Abstract
Rationale
The long-term course and outcome of de-novo autoimmune hepatitis (DAIH) is unknown. A retrospective multi-center study assessing associations and long-term consequences of DAIH developing in a transplanted allograft is presented.
Methods
Children with DAIH were followed from diagnosis until death, re-LT, or transfer of care and for a minimum of 1 year.
Results
31 patients of 1833 (1.7%) LT were identified; 29 followed for a median of 7.1 years (range, 1.6–15); 52% had no rejection preceding diagnosis of DAIH. Transminases fell following treatment with steroids and antimetabolites (ALT 108 vs. 39 U/L (p = 0.002); AST 112 vs. 52 U/L (p = 0.003); GGT 72 vs. 36 U/L (p=0.03), but this was not universally sustained. Transaminases > 2X ULN observed in 38% of patients at last follow-up; commonly GGT, attributed to bile duct injury and ductopenia. Portal hypertension (PHT) seen in four patients and associated with severe fibrosis and cirrhosis. Re-LT occurred in two patients for chronic rejection (CR) and uncontrolled PHT with gastrointestinal bleeding respectively. No deaths from DAIH reported.
Conclusion
DAIH is an uncommon complication following pediatric LT requiring prolonged and augmented immunosuppression. It is associated with continued allograft dysfunction and may lead to bile duct injury, CR and PHT necessitating re-LT.
Keywords: Allograft dysfunction, Bile duct injury, Chronic rejection, Liver re-transplantation, Portal hypertension
INTRODUCTION
Advances in liver transplantation have resulted in improved survival and better outcomes for most patients with liver disease. However, with these advances and longevity of liver allografts come late graft dysfunction that is often difficult to diagnose and represents a significant medical management issue. One of the important causes of chronic liver allograft dysfunction is de novo autoimmune hepatitis (DAIH), defined as late graft dysfunction, associated with autoimmune features in liver transplant patients without previous autoimmune hepatitis, and seen in 2.1%–6.6% of pediatric liver transplant recipients (1–9). Though the underlying etiology and pathogenesis are not well understood, several mechanisms have been proposed to underlie the development of DAIH and more recently, monocyte/macrophage derived cytokines of the IL-12-IFN-γ cluster and IL-6-IL-17 cluster have been demonstrated to be associated with DAIH resulting in a pro-inflammatory milieu that drives paralysis of regulatory function; additionally, regulatory T cells of transplant recipients with DAIH have been reported to display a pro-inflammatory phenotype with functional impairment akin to similar observations in many classical autoimmune diseases (10, 11).
Some of the risk factors associated with development of DAIH include number of acute rejection episodes, steroid dependence (1), human lymphocyte antigen DR3 (HLA DR3) genotype (4), female gender and age ≥ 40 years of the donor (12), and glutathione S-transferase T1 (GSTT1) donor/recipient mismatch (13, 14). The poorly understood pathogenesis and natural history of DAIH has contributed to a lack of consensus on the optimal management of DAIH (15–17).
The concern for long-term allograft health is important for all transplant recipients, particularly those who receive organ transplants as infants and young children as the expectation is for them to maintain the same graft into adulthood. While there are several single center reports highlighting risk factors associated with the development of DAIH and short-mid term patient outcome, to the best of our knowledge, there are no reports of the natural history and long-term consequences of DAIH developing in a transplanted allograft. Given the role DAIH plays as a cause of chronic liver allograft dysfunction in pediatric liver transplant recipients, we sought to characterize the long-term course and outcome of DAIH in terms of complications, graft loss, need for re-transplantation, and management across five large pediatric liver transplant centers, in a multi-center, multi-national, retrospective study of pediatric liver transplant recipients.
METHODS
Patients
Children with a diagnosis of DAIH, followed at Yale New Haven Children’s Hospital, Connecticut; Birmingham Children’s Hospital, United Kingdom; Morgan Stanley Children’s Hospital, New York; Hospital for Sick Children, Toronto, Canada; and Ann and Robert H, Lurie Children’s Hospital, Chicago; were followed from diagnosis until their last follow up visit, death, re-liver transplantation (re-LT), or transfer of care and for a minimum of one year. The definition of DAIH was as previously described: a liver transplant recipient without prior history of autoimmune liver disease presenting with graft dysfunction characterized by elevated aminotransferases; elevated serum immunoglobulin G, positive autoantibody titers: anti-nuclear antibody (ANA), anti-smooth muscle antibody (ASMA) or anti-liver kidney microsomal antibody (anti-LKM); and characteristic biopsy findings of dense lymphocytic portal-tract infiltrate with plasma cells and interface hepatitis (1, 18). Each contributing center made the diagnosis of DAIH based on this definition and no set number or combination of criteria was decided a priori for inclusion in the report. Patients were identified following query of individual center databases. Patients were excluded if graft dysfunction was attributable to any of the following causes: acute and chronic rejection, hepatitis B and C infection, Epstein Barr Virus and Cytomegalovirus (EBV, CMV) infections, vascular problems, biliary complication, drug toxicity, sepsis, recurrence of primary disease or post-transplant lymphoproliferative disease.
DAIH exacerbation was defined as elevated liver enzymes with liver biopsy supporting active DAIH (1, 18) with no other liver pathologies such as acute or chronic rejection, vascular or biliary pathology or infectious etiology. The treating physician at the primary center made the final determination of a diagnosis of DAIH exacerbation. Diagnosis of acute or chronic rejection was biopsy proven based on the accepted Banff criteria (19, 20). A local pathologist interpreted liver biopsies at each contributing center.
Demographics and transplant characteristics
In addition to demographic information, information was collected on graft type, use of induction therapy at transplant; vascular and biliary surgical complications preceding diagnosis of DAIH, as well as potential triggers including history of Cytomegalovirus (CMV) infection and disease, Epstein Barr virus (EBV) infection and post transplant lymphoproliferative disease (PTLD) and number of episodes of acute rejection preceding diagnosis of DAIH. Hepatitis E virus infection was not routinely tested for at the time of diagnosis of DAIH in participating centers. Data was also not available on recipient/donor HLA, donor specific antibodies and GSTT1 mismatching, as these were not routinely performed.
The calcineurin inhibitor dose and level, steroid dose, and other additional liver specific medication at time of diagnosis were obtained. Treatment initiated following DAIH diagnosis, number of exacerbations, and treatment at last clinic visit were obtained from the medical record. The calcineurin inhibitor, mTOR (mammalian target of rapamycin) inhibitor, and 6-thioguanine drug levels following treatment initiation and at last follow-up were not obtained. Other outcome measures obtained included: re-listing for transplant, re-transplantation, portal hypertension, PTLD, CMV infection, glomerular filtration rate < 60 ml/min/1.73m2, death and cause of death. Portal hypertension was defined as the presence of hypersplenism, splenomegaly, ascites, or esophageal/gastric varices. Renal function was assessed using Tc-99m diethylene-triamine-penta-acetic acid (DTPA) scan, cystatin C or 24-hour urine creatinine clearance. Estimated glomerular filtration rate (eGFR) was calculated from cystatin C as previously described (21). Lastly, available data on serum transaminases, serum bilirubin, autoantibody titers, serum immunoglobulin G (IgG) levels obtained at time of diagnosis and yearly till last clinic visit were reviewed. The above data was extracted individually by each center. The Institutional Review Board at the participating centers approved this study.
Statistics
Descriptive statistics were employed for data presentation; median, mean, standard deviation, range and 25–75% quintile were used for continuous variables and proportions for categorical variables. Continuous variables were compared using a two-tail student t-test.
RESULTS
Thirty-one (1.7%) patients (58% female) of 1833 liver transplant recipients were diagnosed with DAIH at a median of 5.3 (range 1.2 – 14.9) years after liver transplant. The dates of transplant ranged between 1990 and 2009 and dates of DAIH diagnosis between 1997 and 2013. Follow up data was available for twenty-nine patients with a median (range) follow-up of 7.1-years (1.6 – 15-years) from the diagnosis of DAIH. The demographic and transplant characteristics of the study subjects are as shown in Table 1. 59% had a pre-transplant diagnosis of biliary atresia; Importantly, no patient had a pre-transplant diagnosis of autoimmune liver disease. Median alanine aminotransferase (ALT) at diagnosis was 108 IU/L (IQR: 40–216), gamma glutamyl transferase (GGT) 72 IU/L (IQR: 23–173), IgG 16.7 g/l (IQR: 16.2–19.1) and total bilirubin 0.7 mg/dl (IQR:0.5–0.9) (Table 1). Five patients (17.2%) had a normal ALT and GGT at the time of diagnosis; however aspartate aminotransferase (AST) was elevated. Allograft dysfunction was fully investigated and was not a result of rejection, infection, a vascular or biliary complication, or drug-induced; Additionally, all patients had characteristic biopsy findings of dense lymphocytic portal-tract infiltrate with plasma cells and interface hepatitis at diagnosis. Twenty patients had positive autoantibodies (two anti-liver-kidney-microsomal antibody positivity, twelve anti-nuclear antibody positivity, five anti-smooth muscle antibody positivity and one anti-nuclear antibody + anti-smooth muscle antibody positivity), and the remaining patients had elevated Immunoglobulin G levels at diagnosis. Autoantibody titers ranged between 1:40 and 1:1600 for ANA/ASMA and 1:1280 and 1:5120 for anti-LKM.
TABLE 1.
Demographics and transplant characteristics of study participants
| De novo autoimmune hepatitis (n = 29) |
|
|---|---|
|
| |
| Gender (m/f) | 11/18 |
|
| |
| Ethnicity | |
| Black | 8 |
| White | 15 |
| Hispanic | 2 |
| Asian | 4 |
|
| |
| Age at transplant median (range) years | 1.5 (0.2–12.3) |
|
| |
| Graft type (n): | |
| DD | 24 |
| LD | 4 |
| UNK | 1 |
|
| |
| Induction therapy at time of transplant (n): | |
| OKT3 | 0 |
| ATG | 0 |
| sIL-2R antagonist | 1 |
| anti-CD52 | 0 |
|
| |
| Pre transplant diagnosis (n): | |
| Biliary atresia | 17 |
| Acute liver failure | 5 |
| Metabolic | 2 |
| Other | 5 |
|
| |
| Duration from transplant at DAIH diagnosis median (range) years: | 5.3 (1.2 – 14.9) |
|
| |
| Liver enzymes at diagnosis median (range): | |
| ALT (5 – 45 IU/L) | 108 (17 – 918) |
| GGT (5 – 32 IU/L) | 72 (16 – 695) |
| Total bilirubin (<1 mg/dl) | 0.7 (0.2 – 12.2) |
|
| |
| Immunoglobulin G at diagnosis median (25–75% quartile): IgG (mg/dl) | 1670 (1620 – 1910) |
|
| |
| Calcineurin inhibitor at diagnosis (n): | |
| Cyclosporine | |
| Tacrolimus | 13 16 |
|
| |
| Number of patients on steroids at diagnosis | 5 |
|
| |
| Treatment once diagnosis made: | |
| Steroid (mg/kg) (mean ± SD) | 0.66 ± 0.68 |
| Azathioprine (n) | 14 |
| Mycophenolate (n) | 1 |
| Sirolimus (n) | 1 |
|
| |
| Number of exacerbations mean ± SD (n=17) | 1.2 ± 1.4 |
|
| |
| Complications pre DAIH diagnosis (n): | |
| Biliary | 6 |
| Arterial | 0 |
| Portal Vein | 2 |
| Hepatic Vein | 1 |
| CMV Infection | 2 |
| CMV Disease | 1 |
| PTLD | 1 |
| Episodes of Acute Cellular Rejection pre | 14 |
| DAIH diagnosis (n): | |
| 0 | 15 |
| 1 | 8 |
| 2 | 1 |
| >2 | 4 |
|
| |
| Complications post DAIH diagnosis (n): | |
| Re-listed | 3 |
| Re-transplanted | 2 |
| Portal hypertension | 4 |
| GFR < 60 ml/min/1.73m2 | 4 |
| PTLD | 0 |
| CMV Infection | 1 |
| Death | 1 |
|
| |
| Duration of follow up median (range) years | 7.1 (1.6 – 15) |
Reference values from Nelson Textbook of Pediatrics, 20th Ed, chapter 727, 3464–3473.
Immunosuppressive therapy at diagnosis
Three patients were on an anti-metabolite at the time of diagnosis (2 Mycophenolate mofetil, 1 Azathioprine). Five patients (17%) received steroids at a dose ranging between 0.1 – 0.42 mg/kg/day, at the time a diagnosis of DAIH was made. The calcineurin inhibitor of choice at diagnosis (prior to any treatment being initiated) was almost equally distributed with sixteen patients (55.1%) being on Tacrolimus and thirteen patients (44.8%) being on Cyclosporine, with median (range) 12-hour trough levels of 6.6 (3 – 17.3) ng/ml (Tacrolimus), 73.5 (42 – 131) ng/ml (Cyclosporine) and 2-hour peak Cyclosporine level of 431.5 (298 – 365) ng/ml (measured in two patients).
Post transplant complications pre-DAIH diagnosis
Fifteen patients (52%) had no episode of acute rejection preceding the diagnosis of DAIH; eight patients (27.5%) had one acute rejection episode, one patient (3.4%) had two, four patients (13.7%) had four or more acute rejection episodes preceding diagnosis of DAIH. We were unable to retrieve information on preceding acute rejection episodes in one patient. Only one patient had an episode of acute rejection preceding the diagnosis of DAIH by less than 6-months; the majority of acute rejection episodes occurred greater than 6-months prior to a diagnosis of DAIH being made; Additionally, the majority of the acute rejection episodes occurred greater than 6-months post liver transplant. Of the eleven patients with trough Tacrolimus levels ≥ 6 ng/ml at the time of diagnosis, seven had ≥ 1 episodes of acute rejection preceding the diagnosis of DAIH.
There was a past history of CMV infection predating the diagnosis of DAIH in two patients with one of the patients developing CMV disease. Early tonsillar PTLD occurred in one patient prior to the diagnosis of DAIH, and leiomyosarcoma in a second patient. Biliary complications (biliary strictures) predated the diagnosis of DAIH in six patients.
DAIH - long-term course and therapy
Twenty-one patients (72%) were placed on steroids once diagnosis was made, with a mean starting dose of 0.66 ± 0.68 mg/kg/day (range: 0.5–2 mg/kg/day). Anti-metabolite therapy with Azathioprine or Mycophenolate Mofetil was initiated in fourteen and one patient respectively once the diagnosis of DAIH was made. The sixteen patients on Tacrolimus at diagnosis remained on Tacrolimus, and the thirteen on Cyclosporine remained on Cyclosporine. The details of the treatment regimens used following diagnosis of DAIH for each patient is shown in Table 2A.
TABLE 2A.
Treatment regimens after diagnosis of DAIH made in all subjects.
| Treatment | Number of subjects |
|---|---|
| Tacrolimus alone | 2 |
| Tacrolimus + Steroid | 3 |
| Tacrolimus + Steroid + Azathioprine | 9 |
| Tacrolimus + Steroid + Mycophenolate | 1 |
| Tacrolimus + Steroid + Sirolimus | 1 |
| Cyclosporine alone | 5 |
| Cyclosporine + Steroid | 3 |
| Cyclosporine + Azathioprine | 1 |
| Cyclosporine + Steroid + Azathioprine | 4 |
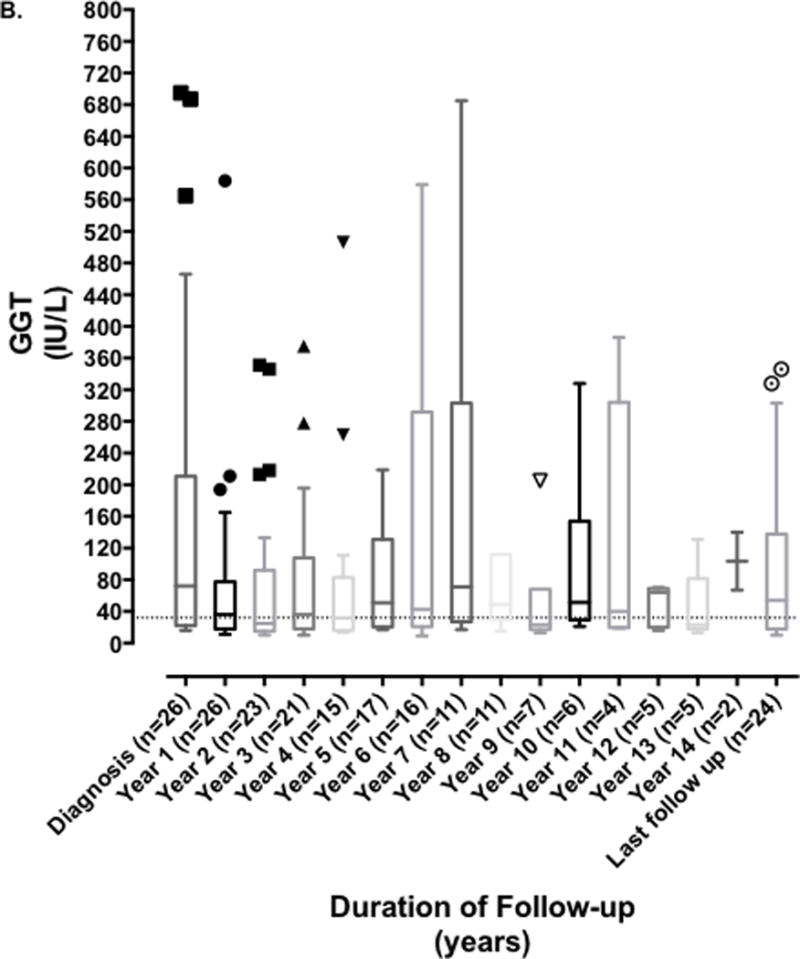
The median (range) of serum ALT, GGT and total bilirubin at years 1, 5, 10, 13 post diagnosis and at last follow-up are shown in Table 2B. Median serum ALT, AST and GGT fell significantly in the first year after diagnosis (ALT 108 vs. 39 U/L (p = 0.002); AST 112 vs. 52 U/L (p = 0.003); GGT 72 vs. 36 U/L (p=0.03), however this was not sustained as serum ALT, AST, and GGT subsequently fluctuated throughout disease course and follow up as shown in Figure 1; with median levels of 30, 37.5 and 54 U/L respectively at last follow-up. We were unable to compare serum IgG levels or autoantibodies levels at diagnosis and follow-up as this was not routinely obtained following the initial level at time of diagnosis at all centers.
TABLE 2B.
Evolution of serum aminotransferases
| ALT | GGT | Total bilirubin | |
|---|---|---|---|
|
| |||
| Year 1 (n=26) | 39 (11 – 199) | 36 (11 – 584) | 0.6 (0.2 – 1.6) |
|
| |||
| Year 5 (n=19) | 44 (13 – 415) | 55 (17 – 837) | 0.7 (0.2 – 3.9) |
|
| |||
| Year 10 (n=6) | 48 (26 – 157) | 51.5 (21 – 328) | 1.2 (0.7 – 1.8) |
|
| |||
| Year 13 (n=5) | 31 (16 – 235) | 23 (13 – 131) | 1.2 (0.7 – 2.0) |
| Last visit (n=25) | 30 (10 – 235) | 54 (10 – 346) | 0.8 (0.2 – 2.9) |
Reference values from Nelson Textbook of Pediatrics, 20th Ed, chapter 727, 3464–3473.
FIGURE 1. Evolution of serum aminotransferases and gamma glutamyl transferase over duration of disease and follow-up. Serum alanine aminotransferase (ALT) and gamma glutamyl transferase (GGT) measured at time of diagnosis of DAIH and subsequently yearly up to last known follow-up.
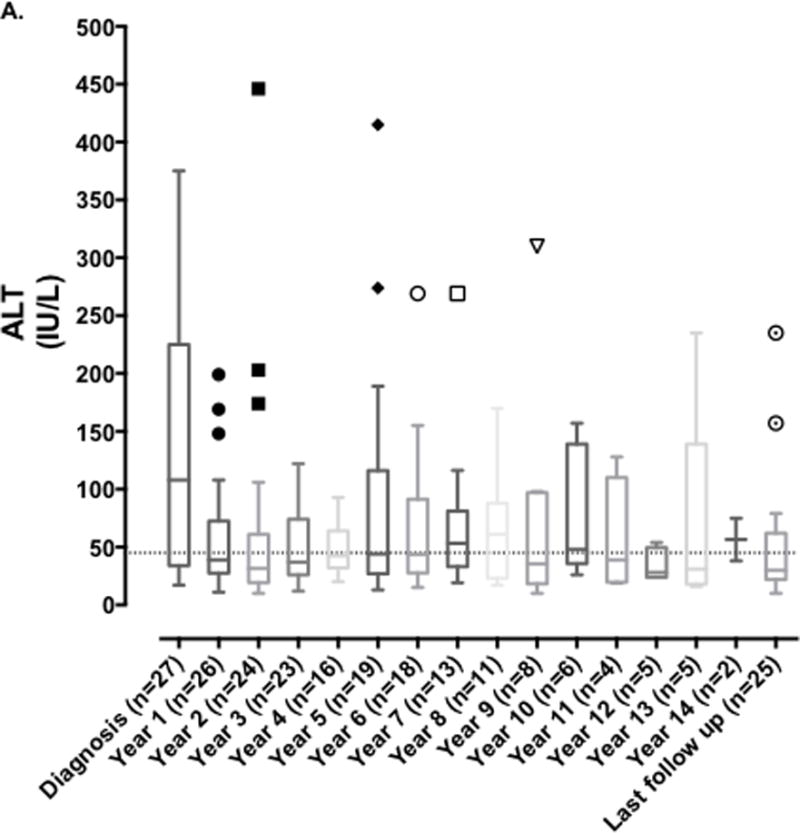
A) ALT levels fluctuated throughout disease course and follow up, remaining above the upper limit of normal.
B) GGT levels fluctuated throughout disease course and follow up, remaining above the upper limit of normal.
Seventeen patients (58%) had a history of disease exacerbation: eight patients (27.5%) had at least one documented episode of disease flare, and nine patients (31%) had two or more documented episodes of disease flare. Disease exacerbations were biopsy proven in all but one patient in whom two episodes of liver enzyme abnormality were attributed to disease flares without biopsy support, thus a repeat liver biopsy was required in 58% of patients following the initial biopsy performed at time of diagnosis.
At the time of last follow-up, eighteen patients (62%) were receiving steroids (seventeen Prednisone, one Budesonide), eight Mycophenolate (27.5%), thirteen Azathioprine (44.8%), and two Sirolimus (6.8%). Sirolimus was complementary (with Tacrolimus) in the two patients. There were more patients on Tacrolimus (68.9%) compared to Cyclosporine (24.1%) suggesting that some patients were converted to Tacrolimus over the follow up period. Despite this, abnormal liver enzymes > 2X ULN was observed in 38% of patients at time of last follow-up, most commonly GGT; of these, two patients were on steroids and Mycophenolate, seven patients were on Tacrolimus, steroids and an anti-metabolite, one patient was on Tacrolimus and Azathioprine, and one patient was on Tacrolimus and Sirolimus. Elevated serum GGT was attributed to bile duct injury in three patients, ductopenia in two patients and biliary stricture in one patient. For the remaining patients, the elevated GGT was thought to be reflective of the liver disease.
Serum aminotransferase and bilirubin levels at time of last follow-up between patients who were off steroids vs. on steroids as well as between patients who were off anti-metabolites vs. on anti-metabolites is shown in Table 3. The small numbers preclude satisfactory statistical analysis. Serum GGT at time of last follow-up tended to be lower if patients were off anti-metabolites vs. being on anti-metabolites (Table 3).
TABLE 3.
Variability in liver enzyme levels on/off treatment at last follow-up.
| On Steroids (n = 18) |
Off Steroids (n = 11) |
On anti-metabolites (n = 21) |
Off anti-metabolites (n = 8) |
|
|---|---|---|---|---|
| Median ALT | 37 | 22 | 36 | 24.5 |
| Median GGT | 71 | 27 | 69 | 21.5 |
| Median TB | 0.75 | 0.9 | 0.7 | 1.3 |
Reference values from Nelson Textbook of Pediatrics, 20th Ed, chapter 727, 3464-3473.
DAIH - Long-term complications
Despite a higher dose of IS, there were no instances of PTLD and CMV infection was documented in only one patient following diagnosis of DAIH. Moderately reduced kidney function as evidenced by a GFR < 60-ml/min/1.73 m2 was observed in four patients (13.7%). No patient was observed to have severely reduced kidney function or end-stage kidney failure. The moderately reduced kidney function observed was likely a consequence of prolonged calcineurin inhibitor exposure as these patients received their transplants between 1990 and 2009 and thus would have had a minimum of 5-years exposure to calcineurin inhibitors.
Four patients (13.7%) developed portal hypertension; three (10.3%) were re-listed for liver transplantation and two were re-transplanted at the time of manuscript preparation. Portal hypertension was associated with cirrhosis in two patients, severe fibrosis in one patient and stage 3-fibrosis with mild bile duct injury and epithelial reactive changes in one patient. Importantly, portal hypertension was not associated with biliary obstruction in the four patients; however one of the four patients had portal vein thrombosis.
The indications for re-transplantation included chronic rejection with portal hypertension in one patient and portal vein thrombosis with uncontrolled portal hypertension and gastrointestinal bleeding in the second patient. The occurrence of chronic rejection with portal hypertension was attributed to DAIH. The explant of the second patient showed replicative senescence of cholangiolar ductal epithelium with focal sclerosing cholangitis-like changes and stage 3-fibrosis (similar to changes seen on liver biopsy before re-transplantation). Of note, the new nomenclature and diagnostic criteria proposed by the BANFF working group on liver allograft pathology for DAIH includes lymphocytic cholangitis (inflammatory bile duct damage) (22). One patient died from a non-DAIH related pathology.
DISCUSSION
This retrospective multi-center study describes observational associations, natural history and long-term consequences of DAIH developing in a transplanted allograft in a cohort of pediatric liver transplant recipients followed for up to 15-years following a diagnosis of DAIH. To the best of our knowledge, this is the first such report detailing these aspects over a prolonged period [median (range) follow up – 7.1 years (1.6 – 15-years)] in a large multicenter cohort. In our multicenter cohort, DAIH was diagnosed in 1.7% of pediatric liver transplant recipients close to the lower range quoted in single center reports of 2.1% – 6.6%(1–9).
With regards to events preceding the diagnosis of DAIH, one could argue that the Cyclosporine trough and 2-hour peak levels at the time of diagnosis are fairly representative of the median duration from transplantation i.e. 5.3-years, however, the Tacrolimus trough levels appear somewhat higher than would be expected at this time from transplant. To address this discrepancy, we investigated for immunological events preceding the diagnosis of DAIH, specifically the occurrence of acute rejection.
Even though the number of acute rejection episodes has been previously reported to be a risk factor associated with development of DAIH, we found within our cohort that only forty-eight percent of patients developed acute rejection pre-DAIH diagnosis; and only one patient experienced acute rejection in the six months preceding the diagnosis of DAIH. Within the SPLIT (Studies of Pediatric Liver Transplantation) cohort, sixty-six percent of patients had one or more documented episodes of acute rejection within the first 10-years after primary liver transplant (23).
With regards to other possible triggers, CMV infection or PTLD were not common in our cohort before the diagnosis of DAIH.
Following the diagnosis of DAIH there was a rapid fall in serum aminotransferase levels which then fluctuated throughout the period of follow-up (Figure 1). This observation draws attention to the currently accepted treatment paradigm and suggests we are either not optimizing our treatments, or perhaps the current treatment options may not be optimal and new treatment approaches should be studied. This is also supported by the fact that disease exacerbation was reported in a little over 1/2 of the patients, and despite ~2/3rds of patients remaining on steroids and ~ 3/4 remaining on an anti-metabolite at last follow-up, the median GGT levels remained higher than the ULN (Table 3). Another possibility is that centers may have been more willing to tolerate aminotransferases in the 40’s to 50’s rather than risk consequences of further increased immunosuppression.
We next questioned if remaining on steroids or anti-metabolites long-term would influence liver enzyme levels; however the serum aspartate and alanine aminotransferases did not differ at time of last follow-up between patients who remained on/off steroids or anti-metabolites. Serum GGT tended to be lower in those off anti-metabolites vs. those who remained on anti-metabolites at last follow-up suggesting these may have been patients who had done well and were weaned off anti-metabolites. These findings may be related to guarded efficacy of the current therapies for DAIH however considering the retrospective study design it may simply reflect patient specific tailoring of immunosuppressive medications where more stable patients are weaned off steroids or antimetabolites. We also acknowledge that as immunosuppressive modifications were done according to contemporaneous local practice, it is somewhat difficult to determine the efficacy of additional steroids or antimetabolites.
Liver biopsies were not done routinely in our patients and the correlation between abnormal liver enzymes and histological changes is unclear. It is possible that routine surveillance liver biopsy in patients with DAIH will provide a better tool for follow up and optimization of therapy.
Although most of the patients with DAIH remained stable with normal graft function the consequence of this persistent biochemical abnormality with presumed continued inflammation in the allograft was the development of chronic graft histological changes of progressive fibrosis – cirrhosis with portal hypertension (n=4) necessitating liver re-transplantation in 2 patients. Portal hypertension as a direct consequence of DAIH has not been previously reported in published series however none of the published series had followed the outcomes of patients up to 15-years after the diagnosis of DAIH (1–3, 6, 7, 9, 13, 27, 28). It is unlikely that portal hypertension was secondary to a biliary or vascular problem as none of the patients with portal hypertension had biliary obstruction and even though one of the four patients with portal hypertension had portal vein thrombosis (3.4%), the remaining patients had no vascular complication. Within the SPLIT cohort, portal vein thrombosis was reported in 7% of patients (29). Re-liver transplantation was seen in 6.9% of patients in our cohort, compared to 12% of 10-year survivors in the SPLIT cohort (23).
It is interesting that elevated serum GGT levels appeared to be the commonest liver enzyme abnormality noted at last follow-up and was attributed to biliary stricture in only one patient, and bile duct injury and ductopenia in another 1/4 th and 1/5 th of patients respectively; Importantly, chronic rejection was a cause of late graft loss in one patient. DAIH has always been recognized histologically to comprise of a plasma cell rich mononuclear portal inflammatory infiltrate with varying degrees of interface hepatitis, in the absence of vascular or bile duct involvement (30). It is tempting to speculate if the subgroup of patients with persistent GGT elevation has a more aggressive variant of the disease and if on more extended follow-up of 20 – 25-years, the majority of this subgroup would have developed chronic bile duct damage leading to chronic rejection and portal hypertension with a need for re-LT. More recently, the BANFF working group on liver allograft pathology has proposed a change in the nomenclature of DAIH to plasma cell-rich rejection (22), with the criteria for diagnosis being: (i) portal and/or perivenular plasma cell-rich infiltrates with easily recognizable periportal/interface and/or perivenular necro-inflammatory activity involving a majority of portal tracts and/or central veins, (ii) lymphocytic cholangitis usually present but not absolutely required (inflammatory bile duct damage might be a relatively minor component), and (iii) original disease other than autoimmune hepatitis. The working group proposes a mixed T cell mediated rejection/Antibody mediated rejection etiology overlapping with autoimmunity supports designation as “plasma cell-rich rejection” in patients without an autoimmune hepatitis original disease diagnosis (22). One may wonder if the lymphocytic cholangitis component in the diagnosis of plasma cell rich rejection may contribute and lead overtime to chronic damage to bile ducts and elevated GGT. It is worth mentioning that elevated serum GGT at the levels observed in Figure 1B, is not a characteristic of autoimmune hepatitis in non-transplanted children.
There are several limitations to our study including the absence of histological data to correlate with liver enzymes; however, as this was not a prospective research study, the providers at each center dictated clinical care. Importantly, vascular or biliary complications that could contribute to elevated liver enzymes were sought and not found to be the cause of liver enzyme elevation in the overwhelming majority of the patients as only one patient had a biliary stricture, and another patient had portal vein thrombosis. Secondly, C4d stains were not performed on all biopsies as recommended by the BANFF working group on liver allograft pathology (22), neither were IgG and IgG4 stains performed and measurement of donor specific antibodies as this was not part of routine clinical care in most of the centers over the study period. Thirdly, the amount of steroid exposure over 15-years would have provided helpful information for care providers however due to the retrospective nature of this report, we were unable to capture this accurately. Fourthly, as we did not include a comparison group without DAIH, we are unable to comment on the potential contribution of biliary strictures, CMV viremia and EBV driven disease to development of DAIH, however, the very small numbers reported suggest they likely are not contributory to development of DAIH.
Fifthly, comparison of liver enzyme fluctuation over 15-years in age, gender, and pre-transplant diagnosis matched controls without DAIH, would have further strengthened our observation that despite treatment, ~38% of patients with DAIH have liver enzyme elevations >2X ULN. This last point notwithstanding, our data suggests there may be a need to explore alternative treatment approaches with construction of a long-term immunosuppression policy, especially in those recipients who fail to respond to the first line of treatment with steroids and anti-metabolites. Lastly, it can be argued that the treatment schedules used by participating centers showed high variability making it challenging to reach a conclusion on lack of response to treatment, however, as is demonstrated in Figure 1, liver enzymes remained >2X ULN in a little over 1/3rd of the patients over the course of the disease supporting the conclusion that their disease was not responsive to the treatment being given.
CONCLUSION
DAIH is an uncommon chronic complication following pediatric liver transplantation that requires prolonged and augmented immunosuppressive treatment. Our report suggests long-term outcomes are not benign as DAIH is associated with continued allograft dysfunction that may lead overtime to bile duct injury, chronic graft histological changes of progressive fibrosis – cirrhosis and portal hypertension necessitating liver re-transplantation in small number of patients. This is the first report documenting bile duct injury and portal hypertension as complications of DAIH. The role of routine surveillance liver biopsies in the treatment of DAIH and tailoring of IS based on these biopsies should be further explored in a prospective study setting. Importantly, improved understanding of the very early determinants of bile duct injury along with surrogate markers of aberrant inflammatory processes should be sought in an attempt to uncover truly effective therapy.
Acknowledgments
GRANTS AND FINANCIAL SUPPORT: 1CTSA Grant Number UL1 TR000142 from the National Center for Advancing Translational Science (NCATS), a component of the National Institutes of Health.
1National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number P30KD034989.
ABBREVIATIONS
- ALT
alanine aminotransferase
- ANA
anti-nuclear antibody
- ASMA
anti-smooth muscle antibody
- AST
aspartate aminotransferase
- ATG
anti-thymocyte globulin
- DAIH
de novo autoimmune hepatitis
- DTPA
Tc-99m diethylene-triamine-penta-acetic acid
- eGFR
estimated glomerular filtration rate
- GGT
gamma glutamyl transferase
- GSTT1
glutathione S-transferase T1
- HLA
human lymphocyte antigen
- LKM
anti-liver kidney microsomal antibody
- mTOR
mammalian target of rapamycin
- OKT3
muromonab-CD3
- PTLD
post-transplant lymphoproliferative disorder
Footnotes
AUTHOR CONTRIBUTION
UDE, Dept. of Pediatrics, Yale University – concept/design, data analysis/interpretation, drafting article, critical revision of article, approval of article.
PM, Dept. of Pediatrics, University of Pittsburgh – data collection, critical revision of article, approval of article.
MM, Dept. of Pediatrics, Columbia University – data collection, critical revision of article, approval of article.
SL, Dept. of Pediatrics, Columbia University – data collection, critical revision of article, approval of article.
DK, Dept. of Pediatrics, University of Birmingham – data collection, critical revision of article, approval of article.
VN, Dept. of Pediatrics, University of Toronto – data collection, critical revision of article, approval of article.
EA, Dept. of Pediatrics, Northwestern University – data collection, critical revision of article, approval of article.
YA, Dept. of Pediatrics, University of Toronto - concept/design, data analysis/interpretation, statistics, drafting article, critical revision of article, approval of article.
References
- 1.Venick RS, McDiarmid SV, Farmer DG, et al. Rejection and steroid dependence: unique risk factors in the development of pediatric posttransplant de novo autoimmune hepatitis. Am J Transplant. 2007;7:955–963. doi: 10.1111/j.1600-6143.2006.01717.x. [DOI] [PubMed] [Google Scholar]
- 2.Riva S, Sonzogni A, Bravi M, et al. Late graft dysfunction and autoantibodies after liver transplantation in children: preliminary results of an Italian experience. Liver Transpl. 2006;12:573–577. doi: 10.1002/lt.20673. [DOI] [PubMed] [Google Scholar]
- 3.Kerkar N, Hadzic N, Davies ET, et al. De-novo autoimmune hepatitis after liver transplantation. Lancet. 1998;351:409–413. doi: 10.1016/S0140-6736(97)06478-7. [DOI] [PubMed] [Google Scholar]
- 4.Salcedo M, Vaquero J, Banares R, et al. Response to steroids in de novo autoimmune hepatitis after liver transplantation. Hepatology. 2002;35:349–356. doi: 10.1053/jhep.2002.31167. [DOI] [PubMed] [Google Scholar]
- 5.Miyagawa-Hayashino A, Haga H, Egawa H, et al. Outcome and risk factors of de novo autoimmune hepatitis in living-donor liver transplantation. Transplantation. 2004;78:128–135. doi: 10.1097/01.tp.0000132328.33460.43. [DOI] [PubMed] [Google Scholar]
- 6.Hernandez HM, Kovarik P, Whitington PF, Alonso EM. Autoimmune hepatitis as a late complication of liver transplantation. J Pediatr Gastroenterol Nutr. 2001;32:131–136. doi: 10.1097/00005176-200102000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Gupta P, Hart J, Millis JM, Cronin D, Brady L. De novo hepatitis with autoimmune antibodies and atypical histology: a rare cause of late graft dysfunction after pediatric liver transplantation. Transplantation. 2001;71:664–668. doi: 10.1097/00007890-200103150-00016. [DOI] [PubMed] [Google Scholar]
- 8.Spada M, Bertani A, Sonzogni A, et al. A cause of late graft dysfunction after liver transplantation in children: de-novo autoimmune hepatitis. Transplant Proc. 2001;33:1747–1748. doi: 10.1016/s0041-1345(00)02826-8. [DOI] [PubMed] [Google Scholar]
- 9.Andries S, Casamayou L, Sempoux C, et al. Posttransplant immune hepatitis in pediatric liver transplant recipients: incidence and maintenance therapy with azathioprine. Transplantation. 2001;72:267–272. doi: 10.1097/00007890-200107270-00018. [DOI] [PubMed] [Google Scholar]
- 10.Edmunds C, Ekong UD. Autoimmune Liver Disease Post Liver Transplantation-A Summary and Proposed Areas for Future Research. Transplantation. 2016;100:515–524. doi: 10.1097/TP.0000000000000922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arterbery AO-AA, Avitzur Y, Ciarleglio M, Deng Y, Lobritto S, Martinez M, Hafler D, Kleinewietfeld M, Ekong UD. Production of Proinflammatory Cytokines by Monocytes in Liver-Transplanted Recipients with De Novo Autoimmune Hepatitis Is Enhanced and Induces TH1-like Regulatory T Cells. Journal of immunology. 2016;196:4040–4051. doi: 10.4049/jimmunol.1502276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montano-Loza AJ, Vargas-Vorackova F, Ma M, et al. Incidence and risk factors associated with de novo autoimmune hepatitis after liver transplantation. Liver Int. 2012;32:1426–1433. doi: 10.1111/j.1478-3231.2012.02832.x. [DOI] [PubMed] [Google Scholar]
- 13.Aguilera I, Wichmann I, Sousa JM, et al. Antibodies against glutathione S-transferase T1 (GSTT1) in patients with de novo immune hepatitis following liver transplantation. Clin Exp Immunol. 2001;126:535–539. doi: 10.1046/j.1365-2249.2001.01682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salcedo M, Rodriguez-Mahou M, Rodriguez-Sainz C, et al. Risk factors for developing de novo autoimmune hepatitis associated with anti-glutathione S-transferase T1 antibodies after liver transplantation. Liver Transpl. 2009;15:530–539. doi: 10.1002/lt.21721. [DOI] [PubMed] [Google Scholar]
- 15.Floreani A, Liberal R, Vergani D, Mieli-Vergani G. Autoimmune hepatitis: Contrasts and comparisons in children and adults - a comprehensive review. J Autoimmun. 2013;46:7–16. doi: 10.1016/j.jaut.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Mieli-Vergani G, Vergani D. Autoimmune liver diseases in children - what is different from adulthood? Best Pract Res Clin Gastroenterol. 2011;25:783–795. doi: 10.1016/j.bpg.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Carbone M, Neuberger JM. Autoimmune liver disease, autoimmunity and liver transplantation. J Hepatol. 2014;60:210–223. doi: 10.1016/j.jhep.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 18.Banff Working G, Demetris AJ, Adeyi O, et al. Liver biopsy interpretation for causes of late liver allograft dysfunction. Hepatology. 2006;44:489–501. doi: 10.1002/hep.21280. [DOI] [PubMed] [Google Scholar]
- 19.Banff schema for grading liver allograft rejection: an international consensus document. Hepatology. 1997;25:658–663. doi: 10.1002/hep.510250328. [DOI] [PubMed] [Google Scholar]
- 20.Demetris A, Adams D, Bellamy C, et al. Update of the International Banff Schema for Liver Allograft Rejection: working recommendations for the histopathologic staging and reporting of chronic rejection. An International Panel. Hepatology. 2000;31:792–799. doi: 10.1002/hep.510310337. [DOI] [PubMed] [Google Scholar]
- 21.Wagner D, Kniepeiss D, Stiegler P, et al. The assessment of GFR after orthotopic liver transplantation using cystatin C and creatinine-based equations. Transpl Int. 2012;25:527–536. doi: 10.1111/j.1432-2277.2012.01449.x. [DOI] [PubMed] [Google Scholar]
- 22.Demetris AJ, Bellamy C, Hubscher SG, et al. Comprehensive Update of the Banff Working Group on Liver Allograft Pathology: Introduction of Antibody-Mediated Rejection. Am J Transplant. 2016;2016 doi: 10.1111/ajt.13909. [DOI] [PubMed] [Google Scholar]
- 23.Ng VL, Alonso EM, Bucuvalas JC, et al. Health status of children alive 10 years after pediatric liver transplantation performed in the US and Canada: report of the studies of pediatric liver transplantation experience. J Pediatr. 2012;160:820–826 e823. doi: 10.1016/j.jpeds.2011.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suciu-Foca N, Ciubotariu R, Liu Z, Ho E, Rose EA, Cortesini R. Persistent allopeptide reactivity and epitope spreading in chronic rejection. Transplant Proc. 1998;30:2136–2137. doi: 10.1016/s0041-1345(98)00564-8. [DOI] [PubMed] [Google Scholar]
- 25.Lohse AW, Weiler-Norman C, Burdelski M. De novo autoimmune hepatitis after liver transplantation. Hepatol Res. 2007;37(Suppl 3):S462. doi: 10.1111/j.1872-034X.2007.00287.x. [DOI] [PubMed] [Google Scholar]
- 26.Demetris AJ, Sebagh M. Plasma cell hepatitis in liver allografts: Variant of rejection or autoimmune hepatitis? Liver Transpl. 2008;14:750–755. doi: 10.1002/lt.21518. [DOI] [PubMed] [Google Scholar]
- 27.Heneghan MA, Portmann BC, Norris SM, et al. Graft dysfunction mimicking autoimmune hepatitis following liver transplantation in adults. Hepatology. 2001;34:464–470. doi: 10.1053/jhep.2001.26756. [DOI] [PubMed] [Google Scholar]
- 28.Petz W, Sonzogni A, Bertani A, et al. A cause of late graft dysfunction after pediatric liver transplantation: de novo autoimmune hepatitis. Transplant Proc. 2002;34:1958–1959. doi: 10.1016/s0041-1345(02)03137-8. [DOI] [PubMed] [Google Scholar]
- 29.Studies of Pediatric Liver Transplantation Research G. Studies of Pediatric Liver Transplantation (SPLIT): Year 2000 Outcomes. Transplantation. 2001;72:463–476. doi: 10.1097/00007890-200108150-00018. [DOI] [PubMed] [Google Scholar]
- 30.Hubscher SG. What is the long-term outcome of the liver allograft? J Hepatol. 2011;55:702–717. doi: 10.1016/j.jhep.2011.03.005. [DOI] [PubMed] [Google Scholar]


