Abstract
Objective
ADAMTS13 is primarily synthesized in liver. The biosynthesis and its physiological role in placenta are not known.
Approach and Results
We employed real time polymerase chain reaction (PCR), immunohistochemistry, and Western blotting analyses, as well as proteolytic cleavage of FRETS-VWF73 to determine ADAMTS13 expression in placenta and trophoblasts obtained from individuals with normal pregnancy and patients with severe preeclampsia. We also determined the role of ADAMTS13 in extravillous trophoblasts using a 3-(4,5-Dimethylthiazol-2-yl)- 2,5-diphenyltetrazolium bromide (MTT) assay, wound scratch assay, transwell migration assay, tube formation assay, and tissue outgrowth assays.
We demonstrated that full-length and proteolytically active ADAMTS13 was expressed in normal human placenta, primarily in the trophoblasts and villous core fetal vessel endothelium during pregnancy. Placental expression of ADAMTS13 mRNA, protein, and proteolytic activity was at the highest levels during the first trimester and significantly reduced at the term of gestation. Additionally, significantly reduced levels of placental ADAMTS13 expression was detected under hypoxic conditions and in patients with preeclampsia. In addition, recombinant ADAMTS13 protease stimulated proliferation, migration, invasion, and network formation of trophoblastic cells in culture. Finally, knockdown of ADAMTS13 expression attenuated the ability of tube formation in HTR-8/SVNEO cells and the extravillous trophoblast outgrowth in placental explants.
Conclusions
Our results demonstrate for the first time the expression of ADAMTS13 mRNA and protein in normal and abnormal placental tissues and its role in promoting angiogenesis and trophoblastic cell development. The findings support the potential role of the ADAMTS13-von Willebrand factor pathway in normal pregnancy and pathogenesis of preeclampsia.
Keywords: ADAMTS13, protein synthesis, placenta, trophoblast, and preeclampsia
Graphical abstract
Introduction
Preeclampsia, a complication of pregnancy, occurs in 5–8% pregnancy. Preeclampsia is characterized by hypertension and proteinuria at ≥20 weeks of gestation (1) an associated with iatrogenic preterm delivery, intrauterine growth restriction, placental abruption, and perinatal mortality. Patients with preeclampsia have increased maternal morbidity and mortality rates. (2, 3) Treatment of preeclampsia is limited to symptomatic control and/or early termination of pregnancy. Despite decades of research, the pathogenesis underlying preeclampsia remains poorly understood. (4) While preeclampsia is often considered a multisystem disorder, pathological features include defective trophoblast invasion and placental thrombosis.
Pregnancy is characterized by deep placentation, in which trophoblasts in the placental bed invade not only into the decidua but also into one-third of the thickness of myometrium. (5, 6) As invasive trophoblasts are responsible for transformation of spiral arteries, defects in trophoblasts might result in shallow placentation and impaired transformation of the spiral arteries, leading to preeclampsia. (7) It has been well established that trophoblast invasion is regulated by multiple factors, including cytokines, growth factors, adhesion molecules, proteases, matrix-derived components, and oxygen.(8, 9) A number of factors, both autocrine (trophoblast-derived) and paracrine (decidua-derived), are known to regulate trophoblast proliferation, migration, and invasiveness. (10, 11)
ADAMTS13, a member of the ADAMTS (a disintegrin and metalloprotease with thrombospondin type 1 repeats) family, plays an important role in regulating hemostasis and thrombosis by cleaving von Willebrand factor (VWF) under shear.(12) ADAMTS13 deficiency is associated with thrombotic thrombocytopenic purpura (TTP). Hepatic stellate cells (13-15), endothelial cells (16, 17), megakaryocytes and platelets (18), renal tubular epithelial cells, glomerular endothelial cells and glomerular podocytes (19) are shown to produce ADAMTS13 at various levels. ADAMTS13 mRNA was detected in human placentae (20, 21), but the expression of the protein in the placenta or any specific cell in the placenta has not been investigated. Here, we determined the biosynthesis and secretion of ADAMTS13 in placentae and potential functions of ADAMTS13 in angiogenesis, development of placental tissue, and its association with preeclampsia.
Materials and Methods
(available online as the supplemental materials)
Results
Characteristics of Patients
The baseline characteristics of the study groups are presented in Table 1. Controls are matched with severe preeclampsia cases for maternal age, incidence of nulliparity, and gestational age at delivery. As expected, mean birth weight was significantly lower in the severe preeclampsia group while incidence of fetal growth restriction was obviously higher in the severe preeclampsia group.
Table 1. Clinical characteristics of the patients and controls.
| Clinica parameters | PE | Control | P value |
|---|---|---|---|
| Maternal age (Years) | 30.1 ± 4.3 | 29.8 ± 3.9 | 0.784 |
| Nulliparity, n (%) | 3 (13.6) | 9 (36) | n/a |
| GA at diagnosis (Weeks) | 33.8 ± 2.6 | n/a | n/a |
| GA at delivery (Weeks) | 37.9 ± 1.7 | 38.6 ± 2.3 | 0.221 |
| In-hospital SBP (mmHg) | 159.9 ± 14,3 | 119.7 ± 11.4 | 0.000 |
| In-hospital DBP (mmHg) | 106.0 ± 9.7 | 77.2 ± 6.8 | 0.000 |
| Proteinuria, n (%) | 22 (100) | 0 (0) | 0.000 |
| Birth weight (Grams) | 2906.4 ± 605.7 | 3281.2 ± 378.2 | 0.013 |
| Fetal growth restriction, n (%) | 5 (22.7) | 0 (0) | 0.041 |
PE, severe preeclampsia; GA, gestation age; SBP, systolic blood pressure; DBP, diastolic blood pressure. The data are shown as the mean ± SEM or percentage. n/a, not determined or not applicable
ADAMTS13 is expressed in placenta
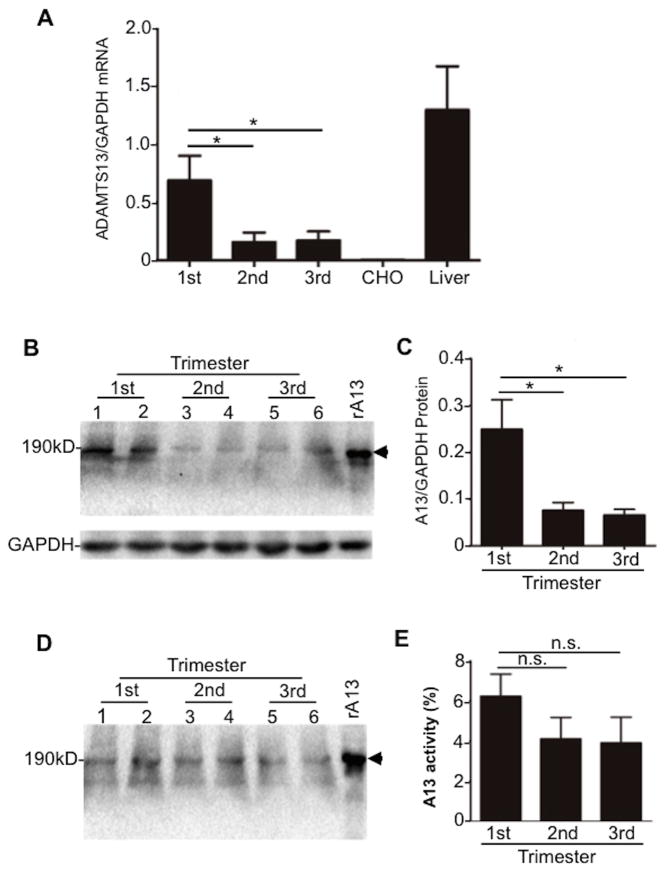
ADAMTS13 mRNA was detected by a real-time polymerase chain reaction (PCR) analysis in placentae throughout pregnancy. The placental ADAMTS13 mRNA levels were highest during the first trimester and reduced during the second and third trimesters (Fig. 1A). The specificity of ADAMTS13 mRNA amplification was demonstrated by the lack of amplification in blank CHO cells and the positive amplification in human liver tissues that are known to express ADAMTS13 mRNA (Fig. 1A).(14) Glycerinaldehyd-3-phosphat-Dehydrogenase (GAPDH) was used to normalize total RNA added during the amplification. The results were expressed as the ratio of ADAMTS13 to GAPDH (Fig. 1A). Immunoblots using a polyclonal anti-ADAMTS13 IgG demonstrated 120-kDa and 190-kDa protein bands in the placental lysates (Fig. 1B & 1C) and in the conditioned medium of cultured placental tissues (Fig. 1D). Consistent with the dynamic changes of mRNA, ADAMTS13 protein was also at the highest levels in placentae during the first trimester and decreased during the second and third trimesters. A positive control consisted of a recombinant full-length ADAMTS13 expressed from CHO cells by transient transfection of an ADAMTS13 plasmid. A negative control was the cell lysate of untransfected CHO cells, showing no detectable bands (data not shown). ADAMTS13 activity was detected in the concentrated conditioned media of placental culture explants from first (mean 6.2%, range 3.9–9.3%), second (mean 4.1%, range 1.2-6.2%), and third (mean 3.9%, range 1.1–6.5%) trimester (Fig. 1E). Together, our results demonstrate that a full-length and proteolytically active ADAMTS13 protein is synthesized and secreted from placental tissues throughout normal pregnancy.
Fig. 1. ADAMTS13 expression in human placenta.
A. Real-time PCR detected the relative abundance of ADAMTS13 mRNA/GAPDH in human placental villous tissues from first (1st) (n=10), second (2nd) (n=3), and third (3rd) (n=10) trimester of pregnancy. Human liver expressing normal ADAMTS13 mRNA (Liver) was used as a positive control and blank CHO cell mRNA was used as a negative control. B and C. ADAMTS13 protein in the placental villous tissue lysate by Western blotting with a rabbit anti-ADAMTS13 IgG. D and E. ADAMTS13 protein and activity detected by Western blotting and FRETS-VWF73, respectively, in the conditioned medium of placental villous tissues. All results were presented as the mean ± SEM. * indicates a p value < 0.05. n.s. indicates a p value >0.05.
Localization of ADAMTS13 in placental tissues
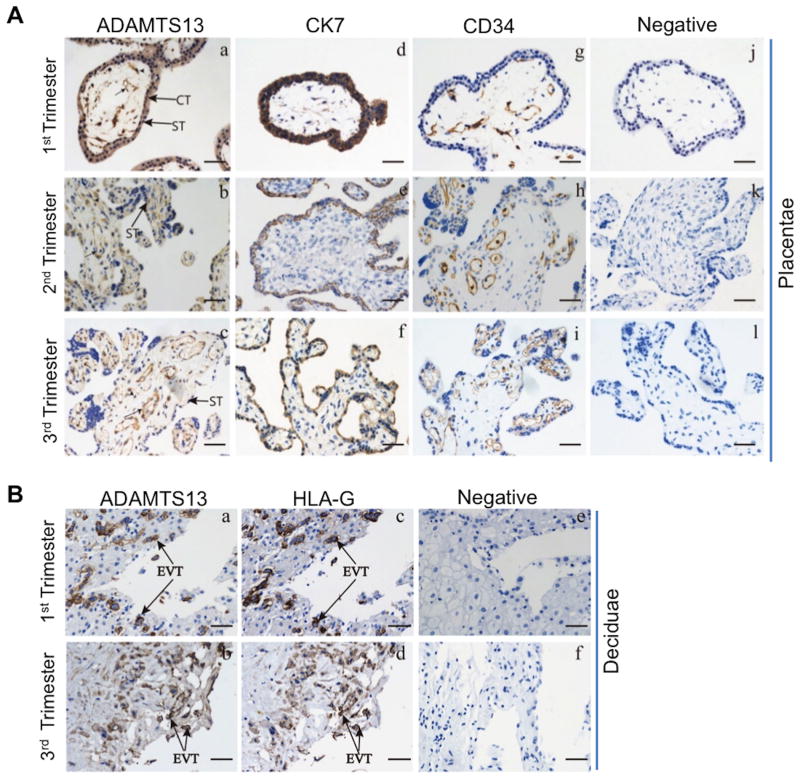
Immunohistochemistry using the polyclonal anti-ADAMTS13 IgG demonstrated that ADAMTS13 protein was expressed in both placental and decidual tissues throughout the gestation. Anti- cytokeratin (CK) 7, anti-human leukocyte antigen (HLA)-G, and anti-CD34 marked the cytotrophoblasts (or syncytiotrophoblasts), extravillous trophoblasts, and villous core fetal blood vessel endothelium, respectively. ADAMTS13-positive staining was observed in the villous cytotrophoblasts and syncytiotrophoblasts (Fig. 2A-a, b, & c), as well as in the extravillous trophoblasts (Fig. 2B-a & b), which were marked by anti-CK7 (Fig. 2A-d, e, & f) and anti-HLA-G (Fig. 2B-c & d), respectively. ADAMTS13 expression was also detected in fetal blood vessel endothelium marked by anti-CD34 and some stromal cells in the villous core (Fig. 2A-a, b, c, j, h, & i). No staining was detected in the tissues after incubation with only secondary antibodies (Fig. 2A-j & k, l and Fig. 2B-e & f). These results demonstrate for the first time that ADAMTS13 is expressed in placental trophoblasts and fetal villous vascular endothelium.
Fig. 2. Cellular expression of ADAMTS13 protein in human placentae and deciduae throughout pregnancy.
Immunoreactivity of anti-ADAMTS13 IgG in fixed trophoblasts (Aa-c) and extravillous trophoblasts in deciduae (Ba-b). CK7 was used as a marker for trophoblasts (Ad-f). HLA-G was used for a maker for extravillous trophoblasts (Bc-d) and CD34 for villous core blood vessel endothelium (Ag-i). Negative controls containing only the secondary antibody were all negative (Aj-l & Be-f). Bar=50 μm. Arrows in Aa-c indicate villous core blood vessel endothelium. Arrowheads in Aa-c indicate stroma cells. CT, cytotrophoblast; ST, syncytiotrophoblast; EVT, extravillous trophoblasts.
ADAMTS13 expression in the placentae of patients with severe preeclampsia
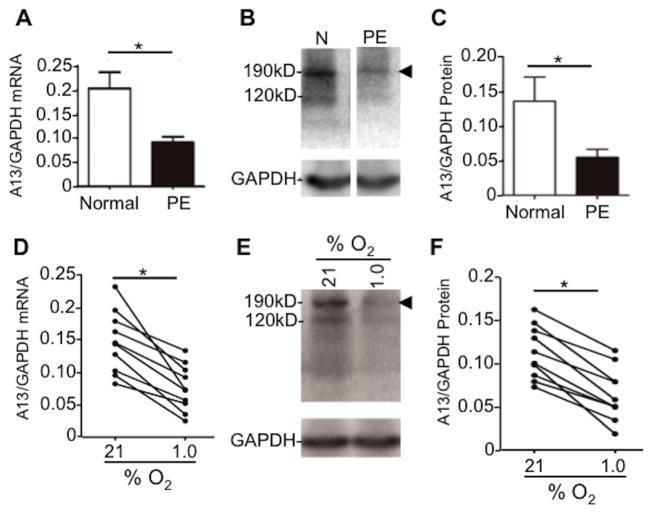
To assess if ADAMTS13 expression is affected during placental ischemia, the levels of ADAMTS13 mRNA in placental tissue were determined in patients with severe preeclampsia and normal pregnant controls. As shown, ADAMTS13 mRNA in the placentae of patients with severe preeclampsia was dramatically reduced as compared to that in the healthy controls (Fig. 3A). Western blotting demonstrated the significantly reduced levels of ADAMTS13 protein in the placental villous tissues in patients with severe preeclampsia compared with those in the controls (Fig. 3B & 3C).
Fig. 3. ADAMTS13 expression is down-regulated in placentase from patients with severe preeclampsia.
A. Relative ratios of ADAMTS13 to β-actin mRNA in patients with severe preeclampsia (PE, n=22) and in normotensive pregnany (Control, n=25) placentae. B. ADAMTS13 protein detected by Western blotting in placental villous tissues from patients with severe preeclampsia (n=22) and normotensive pregnancy (n=25). C. Quantification of the relative aboundance of ADAMTS13 protein in severe preeclamptic placentae and control placentae is shown. D and E. ADAMTS13 mRNA by real-time PCR and ADAMTS13 protein by Western blotting in placental explant cultured under normal and hypoxic conditions. F. Quantification of the immunoreactivity of a full-length ADAMTS13 is shown. All results are presented as the mean ± SEM (n=10). * indicates the p valule <0.05.
To determine whether the reduction of placental expression of ADAMTS13 was caused by tissue ischemia, we determined the levels of placental ADAMTS13 mRNA and protein under normoxic and hypoxic conditions in culture. The results showed that both ADAMTS13 mRNA and protein in placental villous tissues were significantly reduced after being exposed to 2% O2 (hypoxic) for 48 hours compared to 21% O2 (normoxic) (Fig. 3D, 3E, & 3F). These results suggest that the reduction of ADAMTS13 synthesis and secretion in the placentae of patients with severe preeclampsia may be associated with hypertension-related placental ischemia and tissue hypoxia.
ADAMTS13 expression in placental trophoblast cells
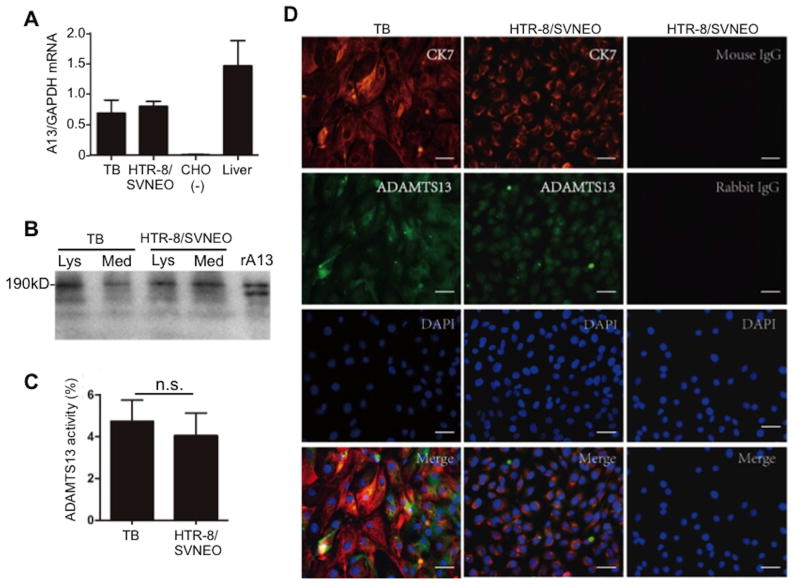
To confirm the specific cell types in placental villous tissues that express ADAMTS13, we isolated primary placental trophoblasts using an enzyme digestion technique and HTR-8/SVNEO cell line for the study. By real-time PCR, Western blotting, and FRETS-VWF73, we were able to detect ADAMTS13 mRNA (Fig. 4A), protein (Fig. 4B), and proteolytic activity (Fig. 4C) in the isolated primary trophoblasts and immortalized trophoblast cell line. Immunofluorescent microscopy using the polyclonal anti-ADAMTS13 IgG demonstrated the positive signal in those cells, which were also positive for anti-CK7 IgG, marking the placental trophoblasts (Fig. 4D). Together, our results indicated that both isolated primary trophoblasts and immortalized trophoblastic cell line produce ADAMTS13 transcripts and functional protein.
Fig. 4. Expression of ADAMTS13 in primary placental trophoblasts and HTR-8/SVNEO cells.
A. Relative ratios of ADAMTS13 to GAPDH mRNA levels in placental trophoblasts. Human liver ADAMTS13 mRNA was used as a positive control and blank CHO cell mRNA was used as a negative control. B. Western blotting demonstrates a full-length ADAMTS13 protein in the cell lysate (Lys) and conditioned medium (Med) of primary trophoblast (TB) and HTR-8/SVNEO cells. Recombiannt ADAMTS13 (rA13) expressed in CHO cells transiently transfected with ADAMTS13 plasmid was used as a positive control. C. ADAMTS13 activity (mean ± SEM) in the conditioned media of primary trophoblasts (TB) and HTR-8/SVNEO cells is shown. D. Immunofluoreceent staining of primary trophoblasts (TB) and HTR-8/SVNEO cells with monoclonal anti-CK7 (10μg/ml) and rabbit anti-ADAMTS13 IgG (20μg/ml) as indicated in the photographs (Bar=50μm). CK7 is a marker for placental trophoblasts.
ADAMTS13 promotes trophoblast cell proliferation, migration, invasion, and tube formation
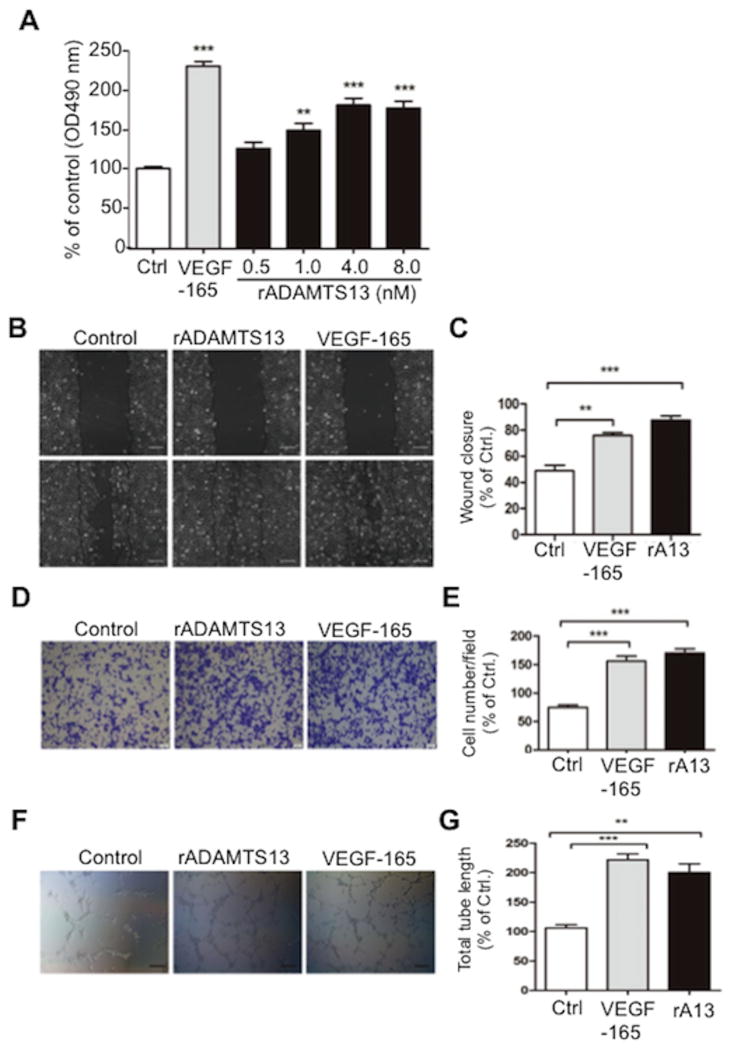
Previous studied demonstrated that ADAMTS13 might be a pro-angiogenetic or anti-angiogenetic factor, depending on its local microenvironment.(22, 23) Placenta is an excellent model tissue to study angiogenesis and cell invasion. Incubation of HTR-8/SVNEO cells with 0-8 nM of recombinant full-length ADAMTS13 for 48 hours resulted in significantly increased proliferation of the trophoblastic cell line in a dose-dependent manner (Fig. 5A); additionally, the incubation of HTR-8/SVNEO cells with recombinant ADAMTS13 (8 nM) resulted in significantly increased cell migration (Fig. 5B & 5C), invasion (Fig. 5D & 5E), and tube formation (Fig. 5F & 5G).
Fig. 5. ADAMTS13 promotes trophoblast cell proliferation, migration, invasion and network formation.
A. Proliferation of HRT-8/SVNEO trophoblasts after treatment for 48 hours with a buffer or VEGF165 (5 nM) or recombiannt ADAMTS13 (0-8 nM). Each experiment was performed in 6 replicates. ANOVA determined the statistical signfiacance of the difference among the groups. B and C. Migration of HTR-8/SVNEO cells after incubation with VEGF165 (5 nM) and recombinant ADAMTS13 (8 nM) for 0 and 24 hours as indicated by a wound scratch assay (Bar=100μm). D and E. The effect of 24 hours treatment of VEGF165 (5 nM) and recombinant ADAMTS13 (8 nM), respectively, on the invasion of HTR-8/SVNEO cells (Bar= 20 μm) by a transwell assay. F and G. The formaiton of tube network in HTR-8/SVNEO cells treated without and with recombinant ADAMTS13 (8 nM), respectively, for 8 hours (Bar=100μm) assessed by the Matrigel assay. The figure shows the representative images of three independent experiments and data are expressed as % of control. ** and *** indicate p values <0.01 and <0.001, respectively.
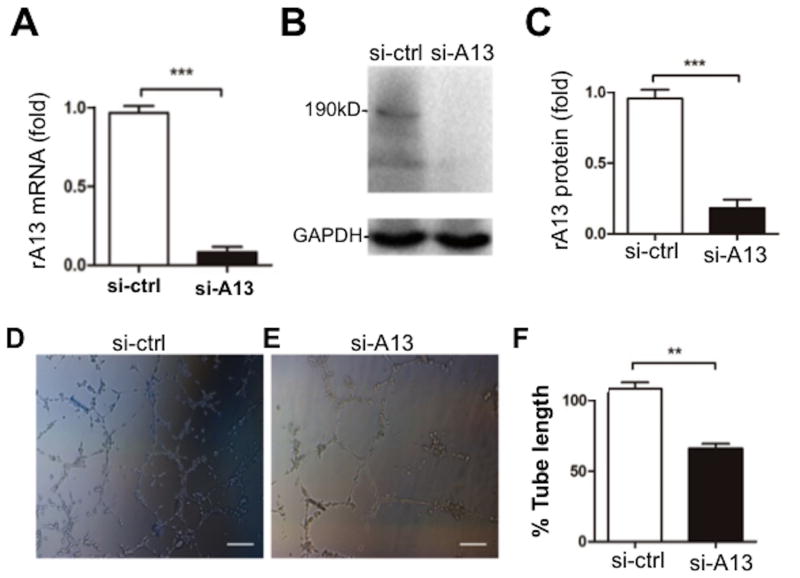
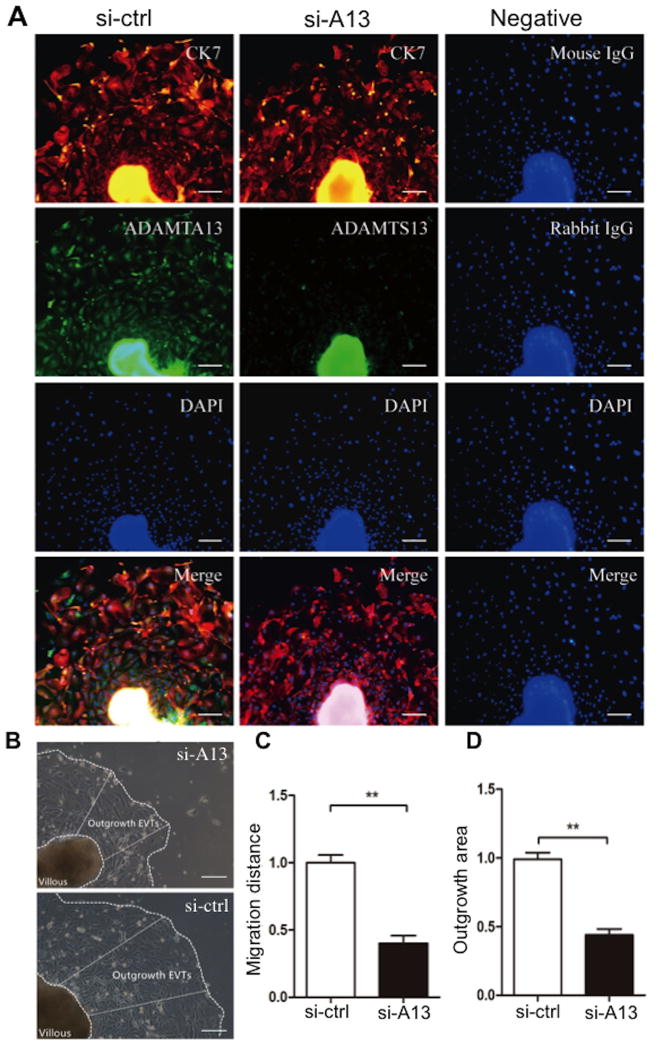
To corroborate these effects of ADAMTS13, we performed siRNA-mediated ADAMTS13 knockdown. Transfection of ADAMTS13 siRNA into cells resulted in significant reduction of ADAMTS13 mRNA and protein expression in HTR-8/SVNEO cells compared with the transfection of the control scramble siRNA (Fig. 6A-C). Knockdown of ADAMTS13 expression in HTR-8/SVNEO cells led to significantly reduced levels of tube formation in a matrigel assay (Fig. 6D-F). Immunofluorescent microscopy demonstrated the significant reduction of ADAMTS13 signal in the outgrowing cells in the explants when transfected with ADAMTS13 siRNA compared with those in the control siRNA (Fig. 7A, column 2). The migration distance and area of the outgrowing cells in ADAMTS13 knockdown explants were dramatically decreased compared with the controls (Fig. 7B-D). Together, our results support that ADAMTS13 may have a role in promoting placental angiogenesis and trophoblast proliferation.
Fig. 6. Silencing of ADAMTS13 expression inhibits the network formation in HTR-8/SVNEO cells.
A, B, and C. Real-time PCR, Western blotting, and quantitation of ADAMTS13 protein demonstrate the signficant reduction of ADAMTS13 mRNA and protein in HTR-8/SVNEO transfected with siRNA specific for ADAMTS13 (50 nM) compared with those with control siRNA (50 nM). D, E, and F. the network formation assessed by matrigel assay in HTR-8/SVNEO cells transfected with siRNA to ADAMTS13 (si-A13, 50 nM) or control siRNA(si-Ctrl, 50 nM) for 48 hours (Bar=100μm). The figure shows the representative images of six independent experiments and data are expressed as % of control. ** and *** indicate the p values of <0.01 and < 0.001, respectively.
Fig. 7. Silencing of ADAMTS13 suppresses the outgrowth of EVTs from human placental explant in culture.
A. Whole mount immunofluorescent assay indicating the silencing efficiency of 72 hours of treatment with ADAMTS13 siRNA (100 nM) in the outgrowing EVTs (Bar=100μm). The antibodies used for immunofluorescence staining are indicated on the images. B. Representative bright field images show the outgrowth of the placental explants after 72 hours of cululture treated with 100 nM of siRNA specific for ADAMTS13 (si-A13) and control siRNA (si-Ctrl). The outgrowth area and distance of the explants under different treatments are indicated by the dashed lines and white arrows. Quantiation of the outgrowth distance (C) and the average surface area of EVT outgrowth (D) is shown. The figure shows the representative images of nine independent experiments and data are expressed as % of control.** indicates the p valule <0.01.
Discussions
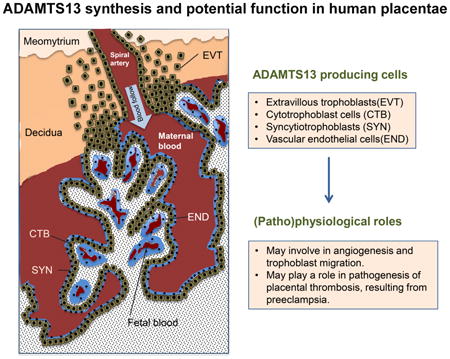
The current study demonstrates that ADAMTS13 mRNA and protein are expressed in human normal placentae and deciduae throughout the pregnancy; in the placenta, ADAMTS13 is mainly expressed in trophoblast and fetal blood vessel endothelium; throughout the pregnancy human placenta synthesizes and secretes ADAMTS13 protein that is proteolytically active; the placental ADAMTS13 is produced at the highest levels in the first trimester, and declines in the second and third trimester of gestation, consistent with the progressive decline in plasma ADAMTS13 antigen during pregnancy. (24) ADAMTS13 synthesis is significantly reduced in placental villous tissues in patients with severe preeclampsia, likely resulting from placental ischemia. This hypothesis is supported by the reduction in ADAMTS13 synthesis after placental explant exposed to the hypoxic conditions; finally, ADAMTS13 promotes proliferation, migration, invasion and network formation of trophoblasts, suggesting a role of ADAMTS13 protease in normal pregnancy and a potential involvement in the pathogenesis of pregnancy-associated complications.
Several members of ADAMTS family are known to express at low levels in human placentae or uterine tissues(25). This suggests a potential role of other matrix metalloproteinases in implantation and placentation during human pregnancy. This may also explain why ADAMTS13 null mice are viable and fertile.(26, 27) Such redundancy in gene families is common. (28-30) Indeed, there is increasing evidence of both redundant and non-redundant roles for distinct ADAMTS subtypes in follicular growth and ovulation and in the development and regression of the corpus luteum in the rodent and bovine ovary. (30-33) While previous study demonstrated the presence of ADAMTS13 mRNA in placentae,(21) its cellular origin and expression levels, as well as biological functions are not known. Here, we demonstrate that ADAMTS13 mRNA and protein are primarily expressed in the trophoblasts and fetal blood vessel endothelium of human placentae; some stromal cells were also positive for ADAMTS13 protein, likely due to the release of ADAMTS13 into the local tissue environment or potential non-specific binding of the polyclonal anti-ADAMTS13 antibody.
Previous studies demonstrated that maternal plasma levels of ADAMTS13 activity decrease progressively to 23% of normal in the third trimester.(24) Plasma ADAMTS13 activity begins to decrease at 12-16 gestational weeks.(34) Our results suggest that ADAMTS13 produced in placental trophoblasts and endothelium may contribute in part to the levels of maternal plasma ADAMTS13 activity during pregnancy in addition to the potential role in maintenance of local environment. Therefore, the change in maternal plasma ADAMTS13 activity or antigen may reflect the health conditions of placental trophoblasts and villous endothelium during normal and abnormal pregnancy.
ADAMTS13 may play a role in pathophysiology of preeclampsia. Aref et al reported a significant reduction of plasma ADAMTS13 activity and increase of plasma VWF in women who were pregnant compared with those who were not.(35) In addition, Stepanian et al demonstrated an association between low ADAMTS13 activity and preeclampsia, independently of VWF. The decrease in plasma ADAMTS13 activity may be related to placental inflammation.(36) In our study, we demonstrated the dramatically reduced levels of placental expression of ADAMTS13 mRNA and protein, in patients with severe preeclampsia. This may explain why maternal plasma ADAMTS13 levels are significantly reduced in patients with preeclampsia. The imbalance between ADAMTS13 and VWF during normal pregnancy and preeclampsia may posses a risk for thrombosis in the intervillous and spiral vessel of placentae, which affects uteroplacental circulation and fetal distress. Consequently, placental ischemia and hypoxia may lead to further decline of local synthesis of ADAMTS13 in the placental villous tissues. This vicious cycle may be associated with the pathogenesis and outcome of preeclampsia.
In addition to the well-established role of ADAMTS13 in hemostasis, ADAMTS13 may involve in inflammation, angiogenesis, and tissue remodeling. VWF is upregulated in the microvascular endothelial cells in the area of necrosis and fiber-like structure during liver injury.(37) Therefore it was speculated that ADAMTS13 release at the time of tissue injury may prevent excessive matrix protein deposition (38) and development of fibrosis in the liver.(39, 40) More recently, Manea et al proposed that ADAMTS13 expressed in tubular cells and podocytes may have other functions such as tissue remodeling in addition to simply cleaving VWF.(19, 41) Our results demonstrate the progressive reduction of placental ADAMTS13 levels from first to third trimester, suggesting the participation of ADAMTS13 proteolysis in human embryo implantation.
There has been an increased interest in the role of ADAMTS13 in angiogenesis. The disintegrin or cysteine-rich domains in other ADAMTS family members appear to regulate cell adhesion and migration;(42, 43) the active metalloproteinase domain can degrade extracellular matrix components, leading to the release of growth factors and cytokines, and subsequently promote cell proliferation, migration, and angiogenesis. (38, 44, 45) Conversely, the thrombospondin 1 (TSP1) motif is shown to inhibit angiogenesis by regulating interacting with vascular endothelial growth factor (VEGF) receptors, thereby regulating VEGF activity.(46, 47) Lee et al. reported that ADAMTS13 might exert pro- or anti-angiogenic effect depending on the local growth environment. In the absence of excessive VEGF, ADAMTS13 promotes angiogenesis by upregulating VEGF and phosphorylation of VEGFR2.(22, 23) In the presence of excessive VEGF, ADAMTS13 appears to inhibit angiogenesis by competing with VEGF for binding to its receptor VEGFR. Our results are consistent with the proangiogenic effect of ADAMTS13 previously found in vitro.
During a normal pregnancy, uterine blood flow increases and enables a perfusion of intervillous space of the placenta and to support fetal growth. The increased blood flow is achieved by physiological transformation of the spiral arteries of the uterus, a process in which trophoblasts invade the arterial wall, destroy the media, and transform the spiral arteries from narrow-diameter to large-diameter vessels. This process enables an adequate perfusion of the placenta.(6) Multiple factors including growth factors, cytokines, and chemokines are involved in this process. In our study, addition of recombinant ADAMTS13 promotes trophoblast cell proliferation, migration, invasion, and tube formation; knockdown of ADAMTS13 expression attenuated the ability of tube formation in HTR-8/SVNEO cells; knockdown of ADAMTS13 in placental explants decreased the migratory and invasive capacity of trophoblast cells. Thus, we propose that the decreased placental ADAMTS13 expression may negatively influence the functions of the trophoblasts, resulting later in the rudimentary remodeling of maternal spiral arteries and abnormal uteroplacental artery blood flow typically associated with preeclampsia.
In summary, our results demonstrate that placental trophoplasts and villous vessel endothelial cells produce a full-length and functional ADAMTS13 protease. Placental expression of ADAMTS13 exhibits a dynamic change during pregnancy, which appears to be inhibited in late pregnancy and in patients with severe preeclampsia, likely due to placental ischemia. Our findings may shed new light on the potential role of ADAMTS13 in placental function, the possible mechanism underlying tissue remodeling during normal pregnancy, and the pathogenesis of placental thrombosis in severe preeclampsia.
Supplementary Material
Highlights.
The current study demonstrates that placental trophoblasts and villous vascular endothelial cells produce a full-length and functional ADAMTS13 protease.
Placental ADAMTS13 expression exhibits dynamic changes during pregnancy, which appears to be significantly reduced in late pregnancy and in patients with severe preeclampsia, likely due to placental ischemia.
Our findings shed new light on a potential role of ADAMTS13 in normal placental function and a possible involvement in preventing placental ischemia in patients with severe preeclampsia.
Acknowledgments
None
Sources of funding: This study was partially supported by the grants from National Science Foundation of China (81200354 to J.X. and 30672243 to S.H.C), National Institute of Health (R01 HL126724 and HL115187 to X.L.Z), and Applied basic research plan of Wuhan (2015060101010037 to S.H.C).
Non-standard Abbreviations and Acronyms
- ADAMTS13
A Disintegrin And Metalloproteinase with ThromboSpondin type 1 repeats, member 13
Footnotes
Disclosures of conflict of interests: X.L.Z. served as a speaker for Alexion, a consultant for Ablynx, and received research support from Alexion and Lee's Pharmaceuticals. All other authors declared no relevant conflict of interests.
References
- 1.Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122:1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 2.Herzog EM, Eggink AJ, Reijnierse A, Kerkhof MA, de Krijger RR, Roks AJ, Reiss IK, Nigg AL, Eilers PH, Steegers EA, Steegers-Theunissen RP. Impact of early- and late-onset preeclampsia on features of placental and newborn vascular health. Placenta. 2017;49:72–79. doi: 10.1016/j.placenta.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 3.Pauli JM, Repke JT. Preeclampsia: Short-term and Long-term Implications. Obstet Gynecol Clin North Am. 2015;42:299–313. doi: 10.1016/j.ogc.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Staff AC, Benton SJ, von Dadelszen P, Roberts JM, Taylor RN, Powers RW, Charnock-Jones DS, Redman CW. Redefining preeclampsia using placenta-derived biomarkers. Hypertension. 2013;61:932–942. doi: 10.1161/HYPERTENSIONAHA.111.00250. [DOI] [PubMed] [Google Scholar]
- 5.Brosens I, Pijnenborg R, Vercruysse L, Romero R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol. 2011;204:193–201. doi: 10.1016/j.ajog.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brosens I, Robertson WB, Dixon HG. The physiological response of the vessels of the placental bed to normal pregnancy. J Pathol Bacteriol. 1967;93:569–579. doi: 10.1002/path.1700930218. [DOI] [PubMed] [Google Scholar]
- 7.Zhou Y, Damsky CH, Fisher SJ. Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype. One cause of defective endovascular invasion in this syndrome? J Clin Invest. 1997;99:2152–2164. doi: 10.1172/JCI119388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knofler M. Critical growth factors and signalling pathways controlling human trophoblast invasion. Int J Dev Biol. 2010;54:269–280. doi: 10.1387/ijdb.082769mk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lunghi L, Ferretti ME, Medici S, Biondi C, Vesce F. Control of human trophoblast function. Reprod Biol Endocrinol. 2007;5:6. doi: 10.1186/1477-7827-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chakraborty C, Gleeson LM, McKinnon T, Lala PK. Regulation of human trophoblast migration and invasiveness. Can J Physiol Pharmacol. 2002;80:116–124. doi: 10.1139/y02-016. [DOI] [PubMed] [Google Scholar]
- 11.Lala PK, Chakraborty C. Factors regulating trophoblast migration and invasiveness: possible derangements contributing to pre-eclampsia and fetal injury. Placenta. 2003;24:575–587. doi: 10.1016/s0143-4004(03)00063-8. [DOI] [PubMed] [Google Scholar]
- 12.Dong JF, Moake JL, Nolasco L, Bernardo A, Arceneaux W, Shrimpton CN, Schade AJ, McIntire LV, Fujikawa K, Lopez JA. ADAMTS-13 rapidly cleaves newly secreted ultralarge von Willebrand factor multimers on the endothelial surface under flowing conditions. Blood. 2002;100:4033–4039. doi: 10.1182/blood-2002-05-1401. [DOI] [PubMed] [Google Scholar]
- 13.Zhou W, Inada M, Lee TP, Benten D, Lyubsky S, Bouhassira EE, Gupta S, Tsai HM. ADAMTS13 is expressed in hepatic stellate cells. Lab Invest. 2005;85:780–788. doi: 10.1038/labinvest.3700275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uemura M, Tatsumi K, Matsumoto M, Fujimoto M, Matsuyama T, Ishikawa M, Iwamoto TA, Mori T, Wanaka A, Fukui H, Fujimura Y. Localization of ADAMTS13 to the stellate cells of human liver. Blood. 2005;106:922–924. doi: 10.1182/blood-2005-01-0152. [DOI] [PubMed] [Google Scholar]
- 15.Niiya M, Uemura M, Zheng XW, Pollak ES, Dockal M, Scheiflinger F, Wells RG, Zheng XL. Increased ADAMTS-13 proteolytic activity in rat hepatic stellate cells upon activation in vitro and in vivo. J Thromb Haemost. 2006;4:1063–1070. doi: 10.1111/j.1538-7836.2006.01893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shang D, Zheng XW, Niiya M, Zheng XL. Apical sorting of ADAMTS13 in vascular endothelial cells and Madin-Darby canine kidney cells depends on the CUB domains and their association with lipid rafts. Blood. 2006;108:2207–2215. doi: 10.1182/blood-2006-02-002139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turner N, Nolasco L, Tao Z, Dong JF, Moake J. Human endothelial cells synthesize and release ADAMTS-13. J Thromb Haemost. 2006;4:1396–1404. doi: 10.1111/j.1538-7836.2006.01959.x. [DOI] [PubMed] [Google Scholar]
- 18.Liu L, Choi H, Bernardo A, Bergeron AL, Nolasco L, Ruan C, Moake JL, Dong JF. Platelet-derived VWF-cleaving metalloprotease ADAMTS-13. J Thromb Haemost. 2005;3:2536–2544. doi: 10.1111/j.1538-7836.2005.01561.x. [DOI] [PubMed] [Google Scholar]
- 19.Manea M, Kristoffersson A, Schneppenheim R, Saleem MA, Mathieson PW, Morgelin M, Bjork P, Holmberg L, Karpman D. Podocytes express ADAMTS13 in normal renal cortex and in patients with thrombotic thrombocytopenic purpura. Br J Haematol. 2007;138:651–662. doi: 10.1111/j.1365-2141.2007.06694.x. [DOI] [PubMed] [Google Scholar]
- 20.Plaimauer B, Zimmermann K, Volkel D, Antoine G, Kerschbaumer R, Jenab P, Furlan M, Gerritsen H, Lammle B, Schwarz HP, Scheiflinger F. Cloning, expression, and functional characterization of the von Willebrand factor-cleaving protease (ADAMTS13) Blood. 2002;100:3626–3632. doi: 10.1182/blood-2002-05-1397. [DOI] [PubMed] [Google Scholar]
- 21.Zheng X, Chung D, Takayama TK, Majerus EM, Sadler JE, Fujikawa K. Structure of von Willebrand factor-cleaving protease (ADAMTS13), a metalloprotease involved in thrombotic thrombocytopenic purpura. J Biol Chem. 2001;276:41059–41063. doi: 10.1074/jbc.C100515200. [DOI] [PubMed] [Google Scholar]
- 22.Lee M, Rodansky ES, Smith JK, Rodgers GM. ADAMTS13 promotes angiogenesis and modulates VEGF-induced angiogenesis. Microvasc Res. 2012;84:109–115. doi: 10.1016/j.mvr.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Lee M, Keener J, Xiao J, Long Zheng X, Rodgers GM. ADAMTS13 and its variants promote angiogenesis via upregulation of VEGF and VEGFR2. Cell Mol Life Sci. 2015;72:349–356. doi: 10.1007/s00018-014-1667-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feys HB, Canciani MT, Peyvandi F, Deckmyn H, Vanhoorelbeke K, Mannucci PM. ADAMTS13 activity to antigen ratio in physiological and pathological conditions associated with an increased risk of thrombosis. Br J Haematol. 2007;138:534–540. doi: 10.1111/j.1365-2141.2007.06688.x. [DOI] [PubMed] [Google Scholar]
- 25.Zhu H, Leung PC, MacCalman CD. Expression of ADAMTS-5/implantin in human decidual stromal cells: regulatory effects of cytokines. Hum Reprod. 2007;22:63–74. doi: 10.1093/humrep/del356. [DOI] [PubMed] [Google Scholar]
- 26.Banno F, Kokame K, Okuda T, Honda S, Miyata S, Kato H, Tomiyama Y, Miyata T. Complete deficiency in ADAMTS13 is prothrombotic, but it alone is not sufficient to cause thrombotic thrombocytopenic purpura. Blood. 2006;107:3161–3166. doi: 10.1182/blood-2005-07-2765. [DOI] [PubMed] [Google Scholar]
- 27.Motto DG, Chauhan AK, Zhu G, Homeister J, Lamb CB, Desch KC, Zhang W, Tsai HM, Wagner DD, Ginsburg D. Shigatoxin triggers thrombotic thrombocytopenic purpura in genetically susceptible ADAMTS13-deficient mice. J Clin Invest. 2005;115:2752–2761. doi: 10.1172/JCI26007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fata JE, Ho AT, Leco KJ, Moorehead RA, Khokha R. Cellular turnover and extracellular matrix remodeling in female reproductive tissues: functions of metalloproteinases and their inhibitors. Cell Mol Life Sci. 2000;57:77–95. doi: 10.1007/s000180050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Curry TE, Jr, Osteen KG. Cyclic changes in the matrix metalloproteinase system in the ovary and uterus. Biol Reprod. 2001;64:1285–1296. doi: 10.1095/biolreprod64.5.1285. [DOI] [PubMed] [Google Scholar]
- 30.Madan P, Bridges PJ, Komar CM, Beristain AG, Rajamahendran R, Fortune JE, MacCalman CD. Expression of messenger RNA for ADAMTS subtypes changes in the periovulatory follicle after the gonadotropin surge and during luteal development and regression in cattle. Biol Reprod. 2003;69:1506–1514. doi: 10.1095/biolreprod.102.013714. [DOI] [PubMed] [Google Scholar]
- 31.Espey LL, Yoshioka S, Russell DL, Robker RL, Fujii S, Richards JS. Ovarian expression of a disintegrin and metalloproteinase with thrombospondin motifs during ovulation in the gonadotropin-primed immature rat. Biol Reprod. 2000;62:1090–1095. doi: 10.1095/biolreprod62.4.1090. [DOI] [PubMed] [Google Scholar]
- 32.Russell DL, Doyle KM, Ochsner SA, Sandy JD, Richards JS. Processing and localization of ADAMTS-1 and proteolytic cleavage of versican during cumulus matrix expansion and ovulation. J Biol Chem. 2003;278:42330–42339. doi: 10.1074/jbc.M300519200. [DOI] [PubMed] [Google Scholar]
- 33.Richards JS, Hernandez-Gonzalez I, Gonzalez-Robayna I, Teuling E, Lo Y, Boerboom D, Falender AE, Doyle KH, LeBaron RG, Thompson V, Sandy JD. Regulated expression of ADAMTS family members in follicles and cumulus oocyte complexes: evidence for specific and redundant patterns during ovulation. Biol Reprod. 2005;72:1241–1255. doi: 10.1095/biolreprod.104.038083. [DOI] [PubMed] [Google Scholar]
- 34.Sanchez-Luceros A, Farias CE, Amaral MM, Kempfer AC, Votta R, Marchese C, Salviu MJ, Woods AI, Meschengieser SS, Lazzari MA. von Willebrand factor-cleaving protease (ADAMTS13) activity in normal non-pregnant women, pregnant and post-delivery women. Thromb Haemost. 2004;92:1320–1326. doi: 10.1160/TH03-11-0683. [DOI] [PubMed] [Google Scholar]
- 35.Aref S, Goda H. Increased VWF antigen levels and decreased ADAMTS13 activity in preeclampsia. Hematology. 2013;18:237–241. doi: 10.1179/1607845412Y.0000000070. [DOI] [PubMed] [Google Scholar]
- 36.Stepanian A, Cohen-Moatti M, Sanglier T, Legendre P, Ameziane N, Tsatsaris V, Mandelbrot L, de Prost D, Veyradier A, Group ES. Von Willebrand factor and ADAMTS13: a candidate couple for preeclampsia pathophysiology. Arterioscler Thromb Vasc Biol. 2011;31:1703–1709. doi: 10.1161/ATVBAHA.111.223610. [DOI] [PubMed] [Google Scholar]
- 37.Peng Y, Chen Q, Yang T, Tao Y, Lu X, Liu C. Cultured mycelium Cordyceps sinensis protects liver sinusoidal endothelial cells in acute liver injured mice. Mol Biol Rep. 2014;41:1815–1827. doi: 10.1007/s11033-014-3031-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stanton H, Melrose J, Little CB, Fosang AJ. Proteoglycan degradation by the ADAMTS family of proteinases. Biochim Biophys Acta. 2011;1812:1616–1629. doi: 10.1016/j.bbadis.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 39.Kim TH, Mars WM, Stolz DB, Petersen BE, Michalopoulos GK. Extracellular matrix remodeling at the early stages of liver regeneration in the rat. Hepatology. 1997;26:896–904. doi: 10.1002/hep.510260415. [DOI] [PubMed] [Google Scholar]
- 40.Kim TH, Mars WM, Stolz DB, Michalopoulos GK. Expression and activation of pro-MMP-2 and pro-MMP-9 during rat liver regeneration. Hepatology. 2000;31:75–82. doi: 10.1002/hep.510310114. [DOI] [PubMed] [Google Scholar]
- 41.Manea M, Tati R, Karlsson J, Bekassy ZD, Karpman D. Biologically active ADAMTS13 is expressed in renal tubular epithelial cells. Pediatr Nephrol. 2010;25:87–96. doi: 10.1007/s00467-009-1262-2. [DOI] [PubMed] [Google Scholar]
- 42.Ota M, Mochizuki S, Shimoda M, Abe H, Miyamae Y, Ishii K, Kimura H, Okada Y. ADAM23 is downregulated in side population and suppresses lung metastasis of lung carcinoma cells. Cancer Sci. 2016;107:433–443. doi: 10.1111/cas.12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin AC, Cardoso AC, Selistre-de-Araujo HS, Cominetti MR. Recombinant disintegrin domain of human ADAM9 inhibits migration and invasion of DU145 prostate tumor cells. Cell Adh Migr. 2015;9:293–299. doi: 10.4161/19336918.2014.994917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wagstaff L, Kelwick R, Decock J, Edwards DR. The roles of ADAMTS metalloproteinases in tumorigenesis and metastasis. Front Biosci (Landmark Ed) 2011;16:1861–1872. doi: 10.2741/3827. [DOI] [PubMed] [Google Scholar]
- 45.Rodriguez-Manzaneque JC, Fernandez-Rodriguez R, Rodriguez-Baena FJ, Iruela-Arispe ML. ADAMTS proteases in vascular biology. Matrix Biol. 2015;44-46:38–45. doi: 10.1016/j.matbio.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luque A, Carpizo DR, Iruela-Arispe ML. ADAMTS1/METH1 inhibits endothelial cell proliferation by direct binding and sequestration of VEGF165. J Biol Chem. 2003;278:23656–23665. doi: 10.1074/jbc.M212964200. [DOI] [PubMed] [Google Scholar]
- 47.Rodriguez-Manzaneque JC, Lane TF, Ortega MA, Hynes RO, Lawler J, Iruela-Arispe ML. Thrombospondin-1 suppresses spontaneous tumor growth and inhibits activation of matrix metalloproteinase-9 and mobilization of vascular endothelial growth factor. Proc Natl Acad Sci U S A. 2001;98:12485–12490. doi: 10.1073/pnas.171460498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.