Abstract
Objective
Upon activation, platelets increase glucose uptake, glycolysis, glucose oxidation and consume stored glycogen. This correlation between glucose metabolism and platelet function is not well understood and even less is known about the role of glucose metabolism on platelet function in vivo. For glucose to enter a cell it must be transported through glucose transporters. Here we evaluate the contribution of glucose transporter 3 (GLUT3) to platelet function in order to better understand glucose metabolism in platelets.
Approach and Results
Platelet specific knockout of GLUT3 (GLUT3-KO) were generated by crossing mice harboring GLUT3 floxed allele to a Pf4 driven Cre recombinase. In platelets, GLUT3 is localized primarily on α-granule membranes, and under basal conditions facilitates glucose uptake into α-granules to be utilized for glycolysis. Following activation, platelets degranulate and GLUT3 translocates to the plasma membrane, which is responsible for activation-mediated increased glucose uptake. In vivo, loss of GLUT3 in platelets increased survival in a collagen/epinephrine model of pulmonary embolism and in a K/BxN model of autoimmune inflammatory disease, GLUT3-KO mice display decreased disease progression. Mechanistically, loss of GLUT3 decreased platelet degranulation, spreading, and clot retraction. Decreased α-granule degranulation is due in part to an impaired ability of GLUT3 to potentiate exocytosis.
Conclusions
GLUT3-mediated glucose utilization and glycogenolysis in platelets promotes α-granule release, platelet activation and post-activation functions.
Keywords: Glucose, Metabolism, Platelets, thrombosis, and Inflammation
Subject Codes: Metabolism, Platelets, and Inflammation
Introduction
Platelets are small anucleate cells, generated by megakaryocytes and circulate in the blood with the primary function of hemostasis. Stimulation of platelets leads to activation, adherence to matrices, aggregation and secretion of humoral mediators1. Although once thought to be simple cellular fragments, platelets are now known to contain large amounts of RNA, synthesize proteins, and facilitate signaling in multiple disease states independent of thrombosis2. These elaborate functions require energy3. Platelet activation leads to increased glucose uptake, glycolysis and lactic acid production and utilization of the large stores of glycogen4, 5. Additionally, platelet activation is estimated to increase rates of ATP consumption by ~ 2-fold4, 6.
Platelets express glucose transporter 1 (GLUT1), and the more abundant glucose transporter 3 (GLUT3)7 which mediate glucose entry into the platelet via facilitative diffusion. Additionally, RNASeq analysis as well as proteomic analysis did not reveal GLUT2 or GLUT4 transcripts or proteins8–11. Roughly 85% of GLUT3 protein is localized to α-granule membranes, and the remaining 15% is found in the plasma membrane12. Platelet degranulation leads to fusion of α-granule membranes with the plasma membrane, resulting in increased GLUT3 availability, which has been suggested to mediate increased glucose uptake following stimulation. The precise mechanisms mediated by the post-activation surge in glucose utilization is incompletely understood.
It is plausible that activation-mediated glucose uptake may promote platelet activation. Although platelets contain δ- and α-granules, GLUT3 specifically localizes to α-granules. Following stimulation, δ-granules rapidly fuse with the plasma membrane, however α-granule fusion is multiphasic. Immediately following stimulation, a small subset of α-granules fuse with the plasma membrane and release their cargo, potentially regulated by their proximity to the plasma membrane. The remaining α-granules are released through compound exocytosis, where granules fuse with each other to form intracellular networks following which α-granule content release is potentiated13, 14. Here we propose that GLUT3 translocation to the plasma membrane in the initial single exocytosis phase, fuels glucose uptake that generates the energy required for potentiation of exocytosis.
Additionally, platelet degranulation in conjunction with GLUT3 translocation to the plasma membrane may continue to provide glucose for other phenomena such as clot retraction and microparticle formation. Therefore, it is imperative to better understand the spectrum of mechanisms regulating platelet function that are mediated by the activation-induced increase in glucose metabolism.
Currently, the only functional studies of post activation glucose metabolism suggest (in vitro), that glucose in the media is required for clot retraction15 and inhibiting platelet mitochondrial respiration and glycolysis blocks platelet aggregation16. Even less is known about the consequence of metabolism on platelet function in vivo, except for observations showing that systemic administration of glucose increases platelet activation17, 18. However, it is unclear whether these effects are directly attributed to platelet metabolism, or might result from systemic effects.
To understand the contribution of glucose metabolism to platelet function we generated a platelet specific knockout of glucose transporter 3 (GLUT3). This model allowed us to determine the mechanisms by which glucose metabolism regulates activation and post-activation platelet function in vivo.
Materials and Methods
Materials and methods are available in the Data Supplement online.
Results
Deletion of GLUT3 reduces glucose metabolism in platelets
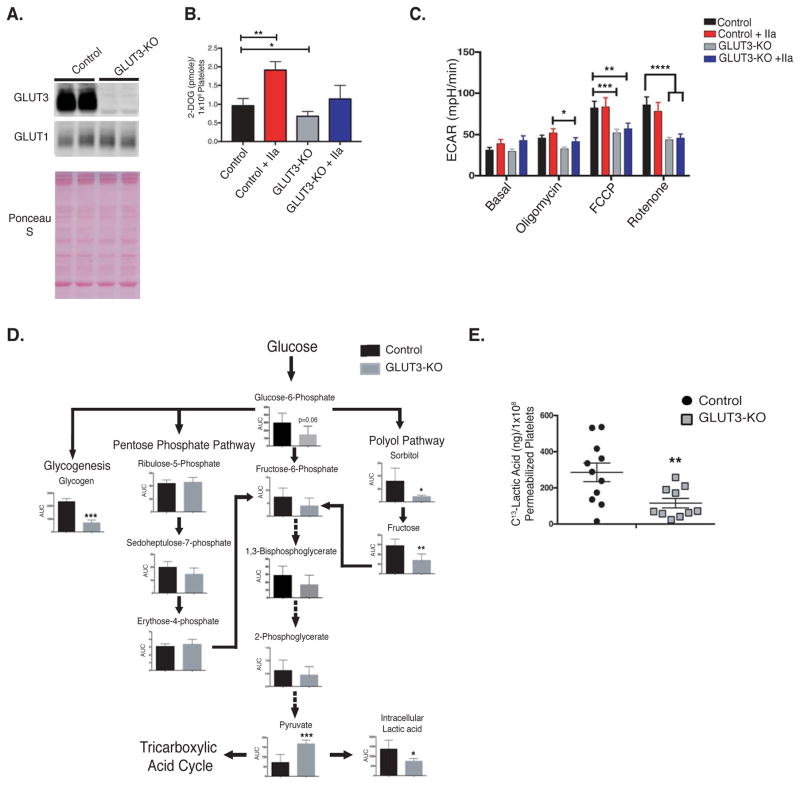
To evaluate the contribution of glucose metabolism to platelet function we generated mice with platelet-specific knockout of GLUT3 (GLUT3-KO). Platelet specific knockout of GLUT3 was verified via western blot analysis of lysates from GLUT3-KO platelets (Figure 1A). In addition, no compensatory increase in GLUT1 protein was observed. Basal total cellular glucose uptake was reduced by 23% in GLUT3-KO platelets relative to littermate controls (Figure 1B). Similar to human platelets 12, 19 thrombin elicited an increase in glucose uptake in control platelets, however this effect was abolished in GLUT3-KO platelets (Figure 1B). These data are consistent with the proposed model that translocation of GLUT3 to the plasma membrane facilitates post-activation glucose uptake12.
Figure 1. GLUT3 deletion reduces platelet glucose utilization.
(A) Western blot analysis of GLUT3 and GLUT1 protein in lysates from control and GLUT3 knockout platelets, n=6. (B) H3-2-deoxy-D-glucose (2-DOG) uptake in platelets was monitored in the presence or absence of 1U/mL thrombin (IIa), n=7. (C) Platelet glycolysis rate, as measured by the extracellular acidification rate (ECAR) was determined using a Seahorse XF24 analyzer. Platelet glycolysis rates with or without 1U/mL thrombin (IIa) were monitored under basal conditions and following treatment with the mitochondrial inhibitors: Oligomycin - ATP synthase inhibitor, Carbonyl cyanide-4 (trifluoromethoxy)phenylhydrazone (FCCP) – mitochondrial uncoupler, and rotenone, mitochondrial complex I inhibitor, n=5. (D) Metabolomics analysis of glycolytic intermediates in non-stimulated freshly isolated platelets were determined and normalized to platelet number, n=5. Glycogen analysis was determined fluorometrically by the enzymatic conversion of glycogen to glucose, n=6. (E) 13C-Lactic acid production by saponin permeabilized platelets, following incubation with 13C-Glucose, n=10. Data are mean±SEM. *P<0.05, **P<0.01,***P<0.001; 2-way ANOVA followed by Tukey’s multiple comparison post hoc test (B and C)); Student’s t test (D and E).
Although glucose uptake was decreased in GLUT3-KO, both basal and thrombin-mediated glycolysis rates were unchanged (Figure 1C). However, following administration of mitochondrial inhibitors, GLUT3-KO platelets failed to increase glycolysis to the same extent as observed in control platelets. These data suggest that resting GLUT3-KO platelets under physiological conditions may sustain glycolysis via GLUT3-independent mechanisms such as increased glycogenolysis.
Metabolomics profiling of freshly isolated, quiescent platelets revealed that the pentose phosphate pathway (PPP) and glycolysis were modestly impacted by deletion of GLUT3. However polyol pathway intermediates fructose and sorbitol were significantly reduced (Figure 1D). Importantly, glycogen content was decreased ~3-fold in GLUT3-KO platelets (Figure 1D).
GLUT3 facilitates platelet α-granule glycolysis
GLUT3 is a bidirectional glucose transporter, which transports glucose by facilitated diffusion. Interestingly, ~85% of GLUT3 resides in α-granule membranes, and the remaining 15% is located in the plasma membrane under non-stimulated conditions12. Because GLUT3 translocates to the plasma membrane following degranulation, and degranulation is a terminal process, we considered the possibility that α-granule GLUT3 may play a role in metabolism prior to degranulation. Proteomic analysis of α-granules, where GLUT3 was used as a specific marker of α-granules and proteomic analysis of platelet releasates, indicate that all proteins required for glycolysis are present in these organelles20–22. To support these observations, we measured the activities of hexokinase and lactate dehydrogenase (LDH) in α-granule enriched subcellular fractions (Figure IA–II). Hexokinase activity was greatest in α-granule enriched pellets. LDH activity was also detected in α-granule pellets, but was also present in other sub-cellular fractions. α-granule enriched fractions also contained mitochondria as evidenced by VDAC immunoreactivity and hexokinase can be associated with mitochondria, (Figure IG). However, we performed additional centrifugations to separate mitochondria from other membranes. Interestingly, the mitochondrial-enriched fraction contained significant GLUT3 contamination suggesting close associations between mitochondria and α-granules. Importantly, fractions enriched for GLUT3 that were devoid of mitochondrial markers retained significant hexokinase activity (Figure IH–II) To test the hypothesis that GLUT3 mediates glucose uptake into α-granules from cytosol, for intra-granular glycolysis, under basal conditions, we employed saponin permeabilization to estimate intragranular glycolysis. Following treatment of platelets with 10ng/mL saponin we detected release of 90% of the platelet lactate dehydrogenase (LDH) into the washed supernatant (Figure IIA), which is a marker of plasma membrane permeabilization. However, at this concentration, α-granules remained intact as determined by the absence of release of soluble α-granule protein PF4 (Figure IIB). Permeabilized GLUT3-KO platelets, with cytosolic proteins washed away, and incubated with 13C-1,6-glucose, displayed a ~2.5-fold decrease in 13C-lactic acid production (Figure 1E), suggesting that α-granule glycolysis is regulated in part by via GLUT3-mediated glucose uptake.
Glucose metabolism in GLUT3-KO megakaryocytes was unchanged
We then investigated if metabolic alterations in platelets arise from dysfunctional megakaryocyte metabolism. Megakaryocytes derived from GLUT3-KO mouse bone marrow demonstrate reduced GLUT3 protein content but normal GLUT1 content (Figure IIIA). This was associated with no changes in basal glycolysis, and unlike GLUT3-KO platelets, GLUT3-KO megakaryocytes displayed no impairment in glycolysis following administration of mitochondrial inhibitors (Figure IIIB). In contrast to platelets, GLUT3-KO megakaryocytes had normal glycogen content (Figure IIIC). Together these data suggest that the metabolic changes in GLUT3 deficient platelets do not arise from dysfunctional glucose metabolism in megakaryocytes.
GLUT3-KO mice exhibit decreased autoimmune inflammatory arthritis
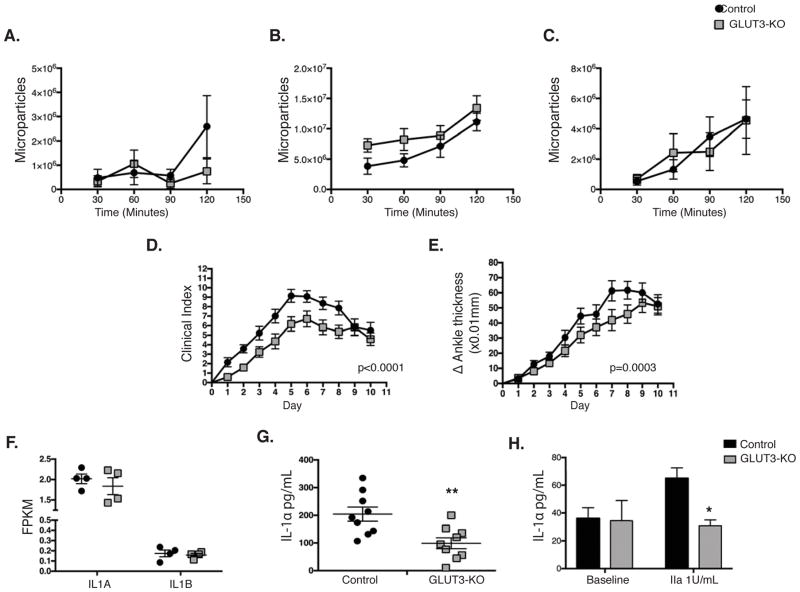
Platelets play an essential role in disease progression of rheumatoid arthritis in a collagen dependent manner, through platelet-derived microparticles and release of IL-123. To explore post-activation functions of GLUT3, we considered the possibility that GLUT3-mediated glucose uptake may be required for microparticle formation. GLUT3 deficient platelets demonstrated no changes in non-stimulated, collagen- or thrombin-induced microparticle formation (Figure 2A–C). However, when mice were subjected to a K/BxN model of autoimmune inflammatory arthritis, we observed a modest but significant and durable reduction in clinical index (p=0.008) and ankle thickness (p=0.0003) (Figure 2D–E). GLUT3-KO platelets displayed no changes in IL1A or IL1B transcripts (Figure 2F), however total platelet IL-1αprotein was significantly reduced (Figure 2G). Furthermore, GLUT3-KO platelets demonstrated decreased thrombin stimulated IL-1α release (Figure 2H), suggesting that the attenuated inflammatory response could be due in part to reduced IL-1α signaling.
Figure 2. GLUT3-KO mice display decreased rheumatoid arthritis disease progression.
Washed platelets from naïve animals were monitored for microparticle formation at the indicated times following stimulation with (A) no agonist, (B) 400ng/mL Convulxin, or (C) 200μM PAR4 peptide, n=6. Mice injected with K/BxN serum to precipitate rheumatoid arthritis were monitored for (D) clinical progression and (E) change in ankle thickness, n=14. (F) RNASeq transcript analysis of GLUT3-KO platelets, data represented as FPKM (fragments per kilobase of exon per million fragments mapped), n=4. (G) Total IL-1α protein in platelets from naïve animals was assessed by ELISA, n=9. (H) IL-1α secreted by platelets 10 minutes following stimulation, n=3. Data are mean±SEM. *P<0.05, **P<0.01; 2-way ANOVA followed by Bonferroni correction (A–E); Student’s t test (F–H).
GLUT3 deficient platelets, demonstrated decreased platelet function
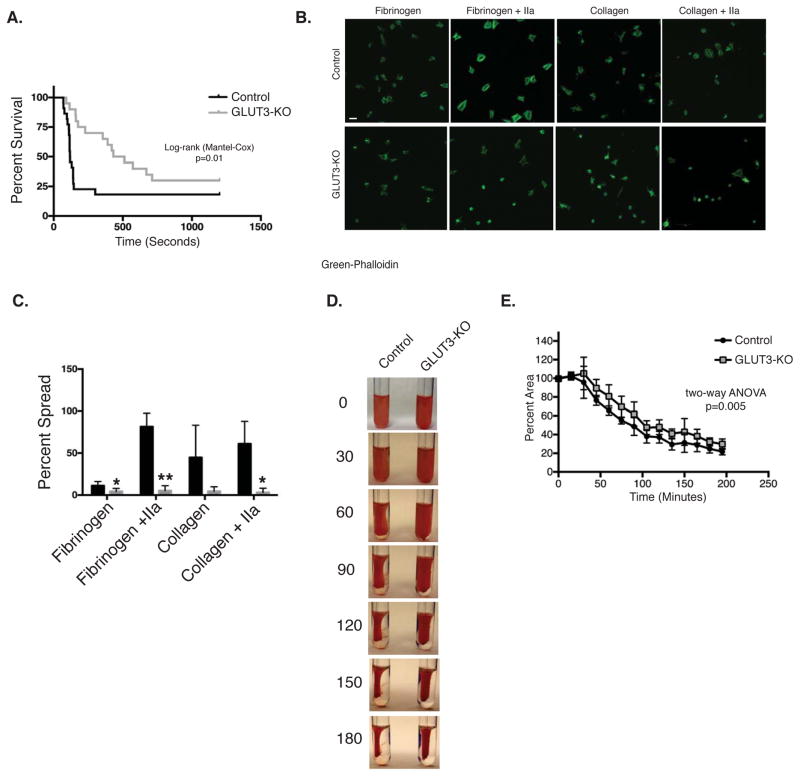
Platelet counts were unchanged in GLUT3-KO mice (Table 1). When GLUT3-KO mice were subjected to collagen/epinephrine induced pulmonary embolism, a significant increase in survival was observed (Figure 3A). In contrast, no changes in hemostasis were observed by tail-bleeding time (Figure IV). Given the evidence for decreased post-activation functions of platelets in vivo, we next examined static adhesion. Platelets were incubated on fibrinogen or collagen and treated with or without thrombin. GLUT3-KO platelets exhibited significantly impaired platelet spreading on both matrixes (Figure 3B and C). Clot retraction occurs following platelet activation and requires glucose metabolism15. Interestingly, blood from GLUT3-KO mice demonstrated reduced clot retraction (Figure 3D and E).
Table 1.
Whole blood analysis by Advia 1200, n=7. P value based on student t-test.
| Sample | Control (Mean ± SD) | DKO (Mean ± SD) | P value |
|---|---|---|---|
| WBC (103 Cells/μL) | 5.3 ± 1.4 | 4.8 ± 0.9 | 0.37 |
| RBC (103 Cells/μL) | 10.1 ± 0.4 | 10.2 ± 0.8 | 0.65 |
| Hemoglobin (g/dL) | 13.8 ± 0.7 | 13.8 ± 0.9 | 0.94 |
| Hematocrit (%) | 49.4 ± 1.4 | 49.6 ± 2.4 | 0.85 |
| MCV (fL) | 49.1 ± 1.3 | 49 ± 1.7 | 0.83 |
| MCH (pg) | 13.6 ± 0.4 | 13.4 ± 0.4 | 0.41 |
| MCHC (g/dL) | 27.6 ± 0.6 | 27.4 ± 0.6 | 0.53 |
| CHCM (g/dL) | 29.1 ± 0.5 | 28.9 ± 0.6 | 0.47 |
| CH (pg) | 14.2 ± 0.4 | 14.1 ± 0.5 | 0.53 |
| RDW (%) | 16.5 ± 1.8 | 16 ± 2.1 | 0.66 |
| HDW (g/dL) | 2 ± 0.1 | 2 ± 0.1 | 0.60 |
| Platelets (103 Cells/μL) | 1062.2 ± 248.1 | 948.8 ± 236.2 | 0.35 |
| Mean Platelet Volume | 7.5 ± 0.3 | 7.8 ± 0.4 | 0.08 |
| Neutrophils (103 Cells/μL) | 0.1 ± 0.1 | 0.2 ± 0.1 | 0.32 |
| Lymphocytes (103 Cells/μL) | 4.9 ± 1.3 | 4.4 ± 1 | 0.38 |
| Monocytes(103 Cells/μL) | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.91 |
| PDW (%) | 41.9 ± 1.9 | 45.1 ± 2.6 | 0.01 |
| PCT (%) | 0.8 ± 0.1 | 0.7 ± 0.2 | 0.62 |
| MPC (g/dL) | 20.7 ± 0.8 | 21.2 ± 1.1 | 0.24 |
| MPM (pg) | 1.4 ± 0.1 | 1.5 ± 0.1 | 3.5×10−3 |
Figure 3. GLUT3 deletion leads to decreased platelet function.
(A) Survival curve following collagen/epinephrine induced pulmonary embolism, n=22. (B) Washed platelets were incubated on collagen or fibrinogen coated chamber slides under static conditions in the presence or absence of 0.1U/mL thrombin (IIa), and stained with phalloidin (green) for cytoskeletal changes, scale 5μm, n=4. (C) Spreading was quantified as summarized in the bar graphs, n=4. (D) Representative image of clot retraction assay performed in diluted whole blood stimulated with thrombin and data quantification (E), n=4. Data are mean±SEM. *P<0.05, **P<0.01 vs. control same treatment; 2-way ANOVA followed by Tukey’s multiple comparison post hoc test (E); Student’s t test (C); Log-rank (Mantel-Cox) test (A.).
Platelet activation is reduced in GLUT3-KO platelets
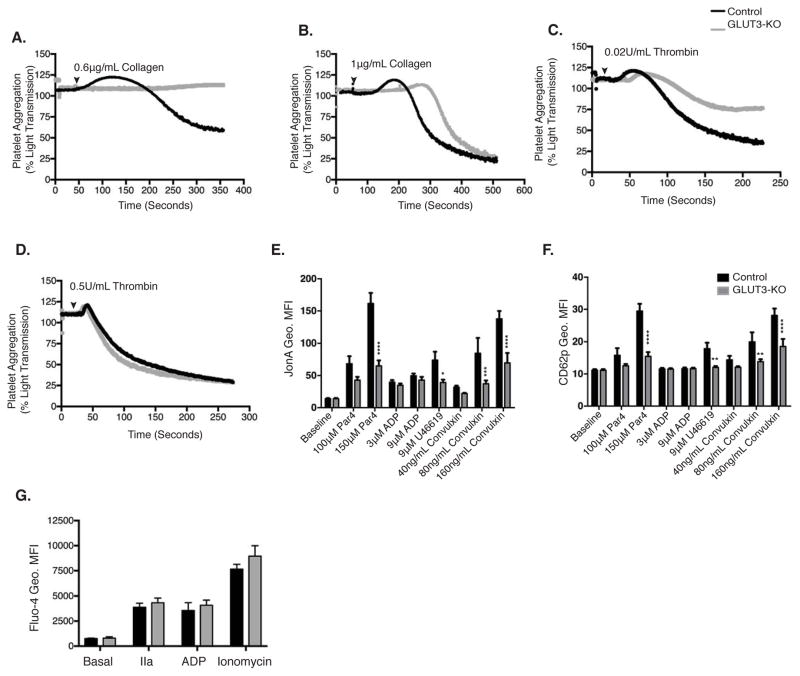
GLUT3-KO platelets displayed impaired aggregation in response to 0.6μg/mL collagen (Figure 4A), however at maximal collagen concentrations (1μg/mL), aggregation was normalized to control levels (Figure 4B). Similarly, in response to 0.02U/mL thrombin, GLUT3-KO platelets exhibited decreased aggregation (Figure 4C), but at maximal concentrations of thrombin (0.5U/mL) aggregation normalized to control levels (Figure 4D). Flow cytometric analysis of diluted whole blood was performed and GPIIbIIIa activation (JonA binding) and α-granule release (CD62p surface binding) monitored. GLUT3-KO platelets did not display changes in baseline activation. However following stimulation with submaximal doses of Par4 peptide, the thromboxane A2 analog U46619 and convulxin, GLUT3-KO platelets exhibited reduced GPIIbIIIa activation (Figure 4E) and CD62p surface translocation (Figure 4F). Importantly thrombin and ADP-mediated Ca2+ mobilization was not impaired in GLUT3-KO platelets (Figure 4G).
Figure 4. GLUT3 deletion leads to decreased platelet activation in vitro.
Representative tracings of washed platelets stimulated with (A) 0.6μg collagen, (B)1μg collagen (C) 0.02U/mL thrombin or (D) 0.5U/mL thrombin, at the indicated times (black arrowhead), n≥3. Diluted whole-blood stimulated with submaximal and maximal agonist concentrations. Platelets were monitored for GPIIbIIIa activation as marked by (E), JONA geometric mean fluorescence (Geo. MFI) and (F), CD62p Geo. MFI, n=6. (G) Platelets loaded with Fluo-4 were analyzed for basal and 1U/mL thrombin (IIa), 9μM ADP or 1μM ionomycin-stimulated cytoplasmic Ca2+ concentration, n=3. Data are mean±SEM. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 vs. control same treatment; 2-way ANOVA followed by Tukey’s multiple comparison post hoc test (E, F, and G).
α-granule release is impaired in GLUT3-KO platelets
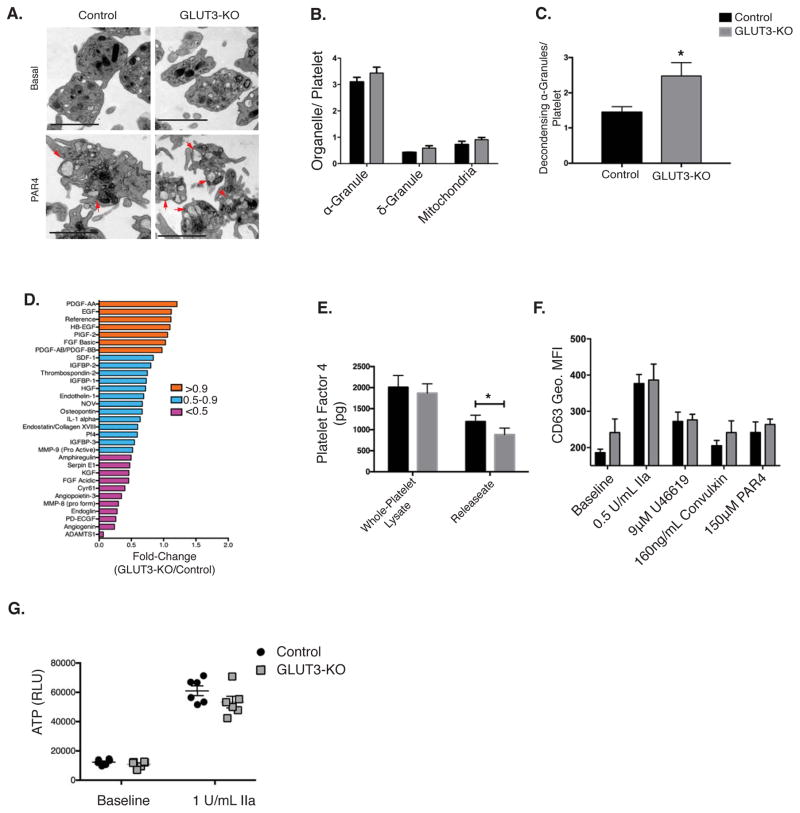
To determine if decreased CD62p surface translocation was due to decreased α-granule number we evaluated platelet ultrastructure. Under basal conditions, electron micrographs exhibited no differences in α-granule, δ-granule, or mitochondrial number relative to controls (Figure 5A–B). However, following stimulation with Par4 peptide, GLUT3-KO platelets displayed a significant accumulation of partially released α-granules (Figure 5A and 5C), which resemble decondensingα-granules14, suggesting a degranulation defect. To determine if these changes were accompanied by impaired α-granule cargo release, the releasate from PAR4 peptide stimulated platelets were evaluated for the protein content of angiogenic factors. The majority of the angiogenesis proteins assayed were reduced in GLUT3-KO releasates (Figure 5D) although a subset of proteins was unchanged. In addition, ELISA quantification of releasate from thrombin-stimulated GLUT3-KO platelets exhibited reduced levels of PF4 (Figure 5E) and this decrease was not attributable to decreased total PF4 content (Figure 5E). This decreased degranulation was specific to α-granules, because following stimulation no change in δ-granule release was observed as measured by CD63 surface translocation and ATP release (Figure 5F–G). Together, these data indicate that GLUT3-KO platelets specifically exhibit decreased α-granule release.
Figure 5. GLUT3 deletion leads to decreased α-granule degranulation in vitro.
(A) Transmission electron micrographs of washed platelets in suspension treated ± 250μM PAR4 peptide. α-granules in the process of degranulation (decondensing) depicted by red arrows, scale bar is 2μm. (B) Organelle density under non-stimulated conditions were quantified and normalized per platelet, n=4. (C) Quantification of α-granules in the process of degranulation (decondensing) in PAR4 stimulated platelets, n=4. (D) Par4 peptide stimulated platelet releasate was monitored using a targeted angiogenesis protein array and expressed as % change relative to control, n=1. (E) ELISA quantification of platelet factor 4 (PF4) in whole platelet lysates and releasate from platelets stimulated with thrombin (1U/mL), n=9. (F) The δ-granule maker CD63 binding (Geo. MFI) was monitored in washed platelets treated with the indicated agonist, n=3. (G) ATP release was monitored, n=6. Data are mean±SEM. *P<0.05 vs. control same treatment; 2-way ANOVA followed by Tukey’s multiple comparison post hoc test (B, E, F, and G). Student’s t test (C).
GLUT3-mediated glucose uptake and glycogenolysis facilitate α-granule fusion
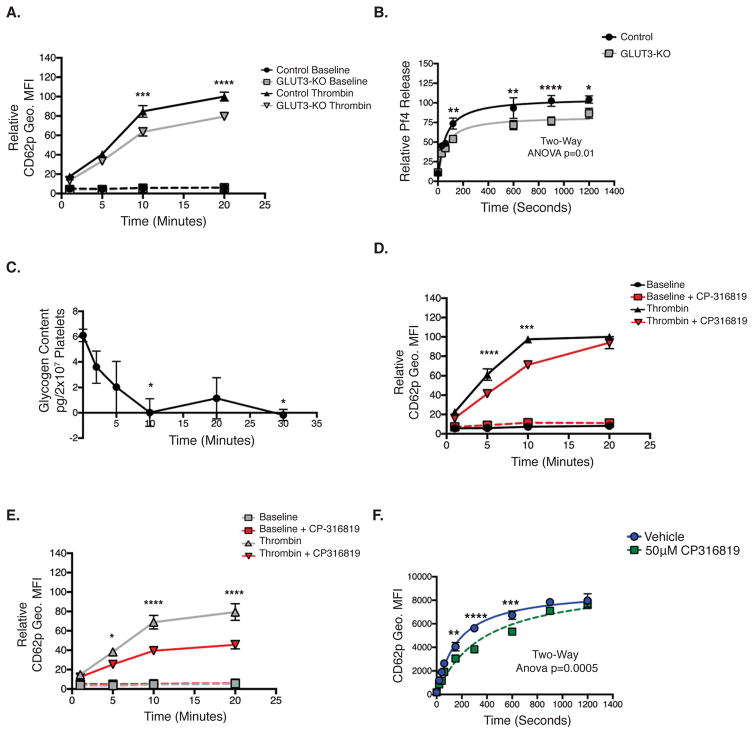
Platelets lacking GLUT3 demonstrate decreased α-granule release. Metabolic characterization of GLUT3-KO platelets demonstrated that GLUT3 facilitates increased agonist-mediated glucose uptake, which occurs once GLUT3 translocates to the plasma membrane. Because α-granule release occurs through both a rapid single exocytosis followed by slower compound exocytosis, we hypothesized that GLUT3 translocation to the plasma membrane by single exocytosis, increases glucose uptake, which then promotes additional α-granule degranulation by increasing glucose-mediated energy generation that potentiates exocytosis. Human PAR1 agonist-mediated CD62p surface translocation requires ~10 minutes24, therefore to test this hypothesis, we examined α-granule degranulation, measured by CD62p surface translocation, for up to 20 minutes following a maximal stimulatory dose of thrombin. Interestingly, GLUT3-KO platelets demonstrated equivalent α-granule release as controls for the first 5 minutes. However, after 10 minutes, GLUT3-KO platelets displayed decreased degranulation relative to controls (Figure 6A). Similar kinetics were observed in Pf4 release (Figure 6B). Platelets contain glycogen stores which are utilized upon stimulation, therefore we examined the rate by which glycogen is consumed following stimulation and found that glycogen is completely depleted by 10 minutes, post stimulation (Figure 6C). Furthermore, we found that in control platelets, the glycogen phosphorylase inhibitor CP-316819 (Figure VA–B) decreased platelet activation during the first 10 minutes following thrombin administration (Figure 6D). Although treatment with the glycogen phosphorylase inhibitor CP-316819, delayed early rates of post-stimulation platelet degranulation (0–10 minutes), degranulation rates eventually normalized to levels of platelets not treated with the glycogen phosphorylase inhibitor, after 20 minutes (Figure 6D). In GLUT3-KO platelets, platelet degranulation following thrombin stimulation increased for 10 minutes, but declined thereafter (Figure 6E). However, following treatment with CP-316819, GLUT3-KO platelets demonstrated dramatically reduced degranulation that was significantly lower than levels observed in GLUT3-KO platelets treated with thrombin alone (Figure 6E). In humans, CP-316819 inhibited α-granule release similar to murine platelets (Figure 6F). Together these data suggest that glycogen mobilization is required for rapid/early degranulation, and GLUT3-mediated glucose uptake mediates later stages of platelet degranulation. In the absence of glycogenolysis, glucose uptake may compensate for and eventually normalize degranulation, and when glycogenolysis and GLUT3-mediated glucose uptake are simultaneously decreased, degranulation is severely inhibited.
Figure 6. Temporal regulation of α-granule degranulation.
(A) Washed platelets stimulated with or without thrombin 1U/mL at T=0, were monitored for CD62p Geo. MFI at the indicated time points, and normalized to control, n=6. (B) Relative Pf4 release in 1U/mL Thrombin- stimulated platelets, at T=0, n=9. (C) Platelets stimulated with 1U/mL thrombin at T=0 then glycogen content was determined at the indicated time points, 10 total mice, n=3. (D) Control or (E) GLUT3-KO platelets were pretreated with or without 50μM CP-316819, then were stimulated with 1U/mL thrombin at T=0, and CD62p Geo. MFI was monitored at the indicated times, n=6. (F) Human platelets pretreated with CP-316819, stimulated with 1U/mL thrombin at t=0 and monitored for CD62p Geo. MFI, n=4. Data are mean±SEM. *P<0.05, **P<0.01,***P<0.001, ****P<0.0001; 2-way ANOVA followed by Tukey’s multiple comparison post hoc test (A, B, D, E and F); 1-way ANOVA followed by Tukey’s multiple comparison post hoc test (C).
Discussion
These studies investigated the contribution of GLUT3-mediated metabolism to platelet function. Here we show that GLUT3 mediates ~20% of basal glucose uptake in quiescent platelets, and we definitively demonstrate that GLUT3 is required for increased glucose uptake in response to platelet activation. Moreover we demonstrate that GLUT3 is required for in vivo thrombosis, platelet activation, degranulation, and clot retraction. These studies provide novel insights into the role of glucose metabolism in the temporal regulation of platelet degranulation, which has in vivo functional consequences.
RNASeq analysis of human and murine platelets and megakaryocytes indicate that GLUT1 and GLUT3 are the only glucose transporters present in platelets8–10. Therefore, our findings suggest that GLUT1 might be the major mediator of basal glucose uptake. Although basal glucose uptake was reduced in the GLUT3-KO platelets, basal glycolysis rates were unchanged. This might indicate an increase in glycogen consumption, a decrease in non-glycolysis mediated metabolism, or a decrease in glucose oxidation into the mitochondria. Glycogen content was decreased 3-fold in the GLUT3-KO platelets and polyol pathway metabolites were significantly reduced. Additionally, mitochondrial respirations were unchanged under basal conditions in the GLUT3-KO platelets, and Seahorse analysis indicated increased mitochondrial reserve capacity in these platelets (Figure VI). These findings support a conclusion that in the absence of GLUT3-mediated glucose uptake, glycolysis is maintained by GLUT1-mediated glucose uptake as well as increased glycogen utilization and decreased glucose flux through non-glycolytic pathways.
~85% of GLUT3 resides in α-granule membranes under non-stimulated conditions. This led us to consider the possibility that GLUT3 facilitates glucose uptake from the cytosol into α-granules for intragranular glycolysis. Proteomic analysis of α-granules, where GLUT3 was used as a specific marker of α-granules and proteomic analysis of platelet releasates, indicate that all proteins required for glycolysis are present in these organelles20–22. Moreover, we now show that subcellular fractions enriched for α-granules exhibited the greatest amount of hexokinase activity and LDH activity was measureable as well. Interestingly LDH activity was detected in other subcellular fractions suggesting that α-granule-derived pyruvate could be converted to lactate by LDH in other subcellular compartments. It is important to note the limitations of platelet subcellular fractionation approaches as evidenced by presence of RANTES in multiple fractions, which could be consistent with breakage of some of the α-granules. Despite this, hexokinase was enriched in particulate fractions. We interpret these findings to indicate that in platelets, the majority of hexokinase is highly associated with (particulate) matter consistent with it being tightly membrane bound. Earlier studies of digitonin-permeabilized platelets, indicated that the pelleted intracellular organelles were enriched for glycolytic intermediary metabolites and glycolytic enzyme activity, consistent with compartmentalization of glycolysis25. Moreover, these studies revealed that only 10% of hexokinase activity was found in the cytosolic supernatant25, 26. Hexokinase is also associated with mitochondria. Our analysis of mitochondrial-enriched fractions revealed hexokinase protein, but we did not detect any hexokinase activity. It is possible that because hexokinase might be loosely associated with the mitochondrial membrane, any activity could have been lost. Importantly, in a GLUT3-enriched membrane fraction that was devoid of mitochondrial contamination, we detected significant hexokinase activity. Taken together, these data support the conclusion that in platelets, hexokinase is not exclusively a soluble enzyme, but rather is tightly associated with particulate compartments such as α-granules.
Using 13C labeled glucose, saponin permeabilized platelets produced readily detectable lactic acid, and deletion of GLUT3 significantly reduced lactate efflux, supporting the hypothesis that GLUT3 facilitates glucose uptake into α-granules where it can then be utilized for glycolysis. These permeabilization experiments do not differentiate α-granules from other intact intracellular organelles, and determination of glycolysis in isolated murine α-granules would clarify this issue. However, isolation of α-granules from murine platelets is technically challenging and could not be achieved. GLUT3 has been described by multiple groups to be specifically localized to α-granules12, 21, 27. The enrichment of GLUT3 in α-granules and our evidence of hexokinase enrichment in this compartment, but the presence of LDH in α-granules and cytosolic fractions raises the possibility that crosstalk may exist between α-granule glucose metabolism and cytosolic metabolism. We speculate that the localization of hexokinase on α-granule membranes could play an important role in the compartmentalization of this process between the cytosol and the α-granule after GLUT3-mediated α-granule glucose uptake occurs.
One potential consequence of a specific reduction in α-granule glycolysis, could be a reduction in platelet ATP generation either in α-granules or in the cytosol. It was not technically feasible monitor ATP production in saponin permeabilized platelets, possibly due to the low quantity of ATP produced by glycolysis and high quantity of stored ATP in δ-granules. Thus, we were unable to directly determine the functional consequence of changes in intragranular glycolysis in α-granules. However in neurons, subcellular localization of glycolysis has been suggested to regulate cellular function28. ATP is an essential cofactor for degranulation, because in permeabilized platelets devoid of cytosol, although degranulation can be initiated with the addition of calcium, ATP addition is an absolute requirement29. It is possible that intra-granule glycolysis or compartmentalization of glycolytic ATP generation via α-granule GLUT3, could regulate platelet degranulation though the luminal production of ATP for the ATPase N-ethylmaleimide-sensitive factor (NSF) which is required for degranulation. In addition to NSF, other ATP-dependent proteins have been identified via α-granule proteomic surveys21 which may be essential platelet granule mobilization or maintenance. Future studies will be required to further elucidate the specific role of α-granule regulated glycolysis to platelet function.
RNASeq analysis of murine megakaryocytes indicate robust expression of both GLUT1 and GLUT3 transcripts9. While significant quantities of GLUT3 are present in platelets, the GLUT1 transcript is expressed at very low levels8, 10. These data suggest that GLUT1 is present earlier in development and might be important for megakaryocyte glucose metabolism, whereas GLUT3 may be induced later and assumes greater importance in platelets. PF4 Cre deletes GLUT3 in the megakaryocyte. However, in contrast to GLUT3-KO platelets, bone marrow derived megakaryocytes displayed no changes in basal or metabolic-stress induced glycolysis or glycogen content. Thus GLUT3 is not a major contributor to glucose metabolism in megakaryocytes. Therefore, the platelet dysfunction observed likely reflects the consequence of impaired GLUT3-mediated metabolism after platelets enter the circulation.
Permeabilized platelets devoid of cytosol maintain the ability to release α-granules, when Ca2+ and ATP are provided30. Interestingly, in response to thrombin stimulation GLUT3-KO platelets exhibited normal cytosolic Ca2+ mobilization, although α-granule release was significantly impaired. These observations support a model in which signaling pathways that lead to platelet activation might be intact in GLUT3-deficient platelets and that impaired ATP production may contribute to the platelet degranulation defect observed. GLUT3-mediated glucose uptake occurs following translocation of GLUT3 containing α-granules to the plasma membrane. Therefore the observation that GLUT3-KO platelets exhibited decreased α-granule degranulation was intriguing. We considered the possibility that decreased glycogen content in GLUT3-KO mice contributed to decreased degranulation. In the presence of an inhibitor of glycogen phosphorylase GLUT3-KO platelets demonstrated an additional reduction in platelet degranulation. These observations reveal a role for GLUT3 mediated glucose uptake in the initial and late release of α-granules. We further explored the possibility that the initial release of α-granules increases GLUT3 translocation to an extent that is sufficient to increase glucose utilization to generate the energy required for completion of platelet degranulation. When we examined the temporal regulation of degranulation, GLUT3-KO platelets initially demonstrated normal degranulation in response to thrombin stimulation, however beyond 10 minutes post stimulation, GLUT3-KO platelets failed to increase degranulation to a similar rate as controls. Additionally, a glycogen phosphorylase inhibitor decreased early degranulation in wildtype and GLUT3 deficient platelets. These data suggest that glycogenolysis, which correlates temporally with single exocytosis, fuels initial degranulation, while GLUT3-mediated glucose uptake is important for the later stages of degranulation, particularly after endogenous glycogen stores are depleted. Moreover, electron micrographs of PAR4 peptide stimulated platelets revealed that 10 minutes post stimulation, an accumulation of α-granules undergoing exocytosis was evident, suggesting early degranulation might be intact but later stages of exocytosis are impaired. We observed a greater number of decondensing α-granules in GLUT3 deficient platelets. Although these findings could indicate granules arrested in compound exocytosis, additional 3D reconstruction image analysis would be needed to rigorously prove this. Together, these relationships support a model in which, as platelets degranulate, surface exposure of GLUT3, increases glucose uptake, that generates the energy required to potentiate exocytosis that sustains platelet activation.
Until now, the significance of increased glucose uptake that occurs after platelets are activated was unclear. Here we demonstrate that fusion of α-granules containing GLUT3 to the plasma membrane is responsible for post activation glucose uptake and α-granule release. Not all α-granule proteins were reduced in releasates from GLUT3-KO platelets. This was interesting because α-granules are thought to be separated into subpopulations based on their content31. Currently we do not know if loss of GLUT3 leads to a specific repression of α-granule subpopulations.
GLUT3 is required for clot retraction. Previous studies have demonstrated that extracellular glucose plays an important role in clot retraction. However, those studies utilized inhibitors that impair glucose uptake via all transporters. Here we demonstrate that although basal glucose uptake is maintained in GLUT3-deficient platelets, the 2-fold increase in glucose uptake following platelet activation that is mediated by GLUT3 is essential for clot retraction.
Activated, platelets generate microparticles, which contribute importantly to the pathogenesis of rheumatoid arthritis disease progression and other immune responses23, 32. Microparticle formation is bioenergetically unfavorable and in vitro is observed for many hours following agonist stimulation33, 34. Thus we considered the possibility that glucose uptake and glycolysis might be the primary energy source that fuels microparticle generation and release. Contrary to our hypothesis, microparticle formation was not impaired in GLUT3 deficient platelets. However, in a model of autoimmune inflammatory arthritis, which is dependent on microparticle formation and IL-1 release, GLUT3-KO mice displayed decreased disease progression. Total cellular IL-1α protein was reduced, along with agonist-mediated IL-1α release, despite normal IL1A and IL1B transcript levels. Unlike IL-1β, which is induced upon activation35, the mechanism of IL-1α synthesis in platelets is incompletely understood 36. Given that GLUT3-deficient megakaryocytes in culture maintain glucose metabolism, our observations suggest that decreased basal glucose metabolism leads to post-transcriptional regulation of IL-1α in the circulating platelets. In human umbilical vein endothelial (HUVEC) cells, IL-1α protein is synthesized by free ribosomes anchored to the cytoskeleton in the cytosol. In platelets IL-1αhas been shown to be located in a punctate distribution however these studies did not localize IL-1α to α-granules36. Our targeted angiogenesis analysis of platelet releasates suggests that IL-1α may also be a α–granule cargo or at least is associated with α-granule release. Thus it is plausible that the reduction in IL-1α content may contribute to decreased arthritis disease progression. Because deletion of GLUT3 in platelets decreases inflammatory arthritis disease progression, and increased survival following in vivo pulmonary embolism, GLUT3 could be considered a therapeutic target, for conditions mediated via inflammatory mediators that are released from activated platelets.
In summary these studies have elucidated the role of GLUT3 mediated glycolysis and glycogenolysis in the temporal regulation of platelet degranulation, post-activation clot retraction and the release of inflammatory mediators that modulate disease progression. They underscore the biological and potential therapeutic significance of subcellular localization of energy metabolism, in a subset of pro-thrombotic and immune platelet functions.
Supplementary Material
Highlights.
In vivo glucose metabolism is required for platelet function.
GLUT3 is required for activation-mediated glucose uptake.
GLUT3-mediated glucose uptake is essential for α-granule release.
GLUT3-mediated glucose uptake contributes to clot retraction.
Acknowledgments
We would like to thank the University of Utah Metabolic Phenotyping Core, and Metabolomics core, the High Resolution Mass spectrometry Facility and the Central Microscopy Research Facility at the University of Iowa and Dr. Steven Lentz for providing feedback. The data presented herein were obtained at the Flow Cytometry Facility, which is a Carver College of Medicine / Holden Comprehensive Cancer Center core research facility at the University of Iowa. The Facility is funded through user fees and the generous financial support of the Carver College of Medicine, Holden Comprehensive Cancer Center, and Iowa City Veteran’s Administration Medical Center.
Author Contribution: TPF, performed experiments, analyzed data, and wrote the manuscript. EM, JR, RB, RS, TF, and NT participated in in vitro and in vivo experiments. EB, ASW, and EDA conceived and supervised the project and wrote the manuscript.
Sources of Funding
GLUT3 floxed mice were generated by the trans-NIH Knock-Out Mouse Project (KOMP) and obtained from the KOMP Repository (www.komp.org). NIH grants to Velocigene at Regeneron Inc. (U01HG004085) and the CSD Consortium (U01HG004080) funded the generation of gene-targeted ES cells for 8500 genes in the KOMP Program which were archived and distributed by the KOMP Repository at UC Davis and CHORI (U42RR024244). RS work was funded by F32 HL128008-01 and T32 DK 091317. JR was funded by K01 GM 103801. EM was funded by T32 HL 105321. RC was funded by T32 DK 007115. ASW was funded by R01 HL 126547-01. EB’s work is supported by a Canadian Institutes of Health Research Foundation grant (to EB), and is recipient of a salary award from the Canadian Institutes of Health Research (CIHR). LB and NT are recipient of a fellowship from The Arthritis Society. This work was supported by NIH Grant U54 HL112311 to ASW and EDA who are both established investigators of the American Heart Association.
Abbreviations
- GLUT3
Glucose Transporter 3
- GLUT1
Glucose Transporter 1
- IIa
Thrombin
Footnotes
The authors have declared no conflict of interest exists.
Disclosure
None.
References
- 1.Denis CV, Wagner DD. Platelet adhesion receptors and their ligands in mouse models of thrombosis. Arteriosclerosis, thrombosis, and vascular biology. 2007;27:728–739. doi: 10.1161/01.ATV.0000259359.52265.62. [DOI] [PubMed] [Google Scholar]
- 2.Weyrich AS, Schwertz H, Kraiss LW, Zimmerman GA. Protein synthesis by platelets: Historical and new perspectives. Journal of thrombosis and haemostasis: JTH. 2009;7:241–246. doi: 10.1111/j.1538-7836.2008.03211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merrick WC. Mechanisms and regulation of eukaryotic protein synthesis. Microbiol Mol Biol Rev. 1992;56:291–315. doi: 10.1128/mr.56.2.291-315.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warshaw AL, Laster L, Shulman NR. The stimulation by thrombin of glucose oxidation in human platelets. The Journal of clinical investigation. 1966;45:1923–1934. doi: 10.1172/JCI105497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karpatkin S. Studies on human platelet glycolysis. Effect of glucose, cyanide, insulin, citrate, and agglutination and contraction on platelet glycolysis. The Journal of clinical investigation. 1967;46:409–417. doi: 10.1172/JCI105542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akkerman JWN, Holmsen H. Interrelationship among platelet responses: Studies on the burst in proton liberation, lactate production, and oxygen uptake during platelet aggregation and Ca2+ secretion. Blood. 1981;57:956–966. [PubMed] [Google Scholar]
- 7.Craik JD, Stewart M, Cheeseman CI. Glut-3 (brain-type) glucose transporter polypeptides in human blood platelets. Thrombosis research. 1995;79:461–469. doi: 10.1016/0049-3848(95)00136-f. [DOI] [PubMed] [Google Scholar]
- 8.Rowley JW, Oler AJ, Tolley ND, Hunter BN, Low EN, Nix DA, Yost CC, Zimmerman GA, Weyrich AS. Genome-wide rna-seq analysis of human and mouse platelet transcriptomes. Blood. 2011;118:e101–111. doi: 10.1182/blood-2011-03-339705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi DS, Smith MC, Campbell RA, et al. Proteasome function is required for platelet production. The Journal of clinical investigation. 2014;124:3757–3766. doi: 10.1172/JCI75247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simon LM, Edelstein LC, Nagalla S, Woodley AB, Chen ES, Kong X, Ma L, Fortina P, Kunapuli S, Holinstat M, McKenzie SE, Dong JF, Shaw CA, Bray PF. Human platelet microrna-mrna networks associated with age and gender revealed by integrated plateletomics. Blood. 2014;123:e37–45. doi: 10.1182/blood-2013-12-544692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burkhart JM, Vaudel M, Gambaryan S, Radau S, Walter U, Martens L, Geiger J, Sickmann A, Zahedi RP. The first comprehensive and quantitative analysis of human platelet protein composition allows the comparative analysis of structural and functional pathways. Blood. 2012;120:e73–82. doi: 10.1182/blood-2012-04-416594. [DOI] [PubMed] [Google Scholar]
- 12.Heijnen HFG, Oorschot V, Sixma JJ, Slot JW, James DE. Thrombin stimulates glucose transport in human platelets via the translocation of the glucose transporter glut-3 from alpha-granules to the cell surface. J Cell Biol. 1997;138:323–330. doi: 10.1083/jcb.138.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eckly A, Rinckel JY, Proamer F, Ulas N, Joshi S, Whiteheart SW, Gachet C. Respective contributions of single and compound granule fusion to secretion by activated platelets. Blood. 2016;128:2538–2549. doi: 10.1182/blood-2016-03-705681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pokrovskaya ID, Aronova MA, Kamykowski JA, Prince AA, Hoyne JD, Calco GN, Kuo BC, He Q, Leapman RD, Storrie B. Stem tomography reveals that the canalicular system and alpha-granules remain separate compartments during early secretion stages in blood platelets. Journal of thrombosis and haemostasis: JTH. 2016;14:572–584. doi: 10.1111/jth.13225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murer EH. Clot retraction and energy metabolism of platelets. Efffects and mechanism of inhibitors. Biochimica et biophysica acta. 1969;172:266–276. doi: 10.1016/0005-2728(69)90069-3. [DOI] [PubMed] [Google Scholar]
- 16.Akkerman JWN, Holmsen H, Driver AH. Platelet aggregation and ca secretion are independent of simultaneous atp production. FEBS letters. 1979;100:286–290. doi: 10.1016/0014-5793(79)80353-1. [DOI] [PubMed] [Google Scholar]
- 17.Bridges JM, Dalby AM, Millar JHD, Weaver JA. An effect of d-glucose on platelet stickiness. The Lancet. 1965;1:75–77. doi: 10.1016/s0140-6736(65)91656-9. [DOI] [PubMed] [Google Scholar]
- 18.Gresele P, Guglielmini G, De Angelis M, Ciferri S, Ciofetta M, Falcinelli E, Lalli C, Ciabattoni G, Davì G, Bolli GB. Acute, short-term hyperglycemia enhances shear stress-induced platelet activation in patients with type ii diabetes mellitus. The American journal of cardiology. 2003;41:1013–1020. doi: 10.1016/s0735-1097(02)02972-8. [DOI] [PubMed] [Google Scholar]
- 19.Ferreira IA, Mocking AI, Urbanus RT, Varlack S, Wnuk M, Akkerman JW. Glucose uptake via glucose transporter 3 by human platelets is regulated by protein kinase b. The Journal of biological chemistry. 2005;280:32625–32633. doi: 10.1074/jbc.M507221200. [DOI] [PubMed] [Google Scholar]
- 20.Coppinger JA, Cagney G, Toomey S, Kislinger T, Belton O, McRedmond JP, Cahill DJ, Emili A, Fitzgerald DJ, Maguire PB. Characterization of the proteins released from activated platelets leads to localization of novel platelet proteins in human atherosclerotic lesions. Blood. 2004;103:2096–2104. doi: 10.1182/blood-2003-08-2804. [DOI] [PubMed] [Google Scholar]
- 21.Maynard DM, Heijnen HFG, Horne MK, White JG, Gahl WA. Proteomic analysis of platelet a-granules using mass spectrometry. Journal of thrombosis and haemostasis : JTH. 2007;5:1945–1955. doi: 10.1111/j.1538-7836.2007.02690.x. [DOI] [PubMed] [Google Scholar]
- 22.Piersma SR, Broxterman HJ, Kapci M, de Haas RR, Hoekman K, Verheul HM, Jimenez CR. Proteomics of the trap-induced platelet releasate. Journal of proteomics. 2009;72:91–109. doi: 10.1016/j.jprot.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 23.Boilard E, Nigrovic PA, Larabee K, Watts GF, Coblyn JS, Weinblatt ME, Massarotti EM, Remold-O’Donnell E, Farndale RW, Ware J, Lee DM. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science. 2010;327:580–583. doi: 10.1126/science.1181928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flaumenhaft R, Dilks JR, Rozenvayn N, Monahan-Earley RA, Feng D, Dvorak AM. The actin cytoskeleton differentially regulates platelet alpha-granule and dense-granule secretion. Blood. 2005;105:3879–3887. doi: 10.1182/blood-2004-04-1392. [DOI] [PubMed] [Google Scholar]
- 25.Akkerman JWN, Ebberink RHM, Lips JPM, Christiaens GCML. Rapid separation of cytosol and particle fraction of human platelets by digitonin-induced cell damage. British journal of haematology. 1980;44:291–300. doi: 10.1111/j.1365-2141.1980.tb01211.x. [DOI] [PubMed] [Google Scholar]
- 26.Rijksen G, Staal GEJ, Beks PJ, Streefkerk M, Akkerman JWN. Compartmentataion of hexoskinase in human blood cells characterization of soluble and particulate enzymes. Biochimica et biophysica acta. 1982;719:431–437. doi: 10.1016/0304-4165(82)90230-6. [DOI] [PubMed] [Google Scholar]
- 27.tot Pannerden HN, de Haas F, Geerts W, Posthuma G, van Dijk S, Heijnen HF. The platelet interior revisited: Electron tomography reveals tubular alpha-granule subtypes. Blood. 2010;116:1147–1156. doi: 10.1182/blood-2010-02-268680. [DOI] [PubMed] [Google Scholar]
- 28.Zala D, Hinckelmann MV, Yu H, Lyra da Cunha MM, Liot G, Cordelieres FP, Marco S, Saudou F. Vesicular glycolysis provides on-board energy for fast axonal transport. Cell. 2013;152:479–491. doi: 10.1016/j.cell.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 29.Ren Q, Wimmer C, Chicka MC, Ye S, Ren Y, Hughson FM, Whiteheart SW. Munc13-4 is a limiting factor in the pathway required for platelet granule release and hemostasis. Blood. 2010;116:869–877. doi: 10.1182/blood-2010-02-270934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flaumenhaft RF, Furie B, Furie BC. Alpha-granule secretion from alpha-toxin permeavilized, mgatp-exposed platelets is induced independently by H+ and Ca2+ Journal of cellular physiology. 1999;179:1–10. doi: 10.1002/(SICI)1097-4652(199904)179:1<1::AID-JCP1>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 31.Italiano JE, Jr, Richardson JL, Patel-Hett S, Battinelli E, Zaslavsky A, Short S, Ryeom S, Folkman J, Klement GL. Angiogenesis is regulated by a novel mechanism: Pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood. 2008;111:1227–1233. doi: 10.1182/blood-2007-09-113837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burger D, Schock S, Thompson CS, Montezano AC, Hakim AM, Touyz RM. Microparticles: Biomarkers and beyond. Clinical science. 2013;124:423–441. doi: 10.1042/CS20120309. [DOI] [PubMed] [Google Scholar]
- 33.Jobe SM, Wilson KM, Leo L, Raimondi A, Molkentin JD, Lentz SR, Di Paola J. Critical role for the mitochondrial permeability transition pore and cyclophilin d in platelet activation and thrombosis. Blood. 2008;111:1257–1265. doi: 10.1182/blood-2007-05-092684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Italiano JE, Jr, Mairuhu AT, Flaumenhaft R. Clinical relevance of microparticles from platelets and megakaryocytes. Current opinion in hematology. 2010;17:578–584. doi: 10.1097/MOH.0b013e32833e77ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindemann S, Tolley ND, Dixon DA, McIntyre TM, Prescott SM, Zimmerman GA, Weyrich AS. Activated platelets mediate inflammatory signaling by regulated interleukin 1beta synthesis. J Cell Biol. 2001;154:485–490. doi: 10.1083/jcb.200105058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thornton P, McColl BW, Greenhalgh A, Denes A, Allan SM, Rothwell NJ. Platelet interleukin-1alpha drives cerebrovascular inflammation. Blood. 2010;115:3632–3639. doi: 10.1182/blood-2009-11-252643. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.