Abstract
OBJECTIVE
The chromatin remodeling enzyme BRG1 transcriptionally regulates target genes important for early blood vessel development and primitive hematopoiesis. However, because Brg1 deletion in vascular progenitor cells results in lethal anemia by embryonic day 10.5 (E10.5), roles for BRG1 in embryonic vascular development after midgestation are unknown. In this study, we sought to determine whether endothelial cell BRG1 regulates genes important for vascular development or maintenance later in embryonic development.
APPROACH AND RESULTS
Using mice with temporally inducible deletion of endothelial BRG1 (Brg1fl/fl;Cdh5(PAC)-CreERT2), we found Brg1 excision between E9.5–11.5 results in capillary dilation and lethal hemorrhage by E14.5. This phenotype strongly resembles that seen when the serum response factor (SRF) transcription factor is deleted from embryonic endothelial cells. Although expression of Srf and several of its known endothelial cell target genes are downregulated in BRG1-depleted endothelial cells, we did not detect binding of BRG1 at these gene promoters, indicating that they are not direct BRG1 target genes. Instead, we found BRG1 binds to the promoters of the SRF cofactors myocardin-related transcription factors A and B (Mrtfa and Mrtfb) in endothelial cells, and these genes are downregulated in Brg1-deficient endothelial cells.
CONCLUSIONS
BRG1 promotes transcription of endothelial Mrtfa and Mrtfb, which elevates expression of SRF and SRF target genes that establish embryonic capillary integrity. These data highlight a new and temporally specific role for BRG1 in embryonic vasculature and provide novel information about epigenetic regulation of Mrtf expression and SRF signaling in developing blood vessels.
Keywords: BRG1, SRF, MRTF-A, MRTF-B
Subject Codes: Developmental Biology, Endothelium, Vascular Biology, Basic Science Research, Epigenetics, Gene Expression and Regulation, Genetically Altered and Transgenic Models
INTRODUCTION
ATP-dependent chromatin remodeling complexes utilize energy derived from ATP hy-drolysis to transiently displace nucleosomes at gene regulatory regions and thereby im-pact target gene expression.1 These nimble epigenetic machines are well suited for facilitating dynamic gene expression changes such as those that occur during embryonic development.2 Global deletion of the ATPase catalytic subunits of these complexes in mouse embryos can result in early and lethal developmental de-fects due to misexpression of genes required for cell lineage commitment or survival.3,4,5 Cell-specific deletion of these ATPases further indicates their capacity to mediate consequential target gene expression changes at specific times and locations in a developing embryo.6,7,8
Deletion of the gene encoding the mammalian SWItch/Sucrose Non-Fermentable (SWI/SNF) chromatin remodeling complex ATPase BRG1 (also known as Smarca4) using a Tie2-Cre+ transgene that is ex-pressed in endothelial and hematopoietic cells has revealed roles for this chromatin re-modeling enzyme in early vascular patterning and venous specification.9,10,11 BRG1 promotes transcription of Wnt receptors and target genes in yolk sac endothelial cells, and Brg1fl/fl;Tie2-Cre+ embryos have abnormal yolk sac vascular patterning by embry-onic day 9.5 (E9.5) due to downregulation of these Wnt signaling pathway genes.10 In addition, BRG1 promotes expression of the transcription factor COUP-TFII, which is required for embryonic venous specification, and Brg1fl/fl;Tie2-Cre+ mutants display aberrant arterial markers on their veins by E10.5.11 However, excision of Brg1 from Tie2+ primitive erythrocytes results in primitive erythrocyte apoptosis, anemia, and lethality by E11.5,9 so Brg1fl/fl;Tie2-Cre+ embryos cannot yield information about roles for BRG1 in later stages of vascular development.
To bypass this lethality and study the role of BRG1 in vascular development beyond E11.5, we previously used a constitutive VE-Cadherin-Cre line, which is fully penetrant in endothelial cells by E14.5.12 Brg1fl/fl;VE-Cadherin-Cre+ embryos survived development and displayed no con-sistent vascular anomalies, but we did not detect robust embryonic endothelial cell BRG1 depletion in this model.13 Therefore, in the current study, we ex-ploited the Cdh5(PAC)-CreERT2 line, which is expressed in endothelial cells following tamoxifen induction,14 to produce more complete embryonic endothelial cell Brg1 deletion. As detailed below, Brg1fl/fl;Cdh5(PAC)-CreERT2 embryos reveal new information about a temporally specific role for BRG1 in establishing vascular integrity by promoting serum response factor (SRF) signaling in capillary endothelial cells.
MATERIALS AND METHODS
Materials and Methods are available in the online-only Data Supplement.
RESULTS
E12.5 Brg1fl/fl;Cdh5(PAC)-CreERT2 embryos display dilated capillaries
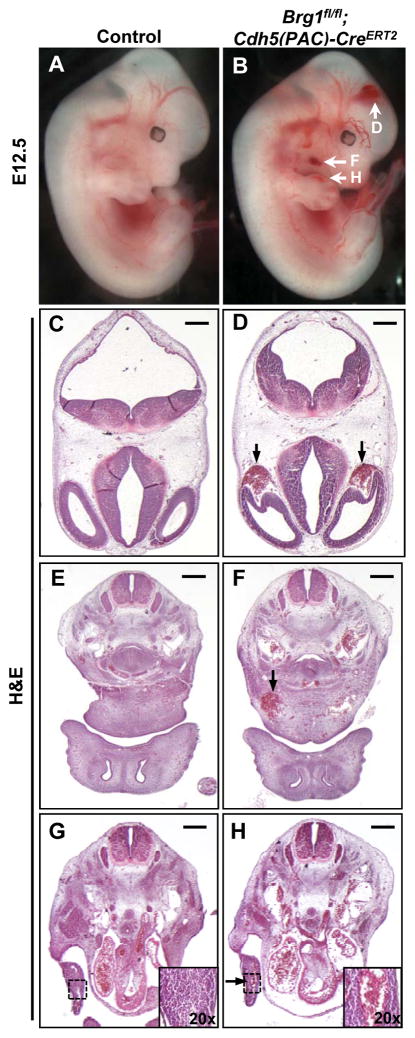
In order to assess the role of BRG1 in developing vasculature after E11.5, we crossed Brg1fl/fl mice onto the Cdh5(PAC)-CreERT2 transgenic line, which is expressed in endothelial cells upon tamoxifen induction.14 Brg1fl/fl females impregnated by Brg1fl/fl;Cdh5(PAC)-CreERT2 males were injected once daily with tamoxifen at E9.5–11.5, and embryos were dissected starting at E12.5. We used the ROSAmT/mG Cre reporter line15 to con-firm that this induction scheme generated largely uniform Cre recombinase activity in endothelial cells throughout the developing embryo (Figure I in the online-only Data Supplement). Unlike their littermate Brg1fl/fl controls, Brg1fl/fl;Cdh5(PAC)-CreERT2 embryos consistently displayed grossly visible superficial pools of blood in the head, neck, and limb buds by E12.5 (Figure 1A,B). Blood was also occasionally observed at the tip of the mutant em-bryonic tails. Transverse histological sections stained with hematoxylin and eosin re-vealed that the pools of blood observed grossly in mutant embryos correlated with di-lated blood vessels rather than vascular rupture (Figure 1C–H).
Figure 1. E12.5 Brg1fl/fl;Cdh5(PAC)-CreERT2 embryos display dilated head, neck, and limb bud capillaries.
Pregnant females carrying control (Brg1fl/fl) and Brg1fl/fl;Cdh5(PAC)-CreERT2 littermate embryos were induced with 1 mg tamoxifen once daily from E9.5–11.5, and embryos were dissected at E12.5. (A,B): E12.5 Brg1fl/fl;Cdh5(PAC)-CreERT2 embryos display grossly visible pools of blood in the head, neck, and limb buds (white arrows, B) compared to littermate controls (A). (C–H): Hematoxylin and eosin (H&E) staining of transverse histological sections from the embryos shown in A and B reveal that Brg1fl/fl;Cdh5(PAC)-CreERT2 embryos have abnormally dilated and blood-filled head (black arrows, D), neck (black arrow, F), and limb bud (black arrow and inset, H) vessels. Images are representative of at least 3 sets of littermate embryos evaluated for each assay. Scale bars: 100 μm (C,D), 200 μm (E–H).
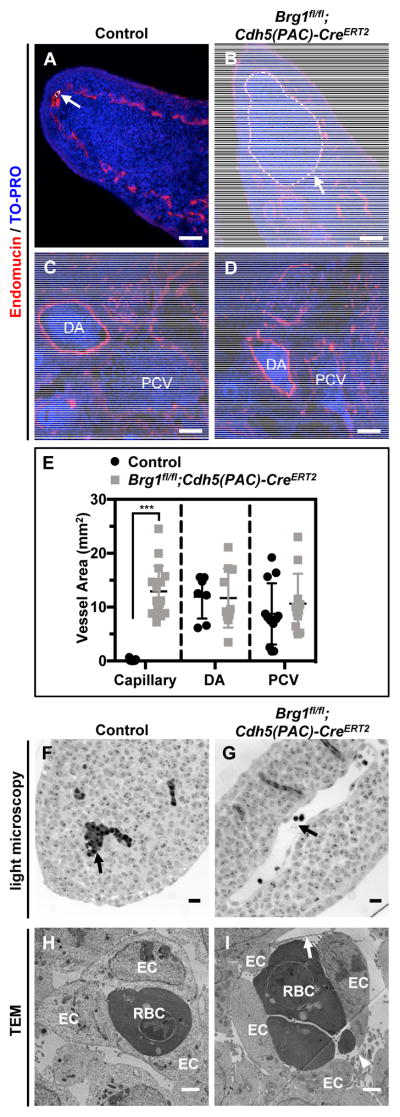
Further analysis of E12.5 embryonic tissue sections immunostained for the endo-thelial cell marker endomucin revealed that capillaries in the neck and limb buds were significantly dilated in Brg1fl/fl;Cdh5(PAC)-CreERT2 embryos, but larger vessels (i.e. the dorsal aortae and posterior cardinal veins) were not aberrantly dilated (Figure 2A–E). Whole-mount immunostaining for the endothelial cell marker PECAM-1 in control and mutant limb buds likewise revealed that mutant vessels were qualitatively disorganized and dilated (Figure II in the online-only Data Supplement). We co-immunostained control and mutant tissue sections for endomucin and the proliferation marker Ki67 to determine if Brg1fl/fl;Cdh5(PAC)-CreERT2 capillary dilation was due to excessive endothelial cell prolif-eration, but we observed no difference in endothelial cell proliferation between control and mutant capillaries (Figure III in the online-only Data Supplement). Closer examina-tion of semithin limb bud sections by light microscopy confirmed dilated but intact mu-tant blood vessels (Figure 2F,G), and transmission electron microscopy revealed dis-tended and attenuated mutant endothelial cells with small discontinuities (Figure 2H,I). These aneurysmal phenotypes became more pronounced and progressed to hemor-rhage at E13.5 and E14.5 (Figure IVA in the online-only Data Supplement), and we re-covered no live Brg1fl/fl;Cdh5(PAC)-CreERT2 em-bryos after E14.5.
Figure 2. Brg1fl/fl;Cdh5(PAC)-CreERT2 limb bud capillaries are dilated and contain thin and discontinuous endothelial cells.
(A–D): E12.5 littermate control (Brg1fl/fl) and Brg1fl/fl;Cdh5(PAC)-CreERT2 embryos were sectioned and immunostained for the vascular marker endomucin (red) and were counterstained with the nuclear marker TO-PRO (blue). Limb bud capillaries are labeled with white arrows and dotted outlines in A and B. Larger dorsal aortae (DA) and posterior cardinal veins (PCV) are labeled in C and D. E: Quantification of capillary, DA, and PCV vessel areas from immunostained sections, such as those shown in A–D. Each circle or square represents a single vessel area; 2 vessels from 3 sections of 3 sets of littermate control and mutant embryos were analyzed for these analyses. Statistical calculations were performed using a two-tailed Student’s t test (***, P<0.001). (F,G): Strikingly dilated blood vessels (black arrow, G) are visible by light microscopy in ultrathin transverse sections of E11.5 Brg1fl/fl;Cdh5(PAC)-CreERT2 limb buds compared to littermate controls (black arrow, F). (H,I): Transmission electron microscopy (TEM) of E11.5 control and mutant limb buds reveals thin (arrow) and discontinuous (arrowhead) endothelial cell (ECs) lining Brg1fl/fl;Cdh5(PAC)-CreERT2 capillaries (J) versus control capillaries (I). RBC=red blood cell. Images are representative of 3 sets of littermate embryos evaluated for A–D and 2 sets for F–I. Scale bars: 50 μm (A–D), 200 μm (F,G), and 500 nm (H,I).
Interestingly, when we initiated tamoxifen induction one day later (E10.5–12.5) and dissected embryos at E13.5, we saw no evidence of dilated blood vessels or other grossly obvious phenotypes in Brg1fl/fl;Cdh5(PAC)-CreERT2 embryos (Figure IVB in the online-only Data Supplement). We questioned whether this phenotypic discrepancy resulted from differences in Brg1 excision between the early (E9.5–11.5) and late (E10.5–12.5) induction schemes. However, when we co-immunostained embryonic tissue sections for endomucin and BRG1, we saw comparable depletion of endothelial BRG1 following both induction schemes (Figure V in the online-only Data Supplement). Therefore, BRG1 expression in endothelial cells from E9.5–10.5 is essential for establishing capillary integrity and embryonic survival beyond E14.5.
Brg1fl/fl;Cdh5(PAC)-CreERT2 embryos resemble mutants with endothelial cell deletion of Srf
In order to generate a list of candidate BRG1 target genes whose misregulation might contribute to the Brg1fl/fl;Cdh5(PAC)-CreERT2 embryonic vascular phenotypes we observed, we searched the literature for other mutants with similar characteristics. We were struck by the phenotypic similarities between Brg1fl/fl;Cdh5(PAC)-CreERT2 embryos and embryos with vascular deletion of the transcription factor Srf. Deletion of Srffl/fl alleles with a Tie1-Cre transgene, which is expressed in developing endothelial cells by E8.0,16 yields capillary dilations and hemorrhage in the head, limb bud, and tail of mutant embryos and lethality by E14.5.17 Similarly, deletion of Srffl/fl alleles with a Tie2-Cre transgene, which is expressed in endothelial cells by E7.5,18 results in dilated head, neck, and yolk sac capillaries and lethality by E14.5.19 These phenotypes likely arise from transcriptional misregulation of SRF target genes involved in endothelial cell junctions and are restricted to capillaries because SRF is not expressed in endothelial cells of larger blood vessels at midgestation.17 Incidentally, we also see stronger expression of BRG1 in endothelial cells of small vessels versus large vessels at E12.5 (Figure VI in the online-only Data Supplement). Since our Brg1fl/fl;Cdh5(PAC)-CreERT2 embryonic phenotypes recapitulated most of the vascular defects seen in Srffl/fl;Tie1-Cre+ and Srffl/fl;Tie2-Cre+ embryos, we hypothesized that Srf and/or its target genes were downregulated in Brg1fl/fl;Cdh5(PAC)-CreERT2 endothelial cells.
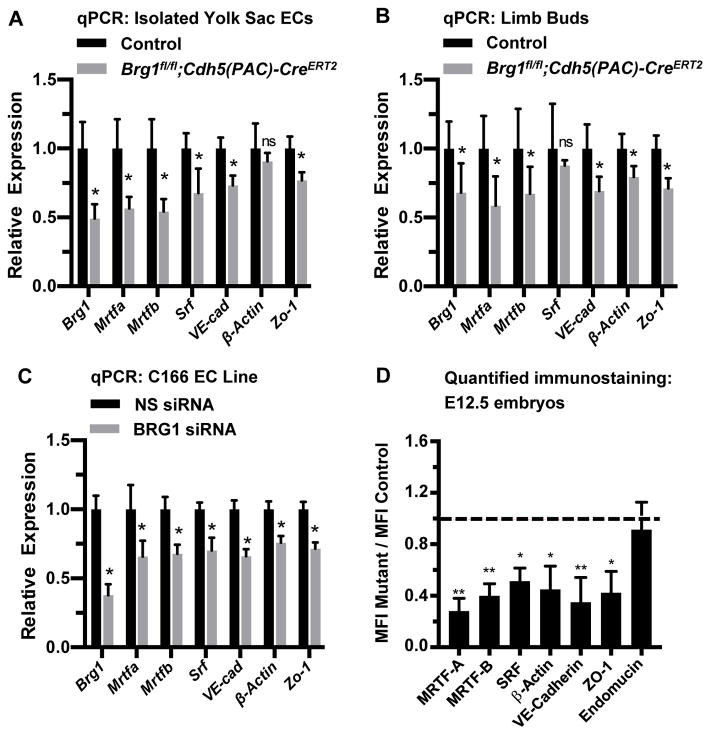
In order to evaluate transcript levels of Srf and its target genes, we isolated primary endothelial cells from E12.5 control and Brg1fl/fl;Cdh5(PAC)-CreERT2 yolk sacs and analyzed relevant transcripts by quantitative RT-PCR (qPCR). We saw significant downregulation of Srf and its target genes VE-Cadherin (also known as Cdh5) and Zo-1 (also known as Tjp1) in mutant endothelial cells (Figure 3A). We likewise saw downregulation of VE-Cadherin, Zo-1, and the endothelial cell SRF target gene β-actin (also known as Actb) in capillary-rich limb buds from Brg1fl/fl;Cdh5(PAC)-CreERT2 embryos (Figure 3B). Since the Myocardin Related Transcription Factor (MRTF) cofactors MRTF-A and MRTF-B are required for SRF-mediated transcription of genes encoding endothelial cell junctional proteins in postnatal mice,20 we also analyzed Mrtfa and Mrtfb transcripts in Brg1fl/fl;Cdh5(PAC)-CreERT2 embryos. We found both transcripts significantly downregu-lated in isolated yolk sac endothelial cells and limb buds from mutant embryos (Figs. 3A and 3B). Finally, when we knocked down BRG1 in the murine C166 yolk sac endothelial cell line with siRNA oligos, we once again detected significant downregulation of tran-scripts for Srf, its coregulators Mrtfa and Mrtfb, and its endothelial cell target genes VE-Cadherin, Zo-1, and β-actin (Figure 3C).
Figure 3. Srf, Mrtfa, Mrtfb, and endothelial cell SRF target genes are downregulated in BRG1-depleted endothelial cells and tissues.
(A–C): Total RNA was collected from freshly isolated yolk sac endothelial cells (ECs; A) or from limb buds (B) of E12.5 control (Brg1fl/fl) and Brg1fl/fl;Cdh5(PAC)-CreERT2 embryos for transcript analysis of Brg1, Srf, SRF co-factors (Mrtfa and Mrtfb), and known endothelial cell SRF target genes (VE-Cadherin, β-actin, and Zo-1) by qPCR. The same transcripts were likewise analyzed in the murine C166 yolk sac EC line following transfection with nonspecific (NS) or BRG1 siRNA oligos for 24 hr (C). For A–C, data were normalized to the relative expression of control samples. Error bars represent S.D. of results from at least three independent experiments or from littermate control and mutant embryos from three different litters. (D) Immunostaining for the vascular marker endomucin and for the gene products of the transcripts analyzed in A–C was performed on sections of E12.5 littermate control and Brg1fl/fl;Cdh5(PAC)-CreERT2 embryos and was quantified (representative immunostaining images used for quantification are shown in Figures VII and VIII in the online-only Data Supplement). Average mean fluorescent intensities (MFI) of mutant capillaries normalized to MFI in control capillaries (dotted line) are shown. Data were compiled from neck and limb bud capillary vessels from 3 images taken from 3 different sets of littermate control and mutant embryos; error bars represent S.D. All statistical calculations were performed using a two-tailed Student’s t test (*, P<0.05; **, P<0.01; ns=not significant).
We next co-immunostained tissue sections from E12.5 control and Brg1fl/fl;Cdh5(PAC)-CreERT2 embryos for endomucin and either MRTF-A, MRTF-B or SRF. We found significant downregulation of both MRTF proteins and of SRF in mutant capillary endothelial cells (Figures 3D and VII in the online-only Data Supplement). We also immunostained for the SRF target gene products β-actin, VE-Cadherin, and ZO-1 and likewise saw significant downregulation of each of these proteins in Brg1fl/fl;Cdh5(PAC)-CreERT2 capillary endothelial cells (Figure 3D and VIII in the online-only Data Supplement). Interestingly, none of these proteins is significantly downregu-lated in E13.5 capillary endothelial cells from Brg1fl/fl;Cdh5(PAC)-CreERT2 embryos that were induced for Brg1 deletion at E10.5–12.5 (Figure IX in the online-only Data Supplement). Therefore, downregulation of SRF, its coregulators MRTF-A/B, and its endothelial cell target genes VE-Cadherin, ZO-1, and β-actin is seen in early-induction Brg1fl/fl;Cdh5(PAC)-CreERT2 mutants with lethal capillary dilation and rupture phenotypes but is not seen in late-induction Brg1fl/fl;Cdh5(PAC)-CreERT2 mutants with no overt vascular phenotypes. These results suggest that misregulated MRTF/SRF signaling underlies the capillary phenotypes in E12.5 Brg1fl/fl;Cdh5(PAC)-CreERT2 mutants.
Mrtfa and Mrtfb are direct targets of BRG1 in embryonic endothelial cells
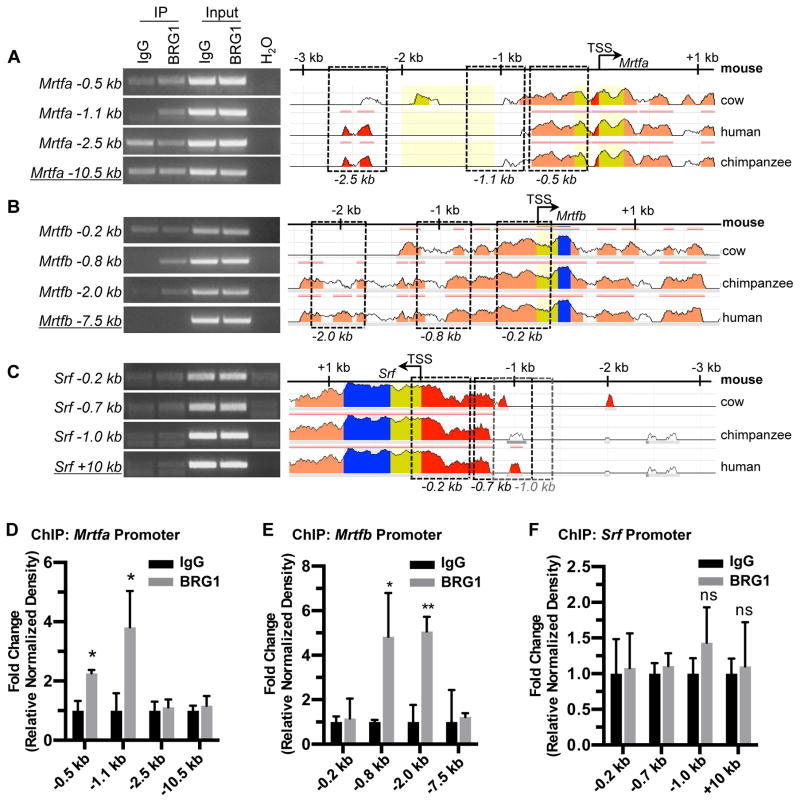
In order to determine if BRG1 directly promotes transcription of Srf, its cofactor genes, or its target genes, we next performed chromatin immunoprecipitation (ChIP) for BRG1 in C166 endothelial cells. We amplified DNA immunoprecipitated with a BRG1 antibody for selected conserved regions of the promoters of Srf, Mrtfa, Mrtfb, and the Srf target genes β-actin, Cx43 (also known as Gja1), VE-Cadherin, and Zo-1. Among these potential BRG1 target genes, we only saw evidence of BRG1 binding at the Mrtfa and Mrtfb pro-moters (Figs. 4 and X in the online-only Data Supplement).
Figure 4. BRG1 interacts with the Mrtfa and Mrtfb promoters in endothelial cells.
Chromatin immunoprecipitation (ChIP) assays were performed in C166 endothelial cells using antibodies against BRG1 or rabbit IgG (as a negative control). (A–C): Immunoprecipitated (IP) DNA was isolated and amplified by PCR to determine whether BRG1 bound conserved regions of the Mrtfa (A), Mrtfb (B), or Srf (C) promoters. Input DNA was isolated from chromatin prior to IP and was used to confirm PCR efficiency. H2O was amplified instead of DNA as a negative control for PCR. Promoter sequence conservation between mouse and other designated species was assessed using the NCBI DCODE website (http://www.dcode.org); peak heights indicate degree of sequence homology. Boxed regions indicate PCR amplicons selected for analysis and are labeled below according to their average distance from each transcription start site (TSS). Negative control amplicons (underlined) are not shown on the sequence alignments but were selected based on their greater distances from each TSS. (D–F): PCR band densities from three independent experiments were quantified using ImageJ, normalized to IgG controls for each experiment, and combined. Data are presented as fold change over the IgG ChIP levels; error bars represent S.D. Statistical calculations were performed using a two-tailed Student’s t test (*, P<0.05; **, P<0.01; ns=not significant).
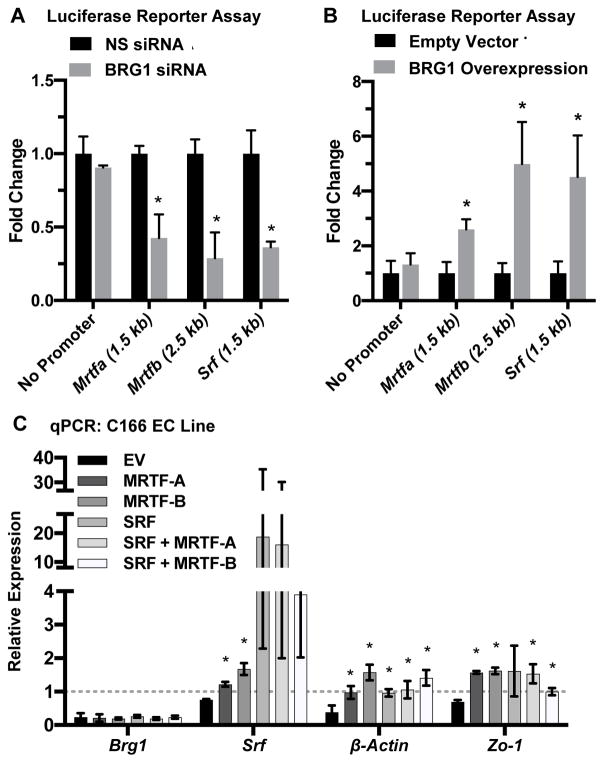
We next employed luciferase reporter assays to test the functional impact of BRG1 ex-pression on Mrtfa, Mrtfb, and Srf transcription. We generated luciferase reporters containing fragments of the Mrtfa or Mrtfb promoters that included the region in which we detected positive BRG1 binding by ChIP. We also made a reporter containing 1.5 kb of the Srf promoter. Upon BRG1 knockdown, we saw a significant decrease in activity for all three of these luciferase reporters (Figure 5A). Conversely, BRG1 overex-pression significantly elevated reporter activity for all three constructs (Figure 5B). The changes seen in the Srf promoter reporter assays surprised us since our ChIP assays did not reveal BRG1 binding to the region of the Srf promoter used in this reporter construct. However, we suspect that these changes are secondary to the transcriptional impact on MRTF-A/B seen with BRG1 knockdown or overexpres-sion, since SRF can autoregulate its own expression,21,22 presumably with the help of MRTF-A/B.
Figure 5. BRG1 expression influences Mrtf transcription.
(A,B): Luciferase reporter constructs containing no promoter (negative control) or fragments of the Mrtfa (1.5 kb), Mrtfb (2.5 kb) or Srf (1.5 kb) promoters upstream of the respective transcription start sites were stably expressed in HEK293T cells. Stable lines were transfected with nonspecific control (NS) or BRG1 siRNA oligos (A) or with a BRG1 expression plasmid or its relevant empty vector control (B). Luciferase activity was measured 24 hr post-transfection and was normalized to activity from control samples. Error bars represent S.D. of results from three independent experiments (with triplicate samples). Statistical calculations were performed using a two-tailed Student’s t test (*, P<0.05). (C): C166 endothelial cells were transfected with nonspecific control or BRG1 siRNA oligos plus an empty vector (EV) or expression plasmids for murine MRTF-A, MRTF-B, and/or SRF for 24 hr. RNA was harvested, and qPCR was performed to detect expression of Brg1, Srf, β-actin, and Zo-1. Data shown represent BRG1 knockdown samples, which were normalized to the relative expression of control nonspecific knockdown samples (dotted line). Error bars represent S.D. of results from three independent experiments. Statistical calculations were performed using a one-way ANOVA comparing overexpression plasmid transfections against EV transfections for each transcript analyzed (*, P<0.05).
Finally, in order to assess whether exogenous expression of MRTF-A/B could rescue expression of Srf and SRF target genes in BRG1 knockdown endothelial cells, we generated MRTF-A and MRTF-B expression vectors and confirmed their ability to generate protein (Figure XI in the online-only Data Supplement). When we overex-pressed these cofactors individually or in combination with SRF in C166 endothelial cells, we were able to rescue expression of Srf and the SRF target genes β-actin and Zo-1, which were diminished in BRG1 knockdown cells (Figure 5C). Altogether, these data indicate that BRG1 regulates expression of Srf and SRF target genes in embryonic endothelial cells by promoting transcription of the SRF co-factors MRTF-A and MRTF-B (Figure 6).
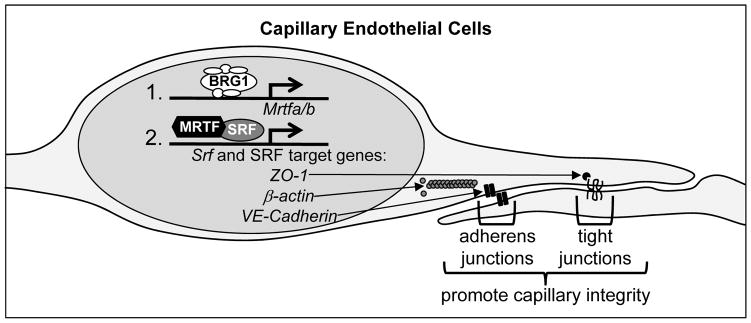
Figure 6. Working model.
Our data indicate that BRG1-containing SWI/SNF chromatin-remodeling complexes promote transcription of Mrtfa and Mrtfb in capillary endothelial cells at E9.5. MRTFA/B are required cofactors that work with SRF to promote expression of Srf itself and of SRF target genes such as ZO-1, β-actin, and VE-Cadherin, which contribute to endothelial cell junctions and capillary integrity. Genetic deletion of Brg1 in endothelial cells at E9.5 results in downregulated Mrtf, Srf, and SRF target gene transcription and subsequent capillary dilation and lethal hemorrhage by E13.5.
DISCUSSION
SRF is a ubiquitously expressed transcription factor that contributes to a variety of cellu-lar processes and is essential for embryonic viability.23 SRF binds DNA at CArG box motifs, which are located in the promoters of more than 200 experimentally validated target genes.24 Although SRF has low transcriptional activity on its own, it can interact with over 60 different cofactors that modulate its transcriptional po-tential in response to cellular stimuli or in specific cell types.25,26 The MRTF-A and MRTF-B coactivators are important modulators of SRF transcriptional activity.27 Rho signaling and actin dynamics regulate the ability of these cofactors to access the nucleus and coactivate SRF-dependent expression of target genes that promote myogenic differentiation and cytoskeletal organization. MRTF-A and MRTF-B have been intensely studied in the context of smooth muscle cells, but knockouts for these broadly expressed genes indicate that they play important roles in coregulating SRF signaling in other cell types as well.28 In endothelial cells, MRTF-A/MRTF-B and SRF are required for normal postnatal retinal angiogenesis and for maintenance of cerebral small vessel integrity.20,29 However, little is understood about what regulates expression of MRTF-A and MRTF-B in endothelial cells or in other cell types. Our new data indicate that the chromatin-remodeling enzyme BRG1 co-activates MRTF-A/MRTF-B expression in capillary endothelial cells at midges-tation to promote SRF signaling but that BRG1 is not required for sustaining MRTF ex-pression after E9.5.
BRG1 has previously been implicated in SRF signaling in the context of smooth muscle cells. BRG1 can bind MRTF-A or the related cofactor myocardin and thereby facilitate increased SRF binding to the promoters of smooth muscle cell-specific genes.30,31 While our new data do not rule out a similar role for BRG1 in promoting SRF-mediated expression of endothelial cell adhesion genes through direct MRTF-A or MRTF-B binding, we do find that overexpression of MRTF-A/B in the C166 yolk sac-derived endothelial cell line is sufficient to elevate expression of SRF target genes even in the absence of BRG1 (Figure 5C). Ideally, we would like to confirm that MRTF-A/B overexpression in midgestational endothelial cells can rescue the vascular phenotypes observed in Brg1fl/fl;Cdh5(PAC)-CreERT2 embryos, but we lack the transgenic mice to perform this in vivo experiment. Likewise, isolated primary embryonic endothelial cells do not lend themselves to robust overexpression studies in our hands. Nevertheless, our in vitro data generated with the C166 endothelial cell line indicate that BRG1 may play a more important role promoting Mrtfa and Mrtfb transcription rather than co-facilitating binding between MRTF-A/B and SRF in embryonic endothelial cells. Further analysis will be re-quired to determine whether BRG1 similarly promotes Mrtfa and Mrtfb expression in additional cell types.
The exquisitely specific timing of the SRF-related Brg1fl/fl;Cdh5(PAC)-CreERT2 embryonic phenotypes is interesting. While induction of Brg1 deletion on days E9.5–11.5 resulted in diminished Mrtf transcription and the phenotypes detailed above, a 24-hour shift in induction (E10.5–12.5) had no effect on Mrtf expression and resulted in no obvious pheno-types (Figures IV and IX in the online-only Data Supplement). This suggests that some other factor compensates for BRG1 to promote Mrtfa/Mrtfb transcription in endothelial cells starting at E10.5. Clearly MRTF-A and MRTF-B continue to be impor-tant cofactors for SRF-mediated transcription of genes required for small vessel development and integrity, as evidenced by the aberrant retinal angiogenesis and intracerebral hemorrhage seen in postnatal vascular Mrtfa/Mrtfb and Srf mutants.20,29 However, postnatal vascular Brg1 mutants do not share these phenotypes,13 which provides further evidence that BRG1 regulates endothelial cell Mrtfa/Mrtfb expression specifically during midgestation.
This newly defined role for BRG1 in developing embryonic capillaries highlights the fascinating temporal/spatial specificity that chromatin remodeling factors exercise in se-lecting and regulating their target genes. In general, we find BRG1 to play transient roles in establishing early steps of blood vessel development and specification at midgestation,9,10,11 but our work has not yet revealed long-term implications for BRG1 in maintaining or recapitulating those roles during postnatal vascular development.13 This observation suggests that BRG1-mediated chromatin remodeling events that promote initial expression of key developmental genes pave the way for establishment of more stable covalent epigenetic marks (i.e. histone acetylation) that sustain long-term gene expression. Indeed, we propose that covalent epigenetic marks provide a more energetically favorable way to sustain long-term gene expression than chromatin remodeling, which relies on ATP hydrolysis. In the context of the present study, we speculate that BRG1 remodels the Mrtf promoters to promote Mrtfa/b transcription in early capillary endothelial cells but that other covalent epigenetic marks maintain the pro-transcriptional status of the Mrtf promoters throughout the rest of embryogenesis and beyond birth. Future analysis of nucleosome condensation and epigenetic marks at the Mrtf promoters in embryonic and postnatal endothelial cells will be required to substantiate this hypothesis.
Supplementary Material
HIGHLIGHTS.
Brg1 excision in embryonic endothelial cells between E9.5–10.5 results in capillary aneurysms and lethal hemorrhage by E14.5—phenotypes that resemble endothelial cell deletion of the transcription factor Srf.
Along with SRF and its target genes, the SRF cofactors MRTF-A/B are transcrip-tionally downregulated in Brg1 mutant endothelial cells.
BRG1 binds the promoters of Mrtfa/Mrtfb in endothelial cells and promotes their expression at midgestation to establish embryonic capillary integrity.
BRG1 is the first identified temporal regulator of Mrtf transcription.
Acknowledgments
We thank Pierre Chambon (Institute for Genetics and Cellular and Molecular Biology) for Brg1-flox mice and Ralf Adams (Max Planck Institute for Molecular Biomedicine, Münster) for Cdh5(PAC)-CreERT2 mice. We also thank Michael McDaniel and the OMRF Imaging Core for confocal microscopy assistance, Layne Rodden for technical assistance, William Berry and James Tomasek for the Mrtfb cDNA subcloning vector and MRTF-B antibody, and the Griffin lab for helpful discussions.
SOURCES OF FUNDING
This work was funded by grants from the National Institutes of Health to C.T.G. (R01HL111178 and R01HL134778) and to C.T.G. and F.L. (P30GM114731).
ABBREVIATIONS
- BRG1
Brahma-related gene 1
- MRTF
myocardin-related transcription factors
- SRF
serum response factor
- EX
Embryonic day X
- VE-Cadherin
vascular endothelial cadherin
- ChIP
chromatin immunoprecipitation
- PECAM-1
platelet and endothelial cell adhesion molecule 1
Footnotes
DISCLOSURES
The authors have nothing to disclose.
References
- 1.Runge JS, Raab JR, Magnuson T. Epigenetic Regulation by ATP-Dependent Chromatin-Remodeling Enzymes: SNF-ing Out Crosstalk. Curr Top Dev Biol. 2016;117:1–13. doi: 10.1016/bs.ctdb.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clapier CR, Cairns BR. The biology of chromatin remodeling complexes. Annu Rev Biochem. 2009;78:273–304. doi: 10.1146/annurev.biochem.77.062706.153223. [DOI] [PubMed] [Google Scholar]
- 3.Bultman S, Gebuhr T, Yee D, La Mantia C, Nicholson J, Gilliam A, Randazzo F, Metzger D, Chambon P, Crabtree G, Magnuson T. A Brg1 null mutation in the mouse reveals functional differences among mammalian SWI/SNF complexes. Mol Cell. 2000;6:1287–1295. doi: 10.1016/s1097-2765(00)00127-1. [DOI] [PubMed] [Google Scholar]
- 4.Stopka T, Skoultchi AI. The ISWI ATPase Snf2h is required for early mouse development. Proc Natl Acad Sci U S A. 2003;100:14097–14102. doi: 10.1073/pnas.2336105100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Shaughnessy-Kirwan A, Signolet J, Costello I, Gharbi S, Hendrich B. Constraint of gene expression by the chromatin remodelling protein CHD4 facilitates lineage specification. Development. 2015;142:2586–2597. doi: 10.1242/dev.125450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de la Serna IL, Ohkawa Y, Imbalzano AN. Chromatin remodelling in mammalian differentiation: lessons from ATP-dependent remodellers. Nat Rev Genet. 2006;7:461–473. doi: 10.1038/nrg1882. [DOI] [PubMed] [Google Scholar]
- 7.Ho L, Crabtree GR. Chromatin remodelling during development. Nature. 2010;463:474–484. doi: 10.1038/nature08911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curtis CD, Davis RB, Ingram KG, Griffin CT. Chromatin-remodeling complex specificity and embryonic vascular development. Cell Mol Life Sci. 2012;69:3921–3931. doi: 10.1007/s00018-012-1023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffin CT, Brennan J, Magnuson T. The chromatin-remodeling enzyme BRG1 plays an essential role in primitive erythropoiesis and vascular development. Development. 2008;135:493–500. doi: 10.1242/dev.010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griffin CT, Curtis CD, Davis RB, Muthukumar V, Magnuson T. The chromatin-remodeling enzyme BRG1 modulates vascular Wnt signaling at two levels. Proc Natl Acad Sci U S A. 2011;108:2282–2287. doi: 10.1073/pnas.1013751108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis RB, Curtis CD, Griffin CT. BRG1 promotes COUP-TFII expression and venous specification during embryonic vascular development. Development. 2013;140:1272–1281. doi: 10.1242/dev.087379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alva JA, Zovein AC, Monvoisin A, Murphy T, Salazar A, Harvey NL, Carmeliet P, Iruela-Arispe ML. VE-Cadherin-Cre-recombinase transgenic mouse: a tool for lineage analysis and gene deletion in endothelial cells. Dev Dyn. 2006;235:759–767. doi: 10.1002/dvdy.20643. [DOI] [PubMed] [Google Scholar]
- 13.Wiley MM, Muthukumar V, Griffin TM, Griffin CT. SWI/SNF chromatin-remodeling enzymes Brahma-related gene 1 (BRG1) and Brahma (BRM) are dispensable in multiple models of postnatal angiogenesis but are required for vascular integrity in infant mice. J Am Heart Assoc. 2015;4:e001972–e001972. doi: 10.1161/JAHA.115.001972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Nakayama M, Pitulescu ME, Schmidt TS, Bochenek ML, Sakakibara A, Adams S, Davy A, Deutsch U, Luthi U, Barberis A, Benjamin LE, Makinen T, Nobes CD, Adams RH. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature. 2010;465:483–486. doi: 10.1038/nature09002. [DOI] [PubMed] [Google Scholar]
- 15.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 16.Gustafsson E, Brakebusch C, Hietanen K, Fassler R. Tie-1-directed expression of Cre recombinase in endothelial cells of embryoid bodies and transgenic mice. J Cell Sci. 2001;114:671–676. doi: 10.1242/jcs.114.4.671. [DOI] [PubMed] [Google Scholar]
- 17.Franco CA, Mericskay M, Parlakian A, Gary-Bobo G, Gao-Li J, Paulin D, Gustafsson E, Li Z. Serum response factor is required for sprouting angiogenesis and vascular integrity. Dev Cell. 2008;15:448–461. doi: 10.1016/j.devcel.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 18.Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- 19.Holtz ML, Misra RP. Endothelial-specific ablation of serum response factor causes hemorrhaging, yolk sac vascular failure, and embryonic lethality. BMC Dev Biol. 2008;8:65. doi: 10.1186/1471-213X-8-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weinl C, Castaneda Vega S, Riehle H, Stritt C, Calaminus C, Wolburg H, Mauel S, Breithaupt A, Gruber AD, Wasylyk B, Olson EN, Adams RH, Pichler BJ, Nordheim A. Endothelial depletion of murine SRF/MRTF provokes intracerebral hemorrhagic stroke. Proc Natl Acad Sci U S A. 2015;112:9914–9919. doi: 10.1073/pnas.1509047112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spencer JA, Misra RP. Expression of the serum response factor gene is regulated by serum response factor binding sites. J Biol Chem. 1996;271:16535–16543. doi: 10.1074/jbc.271.28.16535. [DOI] [PubMed] [Google Scholar]
- 22.Belaguli NS, Schildmeyer LA, Schwartz RJ. Organization and myogenic restricted expression of the murine serum response factor gene. A role for autoregulation. J Biol Chem. 1997;272:18222–18231. doi: 10.1074/jbc.272.29.18222. [DOI] [PubMed] [Google Scholar]
- 23.Arsenian S, Weinhold B, Oelgeschlager M, Ruther U, Nordheim A. Serum response factor is essential for mesoderm formation during mouse embryogenesis. EMBO J. 1998;17:6289–6299. doi: 10.1093/emboj/17.21.6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miano JM, Long X, Fujiwara K. Serum response factor: master regulator of the actin cytoskeleton and contractile apparatus. Am J Physiol Cell Physiol. 2007;292:C70–81. doi: 10.1152/ajpcell.00386.2006. [DOI] [PubMed] [Google Scholar]
- 25.Posern G, Treisman R. Actin’ together: serum response factor, its cofactors and the link to signal transduction. Trends Cell Biol. 2006;16:588–596. doi: 10.1016/j.tcb.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 26.Miano JM. Role of serum response factor in the pathogenesis of disease. Lab Invest. 2010;90:1274–1284. doi: 10.1038/labinvest.2010.104. [DOI] [PubMed] [Google Scholar]
- 27.Parmacek MS. Myocardin-related transcription factors: critical coactivators regulating cardiovascular development and adaptation. Circ Res. 2007;100:633–644. doi: 10.1161/01.RES.0000259563.61091.e8. [DOI] [PubMed] [Google Scholar]
- 28.Mack CP. Signaling mechanisms that regulate smooth muscle cell differentiation. Arterioscler Thromb Vasc Biol. 2011;31:1495–1505. doi: 10.1161/ATVBAHA.110.221135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weinl C, Riehle H, Park D, Stritt C, Beck S, Huber G, Wolburg H, Olson EN, Seeliger MW, Adams RH, Nordheim A. Endothelial SRF/MRTF ablation causes vascular disease phenotypes in murine retinae. J Clin Invest. 2013;123:2193–2206. doi: 10.1172/JCI64201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang M, Fang H, Zhou J, Herring BP. A novel role of Brg1 in the regulation of SRF/MRTFA-dependent smooth muscle-specific gene expression. J Biol Chem. 2007;282:25708–25716. doi: 10.1074/jbc.M701925200. [DOI] [PubMed] [Google Scholar]
- 31.Zhou J, Zhang M, Fang H, El-Mounayri O, Rodenberg JM, Imbalzano AN, Herring BP. The SWI/SNF chromatin remodeling complex regulates myocardin-induced smooth muscle-specific gene expression. Arterioscler Thromb Vasc Biol. 2009;29:921–928. doi: 10.1161/ATVBAHA.109.187229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.