Abstract
Background
Decreased heart rate variability (HRV) is a measure of autonomic dysfunction and brain injury in newborns with hypoxic ischemic encephalopathy (HIE). This study aimed to characterize the relationship between HRV and brain injury pattern by MRI in newborns with HIE undergoing therapeutic hypothermia.
Methods
HRV metrics were quantified in the time domain (αS, αL, and root mean square at short [RMSS] and long [RMSL] time scales) and frequency domain (relative low-[LF] and high-frequency [HF] power) during the time period 24–27 hours of life. Brain injury pattern by MRI was classified as no injury, pure cortical/white matter injury, mixed watershed/mild basal nuclei injury, predominant basal nuclei or global injury, and died. HRV metrics were compared across brain injury pattern groups using a random effects mixed model.
Results
Data from 74 infants were analyzed. Brain injury pattern was significantly associated with degree of HRV suppression. Specifically, negative associations were observed between pattern of brain injury and RMSS (estimate −0.224, SE 0.082, p=0.006), RMSL (estimate −0.189, SE 0.082, p=0.021), and LF power (estimate −0.044, SE 0.016, p=0.006).
Conclusion
Degree of HRV depression is related to pattern of brain injury. HRV monitoring may provide insights into pattern of brain injury at the bedside.
INTRODUCTION
Hypoxic ischemic encephalopathy (HIE) is a major cause of death and disability in newborns. Although therapeutic hypothermia has improved outcome in infants with moderate and severe encephalopathy, nearly half of these infants continue to have adverse outcomes (1). Biomarkers that can provide early insights to help specify type and pattern of brain injury can provide opportunities for targeted therapies and aid in prognostication.
Currently, MRI is the standard for the assessment of brain injury in newborns with HIE (2, 3). It has been well established that pattern of injury by MRI relates to later developmental outcomes (4–8). Specifically, injury to the basal ganglia and thalamic brain regions has been associated with poor neuromotor outcomes(4, 5), whereas cortical watershed-type injuries relate to school-aged cognitive deficits, particularly lower language-related abilities(6). While MRI provides detailed information on location of injury, its sensitivity for reflecting the extent of brain injury in the first 24 hours of life has been questioned(4). Therefore, its use is generally restricted to the subacute period after completion of therapeutic hypothermia when there are fewer factors limiting transport of the critically-ill newborn to the scanner. Thus, biomarkers that reflect pattern of brain injury after hypoxia-ischemia that can be measured during hypothermia treatment can provide key information to the bedside clinician that is currently lacking.
Heart rate variability (HRV) offers a continuous and quantitative method to assess the autonomic nervous system (ANS) non-invasively and has been proposed as a bedside biomarker in newborns with HIE (9–12). Prior studies have shown that reduced HRV is associated with severity of brain injury by MRI(9), abnormal EEG findings(9, 10), and poor long-term neurodevelopmental outcome (10) (11, 12) in infants with moderate to severe encephalopathy. These studies have related HRV metrics to the presence and severity of brain injury, but not to the topography of injury. Recently, we demonstrated that injury at both the cerebral cortical and brain stem level was associated with ANS dysregulation in newborns with various types of brain injury(13). These findings suggest that autonomic regulation can be mediated by both direct injury to the deep brain structures including the brainstem, as well as higher order cortical centers. The aim of this study was to investigate whether HRV is related to the topography of brain injury by MRI in newborns with HIE. We hypothesized that HRV depression would be observed in infants with watershed (WS) predominant brain injury compared to infants with normal MRI and that the greatest HRV depression would be observed in infants with basal nuclei (BN) predominant pattern of injury.
METHODS
Study population
Infants with HIE undergoing therapeutic hypothermia were included from a prospective observational study evaluating biomarkers of brain injury. Inclusion criteria were based on modified National Institute of Child Health and Human Development criteria: gestational age at birth ≥ 35 weeks, birth weight ≥ 1800 g, metabolic acidosis and/or low Apgar score in setting of a perinatal sentinel event, and moderate to severe encephalopathy based on modified Sarnat criteria (1). Infants were systemically cooled to 33.5°C for 72 hours and rewarmed 0.5°C/hour over 6 hours (Blanketrol II, Cincinnati Sub-Zero, Cincinnati, OH). The Children’s National Health System Institutional Review Board approved the study and informed consent was obtained from the parent of each participant.
Data Collection
Clinical and demographic data were collected from the birth hospital and study site medical records. Continuous electrocardiogram (EKG) recordings were obtained from the bedside cardiorespiratory monitor (Philips IntelliVue MP70, Andover, MA). For infants enrolled before 24 hours of life, EKG recordings were prospectively collected at a sample rate of 1000 Hz using custom software developed in LabView (National Instruments, Austin, TX). Otherwise, EKG data were collected if available from an institutional Research Data Export archive (IntelliVue Information Center, Philips Healthcare, Andover, MA) at a sample rate of 125 Hz. We focused on EKG data from 24–27 hours of life based on our prior work demonstrating this as a key time period during which HRV best discriminates between outcome groups(11).
EKG Processing
To attenuate the noise and to correct for the baseline shifts, the EKG was bandpass filtered between 0.5–60 Hz using Butterworth filter with zero-phase distortion. The R-wave (a wave with maximum amplitude of the cardiac cycle) was identified using a combination of Hilbert transform and adaptive threshold detection approach(14) and beat-to-beat interval (RRi) was calculated. The artifacts in the RRi were cleaned using a recently proposed data-driven approach(15). The artifact-free RRi were partitioned into 10-minute epochs. All analyses were performed off-line using MATLAB (MathWorks, Inc., Natick, MA).
Detrended Fluctuation Analysis
Detrended Fluctuation Analysis (DFA) involves the following four steps: 1) Removing the average value of the data and calculating the profile function, which is a cumulative sum of the data. 2) Dividing the profile function into time windows each containing s- number of beats. 3) Fitting a polynomial function to the profile inside each window and calculating the local fluctuation as the root mean square deviation of the profile from the best fit. 4) Averaging the local fluctuation function over all windows to get an overall fluctuation function. For power-law correlated signals such as RRi, the fluctuation function varies with the window size s. The fluctuation exponent is calculated as the slope of the fluctuation function. In this work, we used a fourth order polynomial to detrend the RRi and calculated the following metrics: αS (15–50 beats), αL (100–150 beats), root mean square at short (RMSS) (15–50 beats) and long time scales (RMSL) (100–150 beats)(16). The α metrics characterize the auto-correlations in the data whereas the RMS metrics characterize the variability in the data. For less variable data RMS and αS values will be low and for more redundant data the αL metric value will be high.
Spectral Analysis
For spectral analysis (SA), RRi in each 10-min epoch were interpolated using cubic spline with a sample rate 4 Hz and converted them into evenly sampled data. To estimate the power spectrum of RRi in a 10-minute window, we used a Welch periodogram approach with a frequency resolution of 0.016 Hz. In this approach, we partitioned the RRi into 1-minute non-overlapping epochs. For the RRi in each epoch, we subtracted the mean and normalized it by the standard deviation to mitigate the effects of non-stationarity as described previously (17). In each window, we calculated the periodgram as the square of the magnitude of the Fourier transform of the RRi. To obtain an estimate of the power spectrum, we averaged the periodograms over all 1-minute epochs. The sum of powers in low frequency (LF) (0.05–0.25 Hz) and high frequency (HF) (0.3–1 Hz) bands were calculated. The normalized or relative powers were calculated by dividing the spectral powers by the total power in order to minimize the effect of changes in total power on the estimations of LF and HF components (18). The total power was defined as the sum of powers in the frequency band of 0.05–2 Hz (11, 16, 17).
In summary, reduced HRV is associated with lower values for the HRV metrics αS, RMSS, RMSL, and LF and higher values for the metrics αL and HF(11, 16, 17, 19).
Magnetic Resonance Imaging
MRI was performed on a 3T scanner (Discovery MR750, GE Healthcare, Milwaukee, WI). In surviving infants imaging was performed at target age 3–5 days and 10–12 days according to our institutional protocol. An experienced neuroradiologist (G. V.) who was blinded to each infant’s clinical status reviewed the MRI scans and scored the images according to a previously defined system(4). The degree of basal ganglia/thalamic injury (BG) was assigned a score from 0 to 4 and the degree of cortical WS injury was assigned a score from 0 to 5. From these scores infants were classified by predominant pattern of injury as previously described(5): Total brain injury (maximum BG and WS score), BN predominant (BG≥WS), or WS predominant (WS>BG) injury. The WS group was further divided into infants with pure cortical/white matter injury (WMI) and infants with mixed WS/mild BN injury. Infants who died prior to hospital discharge were grouped separately. Due to the small number of patients with global injury (n=3), these patients were grouped with the BN predominant infants for the purposes of analysis as these patterns of injury have been linked to more severe outcome phenotypes. The score from the later MRI was used for classification of injury pattern, unless only the early MRI was completed.
Statistical analysis
Clinical characteristics were analyzed using standard measures of central tendency. HRV metrics were compared across the five brain injury pattern groups (0=no injury, 1=pure cortical/WMI, 2=mixed WS/mild BN, 3= predominant BN or global injury, and 4=died) using Kruskal-Wallis tests. Additionally, a random effects mixed model was performed in order to adjust for the repeated measures in each subject and six of the most relevant covariates: gender, gestational age at birth, birth weight, encephalopathy grade, seizures during the study period, and use of vasopressors. The HRV metrics were log transformed to satisfy the normality assumption. Secondarily, a sensitivity analysis was performed excluding patients with documented electrographic seizures during 24–27 hours of life (n=9), as there is evidence that there are heart rate characteristic changes around the time of seizures(20, 21). For those metrics that were significantly associated with brain injury pattern, we also performed receiver operating curve analyses to establish HRV cutpoints that could distinguish infants with death or significant BN injury (groups 3–4) from those without BN injury (groups 0–1). We analyzed data from 74 infants prospectively enrolled in a study evaluating biomarkers of brain injury in HIE. For these analyses, this sample size proved to have 0.87 power to detect significant associations (p<0.001) between brain injury pattern and all DFA metrics and SA metrics. Statistical analyses were performed using SAS 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
Of the 80 infants enrolled 74 had EKG data available during the study period. The remaining infants were excluded due to unavailability of data (n=4) and death before 24 hours of life (n=2). MRI used for classification was performed at a median age of 10 days (range 3–18). Early MRI was used for classification in 14 infants who did not undergo late MRI due to death or discharge prior to the target window. The distribution of brain injury pattern in order of increasing severity was as follows: 34 infants had no injury (46%), 10 had the pure cortical/WMI pattern (14%), 6 had the mixed WS/mild BN pattern (8%), 15 had the predominant BN or global injury pattern (20%), and 9 died (12%). Clinical characteristics of the study population are in Table 1.
Table 1.
Clinical characteristics
| Gestational age, wks (mean ± SD) | 38.7 ± 1.5 |
| Birth weight, kg (mean ± SD) | 3.3 ± 0.7 |
| Male gender (n, %) | 37 (50) |
| Apgar score | |
| 1 min (median, range) | 1 (0–9) |
| 5 mina (median, range) | 3 (0–9) |
| 10 minb (median, range) | 5 (0–10) |
| Initial pH (median, range) | 6.95 (6.50–7.42) |
| Encephalopathy grade at presentation (Modified Sarnat) | |
| Moderate (n, %) | 55 (74) |
| Severe (n, %) | 19 (26) |
| EEG seizures (n, %) | 9 (12) |
| Received phenobarbital (n, %) | 19 (26) |
| Received vasoactives (n, %) | 33 (45) |
| Dopamine (n, %) | 33 (45) |
| Epinephrine (n, %) | 4 (5) |
| Age at MRI (median, range)c | 10 (3–18) |
| Respiratory Support (n, %) | 66 (89) |
| High Frequency Ventilator (n, %) | 7 (9) |
| Conventional Ventilator (n, %) | 39 (53) |
| Other (n, %) | 20 (27) |
| None (n, %) | 8 (11) |
| Respiratory Rate | |
| Conventional Ventilator (median, interquartile range) | 22 (16–28) |
| Spontaneous Breathing (median, interquartile range) | 31 (25–36) |
| HFOV | n/a |
| Heart Rate Overall (median, interquartile range) | 110 (99–128) |
| Group 0 | 103 (96–124) |
| Group 1 | 109 (96–120) |
| Group 2 | 106 (100–135) |
| Group 3 | 116 (107–131) |
| Group 4 | 127 (114–145) |
Documented for 73/74 infants;
Documented for 64/74 infants,
Documented for 65/74 infants,
Detrended Fluctuation Analysis
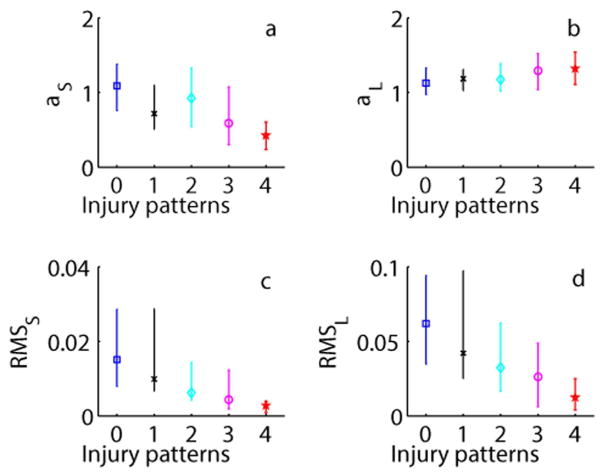
In bivariate analyses, there were significant associations (p<0.001) between brain injury pattern and all DFA metrics (Figure 1). Reduced HRV in the injured groups was shown by a decrease in the value of αS, RMSS, RMSL and an increase in the value of αL across increasing injury severity groups. In the model adjusted for covariates, brain injury pattern remained significantly associated with RMSS (estimate −0.224, SE 0.082, p=0.006) and RMSL (estimate −0.189, SE 0.082, p=0.021). Encephalopathy grade was also significantly associated with HRV metrics across models (p<0.05).
Figure 1. DFA Metrics.
Detrended fluctuation analysis (DFA) metrics: (a) αS, (b) αL, (c) root mean square at short (RMSS) and (d) long (RMSL) time scales presented as median (interquartile range). Injury pattern groups are defined as: 0=no injury, 1=pure cortical/WMI, 2=mixed WS/mild BN, 3= predominant BN or global injury, and 4=died.
Detrended fluctuation analysis (DFA) metrics: (a) αS, (b) αL, (c) root mean square at short (RMSS) and (d) long (RMSL) time scales presented as median (interquartile range). Injury pattern groups are defined as: 0=no injury, 1=pure cortical/WMI, 2=mixed WS/mild BN, 3= predominant BN or global injury, and 4=died.
Spectral Analysis
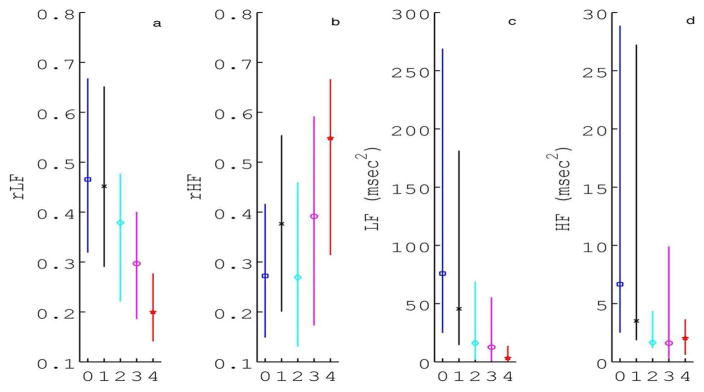
A decrease in relative LF power and an increase in relative HF power was observed across brain injury pattern groups (Figure 2A/B, p<0.001). Bivariate associations were similarly significant when considering absolute LF and HF power, although absolute HF power decreased across brain injury pattern groups (Figure 2C/D, p<0.001). After adjusting for covariates, there was a significant negative association between brain injury pattern and relative LF power (estimate −0.044, SE 0.016, p=0.006). Encephalopathy grade was also significantly inversely associated with relative LF power and directly associated with relative HF power (p<0.05).
Figure 2. SA Metrics.
Spectral analysis (SA) metrics a) relative low frequency (rLF) and b) high frequency (rHF) power presented as median (interquartile range). Absolute c) LF and d) HF power are also shown for comparison. Injury pattern groups are defined as: 0=no injury, 1=pure cortical/WMI, 2=mixed WS/mild BN, 3= predominant BN or global injury, and 4=died.
Spectral analysis (SA) metrics a) relative low frequency (rLF) and b) high frequency (rHF) power presented as median (interquartile range). Absolute c) LF and d) HF power are also shown for comparison. Injury pattern groups are defined as: 0=no injury, 1=pure cortical/WMI, 2=mixed WS/mild BN, 3= predominant BN or global injury, and 4=died.
Impact of Seizures on Brain Injury Pattern and HRV Association
Nine infants were subsequently excluded for electrographic seizures during the study period: 2 with pure cortical/WMI, 4 with mixed WS/mild BN, 1 with predominant BN or global injury pattern, and 2 died. The associations between brain injury pattern and HRV metrics RMSS (estimate −0.225, SE 0.088, p=0.011), RMSL (estimate −0.198, SE 0.086, p=0.022), and LF power (estimate −0.050, SE 0.017, p=0.004) were similar across these reduced models.
HRV Prediction of BN Injury
Receiver operating curve analyses were performed to determine optimal cutpoints of RMSs, RMSL, and relative LF power for distinguishing infants who died or had significant BN injury (Groups 3 and 4) from those with no BN injury (Groups 0 and 1). Area under the receiver operating curve and predictive abilities are summarized in Table 2.
Table 2.
Heart Rate Variability Prediction of Basal Nuclei Injury
| HRV Metric | Sensitivity | Specificity | PPV | NPV | AUC |
|---|---|---|---|---|---|
| RMSS | 0.609 | 0.865 | 0.723 | 0.792 | 0.788 |
| RMSL | 0.678 | 0.750 | 0.734 | 0.696 | 0.771 |
| rLF | 0.717 | 0.680 | 0.740 | 0.655 | 0.776 |
PPV= positive predictive value; NPV= negative predictive value; AUC= area under the receiver operating curve; RMSS= root mean square at short time scales; RMSL= root mean square at long time scales; rLF= relative low frequency power
DISCUSSION
Brain injury pattern is associated with reduced HRV at 24 hours of life in newborns with HIE undergoing therapeutic hypothermia. As expected, global or BN injury was associated with significant HRV depression compared to newborns without brain injury. Reduced HRV was also observed with cortical/WMI alone, although to a lesser degree than that seen with global or BN injury. Brain injury pattern was most associated with HRV metrics reflecting the sympathetic component of the autonomic nervous system (RMSS, RMSL, and LF). These relationships remained significant after adjusting for encephalopathy grade at presentation and after excluding patients with electrographic seizures. These results suggest that monitoring HRV during therapeutic hypothermia may provide bedside insights into the topography of brain injury in newborns with HIE.
Our findings of reduced heart rate variability in infants with brain injury are consistent with other studies of HRV in infants with HIE(9–12). In particular, LF power has been associated with adverse neuroimaging and functional outcomes(10–12). However, our study is the first to evaluate the association between HRV metrics and pattern of injury, not just presence of injury. This functionality may have important clinical implications as it has been suggested that pattern of injury by MRI is more predictive of outcome than assessment of severity of injury to a particular region alone(5). It is recognized, however, that our findings may reflect the relationship between HRV and gradations of overall brain injury severity, as patterns of injury may represent a continuum of injury severity. Similarly, the timing of our assessment of cortical injury by MRI may limit our ability to make firm conclusions about cortical injury in-vivo and its relation to HRV in real-time. However, that these findings could reflect a mechanistic link between regional injury and cerebral inputs regulating autonomic control of heart rate is consistent with animal and human studies that have described the role of the cortical autonomic network in regulation of HRV (22, 23) We speculate that the depression of HRV in infants with isolated cortical injury supports the notion that cerebral cortical inputs are involved in regulation of the autonomic nervous system by term gestation (13). While our findings cannot confirm this mechanistic link, these data can provide ranges for HRV metrics that can reflect risk for BG versus WS involvement for the bedside clinician.
The relationship between HRV and topography of brain injury further strengthens its utility as a bedside monitoring tool. While MRI provides high anatomical resolution, its limited accessibility during the first 24 hours of critical illness limits its temporal resolution(4). Despite improved access in neonatal intensive care units experienced with transport to the MRI suite, its use remains impracticable in the most critically ill patients. HRV analysis, alternatively, allows for real-time assessment of injury severity, and possibly topography of injury, at the bedside. These insights could be helpful to direct therapies for individual patients during the acute and subacute period, optimizing opportunities for intervention within the therapeutic window.
It has been suggested that topography of injury may indicate the etiology of particular insults. For example, while global or BN predominant pattern has been associated with acute profound asphyxia(5, 24, 25), cortical and focal/multifocal WMI has been more commonly described in patients with accompanying chorioamnionitis or placental insufficiency(26). As these variable insults may be amenable to alternative neuroprotective strategies, early stratification of patients by brain injury pattern can have therapeutic implications. Brain injury pattern may in itself indicate responsiveness to cooling, as it has been suggested that alternative cooling regimens may be selectively neuroprotective for cortical versus deep brain structures(27–29). Pattern of injury is also important for prognostication. Basal ganglia and/or thalamic injury have been associated with adverse motor outcomes and cerebral palsy(5, 25). Conversely, injury to the watershed regions has been associated with cognitive deficits at 30 months of age(5) as well as at 4(6) and 11 years of age(30). Thus early insights into pattern of injury may guide expectations for developmental phenotypes(5). We have not yet correlated our brain injury patterns with neurodevelopmental outcome. This correlation is needed and assessment is ongoing. Future analyses will need to assess the additive value of HRV monitoring over standard bedside assessments including clinical exam and EEG.
This study has several limitations. As the exact mechanisms mediating HRV after a hypoxic ischemic insult are complex, the covariates we chose are not all encompassing. For example, intrinsic myocardial dysfunction may be an important factor in HRV depression in babies with HIE. While we adjusted for vasopressor use, we did not account for the dose or cumulative amount of vasopressors required, or other indices of cardiovascular instability. Similarly, it is recognized that the respiratory oscillations can impact heart rate and HRV (31). Given the neonatal respiratory rate falls within the high frequency band interrogated, the relationship between HF power and brain injury observed in our unadjusted analyses may reflect increasing ventilator dependence in the infants with more severe brain injury. We are unable to remove ventilator-related oscillations from the heart rate. While previous studies have used the spectral power around the breathing frequency as the representation of HF power (31, 33), this approach focuses on quantifying ventilator-related oscillations rather than all the components of parasympathetic tone that are believed to reside within the more conventionally defined HF band used in this work. While we are developing methods to isolate respiratory oscillations to remove these effects from quantification of HRV in the HF band, we have to consider the effect of the ventilator oscillations on heart rate as a limitation of this study. As mentioned, our study is unable to differentiate association and causation. Whether regional injury differentially influences autonomic function is unclear, but these data do provide estimates of HRV metrics that correlate with different patterns of brain injury. While we entered into statistical analyses with a large number of observations given the repeated measures over time in subjects, we acknowledge that the sample size was limited particularly in the mixed cortical/mild BN group. Larger studies will need to confirm these findings in order to provide practical benchmark ranges that can signify brain injury categories with reliability. Generalizability of these analyses may be affected by the distribution of injury pattern, however the frequencies of different injury patterns were comparable to other studies in newborns with HIE treated with hypothermia(8, 32). Finally, it should be recognized that MRI was the tool used for assessment of brain injury in this study and that these findings may differ when considering long-term developmental outcome phenotypes.
Conclusions
Pattern of brain injury relates to degree of HRV depression in newborns with HIE. Evaluation of degree and pattern of brain injury may be aided by real-time HRV monitoring in HIE infants undergoing therapeutic hypothermia.
Acknowledgments
Financial Support: This work was supported by the Clinical and Translational Science Institute at Children’s National (UL1TR000075 and 1KL2RR031987-01) and the Intellectual and Developmental Disabilities Research Consortium (NIH P30HD040677). The sponsors had no role in the design and conduct of the study; in the collection, management, analysis and interpretation of data; or in the preparation, review or approval of the manuscript.
Footnotes
Potential Conflicts of interest / Disclosures: All authors have nothing to disclose.
Category of Study: Clinical
References
- 1.Shankaran S, Laptook AR, Ehrenkranz RA, et al. National Institute of Child H, Human Development Neonatal Research N. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 2.Ment LR, Bada HS, Barnes P, et al. Practice parameter: neuroimaging of the neonate: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2002;58:1726–1738. doi: 10.1212/wnl.58.12.1726. [DOI] [PubMed] [Google Scholar]
- 3.Executive summary: Neonatal encephalopathy and neurologic outcome, second edition. Report of the American College of Obstetricians and Gynecologists’ Task Force on Neonatal Encephalopathy. Obstet Gynecol. 2014;123:896–901. doi: 10.1097/01.AOG.0000445580.65983.d2. [DOI] [PubMed] [Google Scholar]
- 4.Barkovich AJ, Hajnal BL, Vigneron D, Sola A, Partridge JC, Allen F, Ferriero DM. Prediction of neuromotor outcome in perinatal asphyxia: evaluation of MR scoring systems. AJNR Am J Neuroradiol. 1998;19:143–149. [PMC free article] [PubMed] [Google Scholar]
- 5.Miller SP, Ramaswamy V, Michelson D, et al. Patterns of brain injury in term neonatal encephalopathy. J Pediatr. 2005;146:453–460. doi: 10.1016/j.jpeds.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 6.Steinman KJ, Gorno-Tempini ML, Glidden DV, Kramer JH, Miller SP, Barkovich AJ, Ferriero DM. Neonatal watershed brain injury on magnetic resonance imaging correlates with verbal IQ at 4 years. Pediatrics. 2009;123:1025–1030. doi: 10.1542/peds.2008-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shankaran S, Barnes PD, Hintz SR, et al. Brain injury following trial of hypothermia for neonatal hypoxic-ischaemic encephalopathy. Arch Dis Child. 2012 doi: 10.1136/archdischild-2011-301524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rutherford M, Ramenghi LA, Edwards AD, et al. Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic-ischaemic encephalopathy: a nested substudy of a randomised controlled trial. Lancet Neurol. 2010;9:39–45. doi: 10.1016/S1474-4422(09)70295-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vergales BD, Zanelli SA, Matsumoto JA, Goodkin HP, Lake DE, Moorman JR, Fairchild KD. Depressed heart rate variability is associated with abnormal EEG, MRI, and death in neonates with hypoxic ischemic encephalopathy. Am J Perinatol. 2014;31:855–862. doi: 10.1055/s-0033-1361937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goulding RM, Stevenson NJ, Murray DM, Livingstone V, Filan PM, Boylan GB. Heart rate variability in hypoxic ischemic encephalopathy: correlation with EEG grade and 2-y neurodevelopmental outcome. Pediatr Res. 2015;77:681–687. doi: 10.1038/pr.2015.28. [DOI] [PubMed] [Google Scholar]
- 11.Massaro AN, Govindan RB, Al-Shargabi T, et al. Heart rate variability in encephalopathic newborns during and after therapeutic hypothermia. J Perinatol. 2014;34:836–841. doi: 10.1038/jp.2014.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matic V, Cherian PJ, Widjaja D, Jansen K, Naulaers G, Van Huffel S, De Vos M. Heart rate variability in newborns with hypoxic brain injury. Adv Exp Med Biol. 2013;789:43–48. doi: 10.1007/978-1-4614-7411-1_7. [DOI] [PubMed] [Google Scholar]
- 13.Schneebaum Sender N, Govindan RB, Sulemanji M, Al-Shargabi T, Lenin RB, Eksioglu YZ, du Plessis AJ. Effects of regional brain injury on the newborn autonomic nervous system. Early Hum Dev. 2014;90:893–896. doi: 10.1016/j.earlhumdev.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Ulusar UD, Govindan RB, Wilson JD, Lowery CL, Preissl H, Eswaran H. Adaptive rule based fetal QRS complex detection using Hilbert transform. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:4666–4669. doi: 10.1109/IEMBS.2009.5334180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Govindan RBA-ST, Metzler M, Andescavage NN, Joshi R, du Plessis A. A spike correction approach for variability analysis of heart rate sick infants. Physica A. 2016;444:35–42. [Google Scholar]
- 16.Govindan RBMAN, Al-Shargabi T, Niforatos Andescavage N, Chang T, Glass P, du Plessis AJ. Detrended fluctuation analysis of non-stationary cardiac beat-to-beat interval of sick infants. Europhysics Letters. 2014;108:40005-p40001–p40006. [Google Scholar]
- 17.Govindan RB, Massaro AN, Niforatos N, du Plessis A. Mitigating the effect of non-stationarity in spectral analysis-an application to neonate heart rate analysis. Comput Biol Med. 2013;43:2001–2006. doi: 10.1016/j.compbiomed.2013.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 19.Malliani A, Pagani M, Lombardi F, Cerutti S. Cardiovascular neural regulation explored in the frequency domain. Circulation. 1991;84:482–492. doi: 10.1161/01.cir.84.2.482. [DOI] [PubMed] [Google Scholar]
- 20.Malarvili MB, Mesbah M, Boashash B. Time-frequency analysis of heart rate variability for neonatal seizure detection. Australas Phys Eng Sci Med. 2006;29:67–72. [PubMed] [Google Scholar]
- 21.Doyle OM, Temko A, Marnane W, Lightbody G, Boylan GB. Heart rate based automatic seizure detection in the newborn. Med Eng Phys. 2010;32:829–839. doi: 10.1016/j.medengphy.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Shoemaker JK, Wong SW, Cechetto DF. Cortical circuitry associated with reflex cardiovascular control in humans: does the cortical autonomic network “speak” or “listen” during cardiovascular arousal. Anat Rec (Hoboken) 2012;295:1375–1384. doi: 10.1002/ar.22528. [DOI] [PubMed] [Google Scholar]
- 23.Cechetto DF. Cortical control of the autonomic nervous system. Exp Physiol. 2014;99:326–331. doi: 10.1113/expphysiol.2013.075192. [DOI] [PubMed] [Google Scholar]
- 24.Okereafor A, Allsop J, Counsell SJ, Fitzpatrick J, Azzopardi D, Rutherford MA, Cowan FM. Patterns of brain injury in neonates exposed to perinatal sentinel events. Pediatrics. 2008;121:906–914. doi: 10.1542/peds.2007-0770. [DOI] [PubMed] [Google Scholar]
- 25.Rutherford M, Srinivasan L, Dyet L, Ward P, Allsop J, Counsell S, Cowan F. Magnetic resonance imaging in perinatal brain injury: clinical presentation, lesions and outcome. Pediatr Radiol. 2006;36:582–592. doi: 10.1007/s00247-006-0164-8. [DOI] [PubMed] [Google Scholar]
- 26.Harteman JC, Nikkels PG, Benders MJ, Kwee A, Groenendaal F, de Vries LS. Placental pathology in full-term infants with hypoxic-ischemic neonatal encephalopathy and association with magnetic resonance imaging pattern of brain injury. J Pediatr. 2013;163:968–995. e962. doi: 10.1016/j.jpeds.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 27.Inder TE, Hunt RW, Morley CJ, Coleman L, Stewart M, Doyle LW, Jacobs SE. Randomized trial of systemic hypothermia selectively protects the cortex on MRI in term hypoxic-ischemic encephalopathy. J Pediatr. 2004;145:835–837. doi: 10.1016/j.jpeds.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 28.Iwata O, Thornton JS, Sellwood MW, et al. Depth of delayed cooling alters neuroprotection pattern after hypoxia-ischemia. Ann Neurol. 2005;58:75–87. doi: 10.1002/ana.20528. [DOI] [PubMed] [Google Scholar]
- 29.Laptook AR, Shalak L, Corbett RJ. Differences in brain temperature and cerebral blood flow during selective head versus whole-body cooling. Pediatrics. 2001;108:1103–1110. doi: 10.1542/peds.108.5.1103. [DOI] [PubMed] [Google Scholar]
- 30.Perez A, Ritter S, Brotschi B, Werner H, Caflisch J, Martin E, Latal B. Long-term neurodevelopmental outcome with hypoxic-ischemic encephalopathy. J Pediatr. 2013;163:454–459. doi: 10.1016/j.jpeds.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 31.Andriessen P, Oetomo SB, Peters C, Vermeulen B, Wijn PF, Blanco CE. Baroreceptor reflex sensitivity in human neonates: the effect of postmenstrual age. J Physiol. 2005;568:333–341. doi: 10.1113/jphysiol.2005.093641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shankaran S, Barnes PD, Hintz SR, et al. Eunice Kennedy Shriver National Institute of Child H, Human Development Neonatal Research N. Brain injury following trial of hypothermia for neonatal hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2012;97:F398–404. doi: 10.1136/archdischild-2011-301524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yiallourou SR, Witcombe NB, Sands SA, Walker AM, Horne RS. The development of autonomic cardiovascular control is altered by preterm birth. Early Hum Dev. 2013;89:145–152. doi: 10.1016/j.earlhumdev.2012.09.009. [DOI] [PubMed] [Google Scholar]