Abstract
Objective
: Signet ring cell carcinoma is a rare subtype of colorectal carcinoma (CRC) with an associated BRAFV600E mutation. We investigated frequencies of BRAF mutation in 28 CRCs containing variable signet ring cell component and their relation with clinicopathologic parameters.
Methods
: According to the presence of signet ring cell component, tumors were categorized into groups as follows: 0%–9%, 10%–24%, 25%–49%, and >50%. Genomic DNA was isolated and analyzed for BRAF V600E gene mutation by polymerase chain reaction-restriction fragment length polymorphism. Eleven of 28 cases (39.3%) showed BRAFV600E mutation, which was also confirmed by Sanger sequencing. To elucidate the importance of existence of signet ring cell component at the molecular level, we separated cases into two groups with cut-off levels of 10% and 50%, which pertain to percentages of signet ring cells.
Results
: Seven of 19 cases (36.8%) under the threshold of 50% and four of nine cases (44.4%) over this threshold value demonstrated BRAF mutation. Three of 7 cases (42.8%) featuring <10% signet ring cell component and eight out of 21 cases (38.1%) showing >10% were BRAF mutated.
Conclusions
: BRAF mutation must be closely associated with the presence of malignant signet ring cells regardless of their percentages.
Keywords: BRAF, mutations, colon cancer, signet ring cell
Introduction
Signet ring cell carcinoma (SRC) is a variant of colorectal carcinoma (CRCs) and defined by the presence of >50% of tumor cells with large mucin vacuole, which abundantly fills the cytoplasm, resulting in compression and eccentric displacement of the nucleus. However, conventional or other specific variants of CRC may also contain variable proportion of signet ring cells with less than 50% of tumor cells. To date, many studies have examined molecular characterizations of SRC-CRC and indicated that most SRC-CRCs share variable molecular alterations, such as high-degree microsatellite instability (MSI-H), frequent CpG island methylator phenotype, and increased methylation level of long interspersed nucleotide element-11-6. Frequent BRAF V600E mutation is also one of the most remarkable molecular abnormalities2,3,5,6. However, to the best of our knowledge, limited information is available on BRAF status in CRCs with less than 50% SRC component, because only several studies assessed the status of BRAF mutation in such tumors2,4,6.
In this study, we investigated frequencies of BRAF gene mutation in 28 cases of sporadic CRCs with various amounts of SRC component and their relationship with clinicopathologic parameters. Percentage values of 50% and 10% for SRC components are comprehensible and sufficient for most practicing pathologists and will provide a good starting point for stratifying cases according to changes at the molecular level for further statistical analysis and interpretation.
Material and methods
Case selection
We retrospectively selected 28 cases of primary and sporadic colorectal adenocarcinomas with variable proportions of SRC among files of the Department of Pathology of Gulhane Military Medical Academy in Ankara. All patients underwent radical surgery and received a close clinical follow-up at 3 months to 144 months after surgery. None of the patients showed history of neoadjuvant chemotherapy or radiotherapy. Tissues were fixed in 10% neutral-buffered formalin, processed routinely, embedded in paraffin, and sections were stained with hematoxylin and eosin (H&E). We reevaluated archival slides of each case to confirm their diagnosis and determine percentages of SRC components, which were scored and categorized into groups as: 0%–9%, 10%–24%, 25%–49%, and >50%.
The design of this study was approved by the local ethical committee of Gulhane Military Medical Academy in Ankara.
Polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) and DNA sequencing
Multiple 4 μm sections were obtained from the most representative blocks of each case. Genomic DNA was manually isolated by using a DNA extraction kit (PureLink Genomic DNA Mini Kit, Invitrogen, Carlsbad, CA, USA) following manufacturer’s protocol.
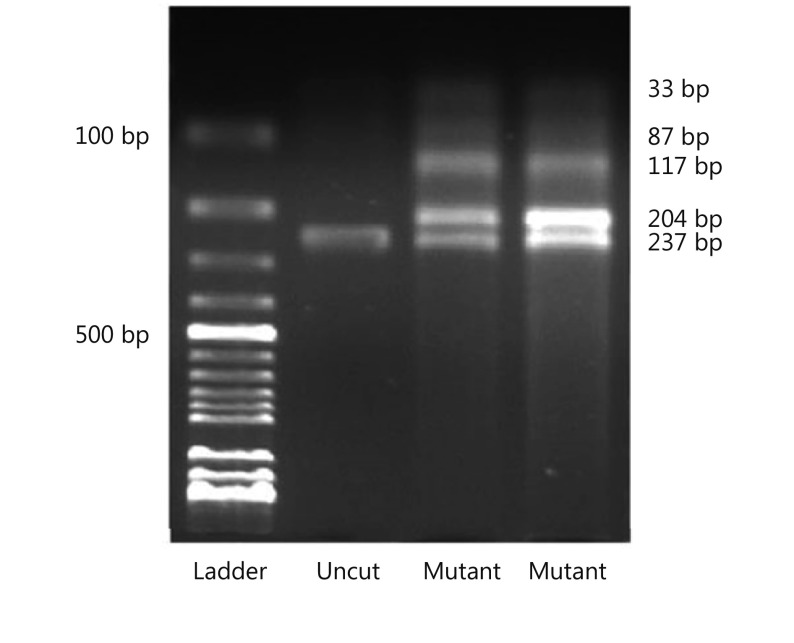
The previously described PCR-RFLP was employed for detection of BRAF V600E. To scan BRAF V600E mutation, a 237 bp fragment of exon 15 of the BRAF gene was amplified using primers: forward 5′-GCTTGCTCTGATAGGAA AATGAG-3′ and reverse 5′-GTAACTCAGCAGCA TCTCAGG-3′. Amplification was carried out in a 50 μL total reaction volume containing 2 mM of dNTPs, 10 pmol each of forward and reverse primers, 2.5 U Taq DNA polymerase, 10× PCR buffer, and 50 ng genomic DNA by using PCR System (CLP Apollo ATC401 Thermalcycler, Apollo Instrumentation, San Diego, CA, USA). PCR cycling conditions were as follows: an initial denaturation step at 95 °C for 5 min, followed by 35 cycles of 94 °C for 20 s, 60 °C for 20 s, 72 °C for 20 s, and final extension step at 72 °C for 5 min. Then, amplified PCR products (237 bp) were digested with restriction endonuclease TspRI (restriction site = NNCASTGNN) (New England Biolabs Beverly, MA, USA) and incubated at 65 °C for 5 h. Undigested and digested PCR products were separated on a 2% agarose gel, stained with ethidium bromide, and visualized using an ultraviolet illuminator (Gel Logic 200 Imaging System, Eastman Kodak Company, Rochester, NY, USA). The 174, 87, or 33 bp fragment alone indicated wild-type BRAF, whereas the 204 and 33 bp bands indicated presence of V600E mutation.
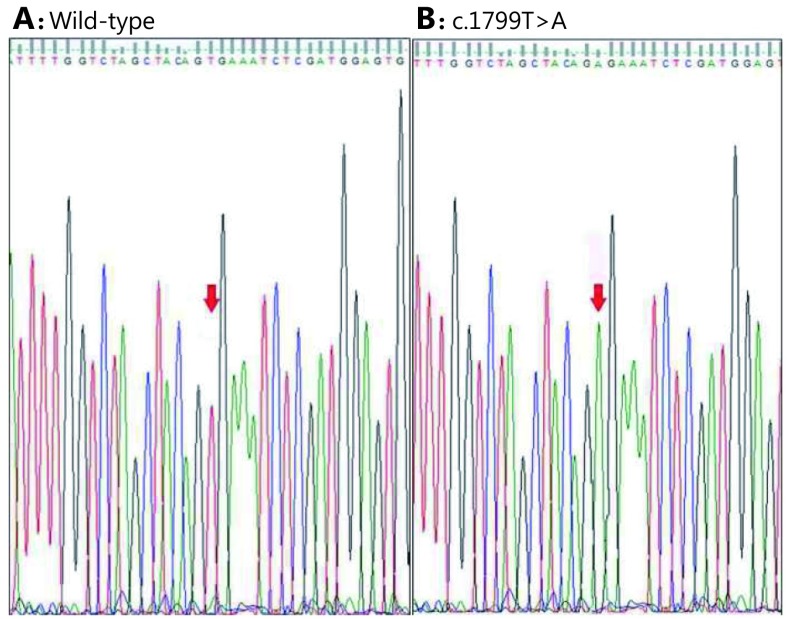
We also performed further DNA direct sequencing on all samples to confirm reliability of PCR-RFLP results. Single-pass sequencing was performed on each template using the forward primer. Fluorescence-labeled fragments were purified from unincorporated terminators by ethanol precipitation. Subsequently, samples were resuspended in distilled water and subjected to electrophoresis in a DNA sequencer (ABI Prism 3730XL DNA Sequencer, Applied Biosystems, Perkin Elmer Corporation, Foster City, USA) in RefGen Ltd.Co. (Ankara University, Technopolis, Ankara, Turkey).
Statistical analysis
All analyses were performed using SPSS for Windows version 17.0 (SPSS, Chicago, IL, USA). Descriptive statistics were presented as mean, minimum-maximum, frequency, and percentage. Chi-square test was used to compare categorical variables. Mann-Whitney U test was used to compare continuous variables. Survival analysis was carried out by Kaplan-Meier method. Cox regression analysis was also performed to determine predictors on overall survival. A P-value of < 0.05 was considered statistically significant.
Results
Mean age of patients totaled 51 years (range: 21–81 years) with a male-to-female ratio of 3.7. Overall, most SRC-CRCs were located in the right colon (46.5%) and rectum (35.7%). According to the T (tumor), N (node), and M (metastasis) staging (TNM) system of colorectal cancer staging, five cases were in stage II (17.9%), 16 in stage III (57.1%), and 7 in stage IV (25.0%). SRC components reached 0%–9% in seven cases (25%), 10%–24% in nine (32.1%), 25%–49% in three (10.8%), and >50% in nine cases (32.1%). In the study group, 19 cases (67.9%) showed <50% SRC component, and the remaining nine cases (32.1%) exhibited SRC component of >50%. SRC component totaled <10% in seven cases (25%), whereas 21 cases (75%) contained >10% of SRCs. In total, regardless of SRC percentages, 11 cases (39.3%) showed BRAF mutation, and the remaining 17 (60.7%) were wild types (Figure 1). Results of DNA sequencing were all consistent with those of PCR-RFLP (Figure 2). Locations of mutant and wild-type tumors were nearly similar to each other. Table 1 summarizes detailed clinicopathologic features and mutation status.
1.
Gel image demonstrates the presence of BRAFV600E mutation in two cases.
2.
Representative electropherograms showing wild-type sequence (A) and mutation (c. 1799T>A) identified in BRAF gene (B). Red arrowheads indicate respective nucleotide changes.
1.
Clinicopathologic features and BRAF status in CRCs with variable signet ring cell component
| BRAF status | BRAF mutant n=11 (%) | BRAF wild–type n=17 (%) | Total n=28 (%) | P | |
| SRC, Signet-ring cell; pTNM, Pathological TNM. | |||||
| Sex | 0.613 | ||||
| Male | 9 (81.8) | 13 (76.5) | 22 (78.6) | ||
| Female | 2 (18.2) | 4 (23.5) | 6 (21.4) | ||
| Age, mean | 48.7±20.9 | 45.6±20.7 | 0.705 | ||
| Location | 0.997 | ||||
| Right colon | 5 (45.4) | 8 (47.1) | 13 (46.5) | ||
| Left colon | 2 (18.2) | 3 (17.6) | 5 (17.8) | ||
| Rectum | 4 (36.4) | 6 (35.3) | 10 (35.7) | ||
| SRC component (%) | 0.957 | ||||
| 0%–9% | 3 (27.3) | 4 (23.5) | 7 (25.0) | ||
| 10%–24% | 3 (27.3) | 6 (35.3) | 9 (32.1) | ||
| 25%–49% | 1 (9.1) | 2 (11.8) | 3 (10.8) | ||
| ≥50% | 4 (36.4) | 5 (29.4) | 9 (32.1) | ||
| pTNM | 0.538 | ||||
| T3 | 2 (18.2) | 5 (29.4) | 7 (25.0) | ||
| T4 | 9 (81.8) | 12 (70.6) | 21 (75.0) | 0.309 | |
| N0 | 2 (18.2) | 4 (23.5) | 6 (21.4) | ||
| N1 | 2 (18.2) | 1 (5.9) | 3 (10.7) | ||
| N2 | 7 (63.6) | 12 (70.6) | 19 (67.9) | 0.581 | |
| M0 | 8 (72.7) | 13 (76.5) | 21 (75.0) | ||
| M1 | 3 (27.3) | 4 (23.5) | 7 (25.0) | ||
| Stage | 0.893 | ||||
| II | 2 (18.2) | 3 (17.6) | 5 (17.9) | ||
| III | 6 (54.5) | 10 (58.9) | 16 (57.1) | ||
| IV | 3 (27.3) | 4 (23.5) | 7 (25.0) | ||
All SRC-CRCs were presented as pT3 (7 cases, 25%) or pT4 (21 cases, 75%) tumors. No SRC-CRC was observed in pT1 or pT2 stage. Although the mutant group featured higher percentage (9 cases, 81.8%) of pT4 tumors than the wild-type group (12 cases, 70.6%), the difference was not statistically significant. A total of 22 out of 28 cases (78.6%) showed metastasis to at least one regional lymph node. Most cases (19 cases, 67.9%) presented four or more lymph node metastases (pN2) in the study. Three (10.7%) of SRC-CRCs exhibited 1 to 3 lymph node metastases (pN1). No lymph node metastasis occurred in 6 cases (21.4%). No statistically significant difference was found between percentages of SRC component and clinicopathologic parameters of BRAF wild-type and mutant cases. As for categorization of cases using 10% and 50% cut-off points for SRC component, we did not observe statistically significant differences involving BRAF mutation status between <10% and >10% groups and <50% and >50% groups. No any relation exists with patients’ age, sex, tumor location, or pTNM. When using the 50% cut-off, 7 of 19 cases (36.8%) under the threshold and 4 of 9 cases (44.4%) over the threshold demonstrated BRAF mutation. However, BRAF mutation was detected in 3 out of 7 cases (42.8%) with <10% SRC and 8 of 21 cases (38.1%) showing >10%.
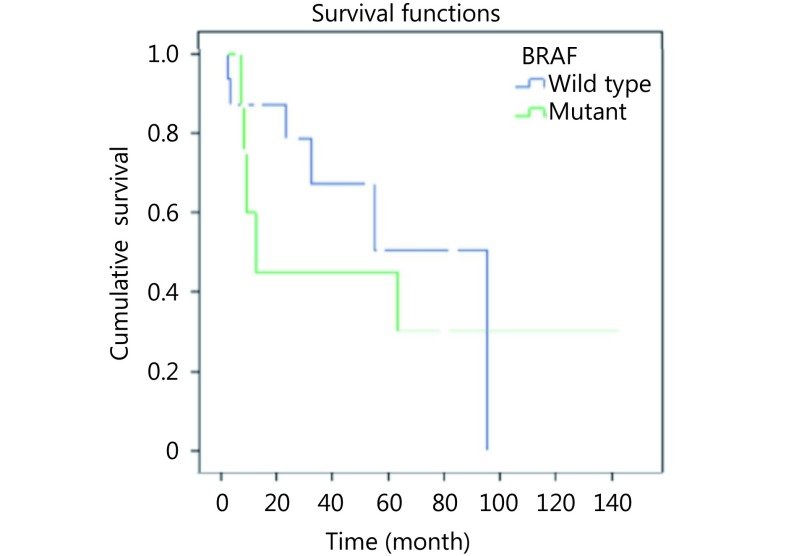
Mean follow-up of disease measured 31.7 months (range: 3–144 months). Eleven cases of SRC-CRCs died because of the disease. At the end of follow-up, 5 among 11 patients died (45%) with BRAF mutant SRC-CRCs, whereas 6 of 17 cases died because of the disease (35%) in wild-type group of BRAF SRC-CRCs. Median survival totaled 12 months in BRAF mutant SRC-CRCs and 95 months in wild-type BRAF SRC-CRCs. Five-year survival reached 45.0% in cases with BRAF mutation and 50.6% in the group with wild-type BRAF (Figure 3). When we adjusted age, gender, percentage of signet ring component, and stage and after Cox regression analysis, we reached no statistically significant increased risk in BRAF mutant group compared with the BRAF wild-type [hazard ratio (HR)=3, 40; 95% confidence interval (CI)=0, 71–16, 29; P=0. 121] (Figure 4).
3.
BRAF mutation status and 5-year survival in signet ring carcinoma.
4.
Histopathology shows CRC with signet cell component (H&E staining, 200×).
Discussion
BRAF is one of the serine/threonine-protein kinases and is a proto-oncogene encoded by the BRAF gene, which is localized to human chromosome region 7p347,8. BRAF is a member of the RAF gene family and participates in the RAS-RAF-MEK-ERK mitogen-activated protein kinase signaling pathway, which mediates cellular responses to serious growth signals9. Activating somatic mutations of BRAF were detected in various tumors, including melanoma, papillary thyroid carcinoma, non-Hodgkin's lymphoma, and adenocarcinoma of the lungs10,11. In 90% of mutated cases, thymine is substituted by adenine at nucleotide 1799. This occurrence leads to substitution of valine (V) by glutamate (E) at codon 600 (V600E) in the activation segment9. BRAF frequency varies; this alteration is shown in nearly all cases of hairy cell leukemia (~100%), cutaneous melanomas (66%), melanocytic nevi (80%), and papillary thyroid carcinoma (30%–70%), and adenocarcinoma of lung (3%)10-14.
BRAF mutation in several subtypes of CRCs has been reported in the range of 5% to 22%10,15-18. BRAF mutation in CRC occurred more commonly in cases with MSI-H but less frequently in patients with germline mutations in one of the mismatch repair genes than in sporadic MSI-H tumors15,18. Mutations of BRAF are associated with MLH1 promoter methylation in sporadic CRC19. Both SRC-CRC and mucinous CRCs are also associated with frequent BRAF mutation compared with nonmucinous CRC2,4,6,20,21.
SRC-CRC is associated with extremely poor prognosis compared with classical ones. BRAFV600E mutation has also been identified as poor prognostic marker in patients with metastatic disease22. To date, many studies investigated molecular characterizations of SRC-CRCs and showed that most SRC-CRCs share various molecular alterations, including MSI-H, high frequency of CpG island methylator phenotype, high methylation level of long interspersed nucleotide element-1, and frequent BRAF mutation1,6. However, to our knowledge, only a few studies assessed the status of BRAF mutation in CRCs with varying SRC components, and they are insufficient for defining such entity as SRC-CRC2,4,6.
Kakar et al.4 reported that BRAF mutations occur more frequently in SRC-CRCs compared with conventional CRCs. They discovered that frequency of BRAF mutation in SRC-CRC reached 33.3% (9 out of 27 cases) but totaled 15.7% (9 out of 57 cases) in CRC. Ogino et al.6 noted that BRAF mutation exhibited a significant adverse effect in a large cohort of more than 600 CRC patients, and CRC with variable SRC component presented more frequent (28%) (9 out of 32 cases) BRAF mutations compared with that without any SRC component (8.6%) (30 out of 348 cases). In this study, frequency of mutation reached 22% in SRC-CRC (tumors with >50% signet ring cell component) and 30% in CRC with less than 50% SRC component6. Inamura et al.2 detected association of high proportion of SRC component with frequent BRAF mutation. The same researchers observed that frequency of BRAF mutation amounted to 35% (6 out of 17 cases) and 33% (38 out of 114 cases) in SRC-CRC and CRC, respectively, with SRC component (less than 50%). Kadowaki et al.23 investigated 811 CRCs and showed that BRAF mutation was associated with shorter survival and independent of MSI status. BRAF mutation frequency totaled 39.3% in CRC containing any SRC component. Our findings agree with results of the abovementioned studies. However, our frequency of BRAF mutation in CRC with more than 50% of SRC component is relatively higher than those in other previously reported studies (44.4%). This discrepancy may be related to geographical and epidemiologic differences. In our study, the 5-year survival reached 45% in cases with BRAF mutation and 50.6% in wild-type group. After adjustment of age, gender, stage, and percentage of SRC component, BRAF mutation was considered not a statistically significant independent predictor. However, hazard ratio was 3.40 times higher in BRAF mutant cases compared with wild-type ones. Thus, BRAF mutation status can be an important survival factor. Lack of statistically significant result can be attributed to the limited number of cases included in our study.
We also investigated significance of extent of SRC component in BRAF mutation. Frequency of BRAF mutation was higher in tumors with >50% SRC component than those with <50% SRC component (44.4% vs. 36.8% for >50% vs. <50%, respectively). However, a significant number of cases (approximately one third of cases) in tumors with <50% SRC component were BRAF mutant. BRAF mutation was also frequent in CRCs with <10% SRC component (42.8% vs. 38.1% for <10% vs. >10%, respectively). These findings strongly indicate that BRAF mutation status is not linked with percentage of SRC, and BRAF mutation not only shows frequently in SRC-CRCs but also in tumors featuring minute population of SRC component. Unfortunately, the number of cases in this group (<10% SRC component) was limited. Thus, the possibility of BRAF mutation in CRC with minor component of SRC should not be considered negligible. Studies on larger series of CRC with focal SRC can further clarify the relation of BRAF mutation status in such tumors. SRC-CRC and CRC with lesser SRC component have been associated with shorter patient survival and are independent of other clinicopathologic and multiple molecular features2,5,20. Most CRCs containing any proportion of SRC were in advanced stage (stage III and IV; 82.1%). When percentages of SRC component and other clinicopathologic parameters (age, sex, location, and outcome) were compared, no statistically significant differences were observed between groups with <50% and >50% SRC and groups with <10% and >10% SRC.
In conclusion, although frequency of BRAF mutation was higher in tumors with >50% SRC component, a significant number of tumor cases (approximately one third of cases) with <50% SRC component were also BRAF mutant. CRCs with a minor (<10%) SRC component presented BRAF mutation rates similar to those of tumors with >10% and >50% SRC. The presence of SRCs in CRCs in minor component (regardless of its percentage) can be an important predictor for BRAF mutation in these tumors. However, the small number of cases poses a serious limitation in our study. Larger studies must be conducted to explore the relation between BRAF mutation and CRCs with <10% and >10% SRC.
Conflict of interest statement
No potential conflicts of interest are disclosed.
References
- 1.Baba Y, Huttenhower C, Nosho K, Tanaka N, Shima K, Hazra A, et al. Epigenomic diversity of colorectal cancer indicated by LINE-1 methylation in a database of 869 tumors. Mol Cancer. 2010;9:125. doi: 10.1186/1476-4598-9-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inamura K, Yamauchi M, Nishihara R, Kim SA, Mima K, Sukawa Y, et al. Prognostic significance and molecular features of signet-ring cell and mucinous components in colorectal carcinoma. Ann Surg Oncol. 2015;22:1226–35. doi: 10.1245/s10434-014-4159-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kakar S, Deng G, Sahai V, Matsuzaki K, Tanaka H, Miura S, et al. Clinicopathologic characteristics, CpG island methylator phenotype, and BRAF mutations in microsatellite-stable colorectal cancers without chromosomal instability. Arch Pathol Lab Med. 2008;132:958–64. doi: 10.5858/2008-132-958-CCCIMP. [DOI] [PubMed] [Google Scholar]
- 4.Kakar S, Deng GR, Smyrk TC, Cun L, Sahai V, Kim YS. Loss of heterozygosity, aberrant methylation, BRAF mutation and KRAS mutation in colorectal signet ring cell carcinoma. Mod Pathol. 2012;25:1040–7. doi: 10.1038/modpathol.2012.44. [DOI] [PubMed] [Google Scholar]
- 5.Kakar S, Smyrk TC. Signet ring cell carcinoma of the colorectum: correlations between microsatellite instability, clinicopathologic features and survival. Mod Pathol. 2005;18:244–9. doi: 10.1038/modpathol.3800298. [DOI] [PubMed] [Google Scholar]
- 6.Ogino S, Brahmandam M, Cantor M, Namgyal C, Kawasaki T, Kirkner G, et al. Distinct molecular features of colorectal carcinoma with signet ring cell component and colorectal carcinoma with mucinous component. Mod Pathol. 2006;19:59–68. doi: 10.1038/modpathol.3800482. [DOI] [PubMed] [Google Scholar]
- 7.Sithanandam G, Druck T, Cannizzaro LA, Leuzzi G, Huebner K, Rapp UR. B-raf and a B-raf pseudogene are located on 7q in man. Oncogene. 1992;7:795–9. [PubMed] [Google Scholar]
- 8.Sithanandam G, Kolch W, Duh FM, Rapp UR. Complete coding sequence of a human B-raf cDNA and detection of B-raf protein kinase with isozyme specific antibodies. Oncogene. 1990;5: 1775 –80. [PubMed] [Google Scholar]
- 9.Tan YH, Liu YQ, Eu KW, Ang PW, Li WQ, Tellez MS, et al. Detection of BRAF V600E mutation by pyrosequencing . Pathology. 2008;40:295–8. doi: 10.1080/00313020801911512. [DOI] [PubMed] [Google Scholar]
- 10.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 11.Garnett MJ, Marais R. Guilty as charged: B-RAF is a human oncogene. Cancer Cell. 2004;6:313–9. doi: 10.1016/j.ccr.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 12.Arcaini L, Zibellini S, Boveri E, Riboni R, Rattotti S, Varettoni M, et al. The BRAF V600E mutation in hairy cell leukemia and other mature B-cell neoplasms . Blood. 2012;119:188–91. doi: 10.1182/blood-2011-08-368209. [DOI] [PubMed] [Google Scholar]
- 13.Puxeddu E, Moretti S, Elisei R, Romei C, Pascucci R, Martinelli M, et al. BRAFV599E mutation is the leading genetic event in adult sporadic papillary thyroid carcinomas . J Clin Endocrinol Metab. 2004;89:2414–20. doi: 10.1210/jc.2003-031425. [DOI] [PubMed] [Google Scholar]
- 14.Paik PK, Arcila ME, Fara M, Sima CS, Miller VA, Kris MG, et al. Clinical characteristics of patients with lung adenocarcinomas harboring BRAF mutations . J Clin Oncol. 2011;29:2046–51. doi: 10.1200/JCO.2010.33.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajagopalan H, Bardelli A, Lengauer C, Kinzler KW, Vogelstein B, Velculescu VE. Tumorigenesis: RAF/RAS oncogenes and mismatch-repair status. Nature. 2002;418:934. doi: 10.1038/418934a. [DOI] [PubMed] [Google Scholar]
- 16.Yuen ST, Davies H, Chan TL, Ho JW, Bignell GR, Cox C, et al. Similarity of the phenotypic patterns associated with BRAF and KRAS mutations in colorectal neoplasia . Cancer Res. 2002;62:6451–5. [PubMed] [Google Scholar]
- 17.Zlobec I, Kovac M, Erzberger P, Molinari F, Bihl M P, Rufle A, et al. Combined analysis of specific KRAS mutation, BRAF and microsatellite instability identifies prognostic subgroups of sporadic and hereditary colorectal cancer . Int J Cancer. 2010;127:2569–75. doi: 10.1002/ijc.25265. [DOI] [PubMed] [Google Scholar]
- 18.Wang L, Cunningham JM, Winters JL, Guenther JC, French AJ, Boardman LA, et al. BRAF mutations in colon cancer are not likely attributable to defective DNA mismatch repair . Cancer Res. 2003;63:5209–12. [PubMed] [Google Scholar]
- 19.Koinuma K, Shitoh K, Miyakura Y, Furukawa T, Yamashita Y, Ota J, et al. Mutations of BRAF are associated with extensive hMLH1 promoter methylation in sporadic colorectal carcinomas . Int J Cancer. 2004;108:237–42. doi: 10.1002/ijc.11523. [DOI] [PubMed] [Google Scholar]
- 20.Kawabata Y, Tomita N, Monden T, Ohue M, Ohnishi T, Sasaki M, et al. Molecular characteristics of poorly differentiated adenocarcinoma and signet-ring-cell carcinoma of colorectum. Int J Cancer. 1999;84:33–8. doi: 10.1002/(sici)1097-0215(19990219)84:1<33::aid-ijc7>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 21.Song GA, Deng G, Bell I, Kakar S, Sleisenger MH, Kim YS. Mucinous carcinomas of the colorectum have distinct molecular genetic characteristics. Int J Oncol. 2005;26:745–50. [PubMed] [Google Scholar]
- 22.Di Nicolantonio F, Martini M, Molinari F, Sartore-Bianchi A, Arena S, Saletti P, et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer . J Clin Oncol. 2008;26:5705–12. doi: 10.1200/JCO.2008.18.0786. [DOI] [PubMed] [Google Scholar]
- 23.Kadowaki S, Kakuta M, Takahashi S, Takahashi A, Arai Y, Nishimura Y, et al. Prognostic value of KRAS and BRAF mutations in curatively resected colorectal cancer . World J Gastroenterol. 2015;21:1275–83. doi: 10.3748/wjg.v21.i4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]