Abstract
Background
Long-term survivors of childhood acute lymphoblastic leukemia (ALL) are at risk for neurocognitive impairment, which may be associated with fatigue, sleep problems, systemic inflammation and oxidative stress. We examined these associations among survivors of childhood ALL treated with chemotherapy only.
Methods
Survivors of ALL (N=70, 50% males, mean[SD] age 14.3[4.7] years; 7.4[1.9] years post-diagnosis) completed neurocognitive testing, behavioral ratings, and reported sleep quality and fatigue symptoms at ≥5 years post-diagnosis. Serum was collected concurrently and assayed for interleukin 1-Beta and 6 (IL-1β, IL-6), tumor necrosis factor alpha (TNF-α), high-sensitivity C-reactive protein (hsCRP), malondialdehyde, myeloperoxidase and oxidized low-density lipoprotein. General linear modeling was used to assess associations among biomarkers and functional outcomes, adjusting for age and stratified by sex.
Results
Survivors performed worse than population norms on executive function and processing speed, and reported more behavioral problems (P’s<0.05 adjusted for multiple comparison). In female survivors, fatigue was associated with poor executive function (r=0.41; P=0.02), processing speed (r=0.56; P<0.001) and attention (r=0.36 to 0.55; P’s<0.05). Female survivors with frequent nighttime awakening displayed more inattention (P=0.01), hyperactivity (P=0.03), and aggression (P=0.01). Worse executive function, processing speed and behavioral symptoms were observed in female survivors with higher IL-6, IL-1β and hsCRP (P’s<0.05). Male survivors with high TNF-α demonstrated worse organization (P=0.03), but no significant associations between neurocognitive outcomes and sleep/fatigue measures were observed.
Conclusion
Neurocognitive function in female survivors appears more susceptible to the effects of sleep disturbance and fatigue. Systemic inflammation may play a role in neurocognitive impairment and behavioral symptoms.
Keywords: Childhood acute lymphoblastic leukemia, fatigue, sleep, neurocognitive, behavioral, inflammation, oxidative stress, survivorship
Introduction
Advances in treatment and supportive care over the last several decades have increased survival rates for childhood acute lymphoblastic leukemia (ALL). Although the 5-year survival rate currently approaches 95%1, survivors who do not receive cranial radiation remain at risk for a myriad of late effects, including neurocognitive impairment in the areas of executive function, processing speed and attention2.
Long-term survivors also report significant fatigue and sleep disturbances, including prolonged sleep onset latency, abnormal sleep duration, nighttime and premature awakenings, and daytime sleepiness3. Fatigue and poor sleep quality have an adverse impact on survivors’ neurocognitive and behavioral functions3. Specifically, fatigue is associated with impairments in processing speed, attention and memory in adult survivors of childhood cancer3. However, little is known about potential biological mechanisms underlying these associations.
There is mounting evidence that systemic inflammation is associated with poor sleep quality and fatigue4. Sleep duration has been linked to serum concentrations of high-sensitivity C-reactive protein (hsCRP), tumor necrosis factor - alpha (TNF-α) and interleukin-6 (IL-6)5. Systemic inflammation and oxidative stress have also been associated with neurocognitive problems in the general population6. Activation of inflammatory pathways has emerged as a proposed pathophysiology behind neurocognitive impairment in survivors of adult cancer treated with chemotherapy only7,8. To date, the majority of research on this subject has been conducted among survivors of early-stage breast cancer7–9. There is limited research that evaluates whether inflammation and oxidative stress contribute to neurocognitive problems in long-term survivors of childhood ALL.
The primary objective of this study was to evaluate the impact of sleep and fatigue on neurocognitive function and behavioral symptoms in survivors of childhood ALL. The secondary objective was to evaluate associations of biomarkers of inflammation and oxidative stress with neurocognitive outcomes, as well as sleep and fatigue measures.
Methods
Participants
From 2000 to 2010, 408 children with ALL were treated at St. Jude Children’s Research Hospital (SJCRH) on the Total Therapy XV protocol (ClinicalTrials.gov, NCT00137111)1. Potentially eligible participants were active survivors who were receiving pediatric follow-up care at SJCRH, and at least five years from diagnosis and over eight years of age. 35 patients died prior to recruitment, and 71 survivors were excluded for the following reasons: previous treatment with cranial radiation for central nervous system (CNS) relapse or bone marrow transplantation or additional chemotherapy for a secondary cancer (n=30); pre-existing non-cancer related neurodevelopmental or genetic disorder associated with cognitive impairment or brain injury unrelated to cancer (n=22); lack of proficiency in English (n=1); or not eligible for follow-up (e.g., discharged from active pediatric follow-up care [n=13], under foster care [n=4] or in prison [n=1]). Of the 302 eligible survivors, 218 (72%) participated in a long-term follow-up study beginning in January 1, 20102. Sleep measures and serum collection for inflammation and oxidative stress were introduced into the research protocol on March 15, 2012, at which time 85 survivors were still eligible for recruitment; 70 of these survivors (83%) completed the sleep measures and contributed serum. This study was approved by the institutional review board at SJCRH; informed consent was obtained from the parent or participant, and assent from the participant, as appropriate.
Neurocognitive and behavioral outcomes
All participants completed neurocognitive testing with certified examiners under the supervision of a board-certified clinical neuropsychologist. Measures of executive function, processing speed, intelligence, attention and memory span were examined. Parents of survivors rated their children’s neurobehavioral functioning. Behavioral problems were self-reported by survivors. Neurocognitive and behavioral assessment tools are summarized in Supplementary Material 1.
Fatigue and sleep measures
Measures of sleep quality were completed by parents of survivors aged 8 to 12 years, and self-reported by adolescent survivors (age 13 to 21 years old). Survivors also self-reported their symptoms of fatigue. Sleep quality and fatigue assessment tools are summarized in Supplementary Material 1.
Biomarkers
A total of 5 ml of blood was drawn from all participants on the day of testing and stored until assay according to standard procedures. Serum was assayed in duplicate at the CLIA-approved Cytokine Reference lab at the University of Minnesota using commercially available standardized immunoassay kits. The following biomarkers of inflammation were assayed: interleukin (IL)-1-beta (IL-1β), IL-6 and tumor necrosis factor-alpha (TNF-α) and high-sensitivity C-reactive protein (hsCRP). Biomarkers of oxidative stress included malondialdehyde (MDA), myeloperoxidase (MPO) and oxidized low-density lipoprotein (OxLDL). Details of biomarkers assay are summarized in Supplementary Material 2.
Statistical analysis
Given evidence to suggest sex-specific associations of oxidative stress and inflammation with vascular-related diseases in the general population10, as well as sex differences in reports of fatigue and sleep in cancer patients3, all analyses were stratified by sex. For descriptive purposes, neurocognitive, behavioral, and fatigue outcomes were transformed into age-adjusted Z-scores (mean=0, standard deviation=1.0) using national normative data. Sleep duration was kept on a continuous scale, while all other indices of sleep were treated as categorical variables. Mann-Whitney tests were conducted to identify measures that were statistically different between male and female survivors, adjusted for false discovery rate (FDR). Only those neurocognitive and behavioral measures on which group performance differed from normative samples or between sexes at P<0.05, adjusted for FDR, were included in subsequent analyses.
Associations among fatigue, sleep quality and neurocognitive and behavioral outcomes were evaluated using general linear modeling (GLM). As biomarkers often display a threshold effect, biomarker levels were rank-ordered into tertiles based on sample distribution of the cohort, stratified by sex. Neurocognitive, behavioral and fatigue scores were compared between survivors falling in the top tertile vs other tertiles using GLM. All statistical models were adjusted for current age and each outcome measure was analyzed separately using the raw score of the specific measure to avoid multi-collinearity. Chi-square was used to examine the proportion of survivors with versus without delayed sleep onset, frequent nighttime and premature awakening by tertile grouping.
Results
Survivors demographic and treatment characteristics are presented in Table 1. Neurocognitive performance fell below the population mean on measures of executive function, perceptual intelligence, attention, memory and processing speed (Supplementary Material 3). More parent-reported neurobehavioral symptoms were endorsed compared to population norms. Survivors self-reported more behavioral problems, as well as greater fatigue compared to the general population (Supplementary Material 3). Approximately two-third of survivors reported delayed sleep onset while 30–40% had frequent nighttime and premature awakenings.
Table 1.
Survivor demographics and treatment characteristics
| Male survivors (N=35) | Female survivors (N=35) | ||||||
|---|---|---|---|---|---|---|---|
| N (%) | Mean (SD) | Range | N (%) | Mean (SD) | Range | ||
| Demographics | |||||||
| Race | White | 30 (86) | 26 (74) | ||||
| Black | 5 (14) | 7 (20) | |||||
| Others | 0 (0) | 2 (6) | |||||
| Ethnicity | Hispanics | 2 (6) | 4 (11) | ||||
| Non-Hispanics | 33 (94) | 31 (89) | |||||
| Current age | Years | 14.8 (5.1) | 8.2–25.5 | 13.9 (4.3) | 8.1–25.4 | ||
| Survivor education | Years | 7.6 (3.9) | 2.0–14.0 | 7.4 (3.6) | 2.0–14.0 | ||
| Maternal education | Years | 13.5 (2.4) | 9.0–20.0 | 13.6 (2.3) | 9.0–18.0 | ||
| Paternal education | Years | 13.7 (3.3) | 8.0–22.0 | 13.6 (2.3) | 10.0–19.0 | ||
| Clinical characteristics | |||||||
| Body mass index | Kg/m2 | 23.1 (8.3) | 15.4–46.4 | 22.2 (5.0) | 14.1–36.7 | ||
| Age at diagnosis | Years | 7.0 (4.8) | 1.2–16.5 | 6.8 (4.5) | 1.9–17.7 | ||
| Time since diagnosis | Years | 7.8 (2.0) | 5.1–12.5 | 7.1 (1.7) | 5.1–11.6 | ||
| Cumulative chemotherapy doses | |||||||
| Oral dexamethasone (mg/m2) | 1148.0 (326.2) | 412.3–1690.1 | 1061.0 (204.1) | 444.2–1534.8 | |||
| IV asparaginase (1000 units/m2) | Erwinia-asparaginase | 402.0 (284.6) | 178.9–871.9 | 375.2 (227.8) | 146.0–741.2 | ||
| L-asparaginase | 314.7 (198.5) | 44.4–593.9 | 215.5 (147.0) | 85.9–584.5 | |||
| Peg-asparaginase | 17.1 (15.5) | 2.5–44.7 | 7.8 (3.8) | 2.5–12.9 | |||
| IV cytarabine (g/m2) | Standard-dose | 2.6 (1.8) | 0.6–4.9 | 1.4 (1.6) | 0.3–4.8 | ||
| High-dose | 8.6 (2.7) | 7.7–19.6 | 7.4 (1.6) | 3.9–8.1 | |||
| IV cyclophosphamide (g/m2) | 2.9 (1.8) | 0.9–6.2 | 1.9 (1.6) | 1.0–4.9 | |||
| IV daunorubicin (mg/m2) | 48.8 (9.1) | 24.6–71.0 | 49.0 (10.5) | 25.0–91.9 | |||
| IV doxorubicin (mg/m2) | 127.0 (58.6) | 58.4–191.5 | 90.4 (51.2) | 58.9–209.0 | |||
| IV leucovorin (mg/m2) | 379.1 (269.6) | 75.0–1645.0 | 337.8 (155.0) | 200.0–655.0 | |||
| Methotrexate | IV standard-dose (g/m2) | 5.2 (4.4) | 2.7–29.4 | 3.3 (0.8) | 0.2–5.1 | ||
| IV high-dose (g/m2) Low risk arm |
15 (43) | 11.5 (3.2) | 2.5–17.1 | 26 (74) | 11.6 (1.8) | 0.9–16.4 | |
| IV high-dose (g/m2) Standard risk arm |
20 (57) | 19.5 (4.4) | 7.4–26.3 | 9 (26) | 20.8 (4.8) | 15.6–29.3 | |
| IT (ml) | 180.1 (52.5) | 60.0–288.0 | 148.7 (34.1) | 93.0–276.0 | |||
| IV vincristine (mg/m2) | 58.1 (13.3) | 28.7–74.6 | 59.1 (11.4) | 31.3–73.3 | |||
| IT chemotherapy | Number of counts | 15.6 (4.3) | 11.0–24.0 | 13.1 (3.1) | 9.0–23.0 | ||
Note: IT=intrathecal; IV=intravenous; SD=standard deviation. With the exception of IT chemotherapy, all drugs are presented in cumulative doses. High dose (HD) IV methotrexate was calculated separately from standard dose within each treatment risk stratum. HD IV methotrexate was defined as daily dose of more than 1g/m2 of IV methotrexate
Fatigue and neurocognitive/behavioral outcomes
In females, higher levels of general fatigue were associated with poorer performance on cognitive flexibility, processing speed and multiple measures of attention (P’s<0.05; Table 2). Higher cognitive fatigue was associated with more parent-reported neurobehavioral symptoms in females, as well as more self-reported inattention, hyperactivity, learning problems and aggression (P’s<0.05). In males, higher fatigue was associated with more self-reported inattention problems (P’s<0.05) (Table 2).
Table 2.
Association of Fatigue with Neurocognitive and Behavioral Outcomes
| Cognitive fatigue^ | Sleep-rest fatigue^ | General fatigue^ | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Males | Females | Males | Females | Males | Females | |||||||
| rp | P | rp | P | rp | P | rp | P | rp | P | rp | P | |
| Neurocognitive Function^ | ||||||||||||
| Executive function | ||||||||||||
| Cognitive flexibility | 0.02 | 0.91 | 0.20 | 0.26 | 0.21 | 0.29 | 0.06 | 0.76 | 0.06 | 0.75 | 0.41 | 0.02 |
| Verbal fluency | 0.14 | 0.47 | 0.11 | 0.54 | 0.12 | 0.54 | −0.11 | 0.54 | −0.14 | 0.48 | 0.20 | 0.27 |
| Working memory | −0.43 | 0.02 | 0.17 | 0.35 | −0.12 | 0.54 | 0.19 | 0.30 | −0.18 | 0.37 | 0.02 | 0.91 |
| Organization | −0.42 | 0.03 | 0.06 | 0.77 | −0.31 | 0.11 | −0.08 | 0.66 | −0.08 | 0.68 | 0.20 | 0.28 |
| Abstract reasoning | 0.005 | 0.98 | −0.02 | 0.90 | 0.17 | 0.40 | −0.26 | 0.15 | −0.01 | 0.97 | 0.28 | 0.13 |
| Processing speed | ||||||||||||
| Motor | 0.29 | 0.15 | 0.33 | 0.07 | 0.14 | 0.49 | 0.06 | 0.73 | 0.12 | 0.55 | 0.56 | 0.0009 |
| Visual | −0.05 | 0.80 | 0.19 | 0.30 | −0.26 | 0.19 | 0.04 | 0.81 | −0.28 | 0.16 | 0.11 | 0.57 |
| Visual-motor (coding) | −0.002 | 0.99 | 0.12 | 0.52 | −0.13 | 0.50 | −0.06 | 0.73 | −0.30 | 0.12 | −0.07 | 0.71 |
| Visual-motor (sequencing) | −0.13 | 0.52 | 0.22 | 0.22 | −0.13 | 0.51 | −0.15 | 0.40 | −0.26 | 0.19 | 0.001 | 0.99 |
| Visual-verbal | −0.11 | 0.58 | 0.08 | 0.65 | −0.28 | 0.16 | −0.07 | 0.71 | −0.03 | 0.87 | 0.47 | 0.006 |
| Intelligence | ||||||||||||
| Spatial intelligence | −0.20 | 0.32 | 0.21 | 0.25 | 0.02 | 0.90 | 0.22 | 0.22 | −0.14 | 0.50 | 0.11 | 0.53 |
| Attention | ||||||||||||
| Omission | −0.28 | 0.15 | 0.26 | 0.15 | −0.18 | 0.38 | −0.24 | 0.19 | −0.14 | 0.48 | 0.29 | 0.11 |
| Variability | 0.09 | 0.67 | 0.33 | 0.07 | −0.12 | 0.54 | −0.01 | 0.97 | −0.29 | 0.14 | 0.36 | 0.04 |
| Perseveration | −0.05 | 0.79 | 0.45 | 0.01 | 0.01 | 0.94 | −0.01 | 0.96 | 0.18 | 0.36 | 0.55 | 0.001 |
| Vigilance | −0.01 | 0.97 | 0.31 | 0.08 | −0.20 | 0.31 | −0.04 | 0.82 | −0.29 | 0.15 | 0.39 | 0.03 |
| Memory: | ||||||||||||
| Digit Span | −0.11 | 0.58 | 0.34 | 0.05 | 0.19 | 0.35 | −0.03 | 0.89 | −0.22 | 0.27 | 0.12 | 0.50 |
| Spatial Span | 0.06 | 0.75 | −0.12 | 0.50 | 0.17 | 0.41 | −0.21 | 0.25 | 0.02 | 0.91 | −0.04 | 0.84 |
| Neurobehavioral Symptoms# | ||||||||||||
| Initiate | −0.05 | 0.83 | −0.54 | 0.004 | −0.01 | 0.97 | −0.37 | 0.06 | 0.04 | 0.88 | −0.21 | 0.30 |
| Organization of materials | 0.13 | 0.58 | −0.33 | 0.09 | 0.18 | 0.44 | −0.09 | 0.66 | 0.06 | 0.81 | −0.13 | 0.52 |
| Planning/organize | 0.12 | 0.61 | −0.53 | 0.001 | 0.12 | 0.62 | −0.12 | 0.56 | 0.15 | 0.53 | −0.28 | 0.15 |
| Working memory | −0.14 | 0.55 | −0.49 | 0.01 | −0.10 | 0.66 | −0.18 | 0.37 | 0.13 | 0.57 | −0.25 | 0.21 |
| Behavioral Symptoms# | ||||||||||||
| Inattention | −0.73 | 0.001 | −0.77 | <0.0001 | −0.66 | 0.003 | −0.14 | 0.47 | −0.52 | 0.03 | −0.71 | <0.0001 |
| Hyperactivity/Impulsivity | −0.42 | 0.08 | −0.63 | 0.0004 | −0.31 | 0.21 | −0.20 | 0.32 | −0.47 | 0.05 | −0.73 | <0.0001 |
| Learning problems | −0.61 | 0.01 | −0.64 | 0.0003 | −0.45 | 0.06 | 0.11 | 0.59 | −0.22 | 0.39 | −0.59 | 0.001 |
| Defiance/aggression | −0.17 | 0.49 | −0.44 | 0.02 | −0.30 | 0.22 | −0.07 | 0.72 | −0.10 | 0.69 | −0.61 | 0.0008 |
Higher scores for these measures are indicative of better functioning
Higher scores for these measures are indicative of worse functioning
rp: For descriptive purpose, partial correlation coefficient (rp) was presented to denote the strength of association between fatigue measures, with each neurocognitive and behavioral outcome, adjusted for age at evaluation.
Sleep and neurocognitive/behavioral outcomes
Longer sleep duration was associated with poorer executive function in females (P’s<0.01)(Figure 1). More frequent nighttime awakening was associated with more self-reported behavioral symptoms in females (Figure 2), and lower performance on cognitive flexibility (P=0.04) and processing speed (P<0.05) in males (Supplementary Material 4). Premature awakening was also associated with poorer processing speed (P’s<0.05) and abstract reasoning (P=0.003) in females (Supplementary Material 4). No association between sleep latency and neurocognitive or behavioral outcomes was found.
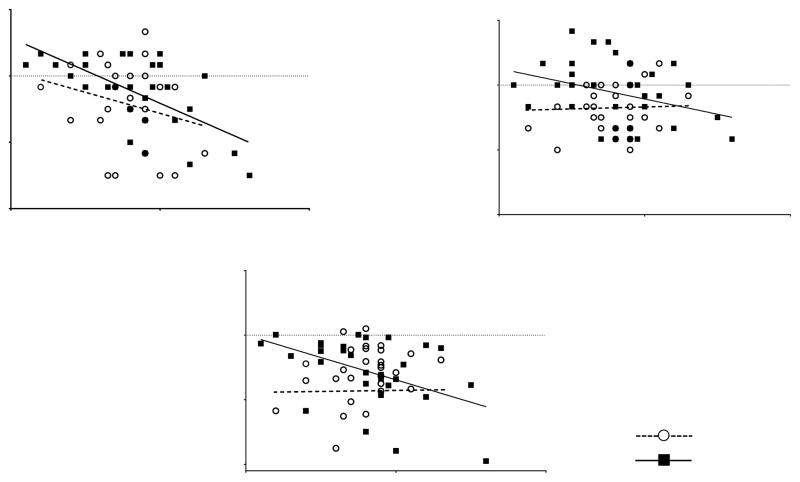
Figure 1. Association between Sleep Duration and Executive Function.
Longer duration of sleep was associated with worse executive function in female survivors but less consistently in male survivors. Associations between sleep duration and other neurocognitive/behavioral measures are presented in Supplementary Material 4.
A: Cognitive flexibility (Males: r=−0.47, P=0.01; Females: r=0.68, P<0.0001);
B: Verbal fluency (Males: r=0.08, P=0.68; Females: r=−0.50, P=0.004).
C: Organization (Males: r=−0.04, P=0.83; Females: r=−0.47, P=0.01).
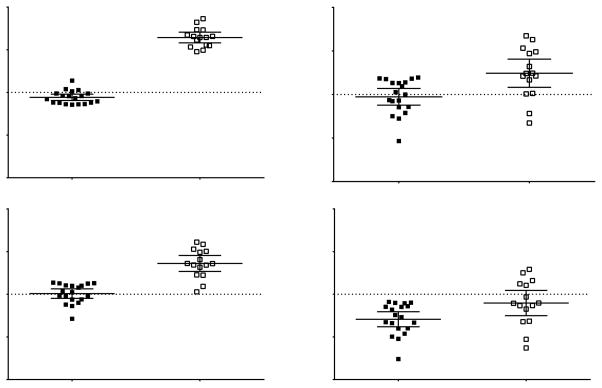
Figure 2. Association between Frequent Nighttime Awakening and Behavior in Female Survivors.
Female survivors who reported frequent nighttime awakening displayed more behavioral symptoms than those who did not, in (A) inattention, P=0.01; (B) hyperactivity/impulsivity, P=0.03; (C) learning problems, P=0.01; (D) defiance/aggression, P=0.04. Such associations were not observed in male survivors; associations between sleep variables and other neurocognitive/behavioral measures are presented in Supplementary Material 4.
Biomarkers and functional outcomes
Distribution and descriptive data for each biomarker is presented in Supplementary Material 5. Age was negatively correlated with TNF-α in both males (P=0.05) and females (P=0.009) (Supplementary Material 6). Body mass index was positively correlated with hsCRP and IL-6 for both male and female survivors. Body mass index (P=0.0009) and age (P=0.01) were correlated with OxLDL in females, but not in males.
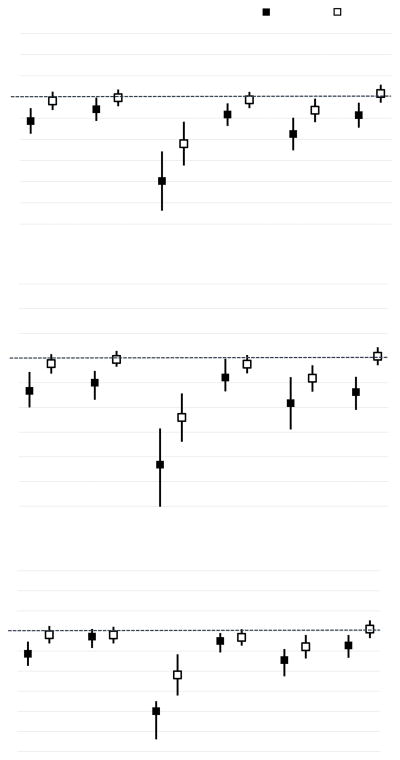
Lower scores on cognitive flexibility, organization and visual-motor processing speed were observed in females with higher IL-6, IL-1β and hsCRP levels (P’s<0.05) (Figure 3). Females with high TNF-α also had worse organization (P=0.04) and abstract reasoning (P=0.02) (Supplementary Material 7). Higher IL-1β was associated with more self-reported behavioral symptoms (P’s<0.05), cognitive fatigue (P=0.002) and longer sleep duration (P<0.001) (Supplementary Material 7).
Figure 3. Association between Inflammatory Biomarkers and Executive Function/Processing Speed in Female Survivors.
Comparison of each executive function/processing speed measure was conducted between female survivors within the top tertile, versus those within other tertiles, for IL-6 (A), IL-1β (B) and CRP (C). Female survivors within the top tertile demonstrated worse performance on multiple measures of executive function and processing speed, as compared to those within other tertiles. Associations between inflammatory biomarkers and other neurocognitive/behavioral measures are presented in Supplementary Material 7.
Males with high TNF-α demonstrated worse parent-reported organization skills (P=0.03), as well as more self-reported inattention (P=0.05) and learning problems (P=0.04; Supplementary Material 7).
Males with higher OxLDL displayed more parent-reported problems with initiation (P=0.04). No other associations between markers of oxidative stress and functional outcomes were identified in males and females (Supplementary Material 8).
We did not identify any associations between serum biomarkers and sleep latency, frequent nighttime and premature awakening for either males or females (Supplementary Material 9).
Discussion
This study explored the impact of sleep and fatigue on neurocognitive and behavioral outcomes among survivors of ALL treated on a chemotherapy-only protocol. Sex differences were observed for sleep, fatigue, neurocognitive and behavioral outcomes. Sleep and fatigue were associated with multiple neurocognitive measures in female survivors, but less so in male survivors. Females with high levels of hsCRP, IL-6 and IL-1β demonstrated worse neurocognitive and behavioral functioning. Higher levels of IL-1β were also associated with greater cognitive fatigue and longer sleep duration in females.
Sex differences for fatigue, sleep duration and neurocognitive measures were somewhat surprising, and might be attributed to more prevalent self-reported symptoms of fatigue in females3. Previous studies have demonstrated the sex-specificity of late effects of cancer treatment11, particularly in neurocognitive function among cancer survivors12. The underlying mechanisms of neurocognitive impairment may differ by sex in cancer survivors, such that females may be more sensitive to the effects of fatigue and sleep disturbances than males. Current evidence in the literature has highlighted that distinct hormonal and physical changes at specific time points in the lifespans of males and females, such as puberty, may confer differential risk for sleep-related disorders and health outcomes.13 Since both sleep and fatigue are highly modifiable health outcomes, an approach targeted at sex-specific pathophysiology may have direct benefit on neurocognitive function in this population. For example, female survivors who exhibit sleep problems may be in greater need for preventive strategies, such as psychological and cognitive behavioral therapy, as well as education or information interventions to improve sleep hygiene.14,15
Contrary to hypotheses and prior studies linking sleep deprivation to poor functional outcomes, we found that longer sleep duration was associated with worse neurocognitive performance in cancer survivors. Similar results were also observed in a study that examined sleep problems in healthy urban adolescents16. Longer sleep duration does not necessarily reflect higher quality sleep but instead may serve as a marker of poor sleep efficiency16. In our study, longer sleep duration was associated with poorer executive functioning, cognitive abilities subserved by the frontal lobes. Recent experimental studies in healthy controls involving sleep quality and sleep fragmentation reported impaired performance on tasks of frontal lobe or executive function, including measures of verbal fluency, creativity and planning skills17,18. We have previously reported that chemotherapy exposure was associated with white matter abnormalities in the frontal brain regions of ALL survivors, suggesting that frontal brain regions and executive functions are more prone to long-term pathology2. Significant associations between sleep and neurocognitive function were observed more consistently in females but not males. To note, studies conducted on patients with traumatic brain injury also suggested that females may be more susceptible to the post-injury sleep disturbances and fatigue than males19,20. Collectively, our results suggest that following treatment-related disruption of frontal lobe function, female survivors may be more sensitive than males to the effects of poor sleep quality on cognitive abilities subserved by frontal lobes.
Behavioral abnormalities are often observed in children and adolescents that demonstrate sleep problems21. In our study, female survivors who had frequent nighttime awakening reported more inattention and learning problems. Self-reported cognitive fatigue and general fatigue were also strongly associated with behavioral problems in both male and female survivors. Fatigue is commonly clustered with sleep and behavioral problems in childhood cancer survivors undergoing active chemotherapy 22, and may share a common pathophysiology that involves dysregulation in neurotransmitters such as dopamine, serotonin, and/or norepinephrine23. Within the general population, inadequate sleep may cause deleterious effects on cardiovascular, immune and various metabolic systems 24. These findings concur with current evidence that interventions improving survivors’ sleep quality may improve their behavioral functioning and ameliorate health outcomes in cancer survivors25.
There is limited research that evaluates associations of biomarkers of inflammation and oxidative stress on neurocognitive function in survivors of childhood cancer. Our results suggest that survivors with higher levels of IL-6, IL-1β and CRP tended to have worse executive function and processing speed. Studies conducted in breast cancer survivors also found weak to moderate correlations between IL-1β, IL-6, TNF-α levels, and different degrees of cognitive impairment8. Higher baseline soluble tumor necrosis factor receptor type II in breast cancer patients treated with chemotherapy was significantly associated with increased self-reported memory complaints, while higher levels of TNF-α were associated with lower left hippocampal volume7,9.
We observed more consistent associations between inflammatory biomarkers and neurocognitive outcomes in female as compared to male survivors. There is mounting evidence in the literature that supports inflammatory mediators and immune cells as principle regulators of brain sexual differentiation, other than just neurotransmitters and sex hormones26. Acute and chronic stress can induce microglial cell mediated immune activation and inflammation in the brain, and recent studies have demonstrated sex differences in microglial density, function, and morphology in several brain regions27. Sex-specific association of oxidative stress and inflammation is also observed in other vascular-related diseases10. Since the levels of biomarkers were similar between males and females in our sample, it is plausible that female survivors of ALL may be more sensitive, with a lower physiologic threshold to the effects of chronic inflammation. Understanding the underlying pathophysiology of these sex-specific risks will facilitate potential application of sex-specific interventions to avoid poor long-term adverse outcomes. To note, studies have shown the anti-inflammatory effects of intensive aerobic activity and strength training, as well as healthy diets that are high in anti-oxidants such as flavonoids and triterpenes, in survivors of cancer.28,29 Female survivors may benefit from intensive administration of these behavioral therapies to minimize the impact of chronic inflammation on health outcomes.
Relatively robust trends between hsCRP and multiple measures of executive function, processing speed and attention were observed. Being one of the most sensitive markers of low-grade inflammation, serum CRP is linked to cerebral microstructural integrity and cognitive function30,31. One study found higher CRP values to be associated with worse performance in executive function in a large cohort of elderly individuals 30. Elevated CRP is also a predictor of vascular injury and cardiovascular complications in the general population31. Whether lowering CRP can prevent cognitive decline and/or microstructural white matter alterations in long-term survivors of ALL needs to be addressed in future studies.
Higher IL-1β levels were associated with greater cognitive fatigue in female survivors. A meta-analysis revealed a positive correlation between general or physical fatigue and serum cytokine levels of IL-6 and IL-1 receptor antagonist in adult cancer patients 4. Cancer-related fatigue is a multidimensional construct that includes physical, sleep-related and/or cognitive tiredness32; our data suggests that not all facets of fatigue may be equally sensitive to changes in biomarkers. Multiple biologic processes can contribute to fatigue symptoms, including immune response, inflammation, metabolic and neuroendocrine function, hypothalamic–pituitary–adrenal (HPA) axis, and genetics23. Consistent with the literature that reported positive correlations between hours of sleep and circulating inflammatory markers33, survivors within the top tertiles of IL-1β and IL-6 also reported longer sleep duration. Increased serum cytokine levels are reported in cancer patients with dampened circadian rhythms, with disordered sleep inducing an increase in IL-6 and transforming growth factor-α34. These cytokines, termed nocturnal cytokines, affect the sleep-wake cycle, causing a disruption in the neuroendocrine control of cortisol release, resulting in further release of pro-inflammatory cytokines35. Our data, albeit preliminary, suggests that fatigue and sleep symptoms may be exacerbated by the impact of immune activation and disruption of circadian rhythms secondary to cancer treatment-related adrenocortical insufficiency.
Limitations should be considered in interpretation of these results. Our study sample size was relatively small and did not include an age-matched healthy comparison control group. However, our results are consistent with the robust literature that demonstrates associations among sleep, fatigue and neurocognitive performance in other diseased populations. Even though the proxy- or self- reported measures of sleep adopted in this study have been previously validated with objective sleep assessments36,37, the reporting style may be influenced by response bias, perceived stress or psychological distress of the rater. The use of subjective measures also did not permit us to explore other sleep-related disorders such as obstructive sleep apnea. Findings from this study should be validated in larger prospective studies with the use of more objective sleep quality measures (e.g., polysomnography and actigraphy). We identified associations between inflammatory biomarkers and body mass index, implying that physical activity and diet may affect the inflammatory and oxidative stress status in survivors. However, these lifestyle data were not collected in this current report and should be investigated in future studies. Lastly, the dosages and schedule of chemotherapy utilized in this study is different from those conducted at other institutions (e.g., COG protocols). However, the current results should be generalizable as the specific chemotherapeutic agents (e.g. methotrexate, corticosteroids, etc.) are similar to those used in other protocols.
Conclusions
Our results support the contribution of poor sleep and fatigue to neurocognitive impairment and behavioral symptoms in adolescent survivors of childhood ALL, and suggest that systemic inflammation may be one of the critical pathophysiological basis linking these functions. Female survivors appear particularly sensitive to the adverse effects of sleep, fatigue and systemic inflammation. Future research should investigate whether the effect of sleep and fatigue on brain structural and functional outcomes may be mediated by the response and reactivity to chronic stress, as well as to evaluate the beneficial effect of pharmacological and lifestyle interventions on reducing systemic inflammation and improving neurocognitive function as well as sleep in survivors of childhood cancer.
Supplementary Material
Acknowledgments
Funding: National Institute of Mental Health (Grant No. MH085849 to K.R.K.), National Cancer Institute (Grant No. CA21765 to C. Roberts; CA195547 to M.M.H and L.L.R), and the ALSAC.
The authors would like to acknowledge Dr. Cara Kimberg, Ms. Cynthia Jones, Ms. Deborah Stewart and Ms. Adrienne Studaway for administering the neurocognitive tests; Ms. Joycelynn Butler for extracting and cleaning the data; Dr. Michael J. Ehrhardt for conducting the biomarker assays.
Footnotes
Disclosures: Dr. Panoskaltsis-Mortari reports a family member employed by BioTechne, which owns the company from which some of the kits used for biomarker analysis were purchased (R&D Systems). All other authors have no conflict of interest to disclose.
Authorship Contributions: KRK had full access to all data and takes responsibility for integrity of the data and data analysis. The following authors have made substantial contributions to the intellectual content of the paper, including conception/design (all), acquisition, analysis, or interpretation of data (all), drafting of the manuscript (KRK, YTC) and critical revision of the manuscript for important intellectual content (all). Contributions also include statistical analysis (YTC, WL, DS), obtaining funding (KRK, LLR, MMH). All authors have approved the final version of this manuscript.
References
- 1.Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360(26):2730–2741. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krull KR, Cheung YT, Fellah S, et al. Chemotherapy Pharmacodynamics, Neuroimaging and Neurocognitive Outcomes in Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia. J Clin Oncol. 2016;34(22):2644–2653. doi: 10.1200/JCO.2015.65.4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clanton NR, Klosky JL, Li C, et al. Fatigue, vitality, sleep, and neurocognitive functioning in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Cancer. 2011;117(11):2559–2568. doi: 10.1002/cncr.25797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schubert C, Hong S, Natarajan L, Mills PJ, Dimsdale JE. The association between fatigue and inflammatory marker levels in cancer patients: A quantitative review. Brain Behav Immun. 2007;21(4):413–427. doi: 10.1016/j.bbi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Mullington JM, Simpson NS, Meier-Ewert HK, Haack M. Sleep loss and inflammation. Best Pract Res Clin Endocrinol Metab. 2010;24(5):775–784. doi: 10.1016/j.beem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Venkataraman K, Khurana S, Tai TC. Oxidative stress in aging-matters of the heart and mind. Int J Mol Sci. 2013;14(9):17897–17925. doi: 10.3390/ijms140917897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kesler S, Janelsins M, Koovakkattu D, et al. Reduced hippocampal volume and verbal memory performance associated with interleukin-6 and tumor necrosis factor-alpha levels in chemotherapy-treated breast cancer survivors. Brain Behav Immun. 2013;30(SUPPL):S109–S116. doi: 10.1016/j.bbi.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheung YT, Lim SR, Ho HK, Chan A. Cytokines as mediators of chemotherapy-associated cognitive changes: Current evidence, limitations and directions for future research. PLoS ONE. 2013;8(12) doi: 10.1371/journal.pone.0081234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ganz PA, Bower JE, Kwan L, et al. Does tumor necrosis factor-alpha (TNF-α) play a role in post-chemotherapy cerebral dysfunction? Brain Behav Immun. 2013;30(SUPPL):S99–S108. doi: 10.1016/j.bbi.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gardner AW, Parker DE, Montgomery PS, et al. Gender and racial differences in endothelial oxidative stress and inflammation in patients with symptomatic peripheral artery disease. J Vasc Surg. 2015;61(5):1249–1257. doi: 10.1016/j.jvs.2014.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armstrong GT, Sklar CA, Hudson MM, Robison LL. Long-term health status among survivors of childhood cancer: Does sex matter? J Clin Oncol. 2007;25(28):4477–4489. doi: 10.1200/JCO.2007.11.2003. [DOI] [PubMed] [Google Scholar]
- 12.Jain N, Brouwers P, Okcu MF, Cirino PT, Krull KR. Sex-specific attention problems in long-term survivors of pediatric acute lymphoblastic leukemia. Cancer. 2009;115(18):4238–4245. doi: 10.1002/cncr.24464. [DOI] [PubMed] [Google Scholar]
- 13.Mallampalli MP, Carter CL. Exploring Sex and Gender Differences in Sleep Health: A Society for Women’s Health Research Report. J Womens Health (Larchmt) 2014;23(7):553–562. doi: 10.1089/jwh.2014.4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garland SN, Johnson JA, Savard J, et al. Sleeping well with cancer: a systematic review of cognitive behavioral therapy for insomnia in cancer patients. Neuropsychiatr Dis Treat. 2014 Jun 18;10:1113–1124. doi: 10.2147/NDT.S47790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berger AM. Update on the state of the science: sleep-wake disturbances in adult patients with cancer. Oncol Nurs Forum. 2009 Jul;36(4):E165–177. doi: 10.1188/09.ONF.E165-E177. [DOI] [PubMed] [Google Scholar]
- 16.Mrug S, Tyson A, Turan B, Granger DA. Sleep problems predict cortisol reactivity to stress in urban adolescents. Physiol Behav. 2016;155:95–101. doi: 10.1016/j.physbeh.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Wu JC, Gillin JC, Buchsbaum MS, et al. Frontal lobe metabolic decreases with sleep deprivation not totally reversed by recovery sleep. Neuropsychopharmacology. 2006;31(12):2783–2792. doi: 10.1038/sj.npp.1301166. [DOI] [PubMed] [Google Scholar]
- 18.Jones K, Harrison Y. Frontal lobe function, sleep loss and fragmented sleep. Sleep Med Rev. 2001;5(6):463–475. doi: 10.1053/smrv.2001.0203. [DOI] [PubMed] [Google Scholar]
- 19.Englander J, Bushnik T, Oggins J, Katznelson L. Fatigue after traumatic brain injury: Association with neuroendocrine, sleep, depression and other factors. Brain injury. 2010;24(12):1379–1388. doi: 10.3109/02699052.2010.523041. [DOI] [PubMed] [Google Scholar]
- 20.Tham SW, Palermo TM, Vavilala MS, et al. The longitudinal course, risk factors, and impact of sleep disturbances in children with traumatic brain injury. Journal of Neurotrauma. 2012;29(1):154–161. doi: 10.1089/neu.2011.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karimzadeh P. Psycho-cognitive behavioral problems in sleep disordered children. Neural Regen Res. 2012;7(8):635–639. doi: 10.3969/j.issn.1673-5374.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hockenberry MJ, Hooke MC, Gregurich MA, McCarthy K, Sambuco G, Krull K. Symptom clusters in children and adolescents receiving cisplatin, doxorubicin, or ifosfamide. Oncol Nurs Forum. 2010;37(1) doi: 10.1188/10.ONF.E16-E27. [DOI] [PubMed] [Google Scholar]
- 23.Saligan LN, Olson K, Filler K, et al. The biology of cancer-related fatigue: a review of the literature. Support Care Cancer. 2015;23(8):2461–2478. doi: 10.1007/s00520-015-2763-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aldabal L, Bahammam AS. Metabolic, endocrine, and immune consequences of sleep deprivation. Open Respir Med J. 2011;5(1):31–43. doi: 10.2174/1874306401105010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keshavarzi Z, Bajoghli H, Mohamadi MR, et al. In a randomized case-control trial with 10-years olds suffering from attention deficit/hyperactivity disorder (ADHD) sleep and psychological functioning improved during a 12-week sleep-training program. World J Biol Psychiatry. 2014;15(8):609–619. doi: 10.3109/15622975.2014.922698. [DOI] [PubMed] [Google Scholar]
- 26.McCarthy MM, Pickett LA, VanRyzin JW, Kight KE. Surprising origins of sex differences in the brain. Horm Behav. 2015;76:3–10. doi: 10.1016/j.yhbeh.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bollinger JL, Bergeon Burns CM, Wellman CL. Differential effects of stress on microglial cell activation in male and female medial prefrontal cortex. Brain Behav Immun. 2016;52:88–97. doi: 10.1016/j.bbi.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bazzan AJ, Newberg AB, Cho WC, Monti DA. Diet and Nutrition in Cancer Survivorship and Palliative Care. Evid Based Complement Alternat Med. 2013;2013:917647. doi: 10.1155/2013/917647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.LaVoy ECP, Fagundes CP, Dantzer R. Exercise, inflammation, and fatigue in cancer survivors. Exerc Immunol Rev. 2016;22:82–93. [PMC free article] [PubMed] [Google Scholar]
- 30.Wersching H, Duning T, Lohmann H, et al. Serum C-reactive protein is linked to cerebral microstructural integrity and cognitive function. Neurology. 2010;74(13):1022–1029. doi: 10.1212/WNL.0b013e3181d7b45b. [DOI] [PubMed] [Google Scholar]
- 31.Miwa K, Tanaka M, Okazaki S, Furukado S, Sakaguchi M, Kitagawa K. Relations of blood inflammatory marker levels with cerebral microbleeds. Stroke. 2011;42(11):3202–3206. doi: 10.1161/STROKEAHA.111.621193. [DOI] [PubMed] [Google Scholar]
- 32.Jean-Pierre P, Figueroa-Moseley CD, Kohli S, Fiscella K, Palesh OG, Morrow GR. Assessment of cancer-related fatigue: Implications for clinical diagnosis and treatment. Oncologist. 2007;12(SUPPL 1):11–21. doi: 10.1634/theoncologist.12-S1-11. [DOI] [PubMed] [Google Scholar]
- 33.Prather AA, Vogelzangs N, Penninx BWJH. Sleep duration, insomnia, and markers of systemic inflammation: Results from the Netherlands Study of Depression and Anxiety (NESDA) J Psychiatr Res. 2015;60:95–102. doi: 10.1016/j.jpsychires.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rich T, Innominato PF, Boerner J, et al. Elevated serum cytokines correlated with altered behavior, serum cortisol rhythm, and dampened 24-hour rest-activity patterns in patients with metastatic colorectal cancer. Clin Cancer Res. 2005;11(5):1757–1764. doi: 10.1158/1078-0432.CCR-04-2000. [DOI] [PubMed] [Google Scholar]
- 35.Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008;26(6):971–982. doi: 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Markovich AN, Gendron MA, Corkum PV. Validating the Children’s Sleep Habits Questionnaire Against Polysomnography and Actigraphy in School-Aged Children. Front Psychiatry. 2014;5:188. doi: 10.3389/fpsyt.2014.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chervin RD, Hedger K, Dillon JE, Pituch KJ. Pediatric sleep questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med. 2000 Feb 01;1(1):21–32. doi: 10.1016/s1389-9457(99)00009-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.