Abstract
Atorvastatin is widely used to lower blood cholesterol and to reduce risk of cardiovascular disease–associated complications. Epidemiological investigations and preclinical studies suggest that statins such as atorvastatin have antitumor activity for various types of cancer. Tuberous sclerosis (TSC) is a tumor syndrome caused by TSC1 or TSC2 mutations that lead to aberrant activation of mTOR and tumor formation in multiple organs. Previous studies have demonstrated that atorvastatin selectively suppressed growth and proliferation of mouse Tsc2 null embryonic fibroblasts through inhibition of mTOR. However, atorvastatin alone did not reduce tumor burden in the liver and kidneys of Tsc2+/− mice as assessed by histological analysis, and no combination therapy of rapamycin and atorvastatin has been tried. In this study, we used T2-weighted magnetic resonance imaging to track changes in tumor number and size in the kidneys of a Tsc1+/− mouse model and to assess the efficacy of rapamycin and atorvastatin alone and as a combination therapy. We found that rapamycin alone or rapamycin combined with atorvastatin significantly reduced tumor burden, while atorvastatin alone did not. Combined therapy with rapamycin and atorvastatin appeared to be more effective for treating renal tumors than rapamycin alone, but the difference was not statistically significant. We conclude that combined therapy with rapamycin and atorvastatin is unlikely to provide additional benefit over rapamycin as a single agent in the treatment of Tsc-associated renal tumors.
Introduction
Atorvastatin, a synthetic inhibitor of 3-hydroxy-3-methyl-glutaryl-CoA reductase, also known as Lipitor, is widely used to lower blood cholesterol and to reduce risk of cardiovascular disease–associated complications [1]. Epidemiological investigations and preclinical studies suggest that statins such as atorvastatin have antitumor activity for various types of cancer including renal carcinoma [2], [3], [4], [5]. Tuberous sclerosis (TSC) is a tumor syndrome caused by TSC1 or TSC2 mutations and characterized by tumor formation in multiple organs [6], [7]. TSC2 is a GTPase activating protein towards Rheb and forms a functional complex with TSC1 that downregulates mTOR (mechanistic target of rapamycin) [8]. TSC-associated tumors exhibit aberrant activation of mTOR. Genetically engineered Tsc1+/− or Tsc2+/− mice spontaneously develop tumors in multiple organs including the liver and the kidneys [9], [10]. Previous studies have demonstrated that atorvastatin selectively suppressed growth and proliferation of mouse Tsc2 null embryonic fibroblasts through inhibition of mTOR [11]. Atorvastatin also inhibited mTOR in normal tissues of Tsc2+/− mice. However, atorvastatin alone did not reduce tumor burden in the liver and kidneys of these mice as assessed by histological analysis [12]. In this study, we used T2-weighted magnetic resonance imaging (MRI) to track changes of tumor number and size in the kidneys of a Tsc1+/− mouse model and assess the efficacy of rapamycin and atorvastatin combination therapy.
Materials and Methods
Animal Procedures
Animal procedures were performed in accordance with the UK Home Office guidelines and approved by the Ethical Review Group of Cardiff University. Tsc1+/− balb/c mice were described previously [9]. Tsc1+/− littermates were randomly allocated into four groups of six, balanced for gender. Animals were treated from the age of 12 months with vehicle by i.p., rapamycin (5 mg/kg) by i.p., atorvastatin (100 mg/kg) by gavage, or rapamycin (5 mg/kg) plus atorvastatin (100 mg/kg) 5 times a week for 2 months and then sacrificed for assessment of tumor burden and analysis of protein expression and phosphorylation in tumors and normal tissues. Rapamycin was purchased from LC Laboratories, Woburn, MA, USA. Atorvastatin (Lipitor) was gift from University Hospital of Wales, Cardiff, UK.
Magnetic Resonance Imaging (MRI)
As described previously [13], MRI scans were carried out using a high-field (9.4 T) small-bore (20 cm) Bruker Biospec 94/20 magnetic resonance spectroscopy (MRI/MRS) spectrometer. Mice were anesthetized with 5% isoflurane in a 40% O2/air mix at 1.2 l/min, injected with 2 × 0.2 ml of 4% glucose/0.18% saline solution subcutaneously, and transferred to specialist MRI bed model #T10532 (Bruker, Ettlingen, Germany) with integrated circulating heated water and an anesthetic nose cone. Isoflurane was reduced to 1.5% to 2% for the maintenance of anesthesia during scanning. Body temperature, breathing rate, and heart rate were monitored throughout using the Model 1025 Monitoring and Gating System (SAI Inc., Stony Brook, NY). Body temperature was maintained during recovery in a warm air V1200 recovery chamber (Peco Services Ltd.) set at 27°C. T2-weighted, respiratory-gated, fat-suppressed RARE scans were performed with an FOV of 10.0 × 4.0 cm, a matrix of 640 × 256, 64 × 0.5–mm coronal slices, a TEeff of 26 milliseconds, a TR of 4100 milliseconds, a RARE factor of 4, and a BW of 100 kHz. The volumes of renal lesions were measured using the software Analyze 10.0 (Analyze Direct, Inc., Overland Park, KS). The measurement was conducted blindly in triplicate.
Histology
Mouse kidneys were fixed in 10% buffered formalin saline (Thermo Scientific, Runcorn, UK) for 24 hours. Fixed kidneys were processed and paraffin embedded according to standard procedures. A series of 5-μm coronal kidney sections was prepared at 200-μm intervals from each kidney. Kidney sections were HE-stained and scanned to create virtual HE slides using an Aperio system (http://www.aperio.com/?gclid = CNXN-8by4a UCFcINfAods3eg1w).
Immunohistochemistry (IHC)
Primary antibodies against phosphorylated S6 ribosomal protein at S235/236, Akt at S473, and Erk1/2 at T202/Y204 were supplied by Cell Signaling Technology (Danvers, MA). Antibodies against Ki67 and phosphorylated RAF1 at S259 were supplied by Abcam (Cambridge, UK). SignalStain Boost Rabbit specific IHC Detection Reagent (Cell Signaling Technology, Danvers, MA) was used to stain antigens.
Western Blot
In addition to primary antibodies described above, primary antibody against β-actin and horseradish peroxidase–conjugated secondary antibody against rabbit were purchased from Cell Signaling Technology (Danvers, MA) for Western blot. Primary antibody against p21 was supplied by Abcam (Cambridge, UK). Extracts of liver tissues were prepared using AllPrep DNA/RNA/Protein Mini Kit (QIAGEN Ltd.-UK, Crawley, UK). Proteins were purified according to the kit supplier's instruction. Twenty micrograms of protein per sample was separated on NuPAGE 4% to 12% Bis-Tris Gels (Fisher Scientific UK Ltd., Loughborough, UK) and transferred onto Amersham Protran Premium 0.45-μm nitrocellulose blotting membranes (GE Healthcare UK Ltd., Little Chalfont, UK). Blots were analyzed with ECL Select Western Detection Kit (GE Healthcare UK Ltd.), and signals were detected using Autochemi Imaging System (UVP, Upland, CA).
Quantitative Real-Time PCR (q-PCR)
Total RNA was isolated from mouse liver tissues using AllPrep DNA/RNA/Protein Mini Kit and TissueRuptor (QIAGEN Ltd.-UK, Crawley, UK). One microgram RNA was used to synthesize cDNA in a 20-μl solution using qScript cDNA SuperMix (Quanta BioSciences, Inc., Gaithersburg, MD). A PCR containing 1 μl cDNA, 2 μl 2.5 μM primer mix, 6.5 μl 2× Perfecta SYBR SuperMix (Quanta BioSciences, Inc., Gaithersburg, MD), and 3.5 μl water was performed in a 7500 Real Time PCR System (Life Technologies Ltd., Paisley, UK). PCR was cycled as 95°C for 3 minutes and 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. The relative quantification of target transcripts was obtained after normalization to Gapdh. Primer sequences of Cdkn1a for real time q-PCR were CCTGGTGATGTCCGACCTG and CCATGAGCGCATCGCAATC.
Statistical Analysis
Wilcoxon rank-sum test was performed for comparisons of treatment efficacy on mouse renal lesions. Analyses were performed using GraphPad Prism 7.01. Two-tailed Student's t test was used for comparison of q-PCR results. P < .05 was considered to be statistically significant.
Results and Discussion
Efficacy of Rapamycin, Atorvastatin, or Combination of Rapamycin and Atorvastatin on Renal Tumors in Tsc1+/− Mice
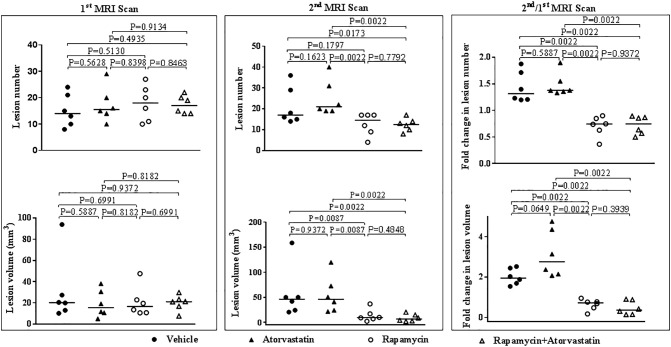
Before starting treatment, Tsc1+/− littermates were randomly allocated into 4 groups of 6, balanced for gender, and subjected to the first MRI scanning at the age of 12 months. Total number and volume of all renal lesions (cystic/papillary/solid) per mouse were determined using Analyze 10.0. As shown in the left panel of Figure 1, total number and volume of all renal lesions varied greatly from mouse to mouse, but no significant difference was detected between any two groups before treatment (Supplementary Table 1). Tsc1+/− mice were then treated with vehicle (i.p.), atorvastatin (100 mg/kg, gavage), rapamycin (5 mg/kg, i.p.), or combination of atorvastatin and rapamycin 5 times a week for 2 months. After treatment, Tsc1+/− mice were subjected to the second MRI scanning to track changes in tumor number and size. First, we directly compared the total number and size of all renal lesions obtained from the second MRI scanning. As reported previously [12], atorvastatin alone did not reduce tumor burden in the kidneys of Tsc1+/− mice (Figures 1 and 2; Supplementary Table 2). We found that combination of rapamycin and atorvastatin but not rapamycin alone reduced tumor number significantly, although both rapamycin alone and rapamycin combined with atorvastatin significantly reduced tumor volume (Figures 1 and 2; Supplementary Table 2). Fold changes in tumor volume over time have been widely used to assess changes in tumor burden in preclinical xenograft models and clinical settings. We compared fold changes of total tumor number and volume (second/first scan). We confirmed that atorvastatin alone did not have any therapeutic efficacy for renal tumors of Tsc1+/− mice and that rapamycin alone or combination of rapamycin and atorvastatin significantly reduced tumor burden in the kidneys of these mice (Figures 1 and 2; Supplementary Table 3). Combination of rapamycin and atorvastatin appeared to have greater therapeutic efficacy for renal tumors, but the difference was not significant (Figure 1; Supplementary Table 3).
Figure 1.
MRI assessment of treatment efficacy on renal tumors of Tsc1+/− mice.
Tsc1+/− mice were subjected to MRI scanning before and after treatment, respectively, in vivo. Total number and volume of all renal lesions (cystic/papillary/solid) per mouse were obtained using Analyze 10.0. Left panel: Comparison of total number and volume of renal tumors between treatment groups before starting treatment at the age of 12 months (the first MRI scan). Middle panel: Comparison of total number and volume of renal tumors between treatment groups after treatment at the age of 14 months (the second MRI scan). Tsc1+/− mice were treated with vehicle, atorvastatin (100 mg/kg), rapamycin (5 mg/kg), or combination of rapamycin (5 mg/kg) and atorvastatin (100 mg/kg) for 2 months before the second MRI scanning. Right panel: Comparison of fold change of total renal tumor number and volume between treatment groups (the second/first scan).
See Supplementary Table 1, Supplementary Table 2, Supplementary Table 3 for statistical details.
See Supplementary Tables 1-3 for statistical details.
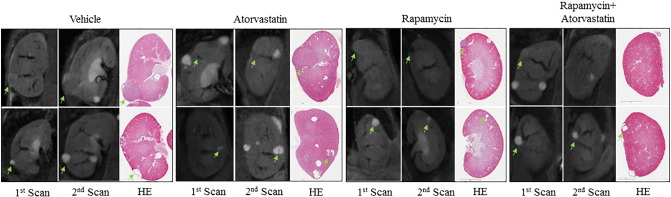
Figure 2.
Changes in renal tumor size of Tsc1+/− mice detected by MRI and histology.
Tsc1+/− mice were scanned using MRI in vivo as in Figure 1 and sacrificed after the second scan for kidney section preparation. Kidney sections were HE-stained. Representative MRI images and HE sections were presented to show size of different tumor types at specified treatment groups.
A major advantage of using MRI instead of traditional histology to assess tumor burden in mice is the use of fewer animals to achieve statistical power. By analyzing changes over time, MRI is particularly useful in genetically engineered mouse models such as Tsc1+/− or Tsc2+/− mice that show great variability in tumor number and size [9], [10].
Effect of Rapamycin, Atorvastatin, or Combination of Rapamycin and Atorvastatin on Cell Proliferation and Oncogenic Signaling in Renal Tumors of Tsc1+/− Mice
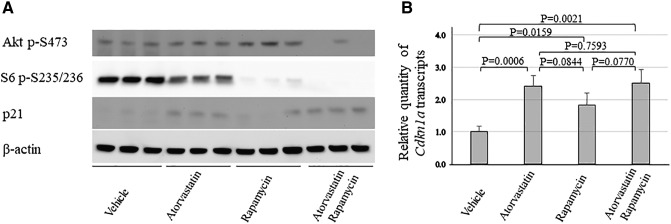
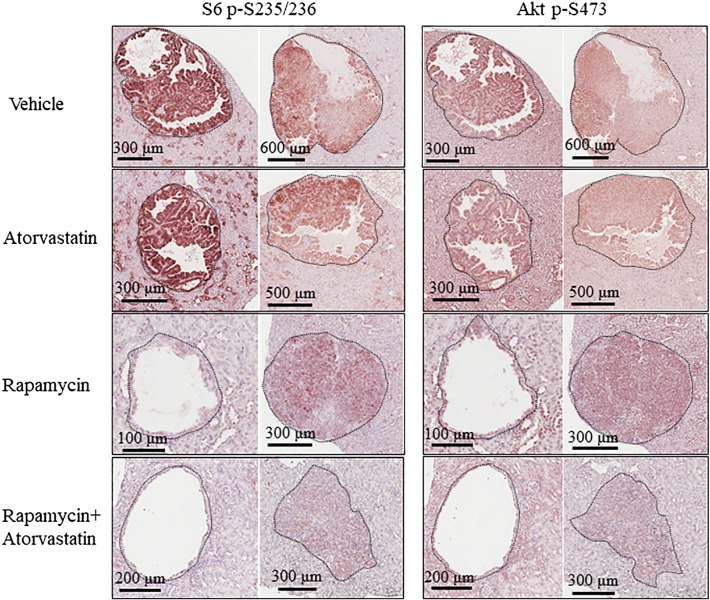
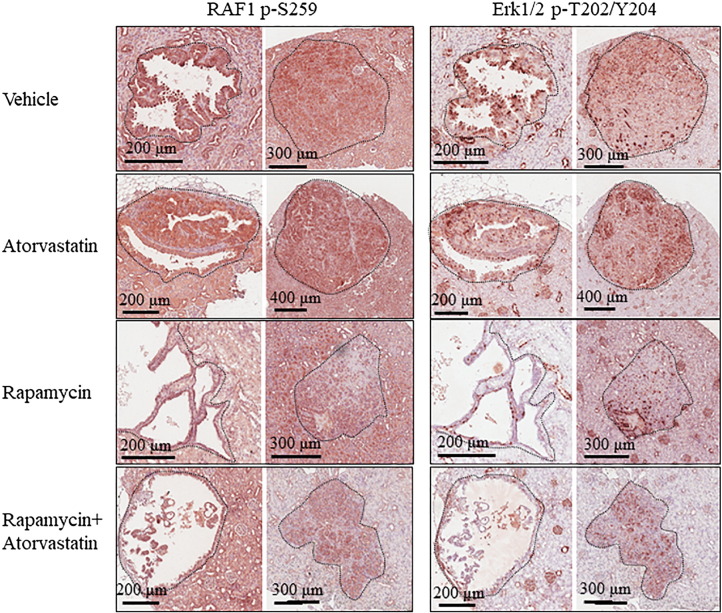
Atorvastatin was reported to selectively suppress growth and proliferation of mouse Tsc2 null embryonic fibroblasts in vitro [11]. In the current study, rapamycin alone or combination of rapamycin and atorvastatin inhibited proliferation of tumor cells as indicated by reduced number of Ki67-positive tumor cells, but atorvastatin alone did not (Figure 3). Atorvastatin was previously shown to significantly inhibit mTOR complex 1 (mTORC1) in normal tissues [11]. We found that atorvastatin slightly inhibited mTORC1 in the liver, whereas rapamycin alone or combination of rapamycin and atorvastatin greatly inhibited mTORC1 as indicated by marked reduction of S6 phosphorylation (Figure 4). In the liver, combination of rapamycin and atorvastatin appeared to reduce phosphorylation of Akt at S473, a marker of mTORC2 activation, but rapamycin or atorvastatin alone did not (Figure 4). As reported previously [12], atorvastatin alone did not inhibit mTORC1 in renal tumors (Figure 5). Rapamycin alone or combination of rapamycin and atorvastatin potently inhibited mTORC1 in renal tumors (Figure 5). In a previous study, atorvastatin was found to effectively delay tumor progression by inhibiting phosphorylation of Akt and Erk proteins in a mouse model of pancreatic cancer [4]. In the current study, we did not observe any effect of atorvastatin on phosphorylation of Akt and Erk1/2 in renal tumors by IHC analysis (Figures 5 and 6). In contrast, rapamycin alone or combination of rapamycin and atorvastatin inhibited phosphorylation of Akt at S473 and Erk1/2 in most solid renal lesions and some renal cysts (Figures 5 and 6) [14], although combination of rapamycin and atorvastatin appeared to have stronger inhibitory effect on phosphorylation at these sites. Previous studies suggest that atorvastatin upregulates expression of p21 [4]. We investigated the expression of p21 by Western and q-PCR analysis to demonstrate the effective delivery of atorvastatin. We found that the levels of p21 protein and Cdkn1a transcripts were significantly increased in the liver treated with atorvastatin (Figure 4). These results suggest that the lack of efficacy of atorvastatin for renal tumors in Tsc mouse models is likely to be associated with insufficient inhibition of mTOR and Erk signaling in the tumors.
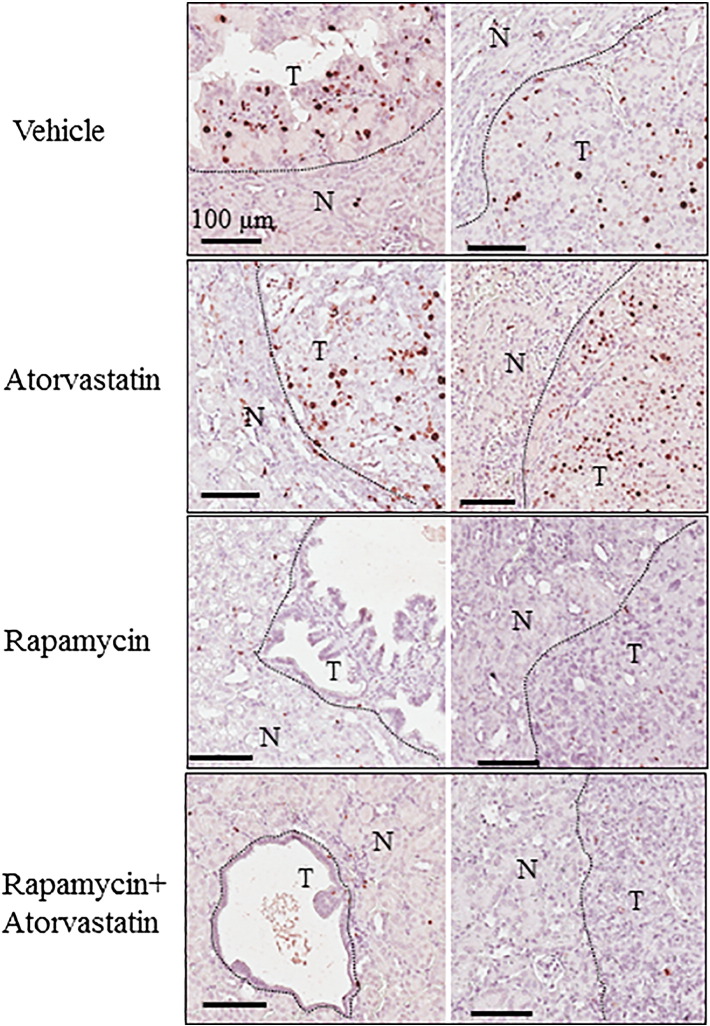
Figure 3.
Treatment effect on proliferation of renal tumor cells in Tsc1+/− mice.
Kidney sections, prepared from 14-month-old Tsc1+/− mice after the second MRI scan, were stained with antibody against Ki67 to assess proliferation of tumor cells. Representative sections were presented to show expression of Ki67 in tumor cells of specified treatment groups.
Figure 4.
Treatment effect on mTOR signaling and expression of p21 in the liver of Tsc1+/− mice.
(A) Western analysis. Proteins were prepared from liver tissues dissected from Tsc1+/− mice treated for 2 months with vehicle, atorvastatin, rapamycin, or atorvastatin/rapamycin combination. β-Actin was used as a loading control. Representative Western blots were presented to show phosphorylation of Akt at S473 and S6 at S235/236 and expression of p21.
(B) q-PCR analysis. Total RNA was isolated from liver tissues dissected from Tsc1+/− mice treated for 2 months with vehicle, atorvastatin, rapamycin, or atorvastatin/rapamycin combination (n = 5 each group). RNA was used to synthesize cDNA, and real time q-PCR was performed to estimate relative quantity of Cdkn1a transcripts.
Figure 5.
Treatment effect on mTOR signaling of renal tumors in Tsc1+/− mice.
Kidney sections, prepared from 14-month-old Tsc1+/− mice after the second MRI scan, were used for IHC analysis. Representative IHC-stained sections were presented to show phosphorylation of S6 at S235/236 and Akt at S473 in renal tumors. Black lines are scale bars.
Figure 6.
Treatment effect on MAPK signaling of renal tumors in Tsc1+/− mice.
Kidney sections, prepared from 14-month-old Tsc1+/− mice after the second MRI scan, were used for IHC analysis. Representative IHC-stained sections were presented to show phosphorylation of RAF1 at S259 and Erk1/2 at T202/Y204 in renal tumors. Black lines are scale bars.
In conclusion, combined therapy of rapamycin and atorvastatin at doses used here is unlikely to provide additional benefit over rapamycin alone in the treatment of TSC-associated renal tumors. Further investigation is needed to test whether higher doses of atorvastatin in combination with rapamycin could bring any additional benefit without causing significant adverse effect for treating renal lesions in these models.
The following are the supplementary data related to this article.
First MRI Assessment of Tumor Burden in Tsc1+/− Mice before Treatment.
(Mann-Whitney Test)
Second MRI Assessment of Tumor Burden in Tsc1+/− Mice after Treatment.
(Mann-Whitney Test)
Fold Change in Lesion Lumber and Volume Obtained by MRI Analysis in Tsc1+/− Mice after Treatment.
(Mann-Whitney Test)
Conflict of Interest Statement
None declared.
Acknowledgments
Acknowledgements
This project was supported by the Wales Gene Park, UK, and the Tuberous Sclerosis Association, UK.
References
- 1.van Leuven SI, Kastelein JJ. Atorvastatin. Expert Opin Pharmacother. 2005;6:1191–1203. doi: 10.1517/14656566.6.7.1191. [DOI] [PubMed] [Google Scholar]
- 2.Demierre MF, Higgins PD, Gruber SB, Hawk E, Lippman SM. Statins and cancer prevention. Nat Rev Cancer. 2005;5:930–942. doi: 10.1038/nrc1751. [DOI] [PubMed] [Google Scholar]
- 3.McKay RR, Lin X, Albiges L, Fay AP, Kaymakcalan MD, Mickey SS, Ghoroghchian PP, Bhatt RS, Kaffenberger SD, Simantov R. Statins and survival outcomes in patients with metastatic renal cell carcinoma. Eur J Cancer. 2016;52:155–162. doi: 10.1016/j.ejca.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Mohammed A, Qian L, Janakiram NB, Lightfoot S, Steele VE, Rao CV. Atorvastatin delays progression of pancreatic lesions to carcinoma by regulating PI3/AKT signaling in p48Cre/+ LSL-KrasG12D/+ mice. Int J Cancer. 2012;131:1951–1962. doi: 10.1002/ijc.27456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shachaf CM, Perez OD, Youssef S, Fan AC, Elchuri S, Goldstein MJ, Shirer AE, Sharpe O, Chen J, Mitchell DJ. Inhibition of HMGcoA reductase by atorvastatin prevents and reverses MYC-induced lymphomagenesis. Blood. 2007;110:2674–2684. doi: 10.1182/blood-2006-09-048033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.European Chromosome 16 Tuberous Sclerosis Consortium Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell. 1993;75:1305–1315. doi: 10.1016/0092-8674(93)90618-z. [DOI] [PubMed] [Google Scholar]
- 7.van Slegtenhorst M, de Hoogt R, Hermans C, Nellist M, Janssen B, Verhoef S, Lindhout D, van den Ouweland A, Halley D, Young J. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science. 1997;277:805–808. doi: 10.1126/science.277.5327.805. [DOI] [PubMed] [Google Scholar]
- 8.Huang J, Manning BD. A complex interplay between Akt, TSC2 and the two mTOR complexes. Biochem Soc Trans. 2009;37:217–222. doi: 10.1042/BST0370217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson C, Idziaszczyk S, Parry L, Guy C, Griffiths DF, Lazda E, Bayne RAL, Smith AJH, Sampson JR, Cheadle JP. A mouse model of tuberous sclerosis 1 showing background specific early post-natal mortality and metastatic renal cell carcinoma. Hum Mol Genet. 2005;14:1839–1850. doi: 10.1093/hmg/ddi190. [DOI] [PubMed] [Google Scholar]
- 10.Onda H, Lueck A, Marks PW, Warren HB, Kwiatkowski DJ. Tsc2(+/) mice develop tumors in multiple sites that express gelsolin and are influenced by genetic background. J Clin Invest. 1999;104:687–695. doi: 10.1172/JCI7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finlay GA, Malhowski AJ, Liu Y, Fanburg BL, Kwiatkowski DJ, Toksoz D. Selective inhibition of growth of tuberous sclerosis complex 2 null cells by atorvastatin is associated with impaired Rheb and Rho GTPase function and reduced mTOR/S6 kinase activity. Cancer Res. 2007;67:9878–9886. doi: 10.1158/0008-5472.CAN-07-1394. [DOI] [PubMed] [Google Scholar]
- 12.Finlay GA, Malhowski AJ, Polizzi K, Malinowska-Kolodziej I, Kwiatkowski DJ. Renal and liver tumors in Tsc2(+/−) mice, a model of tuberous sclerosis complex, do not respond to treatment with atorvastatin, a 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor. Mol Cancer Ther. 2009;8:1799–1807. doi: 10.1158/1535-7163.MCT-09-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang J, Kalogerou M, Gallacher J, Sampson JR, Shen MH. Renal tumours in a Tsc1+/− mouse model show epigenetic suppression of organic cation transportersSlc22a1, Slc22a2 and Slc22a3, and do not respond to metformin. Eur J Cancer. 2013;49:1479–1490. doi: 10.1016/j.ejca.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 14.Yang J, Kalogerou M, Samsel PA, Zhang Y, Griffiths DF, Gallacher J, Sampson JR, Shen MH. Renal tumours in a Tsc2(+/−) mouse model do not show feedback inhibition of Akt and are effectively prevented by rapamycin. Oncogene. 2015;34:922–931. doi: 10.1038/onc.2014.17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
First MRI Assessment of Tumor Burden in Tsc1+/− Mice before Treatment.
(Mann-Whitney Test)
Second MRI Assessment of Tumor Burden in Tsc1+/− Mice after Treatment.
(Mann-Whitney Test)
Fold Change in Lesion Lumber and Volume Obtained by MRI Analysis in Tsc1+/− Mice after Treatment.
(Mann-Whitney Test)