Abstract
The fetus does not reside in a sterile intrauterine environment and is exposed to commensal bacteria from the maternal gut/blood stream which crosses the placenta and enters the amniotic fluid. This intestinal exposure to colonizing bacteria continues at birth and during the first year of life and has a profound influence on lifelong health. Why is this important? Intestinal crosstalk with colonizing bacteria in the developing intestine affects the infant’s adaptation to extrauterine life (immune homeostasis) and provides protection against disease expression (allergy, autoimmune disease, obesity, etc.) later in life. Colonizing intestinal bacteria are critical to the normal development of host defense. Disrupted colonization (dysbiosis) due to maternal dysbiosis, cesarean section delivery, use of perinatal antibiotics or premature delivery may adversely affect gut development of host defense and predispose to inflammation rather than homeostasis leading to increased susceptibility to disease later in life. Babies born by cesarean section have a higher incidence of allergy, type 1 diabetes and obesity. Infants given repeated antibiotic regimens during the first year of life are more likely to have asthma as adolescents. This research breakthrough helps to explain the shift in disease paradigms from infections to immune mediated in children from developed countries. This review will develop this research breakthrough.
INTRODUCTION
Over the last decade, scientists and clinicians alike have recognized the importance of bacteria, particularly bacteria colonizing the gastrointestinal tract, in host metabolic and protective function (1). In fact, the microbiome of the mature human intestine is now considered an ancillary organ of the host providing important contributions to the individual’s health and well being (2). Evidence for this statement includes the observations that the intestinal microbiome weighs one and a half kilograms, the number of bacterial cells which reside in the intestine exceed the number of cells in the body by ten-fold, the number of genes in the intestinal microbiome is one hundred-fold greater than the total genes in the host and the intestinal microbiome is also more metabolically active than the most active body organ, the liver. Accordingly, a better appreciation for how the intestinal microbiome and its metabolites interact with its host is important to our understanding of its role in health and disease.
This principle is particularly important with initial colonization of the infant’s gut. The initial colonization process occurs during gestation and continues until approximately between eighteen months and three years postpartum when a unique signature of bacteria exists within the distal small intestine and colon representing the mature microbiome (details of this process will be discussed later). This neonatal period of postpartum life represents the period of adjustment for metabolic and immunologic development of the infant to its extrauterine environment (3,4). It also represents a time when epigenetic changes in these host functions are very important to the long term health of the child (5). “Epigenetic changes are mechanisms that alter the phenotypic expression without changing the underlying DNA sequence” (6). These changes occur due to environmental factors (nutrients and microorganisms) that influence human genes during development (gestation and the neonatal period). Examples of human disease are obesity and metabolic syndrome occurring in children with inadequate nutrition during gestation as occurred in the Dutch famine in World War II (7) and altered TLR4 expression on intestinal enterocytes in inflammatory bowel disease (8, 9). These changes can affect methylation or acetylation of histones leading to altered transcriptional events and phenotypic expression of disease that can be passed to subsequent generations. It is now clear that initial intestinal bacterial colonization has an important impact on development of these functions in the newborn and infancy periods (10).
This review will consider the process of normal bacterial colonization of the intestine (symbiosis) and its effect on newborn adaptation to the extrauterine environment. It will also underscore the impact of appropriate colonization on the infant’s development of host defense and metabolic function which represent major determinants of health throughout infancy and childhood and into adulthood (11). The impact of disrupted intestinal colonization (dysbiosis) in utero and during infancy on the expression of immune and metabolically-mediated disease during childhood and later in life will also be considered. Finally, strategies to rectify dysbiosis and to prevent disease expression later in life will be considered. These strategies may either decrease the risk for or mitigate the risk for disease later in life.
Initial Intestinal Colonization
The composition of the intestinal microbiome is dependent on many factors including diet, infection and lifestyle changes. Over the last half century with improvement in public health, we have reduced the incidence of infectious diseases in developed countries. Improvement in health (better sanitation, vaccinations, and use of antibiotics, etc.) have affected the exposure to pathogens resulting in less infectious disease (12). However, these measures along with major changes in diet as part of the western lifestyle, have resulted in a paradigm shift in the disease burden to immune-mediated diseases (autoimmunity, allergy, etc.) reflecting less adequate exposure to a balanced colonizing bacteria that can influence the development of protective immunologic functions (13). The original “hygiene hypothesis” suggests that increased exposure to microorganisms in large families with multiple siblings and increased infectious diseases resulted in less allergy in younger siblings (14). The hypothesis has evolved to suggest that a modified microbial antigenic exposure early in life as a result of improved public health measures including increased sanitation, vaccination, and antibiotics has changed the way infants adjust immunologically to the extrauterine environment leading to immune dysfunction and increased inflammatory diseases (15, 16). This observation represents the latest iteration of the so called “Hygiene Hypothesis” (17). This section outlines what constitutes the ideal circumstances for the appropriate initial colonization process. Ideal conditions for initial colonization results in a symbiotic relationship between the colonizing bacteria and intestinal epithelial and lymphoid tissues (TABLE 1) and immune and metabolic homeostasis for the infant.
Table 1.
Phases of intestinal colonization of the infant intestine
| Phase one: Intrauterine period |
| The fetus becomes exposed to maternal microbiota through transplacental passage into amniotic fluid |
| Phase two: 1st week of life |
| The newborn ingests maternal vaginal/colonic microbiota with passage through the birth canal (full term, vaginal delivery) |
| Phase three: Two weeks to four months |
| Introduction of oral liquid feedings |
| Phase four: Four months to one year |
| Period of weaning to solid foods |
| Phase five: One to three years |
| Infant receives table food and intestinal microbiome resembles that of adult intestine (diversity of bacteria with greater than 1000 species) |
It is now known that the fetus does not reside in a sterile environment. Evidence exists that under normal gestational conditions bacteria from the maternal gut passes into mother’s blood stream and can ultimately either reside in the placenta or pass through the placenta and enter the amniotic fluid (18,19). These organisms, far fewer in number than those that inhabit the newborn intestine, can interact with the fetal intestine as the fetus swallows amniotic fluid.
Further, evidence for this conclusion is that maternal gut organisms have been identified in both meconium and cord blood (20, 21). At the point we can only say that microorganisms exist in some normal full term infants (19, 20) as large perspective studies have not yet been done. In addition, their presence is an association and no functional data exists to show cause and effect (21). Data to suggests that intrauterine bacteria interact with the developing fetus is suggested by germ free animal studies (22) in which immune function at birth differs from conventional new born animals. Additional studies in humans are necessary before a stronger claim can be made. Therefore, bacterial-intestinal “crosstalk” begins in utero and represents the new phase one of crosstalk (eg. pre-colonization). Unfortunately, studies are lacking to determine the importance of this process in overall initial colonization and its impact on gut development. Very little information exists regarding bacterial epithelial crosstalk in utero. However, strong evidence exists that initial colonization (phase two) of normal colonization influences immune function in the new born intestine (23,24). These observations principally occur in animal studies using germ free animals. A classic observation made by a Japanese investigator suggests that germ free animals cannot develop tolerance unless their colonization occurs in a newborn (25, 22). In a recent article (26), germ free animals develop natural killer cells (NKC) which influence intestinal (IBD) and lung (asthma) inflammation whereas neonatal colonized infants have a decrease in NKC and develop immune homeostasis (22, 26) again suggesting the importance of early colonization in normal immune function and the absence of disease at a later date.
Under optimal colonizing conditions, a full term, vaginally-delivered newborn ingests a healthy bolus of maternal vaginal/colonic bacteria as it passes through the birth canal. This represents probably the most important phase of initial colonization (phase two). However, additional cause and effect studies need to be done to confirm this statement. With the introduction of oral feedings (breast milk vs. formula — to be discussed later) the intestinal microbiota are further stimulated (phase three). Then with weaning to solid foods (four to six months) (phase four) the gut microbiome has additional stimulus and by about eighteen months to three years (phase five) the gut is colonized with a diverse bacteria (greater than 1000 species) which represents that child’s signature microbiome for life (27) (TABLE 1).
Optimal colonization also requires exclusive breast feeding for the first four to six months. Breast milk contains nutrients and other factors that optimize the proliferation of so called “pioneer” bacteria which uniquely stimulate development of immune factors which favor immune homeostasis over inflammation (28, 29). Those bacteria stimulated by breast feeding (e.g., Bacteroides fragilis, Bifidobacteria infantis and Lactobacillus acidophilus) uniquely stimulate endogenous production of sIgA (30), activation of T regulatory cells (23, 31) and anti-inflammation (24, 32), necessary steps to assure homeostasis. Factors in breast milk which stimulate gut development include oligosaccharides (prebiotics), sIgA, lactoferrin, etc., all of which influence the proliferation of healthy indigenous microbiota (33, 34). In addition to “pioneer bacteria” stimulated by exclusive breast feeding, factors in breast milk can interact directly with the newborn intestine and provide passive protection as well as stimulation to active development of host defense. For example, pIgA and defensins can interfere with pathogen attachment and uptake (33), omega 3 fatty acids (35) and TGF- β (36) can activate enterocytes to cause anti-inflammation and Lactoferrin can interact with the intestine and promote immune homeostasis (28, 33). In contrast, formula-fed newborns produce a more mature microbiota lacking the “pioneer” bacteria uniquely needed for newborn gut development. A study using conventional culture media techniques has shown that breast fed infants have higher levels of Bifidobacterium infantis and Lactobacillus alphadophilus whereas in formula fed infants have increased levels of enterococci and enterobacteria (37). Recently, it has been shown that breast milk has its own microbiome (38) which consists of bacteria from the periauricular alveoli, the infant’s mouth and the maternal gut (39) and contains about 103 microorganisms per cc of breast milk. How maternal gut microbiota get into breast milk is not known but thought to be due to an increase pericellular intestinal transport of maternal gut bacteria taken up orally during the last stages of pregnancy and transmitted to breast milk through macrophages circulating into the breast itself (19). The breast milk macrophages presumably discharge the engulfed bacteria into milk which is ingested by the suckling neonate. Although the composition of breast milk microbiom differs between full term and premature infants (40) and during lactation its contribution to colonizing bacteria and effect on neonatal gut defenses at this point is not understood.
Protective Functions after Initial Colonization
The gastrointestinal tract must be colonized before adequate immune function can develop. Adequate immune function occurs with a balanced innate and adaptive immune response (41). With regard to innate immunity, epithelial and immune cells must react to the penetration of pathogens or noxious antigens to prevent expression of disease but at the same time not overreact to innocuous antigens or commensal bacteria to produce a chronic inflammatory state. Protection from penetration of the mucosal barrier within the mucosal surface requires microbial-intestinal crosstalk of an adequately colonized intestine. Numerous immunologic and non-mmunologic factors contribute to mucosal barrier function (FIGURE 1) (42). These factors are all stimulated by colonizing bacteria. They include the production of anti-bacterial substances (defensins, etc.) by paneth epithelial cells (43), a mucus barrier stimulated by activated MUC-2 genes in goblet cells (44), pattern recognition receptors (e.g. toll-like receptors) on epithelial and immune cells (45) and a specialized epithelium (so called microfold or M-cell) (46) to mediate direct antigen/bacteria access from the intestinal lumen to appropriate lymphoid elements (intraepithelial lymphocytes, macrophages and dendritic cells).
Figure 1.
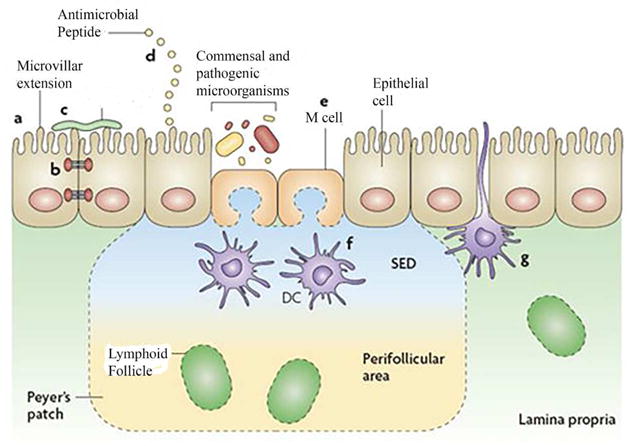
The intestinal epithelial-cell barrier. Simple columnar epithelial cells exhibit physical and biochemical adaptations to microbial colonization to maintain barrier integrity including actin-rich microvillar extensions (a), epithelial-cell tight junctions (b), apically attached and secreted mucins that form a glycocalyx (c) and the production of various anti microbial peptides (d). Specialized intestinal epithelial cells known as M (microfold) cells overlie Peyer’s patches and lymphoid follicles to facilitate luminal sampling. M cells exhibit reduced mucin secretion and have modified apical and basolateral surfaces (e) to promote uptake and transport of luminal contents to professional antigen-presenting cells that inhabit the subepithelial dome (SED) of the Peyer’s patches and lymphoid follicles (f). Specialized dendritic cell (CD) subsets can also extend dendrites between the tight junctions of intestinal epithelial cells to sample luminal contents (g). (Reproduced with permission from reference 16.)
Protective adaptive immunity requires a balanced response of T-helper cells that mediate humoral, cellular and tolerogenic immunity. Full term infants are born with a TH2 bias to protect them from rejection in the womb (47). With initial colonization the TH2 bias becomes a balanced TH1, TH2, TH17 and T-regulatory helper cell response. Germfree animals retain the TH2 bias and colonization is required before a balanced T-helper cell response can develop (48). In fact, colonization must occur in the neonatal period and not later in life for adequate adaptive immunity to develop (22). That is in part why normal initial colonization is essential for immune homeostasis.
Not only do colonizing bacteria per se affect intestinal immune development but metabolites and secreted products from colonizing bacteria interacting with the intestine can help modulate adaptive function. For example, short chain fatty acids (SCFA) produced by fermentation of high fiber diets by “pioneer” bacteria can stimulate a proliferation of FOXP3 T-regulatory cells via a receptor, GFR43, on immune cells (FIGURE 2). This occurs with increased levels of acetate, butyrate and proprionic acid production (49). These SCFA alone given to germfree animals can increase immune tolerance in the absence of active bacteria (50). In like manner, a polysaccharide (PSA) on the surface of Bacteroides fragilis organisms given to uncolonized animals can shift the TH2 to a balanced TH1 and TH2 response and activate a T-regulatory cell response via interaction with TLR2 receptors on dendritic cells (51, 52). Other specific colonizing bacteria (Clostridial species) can interact directly with immune cells to stimulate T-regulatory cells (53). Why is this important? T-regulatory cells and immune tolerance must occur as part of the normal adaptive immune response to prevent increase in autoimmune and allergic diseases that represent the new disease paradigm in developed countries (54). An abnormal colonization process (to be discussed later) can interfere with the development of tolerance and predispose to these conditions (55).
Figure 2.
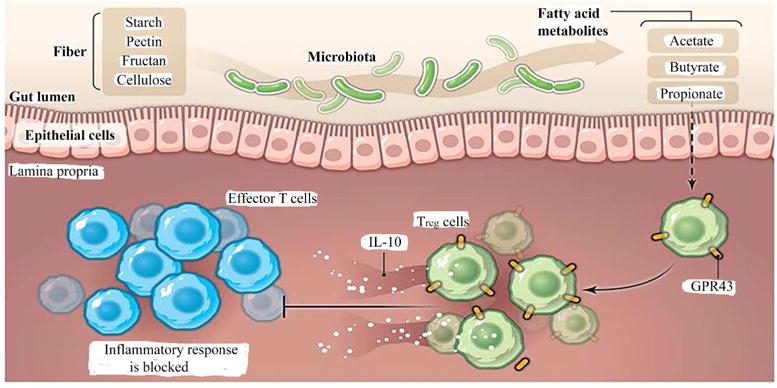
Bacterial metabolites fight intestinal inflammation. Commensal bacteria metabolize fiber and generate short-chain fatty acids. These fatty acids are ligand for GPR43 expressed by Treg cells and stimulate their expansion and immune-suppressive properties such as the production of IL-10, thereby controlling proinflammatory responses in the gut. (Reproduced from reference 29.)
As we have examined the mucosal barrier, we have begun to understand the importance of the mucus coat overlying epithelial cells as an important component of the mucosal barrier (56). We now know that colonizing bacteria can directly stimulate goblet cells to increase the expression of the MUC2 gene, its translation into glycoproteins and production of mucus to cover the epithelia cells surface (57). There is a direct inverse association between the thickness of the mucus coat and intestinal inflammation, particularly in inflammatory bowel disease (56). The mucus coat has an outer less dense component containing protective molecules (sIgA and defensins) and commensal anti-pathogen producing bacteria to protect against pathogen penetration. The inner component of the mucus coat close to the epithelial surface is very dense, contains no microorganisms or protective molecules and acts as a physical barrier to pathogen penetration to the epithelium which can stimulate inflammation (58). This important barrier component is under the control of colonizing bacteria via goblet cell mucous production and its absence has been implicated in chronic intestinal disease. Thus normal initial colonization is critical to the development of intestinal function and protection against expression of disease.
Dysbiosis
When there is a disruption in the composition of intestinal microbiota less diversity and altered ratios of large bacteria taxa occur, a condition called “dysbiosis”. Dysbiosis has been associated with disease states. It’s occurrence can be the result of a disruption to the intestinal function (altered diet, antibiotics, intestinal infection, etc.) or can occur as a result of underlying disease (inflammation, malignancy, chemotherapeutic injury, etc.) Regardless, dysbiosis has been associated with numerous disease states as shown by transplanting the microbiota from either an affected individual or an experimental animal with disease into germfree animals which results in the phenotypic expression of that disease (59). Accordingly, dysbiosis has become an area of clinical and research interest.
Dysbiosis can occur frequently during the initial colonization of the newborn intestine (TABLE 2) (60). Since the neonatal phase of colonization is critical to the initial colonization (e.g., the ingested bolus of maternal vaginal/colonic bacteria) process, any disruption in this step can lead to dysbiosis. This occurs with maternal dysbiosis during pregnancy, delivery by cesarean section, premature delivery and excessive use of antibiotics in the perinatal period. Each circumstance leads to an inadequate phase two of colonization and despite the stimulus of oral feeding and weaning to solid foods final colonization is delayed until four to six years during which time the infant is more susceptible to infection and to immune-mediated diseases (61).
TABLE 2.
Clinical conditions resulting in an atypical (dysbiotic) *intestinal colonization in the perinatal period
| Phase one: Interuterine period |
| Dysbiosis in the maternal intestine (obesity, use of antibiotics) can affect the transplacental passage of microbiota into amniotic fluid |
| Phase two: Sparse inadequate colonization due to maternal dysbiosis, premature delivery, caesarean section delivery or use of perinatal prophylactic antibiotics |
| Phases three and four: Introduction of feeding results in slight modification of the colonization process |
| Phase five: Delayed, incomplete colonization until four to six years |
more susceptibility to pathogens and immune-mediated disease, e.g. atopy
Delivery by c-section can be lifesaving for a distressed fetus. However, in many developed countries the procedure has also become increasingly used for the convenience of the mother or obstetrician. The incidence of elective c-section can be as high as forty percent in certain developed countries (Denmark (62, 63) and Brazil (61). What happens is the fetus is removed from the womb without ingesting maternal vaginal/colonic microbiota which occurs from passage through the birth canal. Instead the infant is exposed to microbiota from mother’s skin or the hospital environment and a less diverse intestinal colonization develops. Many epidemiologic studies have reported a direct association between infants born by c-section, particularly elective c-section, and an increased incidence of autoimmune (IBD, type 1 diabetes) obesity and atopic disease (64–67). However, a recent study from Houston suggests that c-section for clinical emergencies when the fetus has already entered the birth canal and is in distress results in a colonizing microbiota similar to vaginally delivered newborns (68) clarifying discrepancies in c-section studies. A study of allergy-prone infants born initially by c-section showed a striking increase incidence of atopy compared to vaginal delivery (69). This dysbiosis, caused by elective c-sections, particularly in specific patent populations, represents a risk factor for disease.
In like manner, when antibiotics are given to infants in the perinatal and newborn environment, the initial colonization process is disrupted leading to a dysbiotic state (70). Again epidemiologic studies have shown a direct association between the use of antibiotics during the first year of life and the expression of asthma during adolescents (71). The more episodes of antibiotic use in the first year of life and the nature of the antibiotic (broad vs. narrow spectrum) the greater the odds ratio for developing asthma and other diseases (72). Similar observations have been noted for early use of antibiotics and inflammatory bowel disease and type 1diabetes (73). A recently reported experimental study recently reported involving early use of penicillin in an animal model which initially disrupted colonization but it later reverted to a normal colonization pattern despite resulting in the treated newborns gaining excessive body weight leading to adult obesity (74). The tendency for obesity was made worse if the newborn pups were fed a high fat diet.
An infant born prematurely rapidly passes through the birth canal, preventing the ingestion of a bolus of maternal/vaginal microbiota. Just as in birth by c-section, the intestinal colonization process is dysbiotic. This dysbiosis has been reported in association with an increased incidence of necrotizing enterocolitis (NEC) (75). We have studied this condition and have hypothesized that a dysbiotic colonization in association with immature intestinal host defense (inflammation vs. homeostasis) is likely to be important risk factors for NEC (76). We have also reported that the more immature the intestine, e.g., 1500 vs. 1000 gm infants, the more dysbiotic the colonization process becomes, suggesting that intestinal immaturity contributes to intestinal dysbiosis (75). Recently we have reported that when prematures are given mother’s expressed breast milk vs. formula the nature of colonization differs strikingly (77). We speculate that the influence of expressed breast milk, known in part to be the basis for protection against NEC, on intestinal colonization with prematures fed their mother’s expressed breast milk may be in part the basis for prevention of the disease.
Finally, the nature of maternal microbiota during pregnancy can influence initial colonization and may contribute to dysbiosis leading to disease. This have been shown to be true with mothers gaining excessive weight during pregnancy leading to obesity (78). Under these conditions, the mother’s intestinal microbiota becomes dysbiotic and this is passed on to the delivered infant either in utero or at the time of delivery or both (79). As a result the newborn has dysbiotic initial microbiota which favors increased absorption of nutrients and excessive weight gain. These infants gain at a rate much faster than infants born to mothers without the dysbiosis of excessive weight gain during pregnancy. Furthermore, the nature of the weaning diet,(e.g., high fat versus high protein) can contribute to the infant’s excessive weight gain (80). These examples of dysbiosis in initial colonization underscore the importance of normal colonization of the newborn in preventing expression of disease during infancy,, childhood and adulthood. It also strongly supports striving to provide appropriate conditions for normal colonization in infancy — full term vaginal delivery and exclusive breast feeding for four to six months and a balanced diet of weaning food.
How Do We Combat Dysbiosis
The best way to combat dysbiosis is to have a better understanding of the basis for the condition and to recognize how the western lifestyle, particularly the western diet, has affected the composition of microbiota in the gastrointestinal tract (81–83). Once the cause of dysbiosis is established addressing the condition will be easier for the physician. Approaches thus far include dietary changes, prebiotics, probiotics and fecal microbiota transplants (FMT).
Diet
We know that diet can influence the composition of colonizing bacteria from studies described with regard to breast feeding in the neonatal period and its influence on the developing intestine (28). Dietary influence extends beyond that time period. A recent study (84) comparing the composition of intestinal microbiota in children raised in Florence, Italy on a Westernized high fat/high protein dietary intake compared to children raised in a remote village in Africa ingesting complex carbohydrates and a high fiber diet showed that the intestinal microbiota in these two populations was strikingly different as was the nature of disease in these populations. Children in Florence have an increase in immune mediated diseases (atopy, and autoimmune diseases) where as children in Africa have principally infectious disease. At this point this is only an association and other factors such as genetics and geographic disease issues may pertain. Additional cause and effect studies are needed before specific microbial causes can be ascribed to the differences. A clinical study of healthy adults placed on either a high protein, high fat or high carbohydrate diet for prolonged periods of time showed a striking difference in intestinal microbiota (85). Composition of the diet has also shown changes in bacterial genetic expression and in secretions of microbial metabolites (86). These studies suggest that diet influences intestinal microbiota and may be involved in health and disease. The infant when weaned to solid foods at four to six months should be given a healthy balanced diet with fiber, vegetables and fruit and with less processed foods. A recent study has shown that emulsifiers in processed foods affects the mucus layer overlying epithelial cells in the intestine and allows microbes to cause inflammation leading to increased incidence of metabolic syndrome (86).
Prebiotics
Prebiotics are complex carbohydrates (fructo-oligosaccharides, galacto-oligosaccharide, etc.) that are ingested into the intestine and enter the colon intact where they are metabolized by resident microbiota (88–92). The microenvironment created by their metabolism results in proliferation of health promoting indigenous microbiota which positively affect gut function. Proteotypic prebiotics is breast milk which contains eight percent of total milk solids as oligosaccharides, and as stated above, affects intestinal colonization (28). Studies have been done using prebiotics to treat dysbiosis associated with disease (allergy, diarrhea, autoimmune disease) (93–98) with mixed success suggesting that this approach is only partially successful in combating dysbiosis.
Probiotics
Probiotics are live microorganisms isolated from the human intestine which have a health promoting effect beyond their nutritive value (99). Probiotics have been used extensively in pediatrics to prevent the anticipated dysbiosis seen in allergic disease (100), inflammatory bowel disease (101), irritable bowel syndrome, (93) NEC (99) and obesity (102) with mixed results. This approach will become more successful when we identify the specific disruption in intestinal microbiota associated with a specific disease condition. When prebiotics and probiotics are used in combination (symbiotics) correction of dysbiosis has been somewhat more successful (102).
Fecal Microbial Transplant
Fecal microbial transplant uses the intestinal microbiota environment from a healthy individual to treat a patient with a known condition (103). Its use has been primarily for adult patients and success has been shown principally in patients with recurrent C. difficile colitis (104). Trials, with limited success, have also been tried in patients with inflammatory bowel disease (103), diabetes (105) and obesity (106). Unfortunately, because large multi-center clinical trials with an established protocol and a long term follow-up component has not been done to determine potential side effects, the enormous microbial community which is “normal” for one individual may potentially be dangerous for the FMT recipient and the composition of an intestinal microbiota from one donor may differ with another. The approach and methods need to be further standardized before the treatment can be considered for routine therapy. A recent inadequate study claims FMT can affect Autism (68). When the entire intestinal microbial contents from one person is given to another, trillions of microorganisms and their metabolites are transferred some of which could potentially be detrimental to the transplant recipient.
Summary and Conclusions
This review, has underscored the role of initial colonizing intestinal bacteria, as an ancillary body organ, in the appropriate development of immune and metabolic function in the newborn intestine and in determining health and disease in the infant, child and adult. Initial colonization occurs at a time when the newborn infant is adapting to the extrauterine environment and this component of colonizing bacteria on gut function has a lifelong effect. Evidence is provided that dysbiosis, occurring at the time of birth, can have an effect on the expression of disease later in life. Although we do not completely understand the role of intestinal bacterial colonization in phenotypic expression of disease, several current approaches are suggested to combat dysbiosis in order to prevent or to treat disease. When we have a better understanding of the disruption in intestinal microbiota associated with specific disease conditions, we should be more effective in preventing/treating the disease by preventing dysbiosis.
Acknowledgments
Statement of financial support: Research Grants have been obtained from National Institutes of Health, Bethesda, MD, USA (P30 DK040561 and P01 DK033506)
Footnotes
Disclosure: There is no conflict of interest to disclose
References
- 1.Rautava S, Luoto R, Salminen S, et al. Microbial contact during pregnancy, intestinal colonization and human disease. Nat Rev Gastroenterol Hepatol. 2012;9:565–576. doi: 10.1038/nrgastro.2012.144. [DOI] [PubMed] [Google Scholar]
- 2.Shanahan F. Host-flora interactions in inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:S16–S24. doi: 10.1097/00054725-200402001-00004. [DOI] [PubMed] [Google Scholar]
- 3.Renz H, Brandtzaeg P, Hornef M. The impact of perinatal immune development on mucosal homeostasis and chronic inflammation. Nat Rev Immunol. 2012;12:9–23. doi: 10.1038/nri3112. [DOI] [PubMed] [Google Scholar]
- 4.Martin CR, Caicedo RA, Walker WA. Development of intestinal mucosal barrier. In: Neu J, editor. Gastroenterology and Nutrition: Neonatology Questions and Controversies. Elsevier/Saunders; 2012. pp. 39–58. [Google Scholar]
- 5.Alenghat T, Osborne KC, Saenz SA, et al. Histone deacetylase 3 coordinate commensal-bacteria-dependent intestinal homeostasis. Nature. 2013;504:153–157. doi: 10.1038/nature12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zilbauer M, Zellos A, Jenke A, et al. Epigenetics in paediatric gastroenterology, hepatology, and nutrition: present trends and future perspectives. Journal Of Pediatric Gastroenterology And Nutrition. 2016;62(4):521–529. doi: 10.1097/MPG.0000000000001053. [DOI] [PubMed] [Google Scholar]
- 7.Choi S, Friso S. Epigenetics: A new bridge between nutrition and health. Advances In Nutrition. 2010;1(1):8–16. doi: 10.3945/an.110.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barua S, Junaid M. Lifestyle, pregnancy and epigenetic effects. Epigenomics. 2015;(1):85–102. doi: 10.2217/epi.14.71. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi K, Sugi Y, Hosono A, et al. Epigenetic regulation of TLR4 gene expression in intestinal epithelial cells for the maintenance of intestinal homeostasis. J Immunol. 2009;183(10):6522–6529. doi: 10.4049/jimmunol.0901271. [DOI] [PubMed] [Google Scholar]
- 10.Koenig JE, Spor A, Scalfone N, et al. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci. 2011;108:4578–4585. doi: 10.1073/pnas.1000081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mackie RI, Sghir A, Gaskins HR. Developmental microbial ecology of the neonatal gastrointestinal tract. Am J Clin Nutr. 1999;69:1035s–1045s. doi: 10.1093/ajcn/69.5.1035s. [DOI] [PubMed] [Google Scholar]
- 12.Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. NEJM. 2002;347:911–902. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 13.Thorburn AN, Macia L, Mackay CR. Diet, metabolites and “Western-Lifestyle” inflammatory bowel disease. Immunity. 2014;40:833–842. doi: 10.1016/j.immuni.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan J, Shi H, Walker W. The role of microbes in developmental immunologic programming. Pediatric Research. 2011;69(6):465–472. doi: 10.1203/PDR.0b013e318217638a. [DOI] [PubMed] [Google Scholar]
- 15.Guarner F, Bourdet-Sicard R, Rook G, et al. Mechanisms of Disease: the hygiene hypothesis revisited. Nature Clinical Practice Gastroenterology And Hepatology. 2006;(5):275. doi: 10.1038/ncpgasthep0471. [DOI] [PubMed] [Google Scholar]
- 16.Wills-Karp M, Santeliz J, Karp C. The germless theory of allergic disease: revisiting the hygiene hypothesis. Nature Reviews, Immunology. 2001;1(1):69–75. doi: 10.1038/35095579. [DOI] [PubMed] [Google Scholar]
- 17.Shanahan F. The gut microbiota—a clinical perspective on lessons learned. Nat Rev Gastroenterol Hepatol. 2012;9:609–614. doi: 10.1038/nrgastro.2012.145. [DOI] [PubMed] [Google Scholar]
- 18.Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med. 2014;6:237. doi: 10.1126/scitranslmed.3008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donnet-Hughes A, Perez PF, Doré J, et al. Potential role of the intestinal microbiota of the mother in neonatal immune education. Proc Nut Soc. 2010;69:407–415. doi: 10.1017/S0029665110001898. [DOI] [PubMed] [Google Scholar]
- 20.Perez PF, Dore J, Leclerc M, et al. Bacterial imprinting of the neonatal immune system: Lessons from maternal cells? Pediatrics. 2007;119:e725–e732. doi: 10.1542/peds.2006-1649. [DOI] [PubMed] [Google Scholar]
- 21.Ardissone AN, de la Cruz DM, Davis-Richardson A, et al. Meconium microbiome analysis identifies bacteria correlated with preterm birth. PLoS One. 2014;9:e90784. doi: 10.1371/journal.pone.0090784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karlsson M, Kahu H, Hanson L, et al. Neonatal colonization of rats induces immunological tolerance to bacterial antigens. European Journal Of Immunology. 1999;29(1):109–118. doi: 10.1002/(SICI)1521-4141(199901)29:01<109::AID-IMMU109>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz S, Friedberg I, Ivanov IV, et al. A metagenomic study of diet-dependent interaction between gut microbiota and host in infants reveals differences in immune response. Genome Biol. 2012;13:r3. doi: 10.1186/gb-2012-13-4-r32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chichlowski M, De Lartigue G, German JB, et al. Bifidobacteria isolated from infants and cultured on human milk oligosaccharides affect intestinal epithelial function. J Pediatr Gastroenterol Nutr. 2012;55:321–327. doi: 10.1097/MPG.0b013e31824fb899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sudo N, Sawamura S, Tanaka K, et al. The requirement of intestinal bacterial flora for the development of an IgE production system fully susceptible to oral tolerance induction. J Immunol. 1997;159:1739–1745. [PubMed] [Google Scholar]
- 26.Olszak T, An D, Blumberg R, et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;(6080):489–493. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weng M, Walker WA. The role of gut microbiota in programming the immune phenotype. J Developmental Origin of Health and Disease. 2013;4:203–214. doi: 10.1017/S2040174412000712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walker WA, Iyengar RS. Breast milk, microbiota and intestinal immune homeostasis. Pediatr Res. 2015;77:220–228. doi: 10.1038/pr.2014.160. [DOI] [PubMed] [Google Scholar]
- 29.Torrow N, Hornef M. The neonatal window of opportunity: setting the stage for life-long host-microbial interaction and immune homeostasis. The Journal of Immunology. 2017;198:557–563. doi: 10.4049/jimmunol.1601253. [DOI] [PubMed] [Google Scholar]
- 30.Jost T, Lacroix C, Braegger CP, et al. New insights in gut microbiota establishment in healthy breast fed neonates. PLoS One. 2012;7:e44595. doi: 10.1371/journal.pone.0044595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furuta G, Walker WA. Non-immune defense mechanisms of the gastrointestinal tract. In: Blaser MJ, Smith PD, Ravdin JI, Greenberg HB, Guerrant RL, editors. Infections of the Gastrointestinal Tract. Raven Press Ltd; NY: 1995. pp. 89–98. [Google Scholar]
- 32.Rautava S, Walker WA. Breatfeeding — An extrauterine link between mother and child. Breastfeeding Medicine. 2009;4(1):3–10. doi: 10.1089/bfm.2009.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newburg DS, Walker WA. Protection of the neonate by the immune system of developing gut and of human milk. Ped Res. 2007;61:2–8. doi: 10.1203/01.pdr.0000250274.68571.18. [DOI] [PubMed] [Google Scholar]
- 34.Gregory KE, Walker WA. Immunologic factors in human milk and disease prevention in the preterm infant. Curr Pediatr Rep. 2013;1:222–228. doi: 10.1007/s40124-013-0028-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wijendran V, Brenna JT, Walker WA, et al. Long chain poly-unsaturated fatty acids attenuate the IL-1β-induced pro-inflammatory response in human fetal intestinal epithelial cells. Pediatr Res. 2015;78:626–633. doi: 10.1038/pr.2015.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rautava S, Nanthakumar NN, Walker WA, et al. Breast milk transforming growth factor-β2 specifically attenuates IL-1β-induced inflammatory responses in the immature human intestine via a SMAD6 and ERK-dependent mechanism. Neonatology. 2010;99:192–201. doi: 10.1159/000314109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshioka H, Iseki K, Fujita K. Development and differences of intestinal flora in the neonatal period in breast-fed and bottle-fed infants. Pediatrics. 1983;72(3):317–321. [PubMed] [Google Scholar]
- 38.Jeurink P, van Bergenhenegouwen J, Martín R, et al. Human milk: a source of more life than we imagine. Beneficial Microbes. 2013;4(1):17–30. doi: 10.3920/BM2012.0040. [DOI] [PubMed] [Google Scholar]
- 39.Martín R, Heilig G, Zoetendal E, et al. Diversity of the Lactobacillus group in breast milk and vagina of healthy women and potential role in the colonization of the infant gut. Journal Of Applied Microbiology. 2007;103(6):2638–2644. doi: 10.1111/j.1365-2672.2007.03497.x. [DOI] [PubMed] [Google Scholar]
- 40.Cabrera-Rubio R, Collado M, Laitinen K, et al. The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. The American Journal Of Clinical Nutrition. 2012;96(3):544–551. doi: 10.3945/ajcn.112.037382. [DOI] [PubMed] [Google Scholar]
- 41.Martin CR, Walker WA. Gleason & Devaskar: Avery’s Diseases of the Newborn. 9. Elsevier/Saunders; 2011. Innate and mucosal immunity in the developing GI tract: Relationship to early and later disease; pp. 994–1006. [Google Scholar]
- 42.Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat rev Immunol. 2008;8:411–420. doi: 10.1038/nri2316. [DOI] [PubMed] [Google Scholar]
- 43.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol 3. 2003:7190–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 44.Johansson MEV, Larsson JMH, Hansson GC. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc Natl Acad Sci. 2011;108:4659–4665. doi: 10.1073/pnas.1006451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sansonetti PJ. War and peace at mucosal surfaces. Nat Rev Immunol. 2004;4:953–964. doi: 10.1038/nri1499. [DOI] [PubMed] [Google Scholar]
- 46.Neutra MR. M cells in antigen sampling in mucosal tissues. Curr Top Microbial Immunol. 1999;236:17–32. doi: 10.1007/978-3-642-59951-4_2. [DOI] [PubMed] [Google Scholar]
- 47.Hansen CHF, Nielsen DS, Kverka M, et al. Patterns of early gut colonization shape future immune responses of the host. Plos One. 2012;7:e34043. doi: 10.1371/journal.pone.0034043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sudo N, Sawamura S, Tanaka K, et al. The requirement of intestinal bacterial flora for the development of an IgE production system fully susceptible to oral tolerance induction. J Immunol. 1997;159:1739–1745. [PubMed] [Google Scholar]
- 49.Bollrath F, Powrie F. Feed your Tregs more fiber. Science. 2013;341:463–464. doi: 10.1126/science.1242674. [DOI] [PubMed] [Google Scholar]
- 50.Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mazmanian SK, Liu CH, Tzianabos AO, et al. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 52.Mazmanian SK, Kasper DL. The love-hate relationship between bacterial polysaccharides and the host immune system. Nat Rev Imnunol. 2006;6:849–858. doi: 10.1038/nri1956. [DOI] [PubMed] [Google Scholar]
- 53.Atarashi K, Tanoue T, Shima T, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kiss EA, Vonarbourg C, Kopfmann S, et al. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science. 2011;334:1561–1565. doi: 10.1126/science.1214914. [DOI] [PubMed] [Google Scholar]
- 55.Weiner HL, da Cunha AP, Quintana F, Wu H. Oral tolerance. Immunol Rev. 2011;241:241–259. doi: 10.1111/j.1600-065X.2011.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Petersson J, Schreiber O, Hansson GC, et al. Importance and regulation of the colonic mucus barrier in a mouse model of colitis. A J Physiol Gastrointest Liver Physiol. 2011;300:G327–G333. doi: 10.1152/ajpgi.00422.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McAuley JL, Linden SK, Png CW, et al. MUC1 cell surface mucin is a critical element of the mucosal barrier to infection. J Clin Invest. 2007;117:2313–2324. doi: 10.1172/JCI26705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frey A, Giannasca KT, Weltzin R, et al. Role of the glycocalyx in regulating access of microparticles to apical plasma membranes of intestinal epithelial cells implications for microbial attachment and oral vaccine targeting. J Exp Med. 1996;184:1045–1059. doi: 10.1084/jem.184.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ahern PP, Faith JJ, Gordon JI. Mining the human gut microbiota for effector strains that shape the immune system. Immunity. 2014;40:815–823. doi: 10.1016/j.immuni.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Walker WA. Dysbiosis. In: Floch MH, Ringel Y, Walker WA, editors. The Microbiota in Gastrointestinal Pathophysiology: Implications for Human Health, Prebiotics, Probiotics and Dysbiosis. Elsevier Inc; 2016. pp. 227–231. Chapter 25. [Google Scholar]
- 61.Jakobsson HE, Abrahamsson TR, Jenmalm MC, et al. Decreased gut microbiota diversity, delayed Bacteroides colonization and reduced Th1 responses in infants delivered by Caesarean section. Gut. 2013;63:559–66. doi: 10.1136/gutjnl-2012-303249. [DOI] [PubMed] [Google Scholar]
- 62.Chu D, Ma J, Prince A, et al. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nature Medicine. 2017;1:1–5. doi: 10.1038/nm.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sevelsted A, Stokholm J, Bønnelykke K, et al. Cesarean section and chronic immune disorders. Pediatrics. 2015;135(1):e92–e98. doi: 10.1542/peds.2014-0596. [DOI] [PubMed] [Google Scholar]
- 64.Mårild K, Stephansson O, Montgomery S, et al. Pregnancy outcome and risk of celiac disease in offspring: a nationwide case-control study. Gastroenterology. 2012;142:39–45. doi: 10.1053/j.gastro.2011.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Decker E, Engelmann G, Findeisen A, et al. Cesarean delivery is associated with celiac disease but not inflammatory bowel disease in children. Pediatrics. 2010;125:e1433–1440. doi: 10.1542/peds.2009-2260. [DOI] [PubMed] [Google Scholar]
- 66.Mesquita DN, Barbieri MA, Goldani HA, et al. Cesarean section is associated with increased peripheral and central adiposity in young adulthood: cohort study. PloS One. 2013;8:e66827. doi: 10.1371/journal.pone.0066827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thavagnanam S, Fleming J, Bromley A, et al. A meta-analysis of the association between Caesarean section and childhood asthma. Clin Exp Allergy. 2008;38:629–33. doi: 10.1111/j.1365-2222.2007.02780.x. [DOI] [PubMed] [Google Scholar]
- 68.Chu D, Ma J, Prince A, et al. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nature Medicine. 2017 Mar;23(3):314–326. doi: 10.1038/nm.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Penders J, Stobberingh EE, van den Brandt, et al. The role of the intestinal microbiota in the development of atopic disorders. Allergy. 2007;62:1223–1236. doi: 10.1111/j.1398-9995.2007.01462.x. [DOI] [PubMed] [Google Scholar]
- 70.Zeissig S, Blumberg RS. Life at the beginning: perturbation of the microbiota by antibiotics in early life and its role in health and disease. Nature Immunol. 2014;12:307–310. doi: 10.1038/ni.2847. [DOI] [PubMed] [Google Scholar]
- 71.An D, Oh SF, Olszak T, et al. Sphingolipids from a symbiotic microbe regulate homeostasis of host intestinal natural killer T cells. Cell. 2014;13:123–133. doi: 10.1016/j.cell.2013.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marra F, Marra CA, Richardson K, et al. Antibiotic use in children is associated with increased risk of asthma. Pediatrics. 2009;123:1003–1010. doi: 10.1542/peds.2008-1146. [DOI] [PubMed] [Google Scholar]
- 73.Giongo A, Gano KA, Drabb DB, et al. Toward defining the autoimmune microbiome for type 1 diabetes. ISME J. 2011;5:82–91. doi: 10.1038/ismej.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Trasande L, Blustein J, Liu M, et al. Infant antibiotic exposures and early-life body mass. Int J Obes. 2013;37:16–23. doi: 10.1038/ijo.2012.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou Y, Shan G, Sodergren E, et al. Longitudinal analysis of preterm intestinal microbiome prior to necrotizing enterocolitis: A case-control study. Plos One. 2015;10(3):e0118632. doi: 10.1371/journal.pone.0118632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nanthakumar N, Meng D, Goldstein AM, et al. The mechanism of excessive intestinal inflammation in necrotizing enterocolitis: An immature innate immune response. Plos One. 2011;6(3):e17776. doi: 10.1371/journal.pone.0017776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gregory KE, Samuel BS, Houghteling P, et al. Influence of maternal breast milk ingestion on acquisition of the intestinal microbiome in preterm infants. Microbiome. 2016;4:68. doi: 10.1186/s40168-016-0214-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Million M, Lagier J-C, Yahav D, Paul M. Gut bacterial microbiota and obesity. Clin Microbiol Infect. 2013;19:305–313. doi: 10.1111/1469-0691.12172. [DOI] [PubMed] [Google Scholar]
- 79.Galley JD, Bailey M, Dush CK, Schoppe-Sullivan S, Christian LM. Maternal obesity is associated with alterations in the gut microbiome in toddlers. Plos One. 2014;9:e-112026. doi: 10.1371/journal.pone.0113026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Baker JL, Michalesen KF, Rasmussen KM, Sorensen TIA. Maternal prepregnant body mass index, duration of breastfeeding, and timing of complementary food introduction are associated with infant weight gain. Am J Clin Nutr. 2004;80:1579–1588. doi: 10.1093/ajcn/80.6.1579. [DOI] [PubMed] [Google Scholar]
- 81.McFarland L. Use of probiotics to correct dysbiosis of normal microbiota following disease or disruptive events: a systematic review. BMJ Open. 2014;4(8):1. doi: 10.1136/bmjopen-2014-005047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Belizário J, Napolitano M. Human microbiomes and their roles in dysbiosis, common diseases, and novel therapeutic approaches. Frontiers In Microbiology. 2015;6:1050. doi: 10.3389/fmicb.2015.01050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Petersen C, Round J. Defining dysbiosis and its influence on host immunity and disease. Cellular Microbiology. 2014;16(7):1024–1033. doi: 10.1111/cmi.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.De Filippo C, Cavalieri D, Di Paola M, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Nat Acad Sci. 2010;107(33):14691–6. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Faith JJ, Guruge JL, Charbonneau M, et al. The Long-Term Stability of the Human Gut Microbiota. Science. 2013;341:1237439. doi: 10.1126/science.1237439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chassaing B, Koren O, Godrich JK, et al. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. 2015;519:92–96. doi: 10.1038/nature14232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zenhom M, Hyder A, de Vrese M, et al. Prebiotic oligosaccharides reduce proinflammatory cytokines in intestinal Caco-2 cells via activation of PPARγ and preptidoglycan recognition protein 3. J Nutr. 2011;141:971–977. doi: 10.3945/jn.110.136176. [DOI] [PubMed] [Google Scholar]
- 89.Bakker-Zieikzee AM, van Tol EAF, Kroes H, Alles MS, Kok FJ, Bindels JD. Fecal SIgA secretion in infants fed on pre- or probiotic infant formula. Pediatr Allergy Immunol. 2006;17:134–140. doi: 10.1111/j.1399-3038.2005.00370.x. [DOI] [PubMed] [Google Scholar]
- 90.Chichlowski M, DeLartigue G, German JB, Raybould HE, Mills DA. Bifidobacteria isolated from infants and cultured on human milk oligosaccharides affect intestinal epithelial function. J Pediatr Gastro Nutr. 2012;55:321–327. doi: 10.1097/MPG.0b013e31824fb899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Newburg DS, Ko JS, Leone S, Nanthakumar NN. Human milk oligosaccharides and synthetic galactosyloligosaccharides contain 3-, 4-4, and 6-galactosyllactose and attenuate inflammation in human T84, NCM-460, and H4 cells and intestinal tissue ex vivo. J Nutr. 2016;146:358–367. doi: 10.3945/jn.115.220749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ten Bruggencate SJM, Bovee-Oudenhoven IMJ, Letink-Wissink MLG, Kata MB, ven der Merr R. Dietary fructooligosaccarides affect intestinal barrier function in healthy men. J Nutr. 2006;136:70–74. doi: 10.1093/jn/136.1.70. [DOI] [PubMed] [Google Scholar]
- 93.Moro G, Arslanoglu S, Stahl B, Jelinek J, Wahn U, Boehm G. A mixture of prebiotic oligosaccharides reduces the incident of atopic dermatitis during the first six months of age. Arch Dis Child. 2006;91:814–819. doi: 10.1136/adc.2006.098251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lindsay JO, Whelan K, Stagg AJ, et al. The clinical, microbiological and immunological effects of fructo-oligosaccharide in patients with Crohn’s disease. Gut. 2006;55:348–55. doi: 10.1136/gut.2005.074971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gruber C, van Stujvengerg M, Mosca F, et al. Reduced occurrence of early atopic dermatitis because of immunoactive prebiotics among low-atopy-risk infants. J Allergy Clin Immunol. 2010;126:791–797. doi: 10.1016/j.jaci.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 96.Boehm G, Jelinek J, Stahl B, et al. Prebiotics in infant formulas. J Clin Gastroenterol. 2004;38:S76–S79. doi: 10.1097/01.mcg.0000128927.91414.93. [DOI] [PubMed] [Google Scholar]
- 97.Silk DB, Davis A, Vulevic J, Tzortzis G, Gibson GR. Clinical trial: The effects of a trans-galactooligosaccharide prebiotic on faecal microbiota and symptoms in irritable bowel syndrome. Aliment Pharmacol Ther. 2009;29:508–518. doi: 10.1111/j.1365-2036.2008.03911.x. [DOI] [PubMed] [Google Scholar]
- 98.Xia Q, Williams T, Hustead D, Price P, Morrison M, Yu Z. Quantitative analysis of intestinal bacterial populations from term infants fed formula supplemented with fructooligosaccharides. J Pediatr Gastroenterol Nutr. 2013;55:314–320. doi: 10.1097/MPG.0b013e3182523254. [DOI] [PubMed] [Google Scholar]
- 99.Gareau MG, Sherman PM, Walker WA. Probiotics and the gut microbiota in intestinal health and disease. Nature Rev: Gastroenterol & Hepatol. 2010;7:503–514. doi: 10.1038/nrgastro.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kamada N, Chen G, Nunez G. Harnessing pathogen-commensal relations. Nature Med. 2012;18:1190–1191. doi: 10.1038/nm.2900. [DOI] [PubMed] [Google Scholar]
- 101.Mack DR, Michael S, Wei S, McDougall L, Hollingsworth MA. Probiotics inhibit enteropathogenic E coli adherence in vitro by inducing intestinal mucin gene expression. Am J Physiol Gastrointest Liver Physiol. 1999;39:G941–G950. doi: 10.1152/ajpgi.1999.276.4.G941. [DOI] [PubMed] [Google Scholar]
- 102.Thomas DW, Greer FR, et al. Clinical report — probiotics and prebiotics in pediatrics. Pediatrics. 2010;126:1217–1231. doi: 10.1542/peds.2010-2548. [DOI] [PubMed] [Google Scholar]
- 103.Smits LP, Bouter KEC, de Vos WM, Borody T, Nieuwdorp M. Therapeutic potential of fecal microbiota transplantation. Gastroenterology. 2013;145:946–953. doi: 10.1053/j.gastro.2013.08.058. [DOI] [PubMed] [Google Scholar]
- 104.Dutta SK, Girota M, Garg S, et al. Efficacy of combined jejuna and colonic fecal microbiota transplantation for recurrent clostridium difficile infection. Clin Gastroenterol Hepatol. 2014;12:1572–1576. doi: 10.1016/j.cgh.2013.12.032. [DOI] [PubMed] [Google Scholar]
- 105.Hourigan SK, Oliva-Hemker M. Fecal microbiota transplantation in children: a brief review. Pediatr Res. 2016;80:2–6. doi: 10.1038/pr.2016.48. [DOI] [PubMed] [Google Scholar]
- 106.Knaapen M, Kootte RS, Zoetendal EG, et al. Obesity, non-alcoholic fatty liver disease and atherothrombosis: a role for the intestinal microbiota? Clin Microbiol Infect. 2013;19:331–337. doi: 10.1111/1469-0691.12170. [DOI] [PubMed] [Google Scholar]


