Abstract
With more than 9 million new infections and 1.5 million deaths claimed every year, tuberculosis remains one of the major scourges of humankind. The only vaccine available against this disease, the attenuated strain Mycobacterium bovis, BCG is effective against severe forms of the disease in infants, but scarcely effective in protecting adults from the pulmonary form of the disease, thus not stopping transmission. Consequently, the development of an effective anti-tuberculosis vaccine is a major goal for improving global health. The most common concept is that a more effective vaccine should include a first immunization with a live vaccine followed by the administration of an acellular boosting vaccine. In this approach, the live vaccine might be either BCG or a different, more efficient attenuated strain. Recently, we showed that a Mycobacterium tuberculosis mutant missing the gene encoding for the extracellular function sigma factor SigE, is strongly attenuated and is able to induce a more effective protection from M. tuberculosis infection compared to BCG in mice. We now further characterize the protective potential of this novel strain in the guinea pig model of tuberculosis. In the guinea pig, it had limited growth but induced a Th1 immune response and was able to significantly reduce the number of colony forming units as well as prolong survival. Taken together these data provide evidence for the use of the M. tuberculosis sigE mutant as the basis for further development as a vaccine against infection.
Introduction
The only vaccine available against Mycobacterium tuberculosis infection, the attenuated strain of Mycobacterium bovis, Bacillus Calmette Guérin (BCG), is one of the most widely used vaccines currently in use, with about 100 million newborns vaccinated each year (http://www.unicef.org/supply/files/BCG_Supply_Status.pdf). Even if very safe, this vaccine is only effective in preventing invasive diseases such as tubercular meningitis or miliary tuberculosis in infants, but largely ineffective in protecting adults from pulmonary tuberculosis, thus being unable to stop the transmission chain [1]. Consequently, the development of new more potent and effective vaccines against tuberculosis is one of the major goals to improve global health. Currently there are up to sixteen vaccines in the development pipeline that have the potential to be efficacious in humans including both subunit and live attenuated vaccines [2]. Novel live attenuated M. tuberculosis vaccines must come under close scrutiny for their ability to not cause disease. At the same time attenuation cannot be so profound that the new strain is cleared by innate immune reactions and an adaptive immune response is not generated. Thus, there is a fine line between insufficiently attenuated and too attenuated. Currently, the most advanced live vaccines in clinical trials are represented by the ΔureC::hly BCG mutant VPM1002, able to escape from the phagosome, thus affecting improved antigen processing and presentation [3], and MTBVAC, an attenuated strain of M. tuberculosis developed by deleting two independent genes essential for virulence [4–6]. A vaccine should induce immune responses that mimic that of the pathogen, but without the disease caused by the pathogen, and the use of live attenuated strains of M. tuberculosis provides an opportunity to achieve this goal.
Previously, we showed that an M. tuberculosis H37Rv derivative in which the gene encoding the extracytoplasmic sigma factor SigE was deleted, not only was markedly attenuated for growth in macrophages [7] as well as in mice [8], but was also able to elicit a more potent and protective immune response in a murine model of infection when used as a vaccine [9]. Interestingly, this higher protection was more evident when mice were infected with a hyper-virulent strain of M. tuberculosis. Even if the mechanism by which the sigE mutant was able to induce a more potent immune response in mice compared to BCG is still unknown, it might be explained by the recent finding that this mutant is unable to block phagosome maturation [10], suggesting that its antigens might be processed more efficiently. This is not the only M. tuberculosis sigma factor mutant shown to be a promising vaccine candidate. Recently, an M. tuberculosis strain, in which the sigH gene was deleted, was examined as a mucosal vaccine in the non-human primate model and shown to reduce mycobacterial burdens, significantly diminish clinical manifestations and granulomatous pathology [11]. Prior studies had revealed that SigH was essential for resistance to oxidative stress [12] and for induction of immunopathology and lethality in mice [13].
We now assess the M. tuberculosis sigE mutant ST28, previously evaluated in mice, as a vaccine in the guinea pig model of tuberculosis. This mutant was initially used to inoculate guinea pigs and the presence of mycobacteria and cytokine production was subsequently examined. It was then used as a vaccine to determine its ability to reduce the mycobacterial burden and prolong survival after low dose aerosol infection with M. tuberculosis H37Rv.
Material & Methods
Preparation of M. tuberculosis sigE mutant ST28
The H37Rv sigE mutant ST28 [7] was grown at 37°C in LPS-free Midd lebrook 7H9 broth (BD, San Jose, CA) in 150 ml roller bottles with slow rotation (3 rpm) supplemented with 0.2% glycerol, 0.05% Tween-80, 10% AND (Albumin, NaCl, Dextrose). Cultures were grown to the mid-log phase, washed twice with LPS-free Middlebrook 7H9 broth, centrifuged at 20 g for 5 min to remove bacterial clumps and mixed with glycerol up to a final 15% concentration. Finally, bacteria were aliquoted, and maintained at −70°C until used. Before use, some bacterial aliquots were thawed and plated for colony forming units (CFU) determination.
Animals and M. tuberculosis
Outbred, Hartley guinea pigs (450–500 gms, Charles River Lab, MA) were maintained in an ABSL-3 facility at Colorado State University, with sterile chow and water ad libitum. Colorado State University Institutional Animal Care and Use Committee (IACUC) approved all experimental procedures.
M. tuberculosis, H37Rv (TMCC102) used for infection of guinea pigs was grown as described previously [14]. Mycobacterium bovis BCG Pasteur (TMCC1011) strain was grown in Proskaur and Beck (P&B) medium with 0.1% Tween 80 to mid-log phase. Aliquots were stored at −80°C and thawed before use.
Inoculation and infection of guinea pigs
To determine if ST28 grew and induce an immune response, guinea pigs were inoculated with approximately 103 CFU via the intra-dermal route (Supplementary Figure 1A). At days 30 and 60 post-inoculation 5 guinea pigs per time point were euthanized and the lung and spleen examined for growth of the mutant by plating organ homogenates on 7H11 agar. Cytokine mRNA expression and pathology were also investigated.
To assess the ability of the mutant to reduce the mycobacterial burden after low dose aerosol infection, guinea pigs were inoculated as described above and rested for 10 weeks. A saline-treated group and a BCG-vaccinated group (inoculated with 103 CFU via the intradermal route) were included as experimental controls. All guinea pigs were then infected with a low dose aerosol (10–20 CFU) of virulent M. tuberculosis H37Rv using the Madison Aerosol Exposure Chamber (University of Wisconsin, Madison, WI). Five guinea pigs per group were euthanized at day 40 post-infection and the colony forming units (CFU) determined in the lung and spleen (Supplementary Figure 1B).
For the long-term study, ten guinea pigs per group were inoculated with 103 CFU of the ST28, 103 CFU of BCG Pasteur or treated with saline and rested for 10 weeks. All guinea pigs were infected with a low dose aerosol and euthanized when they reached set criteria established by the IACUC, such as being moribund or exceeding acceptable weight loss and/or being affected in their respiratory rate (labored/heavy breathing). Time to euthanasia was used as time to death. Body temperature was measured to aid in tracking clinical progression of disease. For this, guinea pigs received a subcutaneous microchip implant (IPT-300 Bio Medic Data Systems [BMDS], Inc., Seaford, DE) that allowed measurement of temperature and also carried information about experiment number and animal number. The body temperatures of individual guinea pigs were assessed each day at approximately the same time in the afternoon using a DAS-6006/7 scanner transponder (BMDS). At the time of euthanasia, the right caudal lobe of the lung and a piece of the spleen from the guinea pig was utilized to analyze pathological lesions.
Determination of colony forming units from organ homogenates
Lungs and spleens were homogenized in sterile saline and 10-fold serial dilutions plated onto 7H11 agar. Plates were then incubated for up to 21 days at 37°C, at which time colonies were counted. CFU are expressed as Log10 transformed data.
Cytokine mRNA analysis
To determine the extent of immune induction caused by the sigE mutant, a lobe of the lung and a portion of the spleen was collected from 5 animals per group, at days 30 and 60 post-inoculation, placed into TRIZol® (Invitrogen) and immediately homogenized. Samples were processed and cDNA produced using an iScript cDNA synthesis kit (BioRad, Hercules, CA) and quantitative real-time PCR was performed using iQ Syber green Supermix (BioRad) using a CFX Connect™ Real-Time System, with CFX Manager™ Software (Version 3.1, BioRad). Primers to amplify guinea pig TNF-α [15], TGF-β [15], IFN-γ and the housekeeping gene RPLPO for real-time PCR were used to determine the mRNA fold induction for each cytokine (Table S1). The extent of mRNA induction for each cytokine was used and was calculated from the average of the two Cq values for the sample and subtracting it from the housekeeping Cq value, to give a ΔCt value. The ΔCt value for the sample was then subtracted from the ΔCt for the cytokine from naïve animals to equal the ΔΔCt. Fold induction was expressed as 2−ΔΔCt. Cytokine mRNA expression was also determined at day 40 post-infection in the short-term challenge study, but in this study the copy number for cytokine mRNA was calculated. Samples were collected as described above and primers to amplify IFN-γ, TGF-β, TNF-α, IL-17, IL-12 [16], IL-10 [14] and IP-10 [17] for real-time PCR (Table S1 & S2) were used to determine the mRNA copy number for each cytokine (Supplementary material). The extent of mRNA induction for each cytokine was calculated using a standard curve for the specific cytokine, which consisted of a known copy number (Supplementary material). The standard curve was then used to determine the copy number for each unknown sample [18].
Histology
The right caudal lobe of the lung and a piece of liver and of the spleen from the guinea pigs were utilized to analyze pathological lesions. The excised lung lobe was inflated with 10% neutral-buffered formalin and then submerged in the fixative. For processing, the lung lobe, liver and spleen were embedded in paraffin and 5 µm sections cut and stained with hematoxylin and eosin. A pathologist examined the sections, without prior knowledge of the groups, and provided a score based on the extent of lung involvement, inflammation, granuloma formation, necrosis, mineralization, and fibrosis and lesion type as previously described in detail [19].
Analysis of data
The log10-transformed data were used to determine significant differences between groups. Data were assessed for normal distribution prior to analysis. For analysis between multiple groups a one-way ANOVA with a Bonferroni t-test for all pairwise multiple comparisons was applied. Guinea pig survival was plotted using the Kaplan-Meier method, and differences between curves were analyzed using the log-rank test.
Results
Growth of ST28 in guinea pigs
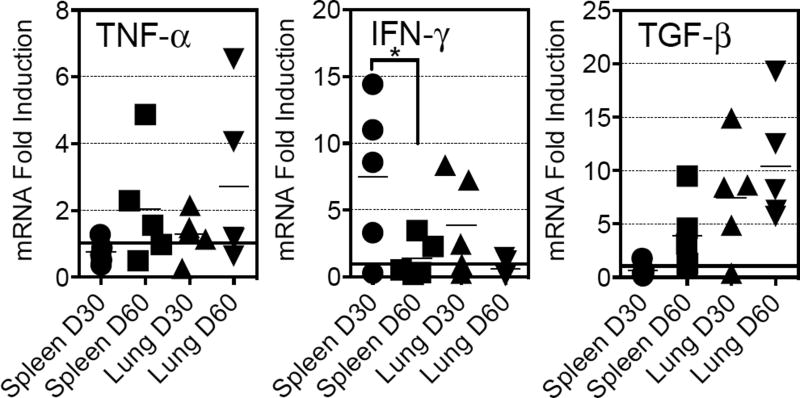
Guinea pigs inoculated via the intradermal route were examined 30 and 60 days-post inoculation for the presence of viable organism in the lungs and spleens. The number of organisms in the lungs at either time point was below the limit of detection for the assay. In the spleen, one guinea pig at day 30 had visible colonies growing on 7H11 agar, but this fell to below the limits of detection at day 60 (Data not shown). To determine if the vaccination protocol induced cytokines the mRNA from the lungs and spleens were examined for the expression of mRNA for TNF-α, IFN-γ and TGF-β (Figure 1). In general, there was a relatively scattered distribution of cytokine mRNA expression, which was anticipated given the outbred nature of the Hartley guinea pig. Specifically, there was no significant difference in TNF-α mRNA between time points in the spleen and lung, although there was a trend towards higher levels at day 60. There was a trend for less IFN-γ mRNA expression in the spleen and lung over time, with a significant decrease in the spleen at day 60 (p<0.05). TGF-β mRNA expression followed a similar pattern to that observed with TNF-α. Taken together, the data suggest that although there was a scatter of responses over time, the attenuated strain induced detectable immune responses. Histological analyses of organs revealed that there were no apparent lesions in the lungs, spleens or livers of any of the guinea pigs. At the later time point there were micro-foci of interstitial and intra-alveolar infiltration of mononuclear leukocytes noted in the lungs of some guinea pigs (data not shown).
Figure 1.
Assessment of the ability of ST28 to mount an immune response in guinea pigs. Guinea pigs were inoculated as described in Material and Methods and in Table 1. At days 30 and 60 after inoculation cytokine mRNA analysis was determined in the lung and spleen. Data are expressed as fold induction of the specific cytokine in relation to the expression of that cytokine in a cohort of naïve guinea pigs. The horizontal bar is set to indicate the 1-fold induction point. N = 5 guinea pigs per time point. Data were analyzed using the Student t-test, p<0.05.
ST28 induced immunity and reduction of colony forming units (CFU) and pathology after pulmonary M. tuberculosis infection
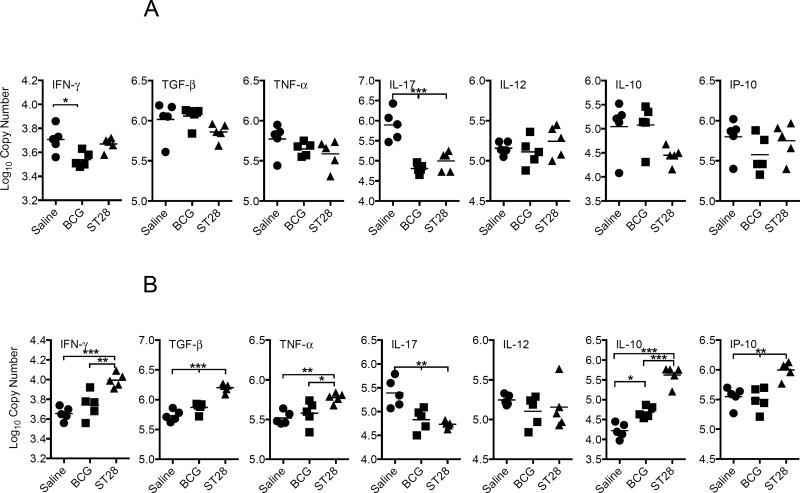
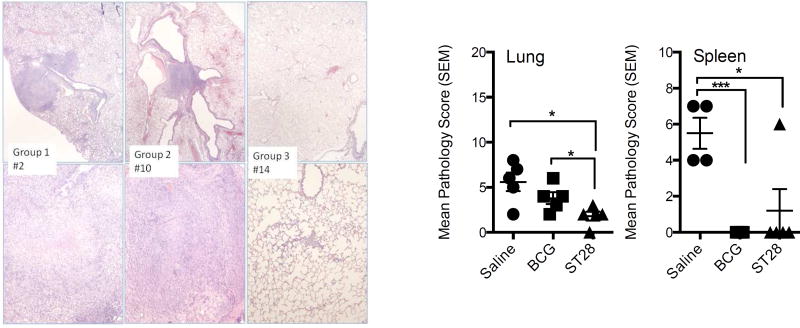
To determine if the immune response generated by inoculation could reduce the number of organisms after infection with a virulent strain of M. tuberculosis, guinea pigs were vaccinated with ST28 as above and then rested for 10 weeks. As controls, a group of guinea pigs was vaccinated with BCG and another group received pyrogen-free saline. At the end of week 10, all guinea pigs were infected with approximately 10–20 CFU of M. tuberculosis H37Rv via the aerosol route. At day 30 post-infection the CFU, mRNA cytokine expression and pathology were determined in the lungs and spleens of 5 animals per group. There was a significant reduction in CFU in the lungs and spleens of the vaccinated guinea pigs (Table 1). Expression of IFN-γ mRNA in the lungs was significantly decreased in the vaccinated groups compared to the saline-treated animals (Figure 2A) and significantly elevated in the spleens of mutant vaccinated animals (Figure 2B). IL-17 mRNA was significantly decreased in both organs of vaccinated guinea pigs, and while TNF-α mRNA expression was significantly elevated in the spleens of ST28-vaccinated animals there was no difference in the lungs. Although IL-10 was significantly elevated in the spleens of ST28-vaccinated guinea pigs, there was a trend to lower levels in the lungs when compared to both saline-treated and BCG-vaccinated animals. Histological examination of organs revealed that ST28-vaccination resulted in a significant decrease in the lung and spleen pathology at day 40 when compared to saline-treated animals, while BCG vaccination resulted in a significant reduction in the spleen only (Figure 3). These data suggest that ST28 was significantly better than BCG at limiting the pulmonary pathology at day 40 post-infection.
Table 1.
Reduction in CFU in guinea pigs vaccinated with ST28 and then infected with a low dose aerosol of M. tuberculosis H37Rv. Guinea pigs were vaccinated with either 103 CFU of the mutant or 103 CFU BCG Pasteur, rested for 10 weeks and then infected with approximately 10–20 CFU of M. tuberculosis H37Rv. CFU was determined in the lungs and spleens at day 30 post infection. N=5 guinea pigs per group. Log10 CFU reduction was determined by subtracting the CFU for the vaccinated group from the CFU for the saline-treated group. A one-way Analysis of Variance (ANOVA) was performed to determine differences between groups.
| Group | Lung | Spleen | ||||
|---|---|---|---|---|---|---|
| Mean Log10 CFU | SD | Log10 CFU Reduction | Mean Log10 CFU | SD | Log10 CFU Reduction | |
| Saline | 4.73 | 0.40 | 4.75 | 0.97 | ||
| BCG | 3.62*** | 0.28 | 1.11 | 2.30** | 0.44 | 2.45 |
| ST28 | 3.22*** | 0.21 | 1.52 | 2.45** | 1.00 | 2.30 |
p<0.01,
p<0.001.
SD = standard deviation.
Figure 2.
Cytokine mRNA expression in the lungs (A) and spleens (B) of guinea pigs inoculated with BCG or ST28 at day 40 after low dose pulmonary infection with M. tuberculosis H37Rv. Data are expressed as Log10 copy number based on a standard curve using a known concentration/copy number of the cytokine amplicon. N = 5 guinea pigs per group. * p<0.05, ** p<0.01, *** p<0.001.
Figure 3.
Histopathology assessment of the lungs in guinea pigs vaccinated with BCG or ST28 at day 40 after low dose aerosol challenge with M. tuberculosis H37Rv. Photomicrographs illustrate the extent of lung pathology for each group, where Group 1 = Saline-treated (guinea pig #2), Group 2 = BCG vaccinated (guinea pig #10) and Group 3 = ST28 inoculated (guinea pig #14). Granulomas with central necrosis were frequent in the saline-group, while granulomatous inflammation without necrosis occurred in some BCG-inoculated animals. Only minimal interstitial leukocyte infiltration occurred in the ST28 inoculated animals. Top panels: 20X, bottom panels: 100X magnification. The bar charts represent the mean pathology score for each group as described in the Materials and Methods. N = 5 guinea pigs per group. * p<0.05, *** p<0.001.
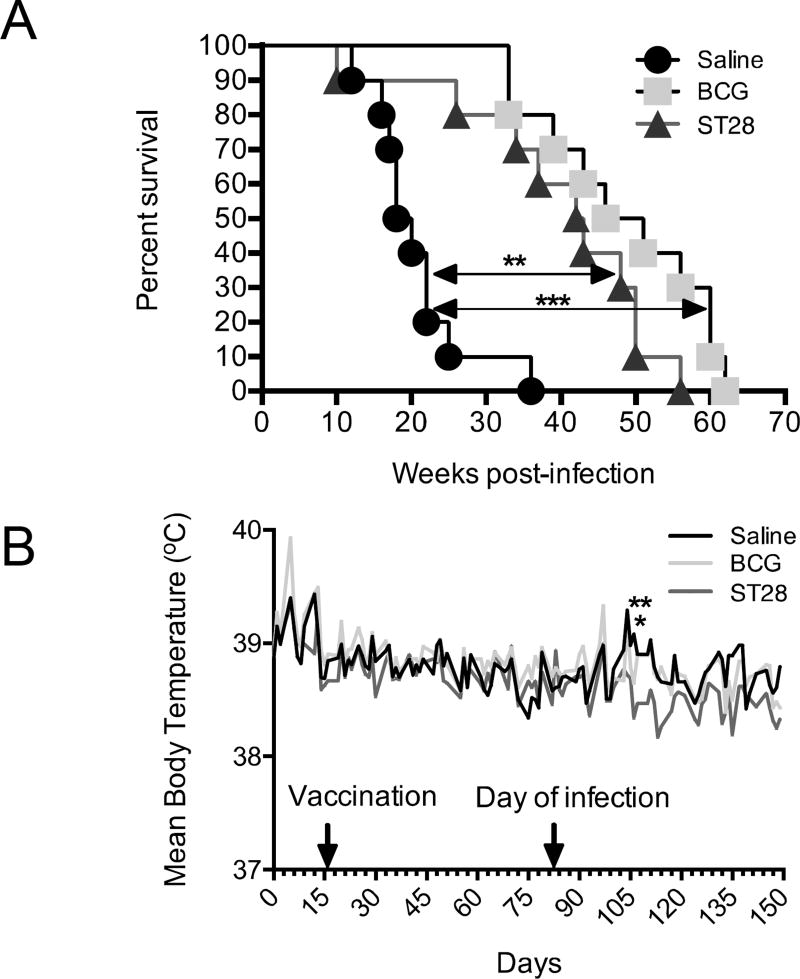
M. tuberculosis sigE mutant significantly prolongs the survival of infected guinea pigs
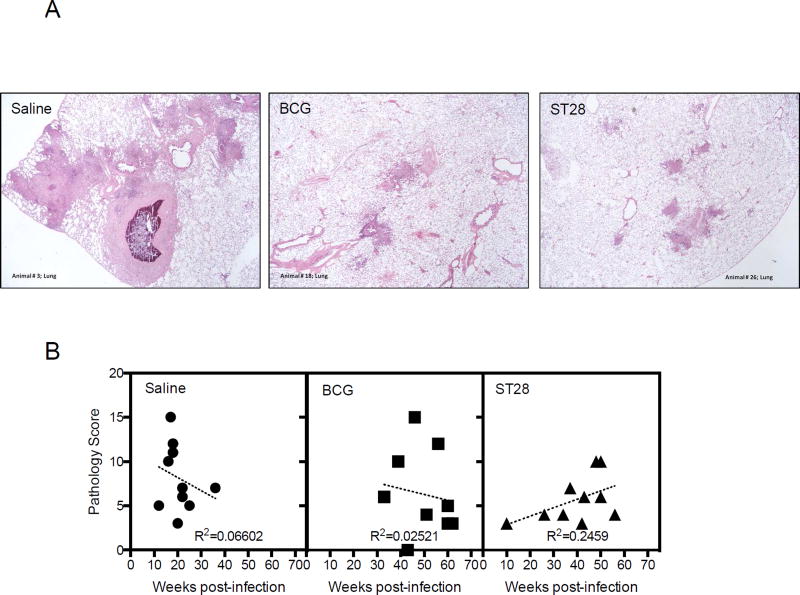
As a measure of vaccine efficacy, the long-term disease based guinea pig model can provide important information about the ability of the vaccine to be able to limit disease. Given that the pathology observed in the guinea pig is similar to that observed in humans, we wanted to determine if ST28, when used as a vaccine had the ability to limit disease and prolong the life of infected guinea pigs. Guinea pigs were vaccinated, infected and followed over time as described above. The sigE mutant ST28 vaccination resulted in a significant prolonged survival of guinea pigs similar to that observed with BCG (Figure 4A) as there was no significant difference between these groups. ST28 significantly prolonged the life of guinea pigs compared to the saline-treated group (p<0.01). In addition, ST28 vaccination significantly prevented the increase in body temperature that is commonly observed in infected guinea pigs at 25 to 30 days post-infections, suggesting that there was limited disease during infection (Figure 4B, day 106, p<0.01), and BCG had a similar but less pronounced effect (Figure 4B, Day 106, p<0.05). There was no significant difference in lung CFU between groups (data not shown). Histological analysis of the lungs, spleens and livers at the time of necropsy revealed that there was a trend towards fewer lesions in the organs of vaccinated animals (Figure 5A) although this amelioration was not significant when quantitated. Analysis of the lung pathology score in relation to time to necropsy revealed no significant correlation between the two outcomes (Figure 5B). Similar correlations were observed in the spleen and liver (Supplementary Figure S2A and B).
Figure 4.
Kaplan-Meier plot (A) of the survival of saline-treated, BCG-vaccinated and ST28 inoculated guinea pigs after low-dose aerosol infection with M. tuberculosis H37Rv. Guinea pigs were monitored daily for body temperature (B) and well-being and weighed weekly to determine their health status. N = 10 guinea pigs per group.
Figure 5.
Representative photomicrographs (A) of lungs from saline-treated, BCG-vaccinated and ST28 inoculated guinea pigs at the time of necropsy, after low-dose aerosol infection with M. tuberculosis H37Rv (40× magnification). The dot plots represent the pathology score for each guinea pig in each group in relation to the time of euthanasia (B), as described in the Materials and Methods. N = 10 guinea pigs per group.
Discussion
Vaccines against M. tuberculosis are urgently needed and there is currently a pipeline of vaccine candidates based on various formulations including the attenuation of M. tuberculosis through specific gene mutations [20]. Others have developed attenuated M. tuberculosis and, indeed, one is based on two independent stable deletion mutations in the virulence genes phoP and fadD26 and is in clinical trials [21]. The current study examined the use, as a vaccine, of an M. tuberculosis mutant in which the sigE gene was disrupted. The mutant strain had minimal growth in vivo, caused limited pathology and induced potent immunity in the guinea pig and, when used as a vaccine, it reduced the growth of virulent M. tuberculosis after pulmonary infection. The sigE mutant significantly prolonged the survival of guinea pigs infected with a low dose aerosol of virulent M. tuberculosis H37Rv. In both studies ST28 was as potent as BCG when used as a vaccine.
ST28 induced a cytokine response in guinea pigs after intradermal inoculation, particularly the day 30 IFN-γ response in the spleen, suggesting the induction of innate and adaptive immunity. Attenuated strains of M. tuberculosis to be used as vaccines have to be assessed for two factors; (1) sufficient attenuation so as not to cause morbidity and mortality in the host and (2) not overly attenuated to the extent that it does not induce a protective immune response. Given the fact that the guinea pig is highly susceptible to infection with M. tuberculosis, this animal model provides us with the ability to determine if an attenuated vaccine can induce an immune response, while not inducing pathology. After pulmonary infection with virulent M. tuberculosis, ST28-vaccinated guinea pigs had significant increases in splenic IFN-γ and TNF-α mRNA, two cytokines that are important for clearance of M. tuberculosis [14], when compared to saline-treated and BCG-vaccinated animals. In the lungs, IFN-γ mRNA was significantly elevated in the saline–treated animals when compared to the BCG-vaccinated group only, suggesting the involvement of other IFN-γ independent mechanisms associated with pulmonary immunity. Lung and spleen IL-17 mRNA expression from BCG and ST28 inoculated guinea pigs was significantly reduced when compared to saline treated animals, suggesting that a reduction in IL-17 may be associated with a reduction of CFU and pathology. Others have shown a role for IL-17, such as in the induction of protective CD4+T cells required for clearance of M. tuberculosis [22], suggesting the need to recruit IL-17-secreting cells into the lung to establish a protective immune response. There is no doubt that IL-17 expression is up-regulated during M. tuberculosis infection, but the question remains as to the importance of this cytokine in vaccine mediated immunity. The importance of IL-17 may also depend on the type of vaccine used and, as we demonstrated, the requirement for IL-17 in an MPL based vaccine for induction of protective immunity was not absolute [23]. IL-10 mRNA expression was significantly up regulated in the spleens of ST28 inoculated animals and may reflect the ability of the mutant to prevent pathology in extra-pulmonary sites. IP-10 is induced by IFN-γ and has been suggested to be an alternative marker for mycobacterial infection in humans [24, 25]. After infection, there was a significant increase in splenic IP-10 in animals vaccinated with the ST28, which correlated with the IFN-γ mRNA expression. IP-10 is known to be involved in effector T cell generation and trafficking and increased expression in the spleen suggests that it may be involved in anti-tuberculosis immunity through the recruitment and activation of T cells [26]. In general, the data show that ST28 elicits a potent immune response resulting in reduced mycobacterial numbers.
Our findings also suggest that use of the ST28 as a vaccine can prolong the life span and reduce disease in M. tuberculosis infected guinea pigs similar to that observed with BCG. As with the testing all tuberculosis vaccines in animal models, the guinea pig model provides us with data to suggest that ST28 can affect the growth of M. tuberculosis resulting in reduction of disease. However, the mechanisms remain unclear and thus moving forward with this vaccine will require a better understanding of whether it kills M. tuberculosis via a different mechanism. It is well recognized that the use of attenuated M. tuberculosis requires the presence of at least two unrelated mutations to prevent the possibility of reversion to the wild-type [27]. However, as with the development of the majority of attenuated M. tuberculosis vaccines, attenuation has been initially tested as a single gene knockout to determine if the attenuation resulted in a mutant that could indeed induce protective immunity, and that it did not induce pathology [5, 11]. Thus, ST28 represents the first stage of development of a novel attenuated M. tuberculosis vaccine that will require further development. The guinea pig model provides an important step in the pre-clinical screening process of these types of vaccines and in vivo assessment provides a potent means to determine if the mutation can truly attenuate a virulent M. tuberculosis, but not to the point where it is cleared too efficiently so as not to actually induce protective immunity.
Supplementary Material
Acknowledgments
The guinea pig studies were supported by the NIH/NIAID program Advanced Small Animal Models for the Testing of Candidate Therapeutic and Preventative Interventions against Mycobacteria (HHSN272201000009I-003, task order 12) at Colorado State University. RM laboratory was supported by the European Community Seventh Framework Programme (FP7/2007–2013) under grant agreements 241745.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andersen P, Doherty TM. The success and failure of BCG - implications for a novel tuberculosis vaccine. Nat Rev Microbiol. 2005;3(8):656–62. doi: 10.1038/nrmicro1211. [DOI] [PubMed] [Google Scholar]
- 2.Delogu G, et al. Mycobacterium tuberculosis virulence: insights and impact on vaccine development. Future Microbiol. 2015;10(7):1177–94. doi: 10.2217/fmb.15.26. [DOI] [PubMed] [Google Scholar]
- 3.Kaufmann SH, et al. The BCG replacement vaccine VPM1002: from drawing board to clinical trial. Expert Rev Vaccines. 2014;13(5):619–30. doi: 10.1586/14760584.2014.905746. [DOI] [PubMed] [Google Scholar]
- 4.Martin C, et al. The live Mycobacterium tuberculosis phoP mutant strain is more attenuated than BCG and confers protective immunity against tuberculosis in mice and guinea pigs. Vaccine. 2006;24(17):3408–19. doi: 10.1016/j.vaccine.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 5.Arbues A, et al. Construction, characterization and preclinical evaluation of MTBVAC, the first live-attenuated M. tuberculosis-based vaccine to enter clinical trials. Vaccine. 2013;31(42):4867–73. doi: 10.1016/j.vaccine.2013.07.051. [DOI] [PubMed] [Google Scholar]
- 6.Spertini F, et al. Safety of human immunisation with a live-attenuated Mycobacterium tuberculosis vaccine: a randomised, double-blind, controlled phase I trial. Lancet Respir Med. 2015;3(12):953–62. doi: 10.1016/S2213-2600(15)00435-X. [DOI] [PubMed] [Google Scholar]
- 7.Manganelli R, et al. The Mycobacterium tuberculosis ECF sigma factor sigmaE: role in global gene expression and survival in macrophages. Mol Microbiol. 2001;41(2):423–37. doi: 10.1046/j.1365-2958.2001.02525.x. [DOI] [PubMed] [Google Scholar]
- 8.Manganelli R, et al. The extra cytoplasmic function sigma factor sigma(E) is essential for Mycobacterium tuberculosis virulence in mice. Infect Immun. 2004;72(5):3038–41. doi: 10.1128/IAI.72.5.3038-3041.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernandez Pando R, et al. Immunogenicity and protection induced by a Mycobacterium tuberculosis sigE mutant in a BALB/c mouse model of progressive pulmonary tuberculosis. Infect Immun. 2010;78(7):3168–76. doi: 10.1128/IAI.00023-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casonato S, et al. Mycobacterium tuberculosis requires the ECF sigma factor SigE to arrest phagosome maturation. PLoS One. 2014;9(9):e108893. doi: 10.1371/journal.pone.0108893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaushal D, et al. Mucosal vaccination with attenuated Mycobacterium tuberculosis induces strong central memory responses and protects against tuberculosis. Nat Commun. 2015;6:8533. doi: 10.1038/ncomms9533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manganelli R, et al. Role of the extracytoplasmic-function sigma factor sigma(H) in Mycobacterium tuberculosis global gene expression. Mol Microbiol. 2002;45(2):365–74. doi: 10.1046/j.1365-2958.2002.03005.x. [DOI] [PubMed] [Google Scholar]
- 13.Kaushal D, et al. Reduced immunopathology and mortality despite tissue persistence in a Mycobacterium tuberculosis mutant lacking alternative sigma factor, SigH. Proc Natl Acad Sci U S A. 2002;99(12):8330–5. doi: 10.1073/pnas.102055799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grover A, et al. Kinetics of the immune response profile in guinea pigs after vaccination with Mycobacterium bovis BCG and infection with Mycobacterium tuberculosis. Infect Immun. 2009;77(11):4837–46. doi: 10.1128/IAI.00704-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allen SS, McMurray DN. Coordinate cytokine gene expression in vivo following induction of tuberculous pleurisy in guinea pigs. Infect Immun. 2003;71(8):4271–7. doi: 10.1128/IAI.71.8.4271-4277.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho H, et al. Recombinant guinea pig tumor necrosis factor alpha stimulates the expression of interleukin-12 and the inhibition of Mycobacterium tuberculosis growth in macrophages. Infect Immun. 2005;73(3):1367–76. doi: 10.1128/IAI.73.3.1367-1376.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rawat KD, et al. Expression of CXCL10 (IP-10) and CXCL11 (I-TAC) chemokines during Mycobacterium tuberculosis infection and immunoprophylaxis with Mycobacterium indicus pranii (Mw) in guinea pig. Infect Genet Evol. 2013;13:11–7. doi: 10.1016/j.meegid.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Dhanasekaran S, et al. Comparison of different standards for real-time PCR-based absolute quantification. J Immunol Methods. 2010;354(1–2):34–9. doi: 10.1016/j.jim.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Drumm JE, et al. Mycobacterium tuberculosis universal stress protein Rv2623 regulates bacillary growth by ATP-Binding: requirement for establishing chronic persistent infection. PLoS Pathog. 2009;5(5):e1000460. doi: 10.1371/journal.ppat.1000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scriba TJ, et al. Vaccination Against Tuberculosis With Whole-Cell Mycobacterial Vaccines. J Infect Dis. 2016;214:659–64. doi: 10.1093/infdis/jiw228. [DOI] [PubMed] [Google Scholar]
- 21.Aguilo N, et al. MTBVAC vaccine is safe, immunogenic and confers protective efficacy against Mycobacterium tuberculosis in newborn mice. Tuberculosis (Edinb) 2016;96:71–4. doi: 10.1016/j.tube.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khader SA, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8(4):369–77. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 23.Esparza-Gonzalez SC, Troy AR, Izzo AA. Comparative analysis of Bacillus subtilis spores and monophosphoryl lipid A as adjuvants of protein-based mycobacterium tuberculosis-based vaccines: partial requirement for interleukin-17a for induction of protective immunity. Clin Vaccine Immunol. 2014;21(4):501–8. doi: 10.1128/CVI.00622-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen KY, et al. Novel biomarker analysis of pleural effusion enhances differentiation of tuberculous from malignant pleural effusion. Int J Gen Med. 2016;9:183–9. doi: 10.2147/IJGM.S100237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blauenfeldt T, et al. Thermostability of IFN-gamma and IP-10 release assays for latent infection with Mycobacterium tuberculosis: A TBnet study. Tuberculosis (Edinb) 2016;98:7–12. doi: 10.1016/j.tube.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 26.Dufour JH, et al. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol. 2002;168(7):3195–204. doi: 10.4049/jimmunol.168.7.3195. [DOI] [PubMed] [Google Scholar]
- 27.Kamath AT, et al. New live mycobacterial vaccines: the Geneva consensus on essential steps towards clinical development. Vaccine. 2005;23(29):3753–61. doi: 10.1016/j.vaccine.2005.03.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.