exposed pregnant mice, whereas there was no difference seen in female offspring. These investigators also found that female offspring born to pregnant mice exposed to diesel exhaust gained 340% more weight following a high fat diet compared to those born to non-exposed pregnant mice; no such differences were found in male offspring. Other research suggested that sex hormones contribute to sex differences induced by pro-inflammatory triggers such as air pollution (Melcangi et al., 2008). For example, a study in rodents found that estrogens may have anti-inflammatory properties mediated by cytokine expression (Shivers et al., 2015).
To our knowledge, only one previous human study has examined the association between prenatal fine particulate matter and anthropometric outcomes in children. Fleisch et al. (Fleisch et al., 2016) found that proximity to major roadway at birth address (<50 m), a surrogate of traffic-related air pollution exposure, was associated with BMI-z score, waist circumference, and skinfold thickness in children aged at 3.3 years as well as at 7.7 years. On the other hand, this same study did not find statistically significant associations between prenatal black carbon or PM2.5 exposure averaged over the 3rd trimester and these measurements nor did they find effect modification by sex (Fleisch et al., 2016). While the methodology used to estimate participant’s exposure to PM2.5 was the same in both studies, Fleisch et al. (Fleisch et al., 2016) assessed exposure in the 3rd trimester only, and thus it is difficult to directly compare the results with our study. Of note, in a recent simulation analysis, our group demonstrated that measuring exposure only in an arbitrarily defined susceptibility window during pregnancy may lead to missed associations (Wilson et al., 2017a). Moreover, the analytical approach used in the present study found that associations between PM2.5 and growth outcomes may be more significant in the 1st and 2nd trimester rather than later in pregnancy. This may also explain the discrepancy between the findings in the two studies, especially in terms of effect modification by sex.
A larger WHR reflects a type of body shape with excess weight around the waist (i.e., central or abdominal obesity, or “apple shape” obesity), whereas increased BMI or fat mass is more of an indicator for increased general body size (Bray and Bouchard 2014). The sex-specific findings in our study suggest that prenatal air pollution exposure may have a stronger association with increased body size in general among boys, whereas it may affect body shape among girls. The observed associations with body composition in these preschool-aged children may have long term health implications in a sex-specific manner. For example, a recent study of 8–16 year-old Hispanic children reported that the association between BMI and insulin resistance, a predictor of type-2 diabetes, was significantly stronger in boys compared to girls; on the other hand, the association between WHR and insulin resistance was significantly stronger in girls compared to boys (Qi et al., 2016). Of note, as the variability of WHR in children is generally small, the estimated effect size of prenatal exposure per unit at each time point on this indicator also likely to be small; however, we were still able to identify a sensitive window and sex difference in estimated cumulative effect over the pregnancy. Further studies are needed to replicate these results in early childhood, elucidate underlying mechanisms, and examine whether these associations are sustained over time. Longitudinal studies assessing air pollution exposures beginning in utero on growth trajectories over the life course will be most informative.
This study has notable strengths. We were able to leverage daily particulate air pollution data estimated by address-specific exposure for each woman over gestation, using a validated state-of-the-art hybrid spatio-temporal LUR model incorporating satellite-derived AOD measures (Kloog et al., 2011), to apply a data-driven advanced statistical approach to more objectively identify sensitive windows of prenatal PM2.5 and more directly examine interactions by child sex. In addition, our study population consists of a lower-SES ethnically mixed inner-city population that is more likely to live in communities with higher traffic-related air pollution exposures as well as being at greater risk for childhood obesity. We also acknowledge some limitations. While we found significant interactions between prenatal PM2.5 exposure and sex for BMI-z, fat mass, and WHR, we did not find statistically significant associations on other anthropometric measures, which may be due to our sample size. Also, while we were able to control for postnatal air pollution exposure as well as several factors known to be important for child growth, we did not have data on dietary or other parenting practices that may influence child growth. However, controlling for SES-related variables including education and race/ethnicity which are correlated with dietary intakes and parenting practices may in part account for these other factors. Finally, our results may be more generalizable to lower SES racial/ethnic minority populations. Larger studies examining sex-specific time-varying effects of prenatal particulate air pollution are thus warranted to corroborate these findings and consider additional confounders or moderators. Moreover, integrating epidemiological findings using analytic methods to more objectively identify sensitive windows with knowledge regarding developmental pathways and processes involved in prenatal programming of obesity that may be disrupted in these time periods as summarized in recent reviews (Berry et al., 2013; Gesta et al., 2007; MacKay and Abizaid 2014) can better inform future mechanistic research in this area.
In summary, this study utilized a data-driven method to objectively elucidate sensitive windows and examined sex differences for the association between prenatal PM2.5 and body composition outcomes in early childhood. We found that increased prenatal PM2.5 exposure, particularly at early-to-mid pregnancy, may be more strongly associated with increased whole body size in boys, whereas it may be more strongly associated with an indicator of body shape (i.e., WHR) in girls. Advanced statistical methods combined with highly temporally resolved exposure data facilitates the more objective identification of susceptibility windows and enhances the ability to detect associations as well as to identify vulnerable groups.
Supplementary Material
Highlights.
Implementation of a novel data-driven approach to objectively identify sensitive windows of prenatal PM2.5 on childhood anthropometry.
Time-dependent associations and effect modification by sex were found based on different anthropometric indices.
Understanding such sex difference in temporal associations may provide insights into underlying mechanisms.
Acknowledgments
The Asthma Coalition on Community, Environment, and Social Stress (ACCESS) project has been funded by NIH grants R01 ES010932, U01 HL072494, R01 HL080674, R01 MD006086; anthropometric phenotyping was supported by R01 MD003963 and P30 ES023515; statistical support was funded by U2C ES026555, T32 ES007142 and P30 ES000002; air pollution modeling was funded by EPA RD83479801. During preparation of manuscript, Chiu was supported by CDC/NIOSH T42 OH008422. The authors declare they have no competing financial interests.
Abbreviations
- AOD
aerosol optical depth
- BDLIM
Bayesian distributed lag interaction model
- BMI-z
body mass index z-score
- CDC
Centers for Disease Control
- DLM
distributed lag model
- LUR
land-use regression
- OS
oxidative stress
- PAHs
polyaromatic hydrocarbons
- ROS
reactive oxygen species
- SES
socioeconomic status
- SS
subscapular skinfold
- TS
triceps skinfold
- WHR
waist-to-hip ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aroor AR, DeMarco VG. Oxidative stress and obesity: the chicken or the egg? Diabetes. 2014;63:2216–2218. doi: 10.2337/db14-0424. [DOI] [PubMed] [Google Scholar]
- Atashi F, Modarressi A, Pepper MS. The role of reactive oxygen species in mesenchymal stem cell adipogenic and osteogenic differentiation: a review. Stem Cells Dev. 2015;24:1150–1163. doi: 10.1089/scd.2014.0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ. Sir Richard Doll Lecture. Developmental origins of chronic disease. Public Health. 2012;126:185–189. doi: 10.1016/j.puhe.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Bell ML, Ebisu K, Belanger K. Ambient air pollution and low birth weight in Connecticut and Massachusetts. Environ Health Perspect. 2007;115:1118–1124. doi: 10.1289/ehp.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry DC, Stenesen D, Zeve D, Graff JM. The developmental origins of adipose tissue. Development. 2013;140:3939–3949. doi: 10.1242/dev.080549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch LL, Parker L, Burns A. Early childhood obesity prevention policies. National Academies Press; 2011. [Google Scholar]
- Bogers RP, Bemelmans WJ, Hoogenveen RT, Boshuizen HC, Woodward M, Knekt P, et al. Association of overweight with increased risk of coronary heart disease partly independent of blood pressure and cholesterol levels: a meta-analysis of 21 cohort studies including more than 300 000 persons. Arch Intern Med. 2007;167:1720–1728. doi: 10.1001/archinte.167.16.1720. [DOI] [PubMed] [Google Scholar]
- Bolton JL, Auten RL, Bilbo SD. Prenatal air pollution exposure induces sexually dimorphic fetal programming of metabolic and neuroinflammatory outcomes in adult offspring. Brain Behav Immun. 2014;37:30–44. doi: 10.1016/j.bbi.2013.10.029. [DOI] [PubMed] [Google Scholar]
- Bolton JL, Smith SH, Huff NC, Gilmour MI, Foster WM, Auten RL, et al. Prenatal air pollution exposure induces neuroinflammation and predisposes offspring to weight gain in adulthood in a sex-specific manner. Faseb j. 2012;26:4743–4754. doi: 10.1096/fj.12-210989. [DOI] [PubMed] [Google Scholar]
- Bray GA, Bouchard C. Handbook of Obesity–Volume 2: Clinical Applications. CRC Press; 2014. [Google Scholar]
- Calderon-Garciduenas L, Franco-Lira M, D’Angiulli A, Rodriguez-Diaz J, Blaurock-Busch E, Busch Y, et al. Mexico City normal weight children exposed to high concentrations of ambient PM2.5 show high blood leptin and endothelin-1, vitamin D deficiency, and food reward hormone dysregulation versus low pollution controls. Relevance for obesity and Alzheimer disease. Environ Res. 2015;140:579–592. doi: 10.1016/j.envres.2015.05.012. [DOI] [PubMed] [Google Scholar]
- Cawley J, Meyerhoefer C. The medical care costs of obesity: an instrumental variables approach. J Health Econ. 2012;31:219–230. doi: 10.1016/j.jhealeco.2011.10.003. [DOI] [PubMed] [Google Scholar]
- CDC. 2000 CDC growth charts: United States. National Center for Health Statistics; 2000. [accessed October 8, 2016]. Available at: http://www.cdc.gov/growthcharts/2000growthchart-us.pdf. [Google Scholar]
- CDC. Vital Signs. U.S. Department of Health and Human Services, Centers for Disease Control; 2013. Progress on Childhood Obesity. [Google Scholar]
- Chiu YH, Hsu HH, Coull BA, Bellinger DC, Kloog I, Schwartz J, et al. Prenatal particulate air pollution and neurodevelopment in urban children: Examining sensitive windows and sex-specific associations. Environ Int. 2016;87:56–65. doi: 10.1016/j.envint.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marchi E, Baldassari F, Bononi A, Wieckowski MR, Pinton P. Oxidative stress in cardiovascular diseases and obesity: role of p66Shc and protein kinase C. Oxid Med Cell Longev. 2013;2013:564961. doi: 10.1155/2013/564961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisch AF, Gold DR, Rifas-Shiman SL, Koutrakis P, Schwartz JD, Kloog I, et al. Air pollution exposure and abnormal glucose tolerance during pregnancy: the project Viva cohort. Environ Health Perspect. 2014;122:378–383. doi: 10.1289/ehp.1307065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisch AF, Luttmann-Gibson H, Perng W, Rifas-Shiman SL, Coull BA, Kloog I, et al. Prenatal and early life exposure to traffic pollution and cardiometabolic health in childhood. Pediatr Obes. 2016 doi: 10.1111/ijpo.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisch AF, Rifas-Shiman SL, Koutrakis P, Schwartz JD, Kloog I, Melly S, et al. Prenatal exposure to traffic pollution: associations with reduced fetal growth and rapid infant weight gain. Epidemiology. 2015;26:43–50. doi: 10.1097/EDE.0000000000000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A, Armstrong B, Kenward MG. Distributed lag non-linear models. Stat Med. 2010;29:2224–2234. doi: 10.1002/sim.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131:242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Hillier TA, Pedula KL, Schmidt MM, Mullen JA, Charles MA, Pettitt DJ. Childhood obesity and metabolic imprinting: the ongoing effects of maternal hyperglycemia. Diabetes Care. 2007;30:2287–2292. doi: 10.2337/dc06-2361. [DOI] [PubMed] [Google Scholar]
- Hsu HHL, Chiu YHM, Coull BA, Kloog I, Schwartz J, Lee A, et al. Prenatal particulate air pollution and asthma onset in urban children. Identifying sensitive windows and sex differences. Am J Respir Crit Care Med. 2015;192:1052–1059. doi: 10.1164/rccm.201504-0658OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imhoff BR, Hansen JM. Extracellular redox environments regulate adipocyte differentiation. Differentiation. 2010;80:31–39. doi: 10.1016/j.diff.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Iyer A, Fairlie DP, Prins JB, Hammock BD, Brown L. Inflammatory lipid mediators in adipocyte function and obesity. Nat Rev Endocrinol. 2010;6:71–82. doi: 10.1038/nrendo.2009.264. [DOI] [PubMed] [Google Scholar]
- Jastreboff AM, Lacadie C, Seo D, Kubat J, Van Name MA, Giannini C, et al. Leptin is associated with exaggerated brain reward and emotion responses to food images in adolescent obesity. Diabetes Care. 2014;37:3061–3068. doi: 10.2337/dc14-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan S, Misra DP, Dvonch JT, Krishnakumar A. Exposures to airborne particulate matter and adverse perinatal outcomes: a biologically plausible mechanistic framework for exploring potential effect modification by nutrition. Environ Health Perspect. 2006;114:1636–1642. doi: 10.1289/ehp.9081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloog I, Koutrakis P, Coull BA, Lee HJ, Schwartz J. Assessing temporally and spatially resolved PM2.5 exposures for epidemiological studies using satellite aerosol optical depth measurements. Atmos Environ. 2011;45:6267–6275. [Google Scholar]
- Kloog I, Melly SJ, Ridgway WL, Coull BA, Schwartz J. Using new satellite based exposure methods to study the association between pregnancy PM(2).(5) exposure, premature birth and birth weight in Massachusetts. Environ Health. 2012;11:40. doi: 10.1186/1476-069X-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo A, Ferrara A, Windham GC, Greenspan LC, Deardorff J, Hiatt RA, et al. Maternal hyperglycemia during pregnancy predicts adiposity of the offspring. Diabetes Care. 2014;37:2996–3002. doi: 10.2337/dc14-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmanan A, Chiu YHM, Coull BA, Just AC, Maxwell SL, Schwartz J, et al. Associations between prenatal traffic-related air pollution exposure and birth weight: Modification by sex and maternal pre-pregnancy body mass index. Environ Res. 2015;137:268–277. doi: 10.1016/j.envres.2014.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavigne E, Ashley-Martin J, Dodds L, Arbuckle TE, Hystad P, Johnson M, et al. Air pollution exposure during pregnancy and fetal markers of metabolic function: The MIREC Study. Am J Epidemiol. 2016;183:842–851. doi: 10.1093/aje/kwv256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang L, Wang F, Li C. Effect of Fine Particulate Matter (PM2.5) on Rat Placenta Pathology and Perinatal Outcomes. Med Sci Monit. 2016;22:3274–3280. doi: 10.12659/MSM.897808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKay H, Abizaid A. Embryonic development of the hypothalamic feeding circuitry: Transcriptional, nutritional, and hormonal influences. Mol Metab. 2014;3:813–822. doi: 10.1016/j.molmet.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell R, Shen E, Gilliland FD, Jerrett M, Wolch J, Chang CC, et al. A longitudinal cohort study of body mass index and childhood exposure to secondhand tobacco smoke and air pollution: the Southern California Children’s Health Study. Environ Health Perspect. 2015;123:360–366. doi: 10.1289/ehp.1307031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcangi RC, Garcia-Segura LM, Mensah-Nyagan AG. Neuroactive steroids: state of the art and new perspectives. Cell Mol Life Sci. 2008;65:777–797. doi: 10.1007/s00018-007-7403-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minghetti L, Greco A, Zanardo V, Suppiej A. Early-life sex-dependent vulnerability to oxidative stress: the natural twining model. J Matern Fetal Neonatal Med. 2013;26:259–262. doi: 10.3109/14767058.2012.733751. [DOI] [PubMed] [Google Scholar]
- MohanKumar SM, Campbell A, Block M, Veronesi B. Particulate matter, oxidative stress and neurotoxicity. Neurotoxicology. 2008;29:479–488. doi: 10.1016/j.neuro.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon-Moran D, Kit BK, et al. Trends in obesity prevalence among children and adolescents in the United States, 1988–1994 through 2013–2014. JAMA. 2016;315:2292–2299. doi: 10.1001/jama.2016.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken E, Huh SY, Taveras EM, Rich-Edwards JW, Gillman MW. Associations of maternal prenatal smoking with child adiposity and blood pressure. Obes Res. 2005;13:2021–2028. doi: 10.1038/oby.2005.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padula AM, Mortimer K, Hubbard A, Lurmann F, Jerrett M, Tager IB. Exposure to traffic-related air pollution during pregnancy and term low birth weight: estimation of causal associations in a semiparametric model. Am J Epidemiol. 2012;176:815–824. doi: 10.1093/aje/kws148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poston L. Developmental programming and diabetes - The human experience and insight from animal models. Best Pract Res Clin Endocrinol Metab. 2010;24:541–552. doi: 10.1016/j.beem.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Qi Q, Hua S, Perreira KM, Cai J, Van Horn L, Schneiderman N, et al. Sex differences in associations of adiposity measures and insulin resistance in US hispanic/latino youth: The Hispanic Community Children’s Health Study/Study of Latino Youth (SOL Youth) J Clin Endocrinol Metab. 2016 doi: 10.1210/jc.2016-2279. jc20162279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundle A, Hoepner L, Hassoun A, Oberfield S, Freyer G, Holmes D, et al. Association of childhood obesity with maternal exposure to ambient air polycyclic aromatic hydrocarbons during pregnancy. Am J Epidemiol. 2012;175:1163–1172. doi: 10.1093/aje/kwr455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush EC, Bristow S, Plank LD, Rowan J. Bioimpedance prediction of fat-free mass from dual-energy X-ray absorptiometry in a multi-ethnic group of 2-year-old children. Eur J Clin Nutr. 2013;67:214–217. doi: 10.1038/ejcn.2012.182. [DOI] [PubMed] [Google Scholar]
- Shivers KY, Amador N, Abrams L, Hunter D, Jenab S, Quinones-Jenab V. Estrogen alters baseline and inflammatory-induced cytokine levels independent from hypothalamic-pituitary-adrenal axis activity. Cytokine. 2015;72:121–129. doi: 10.1016/j.cyto.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strazzullo P, D’Elia L, Cairella G, Garbagnati F, Cappuccio FP, Scalfi L. Excess body weight and incidence of stroke: meta-analysis of prospective studies with 2 million participants. Stroke. 2010;41:e418–426. doi: 10.1161/STROKEAHA.109.576967. [DOI] [PubMed] [Google Scholar]
- Sun Q, Yue P, Deiuliis JA, Lumeng CN, Kampfrath T, Mikolaj MB, et al. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation. 2009;119:538–546. doi: 10.1161/CIRCULATIONAHA.108.799015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton EF, Gilmore LA, Dunger DB, Heijmans BT, Hivert MF, Ling C, et al. Developmental programming: State-of-the-science and future directions-Summary from a Pennington Biomedical symposium. Obesity (Silver Spring) 2016;24:1018–1026. doi: 10.1002/oby.21487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taveras EM, Rifas-Shiman SL, Belfort MB, Kleinman KP, Oken E, Gillman MW. Weight status in the first 6 months of life and obesity at 3 years of age. Pediatrics. 2009;123:1177–1183. doi: 10.1542/peds.2008-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TFoCO. White House Task Force on Childhood Obesity Report to the President. Washington, DC: 2010. Solving the problem of childhood obesity within a generation. [DOI] [PubMed] [Google Scholar]
- Vrijheid M, Casas M, Gascon M, Valvi D, Nieuwenhuijsen M. Environmental pollutants and child health-A review of recent concerns. Int J Hyg Environ Health. 2016;219:331–342. doi: 10.1016/j.ijheh.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Wilson A, Chiu YHM, Hsu HHL, Wright RO, Wright RJ, Coull BA. Potential for bias when estimating critical windows to air pollution in children’s health. Am J Epidemiol. 2017a doi: 10.1093/aje/kwx184. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A, Chiu YHM, Hsu HHL, Wright RO, Wright RJ, Coull BA. Bayesian distributed lag interaction models to identify perinatal windows of vulnerability in children’s health. Biostatistics. 2017b doi: 10.1093/biostatistics/kxx002. Epub ahead of print, 2017 Feb 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RJ, Fisher K, Chiu YH, Wright RO, Fein R, Cohen S, et al. Disrupted prenatal maternal cortisol, maternal obesity, and childhood wheeze. Insights into prenatal programming. Am J Respir Crit Care Med. 2013;187:1186–1193. doi: 10.1164/rccm.201208-1530OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RJ, Suglia SF, Levy J, Fortun K, Shields A, Subramanian S, et al. Transdisciplinary research strategies for understanding socially patterned disease: the Asthma Coalition on Community, Environment, and Social Stress (ACCESS) project as a case study. Cien Saude Colet. 2008;13:1729–1742. doi: 10.1590/s1413-81232008000600008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanovski SZ, Yanovski JA. Obesity prevalence in the United States--up, down, or sideways? N Engl J Med. 2011;364:987–989. doi: 10.1056/NEJMp1009229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye ZW, Zhang J, Townsend DM, Tew KD. Oxidative stress, redox regulation and diseases of cellular differentiation. Biochim Biophys Acta. 2015;1850:1607–1621. doi: 10.1016/j.bbagen.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanobetti A, Wand MP, Schwartz J, Ryan LM. Generalized additive distributed lag models: quantifying mortality displacement. Biostatistics. 2000;1:279–292. doi: 10.1093/biostatistics/1.3.279. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.