Abstract
Objective:
Meningiomas are neoplasms that arise from the meninges of the central nervous system (CNS). They constitute about 25.6% of CNS tumors diagnosed in Egypt. Some morphological variants of meningiomas display aggressive behavior, leading to brain-invasive growth pattern. Although meningiomas are usually treated by complete surgical excision, the risk of postoperative recurrence remains. Hence, additional biomarkers for predicting aggressive behavior must be discovered. This study aims to explore the clinical and biological relevance of the protein expression levels of β-catenin and galectine-3 in meningioma and to understand the pathobiology of this neoplasm.
Methods:
This retrospective study was carried out on 153 cases of meningioma by using tissue microarrays and immunohistochemistry for β-catenin and galectine-3.
Results:
High β-catenin expression was significantly associated with transitional and meningiotheliomatous meningiomas, low tumor grade, low recurrence rate, and low incidence of brain invasion. Meanwhile, high galectin-3 expression was associated with brain invasion, recurrence, high tumor grade, and tumor type. Logistic regression analysis indicated that among all variables included in the model, β-catenin and galactin-3 expression levels were significant predictors of tumor recurrence (P<0.001).
Conclusions:
Galectin-3 and β-catenin are involved in meningioma recurrencebut not in brain invasion. These molecules could be important potential therapeutic targets and predictors for meningiomas.
Keywords: Immunohistochemistry, meningioma, recurrence, brain invasion, galectin-3 and β-catenin
Introduction
Meningiomas are neoplasms that arise from the meninges of the central nervous system (CNS)1,2. Meningiomas constitute 24%–30% of primary intracranial tumors in the USA3 and 25.6% of CNS tumors in Egypt4. Growing meningiomas cause pressure on but do not invade the surrounding brain structures because these tumors are often bound by the pial–glial basement membrane5. However, some morphological variants of meningiomas display aggressive behavior, leading to brain-invasive growth pattern6,7. These variants are characterized by inward projections of tumor into the adjacent brain tissues without an intervening dura and reactive astrocytosis in the adjoining brain tissues and are considered as grade 2 based on the 2016 WHO classification1,5,6.
Complete surgical excision is the preferred treatment for meningiomas, but the risk of postoperative recurrence remains2. The most important factors affecting the recurrence of meningiomas are tumor grade, invasion of the surrounding brain tissues, and extent of surgical resection6,8.
Scholars have studied various biomarkers with predictive and prognostic values for the recurrence and brain invasiveness of meningiomas. These biomarkers include Ki67, Her-2, matrix metalloproteinases, galectin-3, E-cadherin, and β-catenin6,9,10.
β-catenin, one of the four types of catenins, is attached to the cytoplasmic terminal of E-cadherin to form a complex. The deregulation of β-catenin leads to cellular discohesion and changes in cell morphology10 and commonly occurs in gastric, colon, and hepatocellular tumors11,12.
Galectin-3, a member of the family of β-galactoside lectins13, regulates the cell-to-cell and cell-to-matrix interaction by promoting the adherence of tumor cells to the extracellular matrix and endothelium14,15. Galectin-3 also controls cancer metastasis16, but the underlying mechanism remains unclear17. Galectin-3 is expressed in some CNS tumors, such as astrocytomas and meningiomas18,19, and in papillary thyroid carcinomas20. Galectin-3 regulates the expression and nuclear accumulation of β-catenin and activates the Wnt signaling pathway by regulating the phosphorylation of glycogen synthase kinase-3 beta through the PI3K/AKT pathway in colon cancer cells21.
To the best of our knowledge, no studies have examined the concurrent expression of β-catenin and galectin-3 in meningiomas. The present study aims to evaluate the expression of these biomarkers in meningiomas through immunohistochemistry (IHC) analysis and determine their association with pathological type and grade, brain invasion, and postoperative recurrence.
Materials and methods
This retrospective study was conducted on 153 patients diagnosed with meningioma in the Faculty of Medicine, Mansoura University, Egypt, between January 2010 and December 2015. Operative specimens were collected through total, near-total, or subtotal excision. All studied cases were reported as meningioma in the official pathology reports. This study was approved by Mansoura faculty of medicine, pathology department.
Demographic data (age and sex), clinical data (primary tumor site and tumor recurrence following either complete or incomplete excision and determined by CT or MRI studies), and pathological data (histological type and presence of brain invasion) were obtained from the files of the Pathology Department. Sections stained by H&E were retrieved from the archive of the pathology laboratory and reviewed carefully for proper assessment of brain invasion. The infiltration of meningiomas into Robin–Virchow spaces was not considered brain invasion5. The retrieved slides were reexamined to revise the tumor type and grade according to the 2016 WHO classification1. Paraffin-embedded tissue blocks with the most representative microscopic slide were selected for tissue microarray analysis. The tissue microarray blocks were constructed through mechanical pencil tip method22.
The H&E-stained section of each tissue microarray block was examined to ensure adequacy of the prepared blocks. The other sections were mounted on positively charged slides with 3–4 μm thickness for immunohistochemical studies.
Immunohistochemical staining
The prepared sections were deparaffinized using xylene and rehydrated in descending grades of alcohol to water. Antigens were retrieved using 0.01 M citric acid buffer (pH 6.0) and heated in a microwave oven for 10 minutes. The sections were then incubated in 3% H2O2 for 5 minutes to block endogenous peroxidase and washed with distilled water. The sections were added with mouse monoclonal anti-human antibodies against β-catenin (clone; 12F7, 1:500 dilution) and galectin-3 (clone A3A12, 1:100 dilution) antigens (Abcam, Cambridge, UK). Positive controls were examined with each run of immunohistochemical staining by using an adenocarcinoma of the colon for β-catenin and normal colonic mucosa for galectin-3. The antibodies were incubated at room temperature for 1 hour. Immunostaining was conducted by mouse- and rabbit-specific HRP/DAB (ab64264) detection IHC kit (Abcam, Cambridge, UK) according to the manufacturer’s protocols. DAB was then added to the sections for visualization of the im- munoreaction. Mayer hematoxylin was used to counterstain the slides. In each run of immunostaining, a negative control was examined by omitting the primary antibody.
Immunostaining evaluation
The immunostaining intensity of β-catenin was examined under the highest power of a light microscope and scored as 0 (no expression), 1 (yellowish), 2 (imperial yellow), and 3 (brown). The percentage of the stained cells was scored as 0 (<5%), 1 (5%–10%), 2 (11%–50%), 3 (51%–80%), 4 (>80%). The two scores were then multiplied, obtaining values ranging from 0 to 12, and classified as follows: 0 (–), 1–3 (+), 4–6 (++), and >6 (+++). β-catenin was considered to be expressed if it is located in the cytoplasm, membrane, or perinuclear granules10.
The staining pattern of galectin-3 expression is either cytoplasmic or nuclear and scored according to the percentage of the stained tumor cells: – (<10%), + (10%–25%), ++ (26%–50%), and +++ (>50%)23.
Statistical analysis
Data were analyzed using the SPSS program version 16 (Inc., Chicago, IL, USA). Fisher’s exact and Chi-square probability tests were used to examine the association between the different clinicopathological variables and the protein expression of β-catenin and galectin-3. The association between the different clinicopathological parameters and the protein expression of β-catenin and galectin-3 was also studied using binary logistic regression models with Hosmer–Lemeshow goodness-of-fit. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated using brain invasion or recurrence as dependent variable.
Results
Patients’ characteristics
A total of 153 patients diagnosed with meningioma were included in this study. The patients were aged from 6 to 80 years (mean±SD: 47.58±13.92) and were mostly female (73.9%). About 64% of the tumors were located within the hemisphere, and 26.1% of the tumors were located in the skull base. The most prevalent histological types were transitional and meningiotheliomatous meningiomas. About 12% of the cases were classified as grade 2 meningiomas, and only 2% were categorized as malignant variants. Twenty cases were positive for recurrence, and 22 cases showed brain invasion (Table 1).
1.
Clinicopathological characteristics of 153 meningioma cases
| Variables | n (%) |
| Age, years | |
| Ranging from 6 to 80 years (mean±SD) | 47.58±13.92 |
| <30 | 22 (14.4) |
| >30 | 131 (85.6) |
| Gender | |
| Male | 40 (26.1) |
| Female | 113 (73.9) |
| Location | |
| Hemispheric | 99 (64.7) |
| Skull base | 40 (26.1) |
| Spinal | 14 (9.2) |
| Histologic type | |
| Transitional | 60 (39.2) |
| Meningiotheliomatous | 41 (26.8) |
| Psammomatous | 12 (7.8) |
| Fibroblastic | 11 (7.2) |
| Atypical | 18 (11.8) |
| Microcystic | 3 (2) |
| Metaplastic | 2 (1.3) |
| Angiomatous | 3 (2) |
| Malignant | 3 (2) |
| Histologic grade | |
| Grade 1 | 131 (85.6) |
| Grade 2 | 19 (12.4) |
| Grade 3 | 3 (2) |
| Recurrence | |
| No | 133 (86.9) |
| Yes | 20 (13.1) |
| Brain invasion | |
| No | 131 (85.6) |
| Yes | 22 (14.4) |
Correlation of protein expression with other clinicopathological variables
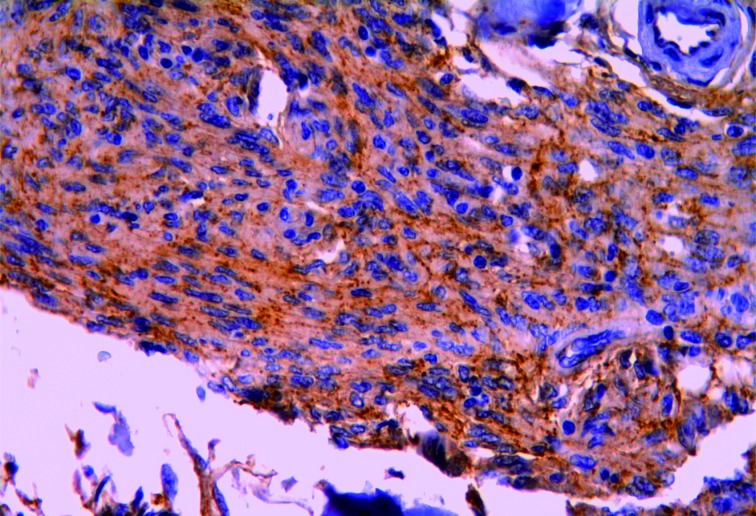
β-catenin was expressed in 115 (75.1%) meningioma cases (Figure 1). High β-catenin expression was significantly associated with transitional and meningiotheliomatous meningiomas, low tumor grade, low recurrence rate, and low incidence of brain invasion (Table 2).
1.
Transitional meningioma showing strong membranous, cytoplasmic, and perinuclear granular expression of β-catenin (IHC staining, 400×).
2.
Relation of β-catenin expression to other clinicopathological variables
| Variables | β-catenin expression, n (%) | χ2 | P | |||
| 0 | + | ++ | +++ | |||
| Age, years | 2.379 | 0.498 | ||||
| <30 | 3 (13.6) | 3 (13.7) | 15 (68.2) | 1 (4.5) | ||
| >30 | 35 (26.7) | 17 (12.9) | 69 (52.6) | 10 (7.6) | ||
| Gender | 0.727 | 0.867 | ||||
| Female | 27 (23.8) | 14 (12.3) | 63 (55.7) | 9 (7.9) | ||
| Male | 11 (27.5) | 6 (15.0) | 21 (52.5) | 2 (5.0) | ||
| Location | 4.971 | 0.548 | ||||
| Hemispheric | 29 (29.2) | 13 (13.1) | 50 (50.5) | 7 (7.0) | ||
| Skull base | 8 (20.0) | 4 (10.0) | 25 (62.5) | 3 (7.5) | ||
| Spinal | 1 (7.2) | 3 (21.4) | 9 (64.3) | 1 (7.1) | ||
| Histologic type | 60.234 | <0.001 | ||||
| Transitional | 5 (8.3) | 8 (13.3) | 42 (70.0) | 5 (8.3) | ||
| Meningotheliomatous | 11 (26.8) | 1 (2.4) | 24 (58.5) | 5 (12.1) | ||
| Psammomatous | 3 (25.0) | 0 | 8 (66.6) | 1 (8.4) | ||
| Fibroblastic | 3 (27.3) | 5 (45.4) | 3 (27.3) | 0 | ||
| Atypical | 11 (61.1) | 5 (27.8) | 2 (11.1) | 0 | ||
| Microcystic | 1 (33.3) | 0 | 2 (66.7) | 0 | ||
| Metaplastic | 1 (50.0) | 0 | 1 (50.0) | 0 | ||
| Angiomatous | 0 | 1 (33.3) | 2 (66.7) | 0 | ||
| Malignant | 3 (100.0) | 0 | 0 | 0 | ||
| Histologic grade | 40.522 | <0.001 | ||||
| Grade 1 | 23 (17.5) | 14 (10.7) | 83 (63.4) | 11 (8.4) | ||
| Grade 2 | 12 (63.1) | 6 (31.5) | 1 (52.6) | 0 | ||
| Grade 3 | 3 (100.0) | 0 | 0 | 0 | ||
| Recurrence | 21.798 | <0.001 | ||||
| No | 27 (20.3) | 14 (10.5) | 81 (60.9) | 11 (8.2) | ||
| Yes | 11 (55.0) | 6 (30.0) | 3 (15.0) | 0 | ||
| Brain invasion | 37.116 | <0.001 | ||||
| No | 23 (17.6) | 14 (10.7) | 83 (63.4) | 11 (8.4) | ||
| Yes | 15 (68.2) | 6 (27.3) | 1 (4.5) | 0 | ||
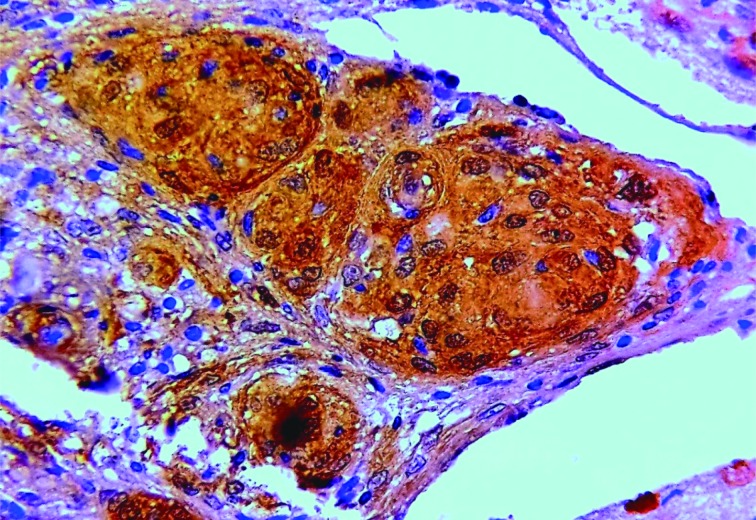
Galectin-3 was expressed in 147 (96.07%) cases (Figure 2). High galectin-3 expression was associated with brain invasion, tumor recurrence, high tumor grade, and atypical and malignant meningiomas (Table 3).
2.
Meningotheliomatous meningioma showing strong cytoplasmic and nuclear expression of galectin-3 (IHC staining, 400×).
3.
Relation of galactin-3 expression to other clinicopathological variables
| Variables | Galectin-3 expression, n (%) | χ2 | P | |||
| 0 | + | ++ | +++ | |||
| Age, years | 1.636 | 0.651 | ||||
| <30 | 0 | 10 (45.5) | 9 (40.9) | 3 (13.6) | ||
| >30 | 6 (4.5) | 63 (48.1) | 41 (31.2) | 21 (16.2) | ||
| Gender | 3.846 | 0.279 | ||||
| Female | 6 (5.3) | 54 (47.7) | 38 (33.6) | 15 (13.2) | ||
| Male | 0 | 19 (47.5) | 12 (3) | 9 (22.5) | ||
| Location | 6.396 | 0.380 | ||||
| Hemispheric | 3 (3.0) | 42 (42.4) | 36 (36.4) | 18 (18.2) | ||
| Skull base | 3 (7.5) | 22 (55.0) | 10 (25.0) | 5 (12.5) | ||
| Spinal | 0 | 9 (64.2) | 4 (28.5) | 1 (7.1) | ||
| Histologic type | 90.118 | <0.001 | ||||
| Transitional | 2 (3.3) | 14 (23.3) | 32 (53.3) | 12 (20.0) | ||
| Meningotheliomatous | 1 (2.5) | 34 (82.9) | 5 (12.2) | 1 (2.4) | ||
| Psammomatous | 2 (66.6) | 1 (33.3) | 0 | 0 | ||
| Fibroblastic | 1 (9.1) | 9 (81.8) | 1 (9.1) | 0 | ||
| Atypical | 0 | 0 | 10 (55.6) | 8 (44.4) | ||
| Microcystic | 0 | 3 (100) | 0 | 0 | ||
| Metaplastic | 0 | 1 (50) | 0 | 1 (50.0) | ||
| Angiomatous | 0 | 2 (66.7) | 0 | 1 (33.3) | ||
| Malignant | 0 | 2 (66.7) | 0 | 1 (33.3) | ||
| Histologic grade | 35.823 | <0.001 | ||||
| Grade 1 | 6 (4.6) | 73 (55.7) | 39 (29.8) | 13 (9.9) | ||
| Grade 2 | 0 | 0 | 9 (47.3) | 10 (52.6) | ||
| Grade 3 | 0 | 0 | 2 (66.6) | 1 (33.4) | ||
| Recurrence | 35.618 | <0.001 | ||||
| No | 6 (4.5) | 73 (54.8) | 41 (30.8) | 13 (9.7) | ||
| Yes | 0 | 0 | 9 (45.0) | 11 (55.0) | ||
| Brain invasion | 39.384 | <0.001 | ||||
| No | 6 (4.6) | 73 (55.7) | 39 (29.8) | 13 (9.9) | ||
| Yes | 0 (0) | 0 (0) | 11 (50.0) | 11 (50.0) | ||
Spearman correlation analysis of galectin-3 and β-catenin showed a negative correlation with mild significance (P = 0.021).
Multivariate logistic regression analysis
The variables included in the models were age, sex, site, tumor type, tumor grade, and expression markers. Of these variables, β-catenin and galectin-3 expression levels were significant predictors of tumor recurrence but not of brain invasion. Tables 4 and 5 show the predictors of brain invasion and recurrence, respectively, determined using multivariate logistic regression models.
4.
Multivariate logistic regression model for brain invasion of meningioma in relation to other parameters
| Variables | P | Odds ratio | 95% CI | |
| Lower | Upper | |||
| Age | 0.880 | 1.113 | 0.2750 | 4.504 |
| Gender | 0.788 | 1.162 | 0.390 | 3.462 |
| Location | 0.083 | 0.432 | 0.167 | 1.115 |
| Histological type | 0.458 | 1.124 | 0.825 | 1.532 |
| Histological grade | 0.586 | 0.615 | 0.106 | 3.549 |
| β-catenin score | 0.930 | 1.025 | 0.595 | 1.765 |
| Galectin-3 score | 0.309 | 0.687 | 0.333 | 1.418 |
5.
Multivariate logistic regression model for meningioma recurrence in relation to other parameters
| Variables | P | Odds ratio | 95% CI | |
| Lower | Upper | |||
| Age | 0.034 | 0.104 | 0.013 | 0.847 |
| Gender | 0.083 | 3.473 | 0.850 | 14.195 |
| Location | 0.248 | 0.367 | 0.067 | 2.013 |
| Histological type | 0.267 | 1.292 | 0.822 | 2.032 |
| Histological grade | 0.336 | 0.351 | 0.042 | 2.961 |
| β-catenin score | 0.003 | 0.183 | 0.059 | 0.568 |
| Galectin-3 score | <0.001 | 13.646 | 3.774 | 49.341 |
Discussion
Although a large majority of meningiomas are classified as benign, their tissue morphologies and treatment outcomes greatly vary24. Patients with invasive meningiomas require postoperative follow-u and adjuvant radiotherapy to increase their survival rate. Hence, assessing brain invasion through microscopic evaluation is important. However, in some instances, this method is difficult to perform on H&E-stained sections. In this regard, additional sections stained by immunohistochemical markers must be examined2,6,9.
Altered β-catenin expression has been identified in several human malignancies, such as gastric, colon, hepatocellular carcinomas, and meningiomas10-12. The occurrence and development of meningiomas are due to intracellular adhesion mediated by E-cadherin and β-catenin25-27.
In this study, the expression level of β-catenin was low in high-grade and aggressive tumors and in cases with brain invasion and tumor recurrence. Similarly, studies that examined cases with similar clinical criteria and pathological grades10,28 showed that the decreased number of cell adhesion molecules was associated with enhanced tumor cell proliferation and might contribute to the invasive ability of meningioma.
Ide et al.29 stated that the percentages of nuclear β-catenin are high in anaplastic meningiomas. The high expression of N-cadherin and the nuclear localization of β-catenin may be involved in the progression of anaplastic meningiomas. However, opposite results were obtained in the present study. The discrepancy in the findings could be due to the type of meningiomas studied. Ide et al.29 tested their biomarkers in canine meningiomas, whereas the present study evaluated human meningiomas. The two studies also employed different clones and techniques.
Zhou et al.10 also tested the expression of adhesion molecules (E-cadherin and β-catenin) in pituitary adenoma. The expression of the two biomarkers was significantly down regulated, consistent with the present results.
Galectin-3 mediates cancer metastasis17, but the underlying mechanism remains unclear18. Galectin-3 is expressed in some CNS tumors, such as astrocytoma and meningioma16,20. Hancq et al.30 reported the high expression of galectin-3 in 64% of benign meningiomas (grade 1) and in 29% of atypical meningioma. However, the staining results were not significantly different between the two groups. Moreover, the expression galectin-3 was similar between recurrence vs. non recurrence group of grade 1 meningiomas31.
Rodriguez et al.32 reported no statistically significant difference between galectin-3 immunoreactivity and WHO grade. Galectin-3 was downregulated during the progression of colon cancer and breast carcinomas; as such, cancer cells can interact with laminin, thereby facilitating cancer invasion and metastases33,34.
In this study, the moderate and strong expression of galectin-3 was prevalent among tumors presenting high grade, recurrence, atypical characteristics, and brain invasion. This finding contradicts those reported by Hancq et al.30 and Rodriguez et al.32.
Miyazaki et al.35 reported the significant expression of galectin-3 in poorly differentiated carcinoma and tumor progression. This finding is similar to the present results but differs from those reported by Okada et al.36; in their study, patients with low immunoreactivity for galectin-3 had a low survival rate.
In this study, an inverse relation was found between the expression of β-catenin and galectin-3. However, Song et al.21 reported that high galectin-3 expression increases the expression of β-catenin. The difference in the results can be attributed to the fact that galectin-3 exhibits other functions, in addition to its role in cell-to-cell and cell-to-matrix interactions as β-catenin prevents tumor spread10,21.
In this study, galectin-3 overexpression was associated with tumor progression. Liu et al.37 explained that galectin-3, similar to Bcl-2, exhibits anti-apoptotic activity, thereby promoting the survival of malignant cells.
Kim et al.18 stated that galectin-3 induces the metastasis of gastric carcinoma by upregulating protease-activated receptor-1(PAR-1) and matrix metalloproteinase-1(MMP-1).
Honjo et al.38 evaluated the cellular localization of galectin-3 and determined its expression in tongue carcinoma and normal tongue mucosa. The expression of nuclear galectin-3 decreased during the transformation to the malignant phenotype, whereas the expression of cytoplasmic galectin-3 increased. Baldus et al.39 reported that nuclear galectin-3 reactivity was higher in diffuse-type cancers than in intestinal-type tumors. Nakahara et al.40 explained the regular movement of galectin-3 between the cytoplasm and nucleus. The change in the localization of this protein suggests its anti-apoptotic action.
The differences in the results of studies regarding the association of galectin-3 with tumor progression and poor prognosis could be attributed to the differences in the number of studied cases, the type of the tested clones, the techniques used, and the cellular localization of galectin-3 (cytoplasmic vs. nuclear).
In this study, galectin-3 and β-catenin were significantly associated with brain invasion but were not independent predictors. Further research should be conducted to confirm this finding considering the few cases with brain invasion employed in the present study or the presence of other molecular factors responsible for brain invasion.
In conclusion, galectin-3 and β-catenin are independent predictors of meningioma recurrence and are potential new targets for treatment of this malignancy. The precise action mechanisms of galectin-3 and β-catenin in meningiomas should be further investigated.
Conflict of interest statement
No potential conflicts of interest are disclosed.
References
- 1.Louis DN, Perry A, Reifenberger G, Von Deimling A, Figarella-Branger D, Cavenee W K, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–20. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 2.Yamasaki F, Yoshioka H, Hama S, Sugiyama F, Arita K, Kurisu K, et al. Recurrence of meningiomas. Cancer. 2000;89:1102–10. doi: 10.1002/1097-0142(20000901)89:5<1102::aid-cncr20>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 3.Panjvani SI, Gandhi MB, Sarvaiya AN, Chaudhari B, Gupta GS. An extracranial invasive meningioma mimicking malignant bone tumor-" Carpet Meningioma”. J Clin Diagn Res. 2013;7:1159–62. doi: 10.7860/JCDR/2013/5394.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zalata KR, El-Tantawy DA, Abdel-Aziz A, Ibraheim AWM, Halaka AH, Gawish HH, et al. Frequency of central nervous system tumors in delta region, Egypt. Indian J Pathol Microbiol. 2011;54:299–306. doi: 10.4103/0377-4929.81607. [DOI] [PubMed] [Google Scholar]
- 5.Backer-Grøndahl T, Moen BH, Arnli MB, Torseth K, Torp SH. Immunohistochemical characterization of brain-invasive meningiomas. Int J Clin Exp Pathol. 2014;7:7206–19. [PMC free article] [PubMed] [Google Scholar]
- 6.Vranic A, Popovic M, Cör A, Prestor B, Pizem J. Mitotic count, brain invasion, and location are independent predictors of recurrence-free survival in primary atypical and malignant meningiomas: a study of 86 patients. Neurosurgery. 2010;67:1124–32. doi: 10.1227/NEU.0b013e3181eb95b7. [DOI] [PubMed] [Google Scholar]
- 7.Saraf S, McCarthy BJ, Villano JL. Update on meningiomas. Oncologist. 2011;16:1604–13. doi: 10.1634/theoncologist.2011-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klinger DR, Flores BC, Lewis JJ, Hatanpaa K, Choe K, Mickey B, et al. Atypical meningiomas: recurrence, reoperation, and radiotherapy. World Neurosurg. 2015;84:839–45. doi: 10.1016/j.wneu.2015.04.033. [DOI] [PubMed] [Google Scholar]
- 9.Das A, Tan WL, Smith DR. Expression of extracellular matrix markers in benign meningiomas. Neuropathology. 2003;23:275–81. doi: 10.1046/j.1440-1789.2003.00512.x. [DOI] [PubMed] [Google Scholar]
- 10.Zhou KY, Wang GT, Wang YR, Jin HH, Yang SX, Liu CB. The potential involvement of E-cadherin and β-catenins in meningioma. PLoS One. 2010;5:e11231. doi: 10.1371/journal.pone.0011231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maruyama K, Ochiai A, Akimoto S, Nakamura S, Baba S, Moriya Y, et al. Cytoplasmic beta-catenin accumulation as a predictor of hematogenous metastasis in human colorectal cancer. Oncol. 2000;59:302–9. doi: 10.1159/000012187. [DOI] [PubMed] [Google Scholar]
- 12.Van Aken E, De Wever O, da Rocha CA, Mareel M. Defective E-cadherin?catenin complexes in human cancer. Virchows Arch. 2001;439:725–51. doi: 10.1007/s004280100516. [DOI] [PubMed] [Google Scholar]
- 13.Konstantinov KN, Robbins BA, Liu FT. Galectin-3, a beta-galactoside-binding animal lectin, is a marker of anaplastic large-cell lymphoma. Am J Pathol. 1996;148:25–30. [PMC free article] [PubMed] [Google Scholar]
- 14.Bartolazzi A, Orlandi F, Saggiorato E, Volante M, Arecco F, Rossetto R, et al. Galectin-3-expression analysis in the surgical selection of follicular thyroid nodules with indeterminate fine-needle aspiration cytology: a prospective multicentre study. Lancet Oncol. 2008;9:543–9. doi: 10.1016/S1470-2045(08)70132-3. [DOI] [PubMed] [Google Scholar]
- 15.Takenaka Y, Fukumori T, Raz A. Galectin-3 and metastasis. Glycoconj J. 2002;19:543–9. doi: 10.1023/B:GLYC.0000014084.01324.15. [DOI] [PubMed] [Google Scholar]
- 16.Matarrese P, Fusco O, Tinari N, Natoli C, Liu FT, Semeraro ML, et al. Galectin-3 overexpression protects from apoptosis by improving cell adhesion properties. Int J Cancer. 2000;85:545–54. [PubMed] [Google Scholar]
- 17.Xin M, Dong XW, Guo XL. Role of the interaction between galectin-3 and cell adhesion molecules in cancer metastasis. Biomed Pharmacother. 2015;69:179–85. doi: 10.1016/j.biopha.2014.11.024. [DOI] [PubMed] [Google Scholar]
- 18.Kim SJ, Shin JY, Lee KD, Bae YK, Choi IJ, Park SH, et al. Galectin-3 facilitates cell motility in gastric cancer by up-regulating protease-activated receptor-1 (PAR-1) and matrix metalloproteinase-1 (MMP-1) PLoS One. 2011;6:e25103. doi: 10.1371/journal.pone.0025103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cay T. Immunhistochemical expression of galectin-3 in cancer: a review of the literature. Turk Patoloji Derg. 2012;28:1–10. doi: 10.5146/tjpath.2012.01090. [DOI] [PubMed] [Google Scholar]
- 20.Sawangareetrakul P, Srisomsap C, Chokchaichamnankit D, Svasti J. Galectin-3 expression in human papillary thyroid carcinoma. Cancer Genom Proteom. 2008;5:117–22. [PubMed] [Google Scholar]
- 21.Song SM, Mazurek N, Liu CM, Sun YJ, Ding QQ, Liu KF, et al. Galectin-3 mediates nuclear β-catenin accumulation and Wnt Signaling in human colon cancer cells by regulation of GSK-3β activity. Cancer Res. 2009;69:1343–9. doi: 10.1158/0008-5472.CAN-08-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shebl AM, Zalata KR, Amin MK. An inexpensive method of small paraffin tissue microarrays using mechanical pencil tips. Diagnost Pathol. 2011;6:117. doi: 10.1186/1746-1596-6-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park YJ, Kwak SH, Kim DC, Kim H, Choe G, Park DJ, et al. Diagnostic value of galectin-3, HBME-1, Cytokeratin 19, high molecular weight cytokeratin, Cyclin D1 and p27kip1 in the differential diagnosis of thyroid nodules . J Korean Med Sci. 2007;22:621–8. doi: 10.3346/jkms.2007.22.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdelzaher E, El Deeb NMF, Gowil AG, Yehya A. Biological and demographic profile of meningiomas in a cohort of egyptian patients: impact on tumor recurrence. ScientificWorldJournal. 2013;2013:375139. doi: 10.1155/2013/375139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akat K, Bleck CK, Lee YM, Haselmann-Weiss U, Kartenbeck J. Characterization of a novel type of adherens junction in meningiomas and the derived cell line HBL-52. Cell Tissue Res. 2008;331:401–12. doi: 10.1007/s00441-007-0512-5. [DOI] [PubMed] [Google Scholar]
- 26.Jagannathan J, Oskouian RJ, Yeoh HK, Saulle D, Dumont AS. Molecular biology of unreresectable meningiomas: implications for new treatments and review of the literature. Skull Base. 2008;18:173–87. doi: 10.1055/s-2007-1003925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimada S, Ishizawa K, Hirose T. Expression of E-cadherin and catenins in meningioma: ubiquitous expression and its irrelevance to malignancy. Pathol Int. 2005;55:1–7. doi: 10.1111/j.1440-1827.2005.01786.x. [DOI] [PubMed] [Google Scholar]
- 28.Utsuki S, Oka H, Sato Y, Kawano N, Tsuchiya B, Kobayashi I, et al. Invasive meningioma is associated with a low expression of E-cadherin and beta-catenin. Clin Neuropathol. 2005;24:8–12. [PubMed] [Google Scholar]
- 29.Ide T, Uchida K, Suzuki K, Kagawa Y, Nakayama H. Expression of cell adhesion molecules and doublecortin in canine anaplastic meningiomas. Vet Pathol. 2011;48:292–301. doi: 10.1177/0300985810389312. [DOI] [PubMed] [Google Scholar]
- 30.Hancq S, Salmon I, Brotchi J, Gabius HJ, Heizmann CW, Kiss R, et al. Detection of S100B, S100A6 and galectin-3 ligands in meningiomas as markers of aggressiveness. Int J Oncol. 2004;25:1233–40. [PubMed] [Google Scholar]
- 31.Hancq S, Salmon I, Brotchi J, De Witte O, Gabius HJ, Heizmann CW, et al. S100 A5: a marker of recurrence in WHO grade I meningiomas. Neuropathol Appl Neurobiol. 2004;30:178–87. doi: 10.1046/j.0305-1846.2003.00525.x. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez FJ, Scheithauer BW, Roncaroli F, Silva AI, Kovacs K, Brat DJ, et al. Galectin-3 expression is ubiquitous in tumors of the sellar region, nervous system and mimics: An immunohistochemical and RT-PCR study. Am J Surg Pathol. 2008;32:1344–52. doi: 10.1097/PAS.0b013e3181694f41. [DOI] [PubMed] [Google Scholar]
- 33.Lotz MM, Andrews CW Jr, Korzelius CA, Lee EC, Steele GD Jr, Clarke A, et al. Decreased expression of Mac-2 (carbohydrate binding protein 35) and loss of its nuclear localization are associated with the neoplastic progression of colon carcinoma. Proc Natl Acad Sci USA. 1993;90:3466–70. doi: 10.1073/pnas.90.8.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castronovo V, Van Den Brûle FA, Jackers P, Clausse N, Liu FT, Gillet C, et al. Decreased expression of galectin-3 is associated with progression of human breast cancer. J Pathol. 1996;179:43–8. doi: 10.1002/(SICI)1096-9896(199605)179:1<43::AID-PATH541>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 35.Miyazaki J, Hokari R, Kato S, Tsuzuki Y, Kawaguchi A, Nagao S, et al. Increased expression of galectin-3 in primary gastric cancer and the metastatic lymph nodes. Oncol Rep. 2002;9:1307–12. [PubMed] [Google Scholar]
- 36.Okada K, Shimura T, Suehiro T, Mochiki E, Kuwano H. Reduced galectin-3 expression is an indicator of unfavorable prognosis in gastric cancer. Anticancer Res. 2006;26:1369–76. [PubMed] [Google Scholar]
- 37.Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nat Rev Cancer. 2005;5:29–41. doi: 10.1038/nrc1527. [DOI] [PubMed] [Google Scholar]
- 38.Honjo Y, Inohara H, Akahani S, Yoshii T, Takenaka Y, Yoshida J, et al. Expression of cytoplasmic galectin-3 as a prognostic marker in tongue carcinoma. Clin Cancer Res. 2000;6:4635–40. [PubMed] [Google Scholar]
- 39.Baldus SE, Zirbes TK, Weingarten M, Fromm S, Glossmann J, Hanisch FG, et al. Increased galectin-3 expression in gastric cancer: correlations with histopathological subtypes, galactosylated antigens and tumor cell proliferation. Tumour Biol. 2000;21:258–66. doi: 10.1159/000030131. [DOI] [PubMed] [Google Scholar]
- 40.Nakahara S, Raz A. Regulation of cancer-related gene expression by galectin-3 and the molecular mechanism of its nuclear import pathway. Cancer Metastasis Rev. 2007;26:605–10. doi: 10.1007/s10555-007-9095-6. [DOI] [PMC free article] [PubMed] [Google Scholar]