Abstract
Providers have expressed a strong desire to have additional clinical decision-support tools to help with interpretation of pharmacogenomic results. We developed and tested a novel disease–drug association tool that enables pharmacogenomic-based prescribing to treat common diseases. First, 324 drugs were mapped to 484 distinct diseases (mean number of drugs treating each disease was 4.9; range 1–37).Then the disease–drug association tool was pharmacogenomically annotated, with an average of 1.8 pharmacogenomically annotated drugs associated/disease. Applying this tool to a prospectively enrolled >1,000 patient cohort from a tertiary medical center showed that 90% of the top ∼20 diseases in this population and ≥93% of patients could appropriately be treated with ≥1 medication with actionable pharmacogenomic information. When combined with clinical patient genotypes, this tool permits delivery of patient-specific pharmacogenomically informed disease treatment recommendations to inform the treatment of many medical conditions of the US population, a key initial step towards implementation of precision medicine.
Adverse drug reactions (ADRs) account for >100,000 deaths in the United States annually, including situations when drugs are appropriately prescribed and administered.1 A systematic review reported that among the 27 drugs frequently cited in ADRs, 60% were associated with at least one drug-metabolizing enzyme with pharmacogenomic (PGx) variation.2 Providing PGx information to healthcare providers could potentially help predict which patients may experience ADRs—or, alternatively, respond favorably—to certain medications.
Some medical centers have already begun adopting PGx testing, making personalized medicine an expanding reality.3–7 However, widespread adoption of PGx testing has been hindered by barriers such as the lack of physician awareness about the availability of tests and how to translate these tests, lack of electronic infrastructures that can store and deliver genomic information, and the need for PGx decision-support.4,7–11 In response to the latter barrier, many groups are developing PGx clinical decision-support (CDS) tools for implementation of PGx results. Additionally, members of the Pharmacogenomics Research Network12 partnered with PharmGKB13 to establish the Clinical Pharmaco-genetics Implementation Consortium (CPIC), which provides peer-reviewed guidelines on how to use PGx results.14 These tools represent important initial steps toward providing decision-support for implementation efforts, and identifying at-risk individuals, key genes, and drugs with PGx relevance.
Providers have expressed a strong desire to have additional CDS tools to help with the interpretation of PGx results, and to provide recommendations during prescribing.10 To our knowledge, no CDS tool has been developed that enables providers to search for disease treatment indications and be provided with a comparative menu of treatment options annotated with PGx information. While significant prior work creating disease–drug knowledge bases has been done,15–22 none of these databases incorporated PGx annotations.
Here we describe the development of a novel disease–drug search engine to enable prescribers to consider PGx information when comparing drug treatments for given clinical indications. This search engine was developed for integration into the Genomic Prescribing System (GPS), an established PGx results delivery system that provides patient-specific PGx information and CDS at the point of care.8 Each CDS concisely translates patient genotypes into clinical recommendations about the use of specific pharmacogenomically affected drugs, including providing dose adjustments and potential alternative medication recommendations when applicable.8 During early adoption of this system, it became clear that the expansion of GPS to include a disease–drug search tool would be attractive—so that disease treatments could be broadly considered with PGx information in mind. We therefore created a disease–drug database with an expanded number of drugs. The disease–drug PGx database was then implemented at an academic tertiary care medical center to determine the potential PGx applicability to prescription decision-support for patients with common medical conditions.
Results
Disease–drug associations
A total of 3,539 disease–drug associations were made, with 324 unique drugs and 1,321 Systemized Nomenclature of Medicine (SNOMED) terms (Supplementary Table S1). The SNOMED terms were then collapsed to 484 distinct diseases, and 2,355 unique disease–drug associations were included in the created database. For disease–drug pairing, the initial interrater agreement score was k = 5 0.81 but reached 1.0 after consensus review for the final constructed database. The average number of disease indications for a given drug was seven (range 1–93), while the average number of drugs to treat a single disease was five (range 1–37). For example, albuterol has seven disease treatment indications: asthma, bronchitis, chronic obstructive pulmonary disease, cough, hyperkalemia, infection-respiratory syncytial virus, and respiratory distress. Conversely, there are 13 drugs that treat chronic obstructive pulmonary disease: albuterol, budesonide, clarithromycin, formoterol, indacaterol, ipratropium, methyl-prednisolone, prednisolone, prednisone, salmeterol, sulfamethoxazole, tiotropium, and trimethoprim.
Figure 1 shows the mappings for 10 common diseases (coronary artery disorder, diabetes mellitus, gastroesophageal reflux disease, hyperlipidemia, hypertension, musculoskeletal—mechanical joint disorders, obesity, osteoporosis, pain-musculoskeletal, and surgery-joint). The red links in the figure depict the connections between one of these 10 diseases to a more specific, yet synonymous SNOMED disease indication. The green links connect the 10 diseases to drugs that are treatment options. “Hypertension” is highlighted as an illustrative example. The synonymous SNOMED terms for hypertension are found in the magnified red text in Figure 1. The green links sprouting from hypertension lead to the drugs that were mapped to treat hypertension. Hence, if “Hypertension” was searched using the disease–drug database, all of the green drugs would appear as treatment options.
Figure 1.
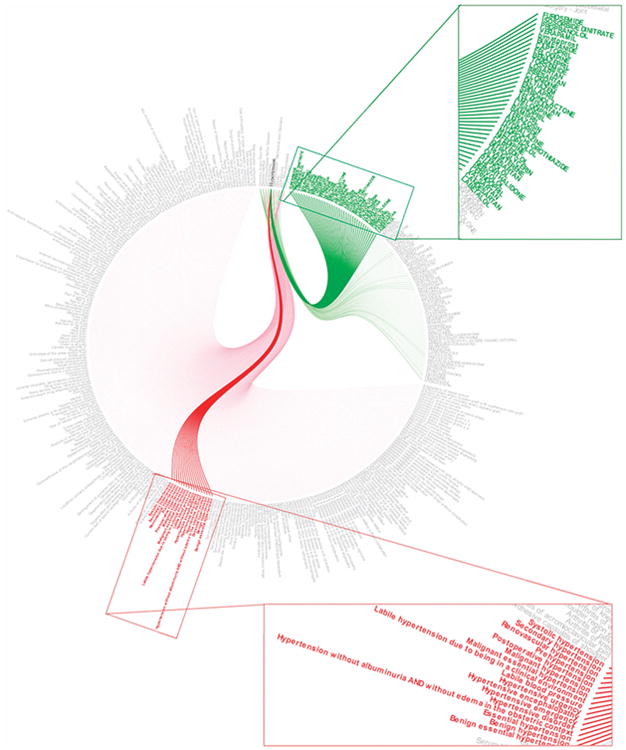
An example of 10 diseases mapped in the disease–drug database, specifically highlighting “hypertension.” Red links represent SNOMED diseases associated with one of the 10 diseases, and green links represent drugs associated with one or more of the 10 diseases.
Disease–drug database comparison to other knowledge bases
We found that the drugs included in our database shared significant commonality with those previously included in MEDI20 and the National Drug File — Reference Terminology (NDF-RT)16 (although there was general lack of overlap with LabeledIn22), suggesting the overall relevance of our database as a drug prescribing resource. Disease overlap was more limited across the compared databases, although significant disease overlap (>33%) with MEDI and ours existed (Supplementary Table S2). The large number of unique disease–drug pairings in our database suggests the potential for significant novelty in our tool. None of the other databases included PGx information.
Pharmacogenomic utility of the disease–drug database
Next, PGx utility evaluation of the disease–drug association tool was undertaken according to three different PGx clinical annotation standards. First, we evaluated 104 drugs with germline PGx US Food and Drug Administration (FDA) label information (these drugs are identified by the FDA as broadly containing— within their drug labels—genetic considerations of any sort that might impact the pharmacology, drug interactions, dosing, or use;23 see Methods for specific description of how the FDA PGx drugs were identified). Second, the 35 drugs with CPIC-A guidelines were assessed (https://www.pharmgkb.org/cpic/pairs; see details in the Methods). CPIC-A guidelines contain PGx result interpretations and prescribing recommendations for drugs deemed by the CPIC as having the highest evidentiary support for being pharmacogenomically clinically actionable (i.e., genetic information should be used to change prescribing of the affected drug).14 Third, we analyzed prescribing utility for the 46 drugs that currently have clinically actionable PGx information within GPS. GPS provides PGx prescribing consultations to providers for drugs for which high-quality clinical outcomes studies have demonstrated a PGx role8,24 (note that GPS has prioritized commonly used drugs, meaning some less commonly used drugs with high-level PGx information are not yet included in GPS). Thus, each of these three sources uses slightly different criteria to identify drugs as pharmacogenomically important. The composite list of drugs from each of the three analyzed sources is shown in Table 1.
Table 1. FDA, CPIC-A, and GPS medications with available, germline pharmacogenomic information.
| FDA drugs (104) | CPIC A drugs (35) | GPS drugs (46) | |
|---|---|---|---|
| Abacavir | Metroclopramide | Abacavir | Amlodipine |
| Amitriptyline | Metoprolol | Allopurinol | Aspirin |
| Aripiprazole | Mipomersen | Amitriptyline | Atenolol |
| Atomoxetine | Modafinil | Azathioprine | Atorvastatin |
| Azathioprine | Mycophenolic Acid | Boceprevir | Azathioprine |
| Capecitabine | Nalidixic Acid | Capecitabine | Benazepril |
| Carbamazepine | Nefazodone | Carbamazepine | Budesonide |
| Carglumic Acid | Nilontinib | Citalopram | Candesartan |
| Carisoprodol | Nitrofurantoin | Clopidogrel | Capecitabine |
| Carvedilol | Nortriptyline | Codeine | Carvedilol |
| Celecoxib | Omeprazole | Desipramine | Clopidogrel |
| Cevimeline | Pantoprazole | Doxepin | Colestipol |
| Chloroquine | Paroxetine | Escitalopram | Doxorubicin |
| Chlorpropamide | Pazopanib | Fluorouracil | Duloxetine |
| Cisplatin | Peg-3350 | Fluvoxamine | Esomeprazole |
| Citalopram | Peginterferon alfa-2b | Imipramine | Felodipine |
| Clobazam | Pegloticase | Irinotecan | Fenofibrate |
| Clomipramine | Perphenazine | Ivacaftor | Fluorouracil |
| Clopidogrel | Phenytoin | Mercaptopurine | Fluticasone Propionate |
| Clozapine | Pimozide | Nortriptyline | Fluvastatin |
| Codeine | Prasugrel | Oxycodone | Hydralazine |
| Dabrafenib | Pravastatin | Paroxetine | Hydrochlorothiazide |
| Dapsone | Primaquine | Peginterferon alfa-2a | Irbesartan |
| Desipramine | Propafenone | Peginterferon alfa-2b | Irinotecan |
| Dexlansoprazole | Propranolol | Phenytoin | Isosirbide dinitrate |
| Dextromethorphan | Protriptyline | Rasburicase | Lansoprazole |
| Diazepam | Pyrazinamide | Ribavirin | Mercaptopurine |
| Doxepin | Quinidine | Simvastatin | Metformin |
| Drospireonone | Quinine Sulfate | Tacrolimus | Metoprolol |
| Eltrombopag | Rabeprazole | Tamoxifen | Montelukast |
| Esomeprazole | Rasburicase | Thioguanine | Nifedipine |
| Ethinyl Estradiol | Rifampin | Tramadol | Omeprazole |
| Fluorouracil | Risperidone | Trimipramine | Ondansetron |
| Fluoxetine | Rosuvastatin | Voriconazole | Pantoprazole |
| Flurbiprofen | Sodium Nitrite | Warfarin | Pegylated Interferon |
| Fluvoxamine | Succimer | Perindopril | |
| Galantamine | Sulfamethoxazole | Pravastatin | |
| Glimperide | Tamoxifen | Rabeprazole | |
| Glipizide | Terbinafine | Ribavirin | |
| Glyburide | Tetrabenazine | Rosuvastatin | |
| Hydralazine | Thioguanine | Sildenafil | |
| Iloperidone | Thioridazine | Simvastatin | |
| Imipramine | Ticagrelor | Sunitinib | |
| Indacterol | Tolterodine | Triamcinolone | |
| Irinotecan | Tramadol | Verapamil | |
| Isoniazid | Trimethoprim | Warfarin | |
| Isosorbide Dinitrate | Trimipramine | ||
| Ivacaftor | Valproic Acid | ||
| Lansoprazole | Venlafaxine | ||
| Lomitapide | Voriconazole | ||
| Mafenide | Vortioxetine | ||
| Mercaptopurine | Warfarin |
Tegafur and telaprevir were removed from the CPIC-A list because they are not approved for use in the United States.
Methylene blue was removed from the FDA PGx list because it is not approved by the FDA.
It is first notable that many of the PGx-informed drugs were identified as such by more than one source. For example, Figure 2 shows that out of the 104 FDA drug labels, 27 also had CPIC-A guidelines, 18 also had GPS guidelines, and seven had guidelines from all three PGx sources. Of the 35 CPIC-A guideline drugs, 10 also had GPS guidelines. Consolidating all three sources, on average there were 1.8 (range 0–18) pharmacogenomically annotated drugs associated with each of the 484 diseases, with compositely 66.9% of the diseases having at least one PGx associated drug (data not shown). As might be expected given the respective PGx criteria for each of the three sources, the FDA-based analysis produced the most liberal disease–drug annotation, with 55.2% of the 484 diseases having at least one PGx-informed drug. GPS, alternatively, linked PGx drugs with 39.7% of the diseases, and CPIC-A with 29.1%.
Figure 2.
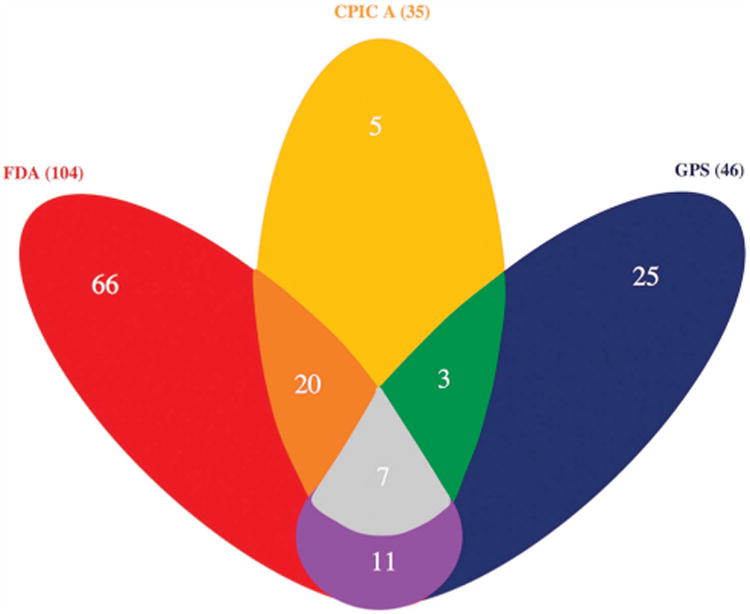
A Venn diagram that displays the amount of drug overlap between the three PGx sources. The FDA has 104 PGx drugs (red), CPIC-A has 35 PGx drugs (yellow), and GPS has 46 PGx drugs (blue). An overlap is shown when two of the three PGx source colors are blended together.
Integration of the disease–drug database into a pharmacogenomic decision-support resource, the GPS
The final disease–drug tool was then implemented into the already established clinical PGx results delivery system at our institution, GPS. As previously described,6,8,24 GPS uses a colored traffic signal system to alert physicians of the different PGx risk levels when prescribing a drug. Red lights signify drugs with a high risk of severe undesirable outcomes, yellow lights signify drugs with increased genetic risk (caution), and green lights signify drugs with a favorable genetic association. Enhancing the disease–drug search engine within GPS using the above-created disease–drug association tool permits GPS users to search for a list of potential drugs available to treat a specific disease. We posit that this increases the versatility and applicability of GPS as a PGx CDS system.
An example of a disease–drug search within GPS is shown in Figure 3. A search for the disease “GERD” auto populates to “GERD—gastroesophageal reflux disease,” and displays the respective potential drug treatments available. Assuming that the specific patient being searched has had their DNA tested preemptively across a panel including all relevant PGx markers (as identified by the FDA, CPIC, or GPS), like the patient in this example has had, then based on the patient's specific PGx results, either esomeprazole or rabeprazole are identified as pharmacogenomically more favorable prescription choices compared to pantoprazole; and omeprazole and lansoprazole are associated with even less favorable genetic risk-related outcomes. The remaining medications that also treat GERD but do not have clinically actionable PGx information known are shown as having “no known PGx information.” By using this novel disease–drug search engine in GPS, we imagine that prescribers could choose a greater number of pharmacogenomically favorable medications the first time, replacing the traditional trial-and-error method. Data on physician behavior and prescribing choices in response to this PGx search functionality are currently being collected and analyzed in the longitudinal phase of the 1200 Patients Project.6
Figure 3.
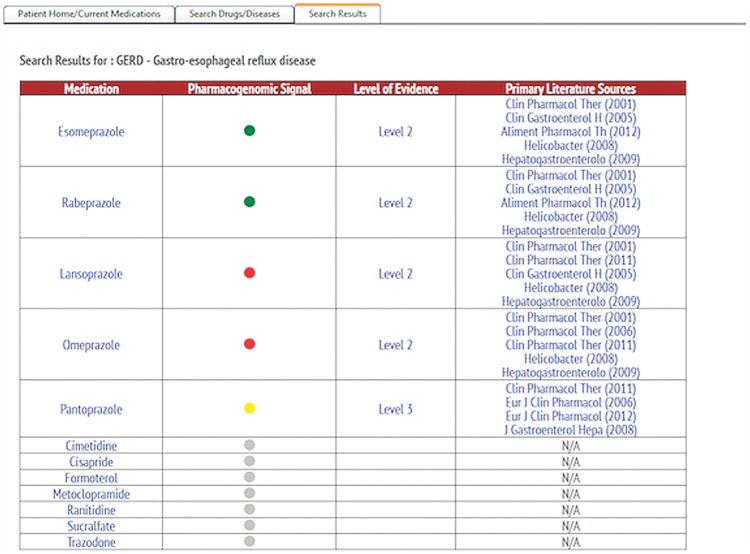
Incorporating the disease–drug search engine in GPS, specifically highlighting the results of the disease search, “GERD-Gastroesophageal reflux disease.” Red lights signify drugs with a high risk of severe undesirable outcomes, yellow lights signify drugs with increased genetic risk (caution), and green lights signify drugs with a favorable genetic association. Level 1 represents data from a well-performed large study including replication, or replicated by two or more large, well-performed studies. Published dosing guidelines or FDA label information likely exists. Level 2 represents data from at least one well-performed study of at least 100 patients; or from several small or moderately sized studies, which show consistent results. Level 3 represents data from a relatively small single study (<100 patients); or several similarly executed contradictory studies exist.
Validation of the disease–drug search tool to inform disease treatment in an existing patient cohort
The newly created disease–drug search database was then tested using the 1,019 outpatients enrolled in our institutional PGx implementation study to determine the applicability of pharmacogenomics-based prescribing in a clinical setting. Table 2a shows the baseline characteristics of this external validation cohort, with patients having an average age of 61 ± 15 years. 56% of patients were females and most were enrolled from a primary care clinic (41%) or a cardiology clinic (20%). The top 25 diseases found within this population is shown in Table 2b, which reflected many of the top leading causes of death in the US based on the World Health Organization and Centers for Disease Control and Prevention.25,26
Table 2a. Baseline characteristics of the external validation cohort.
| Total Patients | 1,019 | |
|---|---|---|
| Average Age – mean (range; years) | 61 ± 15 (20–97) | |
| Gender | Number of Patients | % of Patients |
| Male | 445 | 43.7 |
| Female | 574 | 56.3 |
| Demographics | ||
| White | 616 | 60.5 |
| Black or African American | 309 | 30.3 |
| Unknown | 33 | 3.2 |
| Asian | 32 | 3.1 |
| More Than One Race | 26 | 2.6 |
| American Indian/Alaska Native | 2 | 0.2 |
| Native Hawaiian or Other Pacific Islander | 1 | 0.1 |
| Ethnicity | ||
| Not Hispanic or Latino | 498 | 48.9 |
| Unknown | 481 | 47.2 |
| Hispanic or Latino | 40 | 3.9 |
| Clinics From Which Patients Were Enrolled | ||
| Primary Care | 417 | 40.9 |
| Cardiology | 207 | 20.3 |
| Oncology | 151 | 14.8 |
| Gastroenterology | 81 | 7.9 |
| Hepatology | 56 | 5.5 |
| Pulmonology | 42 | 4.1 |
| Rheumatology | 41 | 4.0 |
| Nephrology | 24 | 2.4 |
Table 2b. Top 25 medical conditions present in the external validation cohort.
| Disease | Number of Patients | % of Patients |
|---|---|---|
| Hypertension | 528 | 51.8 |
| Hyperlipidemia | 412 | 40.4 |
| Musculoskeletal - Mechanical Joint Disorders | 186 | 18.3 |
| Gastroesophageal Reflux Disease | 179 | 17.6 |
| Obesity | 170 | 16.7 |
| Pain - Musculoskeletal | 131 | 12.9 |
| Coronary Artery Disorder | 129 | 12.7 |
| Osteoporosis | 129 | 12.7 |
| Diabetes Mellitus | 127 | 12.5 |
| Surgery - Joint | 118 | 11.6 |
| Hysterectomy/Oophorectomya | 116 | 11.4 |
| Sleep Apnea | 110 | 10.8 |
| Rhinitis | 102 | 10.0 |
| Anemiaa | 102 | 10.0 |
| Hypothyroidism | 101 | 9.9 |
| Cancer - Breast | 98 | 9.6 |
| Asthma | 96 | 9.4 |
| Chronic Kidney Disease | 92 | 9.0 |
| Depression | 90 | 8.8 |
| Surgery - Head And Necka | 90 | 8.8 |
| Polyp - Intestinea | 90 | 8.8 |
| Musculoskeletal Spine Disorders | 78 | 7.7 |
| Tobacco Use | 78 | 7.7 |
| Benign Tumor | 74 | 7.3 |
| Arthritis | 74 | 7.3 |
Medical conditions that were mapped to none of the 324 drugs in the disease–drug database.
A total of 771 unique diseases were extracted from the 1,019 patient charts, with 484 (62%) of those diseases associated with a potential drug therapy (a large number of the top “diseases” were actually surgeries or conditions treated primarily by a surgical intervention, e.g., “hysterectomy/oophorectomy,” “surgery of the head and neck,” and “intestinal polyp”).
The top 21 diseases present in our patient cohort (excluding the above three surgical disorders and anemia) were then examined using the PGx annotations found within the disease–drug database. Figure 4a shows a detailed list of the specific PGx annotations associated with disease–drug pairs. Each row displays a drug that was mapped to at least one of the diseases, and also shows different colored cells for drugs that have PGx information: red cells represent PGx information according to FDA-defined PGx drugs, orange cells represent CPIC-A-defined PGx drugs, blue cells represent GPS-defined PGx associations, and combinational colored cells represent PGx indications that are present according to more than one of our chosen PGx actionability criteria. For example, coronary artery disease (CAD) is associated with 24 potential treatment medications, with GPS-defined PGx drugs (blue cells) including amlodipine and aspirin, in addition to several other drugs (including carvedilol and metoprolol) that have both FDA and GPS-defined PGx guidelines (blue-red cells). As another example, simvastatin has both a CPIC-A and a GPS PGx indication (blue-orange cells), while warfarin and clopidogrel have PGx indications in all three sources (blue-red-orange cells). The dark gray cells contain drugs that treat CAD, but have no PGx information from these three sources.
Figure 4.
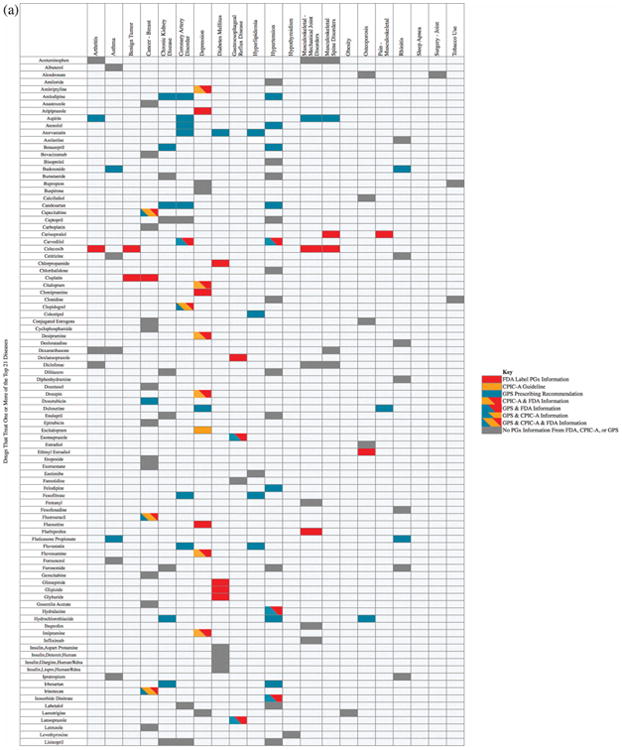
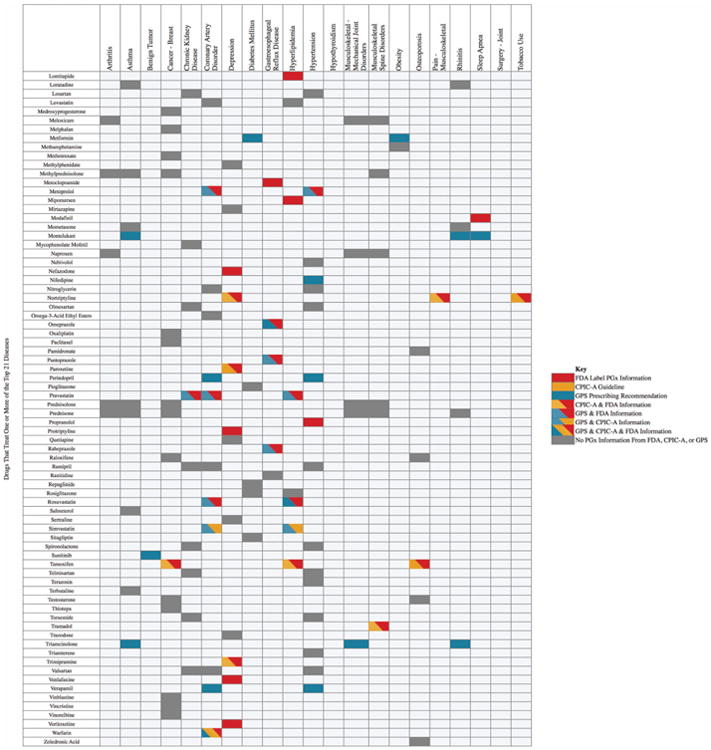
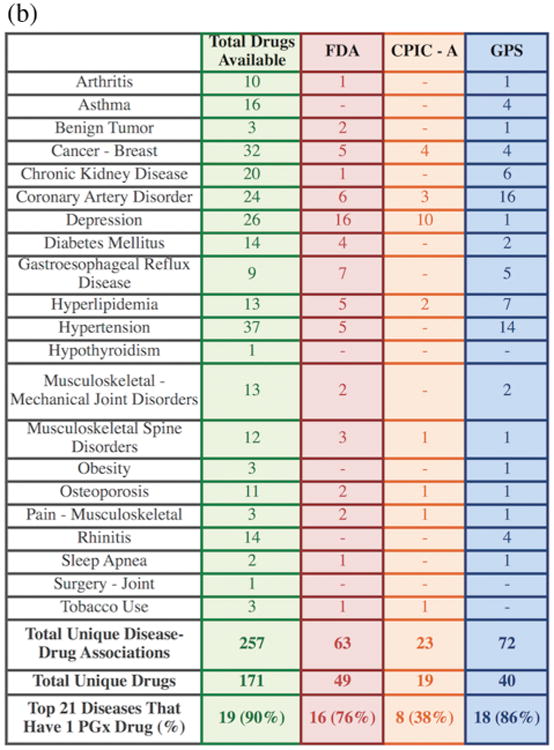
(a) The disease–drug search results for the top 21 diseases found in the external validation cohort. Each row lists a drug that treats one or more of the top 21 diseases. (b) A summary of the disease–drug search results for the top 21 diseases.
The disease–drug associations with PGx annotations for the top 21 diseases present in our validation cohort are summarized in Figure 4b. Overall, 90% of the top 21 diseases can be treated with at least one drug that has actionable PGx information available, with 76% of diseases treatable with at least one PGx-informed drug according to the FDA label criteria, 38% according to CPIC-A criteria, and 86% according to GPS PGx criteria. Since many of our patients have multiple comorbidities, the number of patients in our validation cohort with at least one disease treatable using PGx information would increase to 97.5% (994/1,019) using FDA-supported criteria, 93% (948/1,019) with CPIC-A guidelines, and 97.4% (993/1,019) using GPS information, making this disease–drug search engine highly clinically relevant to typical US patients.
Discussion
We created a novel disease–drug database and search engine that empowers physicians to integrate patient-specific genotype data with clinically actionable PGx data when considering treatment of common medical conditions. Being able to search a disease indication and compare various treatment options based on patient-specific PGx information not only could provide more informed prescribing decisions, but also potentially avoid ADRs and facilitate the ideal of choosing the “right drug at the right dose at the right time.”27 Our validation showed PGx-based prescribing can inform the treatment of the great majority of medical conditions and would potentially affect nearly every patient with some of the most common US medical diseases. This type of system represents an important component of the eventual infrastructure that will be necessary for developing a comprehensive clinical support paradigm for PGx implementation.
Ideally, a disease–drug search engine would be used when healthcare providers are deciding on new prescriptions for their patients. Many current CDS tools alert physicians of a patient's PGx response after they place an order.4,28,29 Then physicians have the option to override the alert or follow the alternate drug or dosing recommendations.4,28,29 In many of those systems, physicians must have already decided what medication they want to prescribe and then the PGx information is supplied in response to that decision. In contrast, our disease–drug search tool enables truly preemptive patient-specific PGx result consideration, at the moment (and decision-level) of consideration of all medications that are available as treatments for a given disease. This closely simulates the way that physicians already operate in routine care—physicians often are well-versed in the available treatments and can compare and contrast their evidentiary usefulness (or they may have equipoise about multiple treatments for a single condition). In the ideal scenario, PGx information would be brought to bear on this complex medical decision-making calculus as well. Our tool permits this possibility. There of course remain a number of critical next steps that will be required for wide dissemination and use of CDS tools for PGx-based prescribing, including agreement on and standardization of actionable PGx genotypes, identification of the relevant parties who should receive and take ownership of addressing actionable results, overcoming provider learning curves and time constraints on the implementation of genomic findings, integration with electronic medical records, and cost considerations.3,7–9,30
Our database was compared with three well-known disease– drug knowledge bases (LabeledIn, MEDI, and NDF-RT), and significant overlap for the included drugs was observed, although ours characterized many unique disease–drug pairs and added the novel component of PGx annotation, which was not included in any of these prior published disease–drug databases. However, there are existing databases that allow searches of PGx information, including CDS data linked to genotypes. PharmGKB (www.pharmgkb.org) has perhaps the most extensive PGx database,31 and disease indication searches are possible in their tool but only drugs with PGx publications are displayed (and drugs with both low-level and clinically actionable PGx information are simultaneously resulted). The clinical implementation program eMERGE has implemented an external database called SPHINX (Sequence Phenotype and Pharmacogenomics Integration Exchange).5 Anyone can search patient-specific (but deidentified) information in this database.5 Enrolled providers of the eMERGE project have access to their patients' specific information, including current drug lists, disease lists, and other demographic information.5 However, this system was primarily built to determine novel PGx associations from the patient data.5 Our disease–drug search engine, in contrast, is designed to help physicians at the point of prescribing.
Our disease–drug database showed extensive relevance and applicability in our validation cohort, with over 93% of patients having at least one disease that could be treated with a PGx-actionable drug. This illustrates both the potential reach and impact of PGx if widely available and creatively delivered into the hands of clinicians, but it also demonstrates the frustrating likelihood that most PGx information is currently being grossly underutilized.32 While definitive prospective (or pragmatic) studies are still required to show that PGx implementation improves patient outcomes, implementation in a wider context is unlikely to do harm for most contexts.33 The lack of wider application is almost certainly in part due to a lack of knowledge or lack of availability of PGx knowledge at the point of care. From a recent survey of over 300 physicians, only 20% stated they consult drug labels or the FDA website when wanting to know more about PGx.10 Another study showed that over 75% of physicians surveyed were unaware of the FDA PGx drug labels.34 With the extensive amount of PGx knowledge available, it seems that physicians have difficulty keeping track of which drugs have, or might have, actionable or clinically relevant PGx information.
Popular existing drug databases also do not consistently report PGx information. Per one recent study, while 95.3% of FDA label PGx information was present in Lexicomp, only 67.7% were present in ePocrates Free.35 Informatics tools need to keep pace with the rapidly expanding PGx evidentiary landscape and also anticipate and address the needs of users for real-world implementation. Improvement of continuing educational PGx tools for healthcare providers may also address this translational gap.
Limitations of our disease–drug search engine are acknowledged. For one, our disease–drug database was initially built containing only 324 commonly prescribed drugs available in the US—a large number (and a number not dissimilar from other published disease–drug databases21,22), but a number representing only a portion of the available drugs on the market. The search tool will additionally need to be regularly updated as new drugs or disease indications are published. Since it was manually curated, there is a chance of human error, and computer algorithms to assign disease indications, SNOMED terms, and synonymous disease groups as well as application of natural language processing technologies might enhance its future functionality.36,37 Extensibility to electronic medical records (EMRs) will be necessary for wide dissemination. Finally, to further expand the database in the future, we are currently considering ways to include both drug–drug interactions and drug–drug–gene interaction information to match as fully as possible the real-life CDS complexities that prescribers face.
It should be acknowledged that, in order for our disease–drug search engine within GPS to truly inform individual patient prescribing, preemptively obtained PGx results for specific patients must be available and integrated with the system. Currently, it is more likely that—with the exception of a few academic medical centers—most providers (and patients) will not have individual genotype information available, and many do not have immediate, easy access to testing. While such availability evolves, our disease–drug search engine could still have an important immediate implementation role if deployed creatively. For example, providers (especially those in training) might use the database to look up treatments for common ailments, and would likely turn to this database after a first-line treatment did not work (simultaneously learning which drugs have actionable PGx interactions). More extensibly, during EMR-based prescribing when any provider enters a drug to be prescribed, a link-out/alert to the disease–drug database could be provided when the prescribed drug has a known PGx interaction. This link-out could trigger the prescriber to review all drugs available for that condition, and the known PGx interactions. The provider might choose to prescribe another drug (e.g., one without a PGx interaction), might order testing, or may still prescribe the drug with a known PGx interaction— withholding testing—while carefully monitoring the patient's clinical response. The PGx-annotated disease–drug tool thus provides the prescriber the opportunity to review several treatment options at the point of care, discuss them with the patient, and make a joint decision about the best treatment or course of action.
In conclusion, we created a novel disease–drug searchable database to allow prescribers to identify drugs treating common diseases that have clinically relevant PGx information. Pharmacogenomic-based disease-level prescribing was shown to have high-predicted applicability in informing the treatment of common diseases in the US population. We believe this system is a key initial step in developing the clinical support paradigm required for precision medicine. This disease–drug tool is currently being utilized in an ongoing, institutional prospective clinical implementation project to assess prescribing behavior changes based on the availability of pharmacogenomic data.
Methods
Developing the disease–drug search database/tool
We created a disease–drug database by manually compiling disease indications for 324 commonly prescribed medications (Supplementary Table S3). Because this database was being designed to incorporate PGx annotations, the list of drugs was comprehensive but specifically included medications known to have clinically relevant PGx data, specifically from FDA labels that have PGx information,23 CPIC-A guidelines,14 and those included in our previously developed GPS.6,8,24 Drugs not included from these preceding three sources but that had 20 or more PGx publications were also included in the prospective drug list. Medications taken by ≥4 patients in our institutional outpatient cohort were also included to capture the most commonly prescribed drugs. Vitamins, minerals, and topical medications were excluded. Once the final drug list was compiled, all generic medication names were mapped to NDF-RT.16
FDA approved and non-FDA approved (alternative) disease indications for each drug were compiled manually from two publicly available sources: Micromedex®38 and UpToDate®.39 Additional disease indications for these drugs were added based on Internet-based analysis of common prescribing patterns. Two physicians (B.B.K. and P.H.O.) completed an independent and then comparative literature reviews of the compiled list. A pharmacist (Y.M.L.) then independently examined the final list of disease–drug indications. The pharmacist and one physician resolved the remaining pairs through discussion and published evidence analysis until 100% consensus was reached.
In order to normalize the disease terms, final disease indications were manually linked to the respective disease terms from SNOMED40 as closely as possible. The final list of disease to SNOMED mappings was reviewed a second time to ensure the terms were correctly paired. Two physicians then grouped synonymous SNOMED terms into a simplified disease group arrangement representing distinct disease conditions.
Comparing the disease–drug database with other disease–drug resources
In order to validate the strength and inclusivity of our disease-drug search engine, it was compared to three publicly available previously published knowledge bases, LabeledIn,22 MEDI,20 and NDF-RT.16
Normalization of ontologies was first performed to cross-compare the databases. Once all drugs and indications in the databases were converted to like reference terminologies (we chose NDF-RT for drugs and SNOMED for diseases), an overlap analysis for the shared number of drugs, diseases, and disease-drug pairs was performed.
Annotation of the novel disease–drug database with pharmacogenomic information
The database medications were then annotated with PGx information denoting potential clinical actionability. Annotations were ascribed using three different PGx standards: 1) drugs with FDA drug labels that included PGx information,23 2) drugs with existing CPIC guidelines,14 and 3) drugs deemed as having clinically deliverable PGx associations based on our previously described institutional PGx implementation project, the 1200 Patients Project.6,8,24
For analysis standard 1) (FDA), drugs with FDA labels containing germline PGx information were included. Of the drug labels identified by the FDA as including “PGx Biomarker” information (available as of April 2015 at http://www.fda.gov/Drugs/ScienceResearch/ResearchAreas/Pharmacogenetics/ucm083378.htm), 142 unique drugs were listed (individual medications used in combination drugs were counted as separate medications), and 105 referenced germline PGx considerations (those excluded contained somatic biomarker considerations for cancer treatment). Methylene blue was also then excluded because it lacks an FDA-approved indication, leaving 104 drugs for the analysis. These 104 drugs were broadly considered as having PGx considerations within their labels, spanning a spectrum, with some drugs having specific dosing guidance based on genotype, while others more generally mentioned the pharmacologic role of given genes in the drug's metabolism, disposition, or in drug interactions.23 For analysis standard 2) (CPIC), only drugs with CPIC-level A guidelines pertaining to germline PGx were included (these level A drugs are identified by CPIC as having the strongest supporting PGx evidence; https://www.pharmgkb.org/cpic/pairs), thus making our analysis by this CPIC standard comparatively the most conservative of the three analysis sources used.41 Of the 44 CPIC-A guidelines available as of April 2015, 37 unique drugs were listed, and 35 were included in this study (tegafur and telaprevir were excluded because they lack US approval). For analysis 3) (drugs already being clinically delivered with PGx decision-support within our existing clinical implementation program at the University of Chicago for the past nearly 3 years), we included all 46 drugs and around 250 CDS summaries that are available within the results delivery informatics system at our institution, GPS.8 The process for a result being delivered clinically within GPS has been previously described.24
External validation of the disease–drug search tool using a clinic patient cohort at a major medical center
To determine the applicability of PGx-based prescribing, the disease– drug search database was integrated into the preexisting PGx results delivery system (GPS), and then tested in an outpatient cohort to determine the prevalence of PGx information available for medications that treat common diseases within the patient cohort. The patients in this cohort were prospectively recruited as part of a preemptive PGx implementation study at the University of Chicago termed the 1200 Patients Project (clinicaltrials.gov identifier NCT01280825, IRB #10-487-A).8
The disease conditions for the enrolled 1,019 patients were manually extracted from the medical center's EMR using a detailed, outpatient chart examination that included a review of and summation of all diseases listed in: 1) ICD-9 diagnoses, 2) the EMR patient-specific “Problems list,” 3) the past medical and surgical history section of an individual outpatient clinic note for each patient, and 4) the assessment and plan section of an individual outpatient clinic note for each patient. All ICD-9 diagnoses and other diseases were manually copied from the EMR, with variations of the same disease, e.g., “hypertension” and “HTN,” harmonized.
All problems/conditions were manually linked to their respective standardized disease terms in SNOMED as closely as possible. Synonymous problem terms within a single defined disease group (described above) were counted only once per patient to avoid double-counting. We then analyzed the performance of this disease–drug PGx search tool by applying it to both the patient-level and aggregate (at the level of the cohort) diseases that were represented.
Supplementary Material
Study Highlights.
What is the Current Knowledge on the Topic?
☑ Providers have expressed a strong desire to have clinical decision support (CDS) tools to assist interpretation of pharmacogenomic (PGx) results. Although the development of CDS tools is expanding, there are no databases, to our knowledge, that currently combine searchable disease–drug indications with PGx clinical annotations for use at the point of prescribing.
What Question Does This Study Address?
☑ We built a disease-drug database with PGx annotations to determine the applicability of PGx-informed prescribing within a prospectively enrolled, institutional patient cohort.
What This Study Adds To Our Knowledge
☑ Our validation testing showed that pharmacogenomic-based prescribing can inform the treatment of the great majority of common medical conditions and would potentially affect nearly every patient having common medical diseases.
How This Might Change Clinical Pharmacology and Therapeutics
☑ This type of PGx-based prescribing decision-support represents an important step in the further implementation of precision medicine.
Acknowledgments
We thank Mr. Ishai Strauss for his contributions to this work. This study was supported by the National Institutes of Health (NIH) grant K23 GM100288-01A1 (PHO), NIH/National Heart, Lung, and Blood Institute grant 5 U01 HL105198-09 (MJR, PHO), The University of Chicago Innovation Fund (KD, MJR, PHO), the Conquer Cancer Foundation of the American Society for Clinical Oncology (MJR), The William F. O'Connor Foundation (MJR), and the Central Society for Clinical and Translational Research Early Career Development Award (PHO).
Footnotes
Additional Supporting Information may be found in the online version of this article.
Conflict Of Interest: K.D., M.J.R., and P.H.O. are coinventors of a pending patent application for the Genomic Prescribing System. M.J.R. is a coinventor holding patents related to pharmacogenetic diagnostics and receives royalties related to UGT1A1 genotyping. No royalties are received from the genotyping performed in this study.
Author Contributions: S.H., K.D., Y.M.L., M.J.R., and P.H.O. wrote the article; S.H., K.D., M.J.R., and P.H.O. designed the research; S.H., B.B.K., K.D., Y.M.L., P.M.G., and P.H.O. performed the research; S.H., B.B.K., K.D., Y.M.L., P.M.G., and P.H.O. analyzed the data; S.H., K.D., P.M.G., and P.H.O. contributed new reagents/analytical tools.
References
- 1.Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279:1200–1205. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- 2.Phillips KA, Veenstra DL, Oren E, Lee JK, Sadee W. Potential role of pharmacogenomics in reducing adverse drug reactions: a systematic review. JAMA. 2001;286:2270–2279. doi: 10.1001/jama.286.18.2270. [DOI] [PubMed] [Google Scholar]
- 3.Manolio TA, et al. Implementing genomic medicine in the clinic: the future is here. Genet Med. 2013;15:258–267. doi: 10.1038/gim.2012.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunnenberger HM, et al. Preemptive clinical pharmacogenetics implementation: current programs in five US medical centers. Annu Rev Pharmacol Toxicol. 2015;55:89–106. doi: 10.1146/annurev-pharmtox-010814-124835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rasmussen-Torvik LJ, et al. Design and anticipated outcomes of the eMERGE-PGx project: a multicenter pilot for preemptive pharmacogenomics in electronic health record systems. Clin Pharmacol Ther. 2014;96:482–489. doi: 10.1038/clpt.2014.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Donnell PH, et al. Adoption of a clinical pharmacogenomics implementation program during outpatient care—initial results of the University of Chicago “1,200 Patients Project. Am J Med Genet C Semin Med Genet. 2014;166C:68–75. doi: 10.1002/ajmg.c.31385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Relling MV, Evans WE. Pharmacogenomics in the clinic. Nature. 2015;526:343–350. doi: 10.1038/nature15817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Donnell PH, et al. The 1200 patients project: creating a new medical model system for clinical implementation of pharmacogenomics. Clin Pharmacol Ther. 2012;92:446–449. doi: 10.1038/clpt.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson JA, Burkley BM, Langaee TY, Clare-Salzler MJ, Klein TE, Altman RB. Implementing personalized medicine: development of a cost-effective customized pharmacogenetics genotyping array. Clin Pharmacol Ther. 2012;92:437–479. doi: 10.1038/clpt.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johansen Taber KA, Dickinson BD. Pharmacogenomic knowledge gaps and educational resource needs among physicians in selected specialties. Pharmacogenom Personal Med. 2014;7:145–162. doi: 10.2147/PGPM.S63715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson JA. Pharmacogenetics in clinical practice: how far have we come and where are we going? Pharmacogenomics. 2013;14:835–843. doi: 10.2217/pgs.13.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pharmacogenomics Research Network (PGRN) [Accessed August 2015]; http://www.pgrn.org/
- 13.PharmGKB: The Pharmacogenomics Knowledgebase. [Accessed August 2015];2015 https://www.pharmgkb.org/
- 14.Relling MV, Klein TE. CPIC: Clinical Pharmacogenetics Implementation Consortium of the Pharmacogenomics Research Network. Clin Pharmacol Ther. 2011;89:464–467. doi: 10.1038/clpt.2010.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salmasian H, Tran TH, Chase HS, Friedman C. Medication-indication knowledge bases: a systematic review and critical appraisal. J Am Med Inform Assoc. 2015;22:1261–1270. doi: 10.1093/jamia/ocv129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carter JS, et al. Initializing the VA medication reference terminology using UMLS metathesaurus co-occurrences. Proceedings / AMIA Annual Symposium AMIA Symposium. 2002:116–120. [PMC free article] [PubMed] [Google Scholar]
- 17.Kuhn M, Campillos M, Letunic I, Jensen LJ, Bork P. A side effect resource to capture phenotypic effects of drugs. Mol Syst Biol. 2010;6:343. doi: 10.1038/msb.2009.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCoy AB, et al. Development and evaluation of a crowdsourcing methodology for knowledge base construction: identifying relationships between clinical problems and medications. J Am Med Inform Assoc. 2012;19:713–718. doi: 10.1136/amiajnl-2012-000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fung KW, Jao CS, Demner-Fushman D. Extracting drug indication information from structured product labels using natural language processing. J Am Med Inform Assoc. 2013;20:482–488. doi: 10.1136/amiajnl-2012-001291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei WQ, Cronin RM, Xu H, Lasko TA, Bastarache L, Denny JC. Development and evaluation of an ensemble resource linking medications to their indications. J Am Med Inform Assoc. 2013;20:954–961. doi: 10.1136/amiajnl-2012-001431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jung K, et al. Automated detection of off-label drug use. PLoS One. 2014;9:e89324. doi: 10.1371/journal.pone.0089324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khare R, Li J, Lu Z. LabeledIn: cataloging labeled indications for human drugs. J Biomed Inform. 2014;52:448–456. doi: 10.1016/j.jbi.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Table of Pharmacogenomic Biomarkers in Drug Labeling. 2015 Apr; http://www.fda.gov/drugs/scienceresearch/researchareas/pharmacogenetics/ucm083378.htm.
- 24.Kaufman AL, et al. Evidence for clinical implementation of pharmacogenomics in cardiac drugs. Mayo Clin Proc. 2015;90:716–729. doi: 10.1016/j.mayocp.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noncommunicable Diseases (NCD) Country Profiles, 2014 — United States of America. [Accessed August 2015]; http://www.who.int/nmh/countries/usa_en.pdf?ua=1.
- 26.Leading Causes of Death. [Accessed August 2015]; http://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm.
- 27.Altman R, Flockhart DA, Goldstein DB. Principles of Pharmacogenetics and Pharmacogenomics. Cambridge University Press; Cambridge, New York: 2012. [Google Scholar]
- 28.Weitzel KW, et al. Clinical pharmacogenetics implementation: approaches, successes, and challenges. Am J Med Genet C Semin Med Genet. 2014;166C:56–67. doi: 10.1002/ajmg.c.31390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crews KR, Hicks JK, Pui CH, Relling MV, Evans WE. Pharmacogenomics and individualized medicine: translating science into practice. Clin Pharmacol Ther. 2012;92:467–475. doi: 10.1038/clpt.2012.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haga SB, O'Daniel JM, Tindall GM, Mills R, Lipkus IM, Agans R. Survey of genetic counselors and clinical geneticists' use and attitudes toward pharmacogenetic testing. Clin Genet. 2012;82:115–120. doi: 10.1111/j.1399-0004.2012.01848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hewett M, et al. PharmGKB: the Pharmacogenetics Knowledge Base. Nucl Acids Res. 2002;30:163–165. doi: 10.1093/nar/30.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Veltman JA, Lupski JR. From genes to genomes in the clinic. Genome Med. 2015;7:78. doi: 10.1186/s13073-015-0200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Altman RB. Pharmacogenomics: “noninferiority” is sufficient for initial implementation. Clin Pharmacol Ther. 2011;89:348–350. doi: 10.1038/clpt.2010.310. [DOI] [PubMed] [Google Scholar]
- 34.Haga SB, Burke W, Ginsburg GS, Mills R, Agans R. Primary care physicians' knowledge of and experience with pharmacogenetic testing. Clin Genet. 2012;82:388–394. doi: 10.1111/j.1399-0004.2012.01908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vaughan KT, Scolaro KL, Anksorus HN, Roederer MW. An evaluation of pharmacogenomic information provided by five common drug information resources. J Med Libr Assoc. 2014;102:47–51. doi: 10.3163/1536-5050.102.1.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coulet A, Shah NH, Garten Y, Musen M, Altman RB. Using text to build semantic networks for pharmacogenomics. J Biomed Inform. 2010;43:1009–1019. doi: 10.1016/j.jbi.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carrell DS, et al. Using natural language processing to improve efficiency of manual chart abstraction in research: the case of breast cancer recurrence. Am J Epidemiol. 2014;179:749–758. doi: 10.1093/aje/kwt441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Micromedex Solutions j Evidence-Based Clinical Decision Support. [Accessed June 2015]; http://micromedex.com/
- 39.UpToDate. [Accessed June 2015]; http://www.uptodate.com/contents/search.
- 40.SNOMED Clinical Terms® (SNOMED CT®) [Accessed June 2015]; https://www.nlm.nih.gov/research/umls/Snomed/snomed_main.html.
- 41.CPIC Genes/Drugs. [Accessed April 2015]; https://www.pharmgkb.org/cpic/pairs.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


