Abstract
BACKGROUND
Patients with inherited bone marrow failure syndromes (IBMFS) may have several risk factors for low bone mineral density (BMD). We aimed to evaluate the prevalence of low BMD in IBMFS and determine associated risk factors.
METHODS
Patients with IBMFS with at least one Dual Energy X-ray absorptiometry (DXA) scan were evaluated. Diagnosis of each IBMFS, Fanconi anemia (FA), dyskeratosis congenita, Diamond-Blackfan anemia, and Shwachman-Diamond syndrome, was confirmed by syndrome-specific tests. Data were gathered on age, height and clinical history. DXA scans were completed at the lumbar spine, femoral neck and forearm. BMD was adjusted for height (HAZ) in children (age ≤20 years). Low BMD was defined as a BMD Z-score or HAZ ≤−2 in adults and children, respectively, in addition to patients currently on bisphosphonate therapy.
RESULTS
Nine of 35 adults (26%) and 11 of 40 children (27%) had low BMD. Adults with FA had significantly lower BMD Z-scores than those with other diagnoses but HAZ did not vary significantly in children by diagnosis. Risk factors included hypogonadism, iron overload and glucocorticoid use.
CONCLUSIONS
Adults and children with IBMFS have high prevalence of low BMD. Prompt recognition of risk factors and management are essential to optimize bone health.
INTRODUCTION
Inherited bone marrow failure syndromes (IBMFS) are a group of rare genetically heterogeneous disorders associated with varying degrees of bone marrow failure (BMF) and a high risk of cancers including leukemia, myelodysplastic syndrome and solid tumors [1]. Fanconi anemia (FA), Diamond-Blackfan anemia (DBA), dyskeratosis congenita (DC) and Shwachman-Diamond syndrome (SDS) comprise the four major IBMFS. Patients with IBMFS may have several risk factors for low bone mineral density (BMD), including chronic glucocorticoid therapy [2] and iron overload from frequent red blood cell transfusions [3]. Endocrine dysfunction with hypogonadotropic hypogonadism, and growth hormone secretory abnormalities as described in FA may also contribute to low BMD [4]. In addition, hematopoietic stem cell transplantation (HSCT) has been shown to be associated with decreased BMD [5].
BMD is usually reported as T-score (compared with standard reference for Caucasian females, aged 20–29 years) but in premenopausal women and men younger than 50 years, Z-scores are preferred; low BMD is defined as a Z-score of −2 or lower [6]. Skeletal sites typically included for determination of BMD using dual-energy X-ray absorptiometry (DXA) scan are postero-anterior lumbar spine (L1–L4), femoral neck or total proximal femur and, in some cases, one-third of the radius of the non-dominant forearm [6]. Low BMD in children is defined as age, gender and height-specific BMD Z-scores (using a pediatric reference) of ≤ −2 at the spine or the proximal femur [7]. Since BMD is only an areal measure, previous studies in adults have looked at bone mineral apparent density (BMaD) as an alternative volumetric measure [8, 9], but that method is not widely used.
BMD has been previously reported in small groups of patients with IBMFS. Twelve of 13 adults with FA had low BMD [4]. Another study noted normal BMD in children with FA after correction for height age (the age that corresponds to the child’s height when plotted at the 50%ile on the growth chart) [10]. Nine of 11 patients with SDS had osteoporosis [11]. Premature osteoporosis was also described in patients with DC [12]. To our knowledge, there are no studies of BMD in DBA. We now report the prevalence of low BMD in children and adults with the four major IBMFS and discuss the associated risk factors.
METHODS
Protocol
The participants were patients with DBA, DC, FA or SDS enrolled in the National Cancer Institute’s (NCI) IBMFS protocol 02-C-0052; (http://www.marrowfailure.cancer.gov); clinicaltrials.gov NCT00027274). The study was approved by the NCI Institutional Review Board. Written informed consent was obtained in accordance with Health and Human Services regulation 45 CFR 46. Diagnosis of each IBMFS was based on standard criteria [1] and confirmed by syndrome-specific tests as described previously [13]. The diagnosis of DC was not confirmed in 3 patients with aplastic anemia and very short telomeres from 2 families in whom the mutated gene was not identified; these patients were designated ‘DC-like’ but classified under DC for this analysis.
Subjects
This report includes 75 patients who had at least one DXA scan completed at the National Institutes of Health (NIH) Clinical Center as part of their outpatient evaluation on the NCI IBMFS protocol. There were 40 children (defined as ≤20 years of age) and 35 adults. Data were gathered on age, treatment history including age at the start of therapy, and duration of therapy with specific reference to glucocorticoid, testosterone, other androgen, estrogen, and bisphosphonate use, and prior HSCT. Data on fracture history, iron overload and endocrine features (clinical and laboratory) suggestive of hypogonadism were evaluated when available. The diagnosis of hypogonadism in post pubertal children and adults was confirmed on the basis of the endocrinology consult note or evidence of clinical and laboratory findings as previously described [4]. Iron overload was diagnosed by high serum ferritin and confirmed by T2*-magnetic resonance imaging of the liver, SQUID (superconducting quantum interference device) or by liver biopsy.
Measurements
Weight was obtained using a digital scale (Life Measurements Instruments, Concord, CA) and height (measured in triplicate to the nearest mm) using a Harpenden stadiometer (Holtain Ltd., Crymych, UK) calibrated before each set of measurements. Height was expressed as a Z-score specific for age and gender, based on National Center for Health Statistics data [14]. The measurements were recorded on the same day as the DXA scan.
BMD was measured by DXA scan (QDR-4500A; Hologic, Bedford, MA) at the postero-anterior lumbar spine (L1–L4) (referred to as ‘spine BMD’), femoral neck, total hip, and forearm (1/3 radius). In subjects with multiple BMD measures, the first available measure was included for this study. Age and gender-specific height-adjusted BMD Z-scores (HAZ) were calculated in children using the online Bone Mineral Density Childhood Study (BMDCS) calculator [15]. We used the age cut-off of 20 years to define children in this study since peak bone mass is achieved after age 20 years (the adult T-score reference standard is a white female 20–29 years [6]) and the BMDCS calculator provides HAZ estimates up to age 20 years [15]. In adults, standard BMD Z-scores adjusted for gender, age and ethnicity were calculated. Bone mineral apparent density (BMaD) scores were computed in adults only, as a volumetric measure of bone density using the formula: Spine BMaD = BMC/(Area)^3/2 and Femoral neck/forearm BMaD = BMC/(Area)^2 [8, 9].
Low BMD in this study was defined as one of the following: HAZ at spine or femoral neck ≤ −2 in children, and spine or femoral neck BMD Z-score ≤ −2 in adults or if patients were already on bisphosphonate therapy for low BMD at the time of DXA scan.
Data Analysis
Data were described by frequency distributions and percentages, and simple descriptive statistics where results are reported as mean ± standard deviation, or median [inter-quartile (25th, 75th percentile) range], and as relevant for informative purposes, range (minimum to maximum). Categorical data were compared using Fisher’s exact test. Comparisons between (i.e., hypogonadism) or among (i.e., syndrome) groups were done by t-test or analysis of covariance (ANCOVA), respectively, with post-hoc pairwise comparisons corrected by the Bonferroni method. Correlation analyses involved Pearson’s correlation coefficient. Univariable (simple) and multivariable (multiple) regression models assessed the predictive relation between relevant covariates and bone parameter outcomes. All data were assessed for their distributional assumptions, and all tests were two-sided with statistical significance set at p<0.05. Where applicable, p-values corrected for multiple comparisons are reported. Data were analyzed using SAS v9.2 (SAS Institute, Inc., Cary, NC).
RESULTS
Patient Characteristics
Fifteen of the 75 patients had DBA, 30 DC, 24 FA and 6 SDS (Table 1). Thirty-four (45%) were females. Forty were children (median age: 13.0 years; range: 7.6–19.8). The median age of adults was 30.4 years (range: 20.6–69.0). Patients with FA and SDS were shorter than patients with DC (p<0.05; Table 1), while those with DBA were similar to DC. Hypogonadism was present in 14/35 (40%) adults (3 DBA, 11 FA) and in 3/40 children (7.5%) (1 DC, 2 FA). Six children (2 DC, 4 FA) and 3 adults (FA) had undergone HSCT. Eight of 75 patients were on oral glucocorticoid therapy (5 DBA, 2 DC, 1 FA) and eight were on inhaled glucocorticoids (3 DC, 3 FA, 2 SDS). Nine (6 DC, 3 FA) patients (3 males, 6 female) were on androgen treatment (details in Supplemental Tables S1 and S2 (online)). Five adult females (2 DC, 3 FA) and 4 girls with DC were on estrogen/progestin therapy for ovarian insufficiency and menorrhagia, respectively.
Table 1.
Demographic and bone mineral density characteristics in patients with IBMFS.
| Parameter | DBA | DC | FA | SDS | All IBMFS |
|---|---|---|---|---|---|
| N (%) | 15 (20%) | 30 (40%) | 24 (32%) | 6 (8%) | 75 |
|
| |||||
| Male: Female | 10: 5 | 20:10 | 8:16 | 3: 3 | 41: 34 |
|
| |||||
| Median Age (IQR) | 21.6 (10.8–42.1) | 17.8 (13.9–27.7) | 24.2 (13.8–33.8) | 12.7 (12–13.5) | 19.2 (12.7–30.2) |
| Range (years) | 8.5–58.4 | 7.6–69 | 8.1–56.6 | 8.2–30.4 | 7.6–69 |
|
| |||||
| Height Z-score* | −0.94 (1.25) | −0.06 (1.12) | −1.62 (1.35) | −2.24 (2.18) | −0.91 (1.51) |
| Mean (±SD) | |||||
|
| |||||
| Adults / Children | Adults / Children | Adults / Children | Adults / Children | Adults / Children | |
|
| |||||
| Number | 8 / 7 | 11 / 19 | 15 / 9 | 1 / 5 | 35 / 40 |
|
| |||||
| Hypogonadism | 3 / 0 | 0 / 1 | 11 / 2 | 0 | 14 / 3 |
|
| |||||
| HSCT | 0 | 0 / 2 | 3 / 4 | 0 | 3 / 6 |
|
| |||||
| Oral glucocorticoids | 0 / 5 | 0 / 2 | 1 / 0 | 0 | 1 / 7 |
|
| |||||
| Inhaled steroids | 0 | 0 / 3 | 2 / 1 | 0 / 2 | 2 / 6 |
|
| |||||
| Androgens | 0 | 2 / 4 | 2 / 1 | 0 | 4 / 5 |
|
| |||||
| Estrogen/progesterone | 0 | 2 / 3 | 3 / 1 | 0 | 5 / 4 |
|
| |||||
| Low BMD N | 2 / 1 | 2 / 6 | 5 / 3 | 0 / 1 | 9 / 11 |
p<0.001 for overall height Z-score differences, and p<0.05 for each of SDS and FA vs. DC (post-hoc comparison)
HSCT Hematopoietic stem cell transplant; BMD Bone mineral density; IBMFS Inherited bone marrow failure syndrome; DBA Diamond-Blackfan anemia; DC Dyskeratosis congenita; FA Fanconi anemia; SDS Shwachman-Diamond syndrome; IQR inter-quartile range (25th percentile, 75th percentile)
BMD distribution
The overall prevalence of low BMD was 27% (20/75). There was no significant difference in the prevalence of low BMD by IBMFS diagnosis (p=0.8), or when stratified by children (p=0.9) and adults (p=0.9) (Table 1).
Eleven of the forty children (28%) had low BMD (Supplemental Table S1 (online)). One child with DC had both spine and femoral neck HAZ ≤ −2, nine children (1 DBA, 5 DC, 2 FA, 1 SDS) had a femoral neck HAZ ≤ −2 but spine BMD HAZ >−2, and one child with FA had a history of vertebral compression fractures and low BMD two years prior to the study; his spine and femoral neck HAZ scores were normal on alendronate therapy at the time of this study. Three of the 11 children with low BMD (2 DC, 1 FA) were on chronic oral glucocorticoid therapy (one for pulmonary fibrosis and the other 2 for BMF) and three had transfusion-associated iron overload (1 DBA, 2 DC); two (with FA) were 4 and 10 years post-HSCT, while three (2 DC, 1 SDS) had not been on any treatment.
Prevalence of low BMD in adults was 26% (9/35) (Supplemental Table S2 (online)). Spine and femoral neck BMD Z-score were ≤ −2 in two adults (1 DC, 1 FA), four had only spine BMD Z-score ≤ −2 (1 DC, 3 FA), and one (DBA) had femoral neck BMD Z-score of −2. Seven of the 9 adults with low BMD had hypogonadism (5 FA, 2 DBA). Four were on bisphosphonates: one each with DBA and FA had been on alendronate treatment for over seven years and had normal BMD Z-scores at the time of study, while two others (both FA) had BMD Z-score ≤ −2 despite alendronate therapy for two years; one of them had also developed vertebral compression fractures. Three of the nine adults with low BMD had iron overload (2 DBA, 1 DC), one (FA) had been on prednisone (plus androgen) for over 10 years, and one (DC) was not on any treatment. All five adults with FA with low BMD and the two with DBA had hypogonadism.
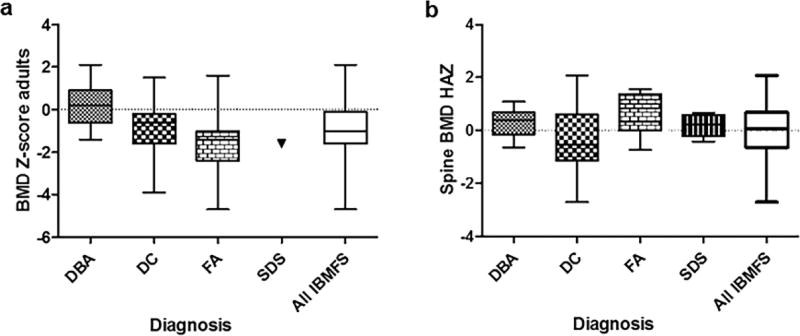
Table 2 shows the distribution of BMD in adults and children by diagnosis and site of BMD measurement and Figure 1 shows the distribution of lumbar spine BMD. There were no statistically significant differences in HAZ at spine (p=0.12), femoral neck (p=0.18), or forearm (p=0.53) in children with the four syndromes. In adults, there were significant differences only in BMD Z-score at the spine (p=0.034, FA vs. DBA driving the difference) but not at the femoral neck (p=0.25) or forearm (p=0.49) by diagnosis.
Table 2.
Bone mineral density data by diagnosis at different sites in adults and children with IBMFS.
| Adults BMD Z-score | Children (Age ≤20 years) HAZ | |||||||
|---|---|---|---|---|---|---|---|---|
| Diagnosis | N | Spine* | Femoral Neck | Forearm | N | Spine | Femoral Neck |
Forearm |
| DBA | 8 | 0.3 (1.1; −1.4 to 2.1) | −0.2 (1.2; −2.0 to 1.3) | −0.4 (1.1; −2.4 to 1.2) | 7 | 0.3 (0.6; −0.6 to 1.1) | −0.6 (1.2; −2.4 to 1.1) | −0.7 (1.3; −2.4 to 0.6) |
| DC | 11 | −0.9 (1.4; −3.9 to 1.5) | −0.6 (0.9; −2.2 to 0.7) | −0.3 (1.2; −2.0 to 2.0) | 19 | −0.3 (1.2; −2.7 to 2.1) | −1.5 (1.1; −4.3 to 0.4) | −0.4 (1.2; −3.1 to 1.6) |
| FA | 15 | −1.6 (1.5; −4.7 to 1.6) | −1.2 (1.3; −4.5 to 1.6) | −1.0 (1.4; −3.3 to 2.1) | 9 | 0.6 (0.8; −0.7 to 1.6) | −0.7 (1.2; −2.0 to 1.8) | −1.0 (0.9; −2.1 to 0.4) |
| SDS | 1 | −1.6 | −0.5 | −0.7 | 5 | 0.2 (0.4; −0.4 to 0.7) | −0.2 (1.8; −2.1 to 2.8) | −0.4 (1.2; −2.1 to 0.7) |
| All IBMFS | 35 | −1.0 (1.5; −4.7 to 2.1) | −0.8 (1.2; −4.5 to 1.6) | −0.7 (1.3; −3.3 to 2.1) | 40 | 0.1 (1.0; −2.7 to 2.1) | −1.0 (1.3; −4.3 to 2.8) | −0.6 (1.1; −3.1 to 1.6) |
Data are mean (SD; range), where range is provided for informative purposes.
p<0.05 only for DBA vs. FA (post-hoc comparison)
Figure 1. Distributional plot of bone mineral density at the lumbar spine by diagnosis (a) in adults and (b) children.
rp is the Pearson Correlation Coefficient
In adults, (a) BMD Z-score was statistically significantly different by diagnosis (p=0.034, FA vs. DBA driving the significant difference). (b) There were no statistically significant differences in BMD HAZ (p=0.12) among diagnoses in children; data at lumbar spine is shown.
Correlation of BMD and BMaD measures with height in adults with IBMFS (N=35)
Spine BMD Z-score was strongly correlated with height (Pearson correlation coefficient, rp=0.55, p<0.001) but spine BMaD was not (rp=−0.01, p=0.95). Similarly, femoral neck BMD Z-score was strongly correlated with height (rp=0.48, p=0.005) but femoral neck BMaD was not (rp=0.13, p=0.46) (Supplemental Figure S1 (online)).
Correlations of multiple site measures
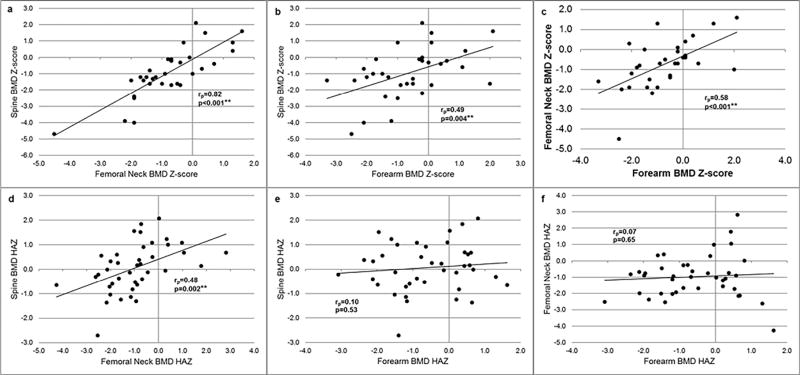
BMD measurements from the 3 different sites were relatively well correlated (Figure 2): Spine and femoral neck BMD Z-scores (rp=0.82, p<0.001) in adults (Fig 2A) were highly correlated, as were spine and forearm BMD Z-scores (rp=0.49, p=0.004; Fig 2B) and forearm and femoral neck BMD Z-scores (rp=0.58, p<0.001; Fig 2C). In children, spine and femoral neck HAZ were correlated (rp=0.48, p=0.002; Fig 2D), but forearm BMD HAZ was not correlated with spine (rp=0.10, p=0.53; Fig 2E) and femoral neck (rp=0.07, p=0.65; Fig 2F) BMD HAZ.
Figure 2. Correlation of bone mineral density (BMD) measurements at different sites.
In adults, (a) femoral neck BMD Z-score vs spine BMD Z-score; (b) forearm BMD Z-score vs spine BMD Z-score; (c) forearm BMD Z-score vs femoral neck BMD Z-score. In children, (d) femoral neck BMD HAZ vs spine BMD HAZ; (e) forearm BMD HAZ vs spine BMD HAZ; (f) forearm BMD HAZ vs femoral neck BMD HAZ.
Factors associated with low BMD
HSCT
Three of 35 (9%) adults, all with FA, had undergone HSCT. Average time since HSCT was 11.5 years (± 5.3, range 5.6 to 15.9). Mean Spine BMD Z-score was −2.4 (± 2.0, range −4.7 to −1.2) in adults with HSCT compared with −0.8 (± 1.4, range −4.0 to 2.1) in non-HSCT adult patients, but this was not statistically significant (p=0.08). However, adults who underwent HSCT had lower femoral neck BMD Z-scores (mean −2.5 ± 1.7, range −4.5 to −1.3 vs. mean −0.6 ± 1.0, range −2.2 to 1.6; p=0.007) than those who did not undergo HSCT. Six of 40 (15%) children underwent HSCT (2 DC and 4 FA); neither mean spine BMD-HAZ nor femoral neck BMD-HAZ was statistically significantly different between the two groups (spine: 0.0 ± 1.0, range 2.7 to 2.1 vs. 0.2 ± 0.8, range −0.7 to 1.2, p=0.7; femoral neck: −1.1 ± 2.1, range −4.3 to 1.8 vs. −0.9 ± 1.2, range −2.6 to 2.8, p=0.8).
Hypogonadism
Fourteen of 35 adults (40%) had hypogonadism (Table 1). Spine BMD-Z score (mean −2.1 ± 1.3, range −4.7 to −0.2) in those with hypogonadism was significantly lower than in those without hypogonadism (mean −0.2 ± 1.1, range −2.2 to 2.1; p=0.001). Femoral neck BMD Z-score was also lower in adults with hypogonadism compared with those without (mean −1.7 ± 1.0, range −4.5 to −0.4 vs mean −0.1 ± 0.9, range −1.3 to 1.5; p<0.001). Several children were pre-pubertal, and conclusive determination of hormonal status was only possible in 18/40 (45%), of whom 3 (17%) had hypogonadism.
Steroids
The number of patients on glucocorticoids (13 children, 3 adults), androgens (5 children, 4 adults) and estrogen/progesterone (4 children and 5 adults) therapy was relatively small. For adults, a multivariable regression model incorporating the predictors of height, HSCT, steroids, diagnosis and hypogonadism, explained 40% of the variability in spine BMD-Z score (p=0.001). These covariates together explained more of the variability in femoral neck Z-score in adults (65%, p<0.001) (Table 3). In children, the small numbers precluded additional analysis.
Table 3.
Results from simple and multiple regression models for outcomes of spine and femoral neck BMD Z-scores in adults with IBMFS.
| Univariable Models | Multivariable Models | |||||||
|---|---|---|---|---|---|---|---|---|
| Spine BMD Z-score | Femoral Neck BMD Z-score |
Spine BMD Z-score | Femoral Neck BMD Z-score |
|||||
| R2 | P-value | R2 | P-value | Adj R2 | P-value | Adj R2 | P-value | |
| All variablesa | 40.5% | 0.001 | 65.1% | <0.001 | ||||
| Height | 20.2% | 0.007 | 22.7% | 0.005 | --- | 0.13 | --- | 0.015 |
| HSCT | 8.9% | 0.081 | 21.1% | 0.007 | --- | 0.043 | --- | <0.001 |
| Glucocorticoids | 4.2% | 0.24 | 2.7% | 0.36 | --- | 0.14 | --- | 0.12 |
| Diagnosis (FA vs non-FA) | 14.1% | 0.026 | 11.9% | 0.0496 | --- | 0.19 | --- | 0.006 |
| Hypogonadism | 36.4% | <0.001 | 42.2% | <0.001 | --- | 0.003 | --- | <0.001 |
Height, HSCT, steroids, diagnosis, hypogonadism.
R2 = coefficient of determination; indicates the percentage of the variability of the outcome that is explained by the model.
HSCT: Hematopoietic stem cell transplantation
FA: Fanconi anemia
DISCUSSION
This study provides the first comprehensive description of bone mineral density in a cohort of patients with IBMFS. Twenty-eight percent of the children have low BMD even after correction of BMD to height-specific Z-scores using a pediatric reference calculator. The median age of children with low BMD was 15.8 years (range: 12.7–19.8 years). There was no difference in the frequency of low BMD by IBMFS diagnosis (DC vs. FA vs. DBA vs. SDS) among children. If we presume femoral neck measurements to be unreliable in children due to significant variability in skeletal development [7], then using the spine BMD alone, the prevalence of low BMD was 7.5% (3/40). Screening DXA scan is generally not recommended prior to 14 years of age unless compelled by clinical evidence of osteopenia or history of long term glucocorticoid use. Identification of low BMD in children with IBMFS is important since some may have vertebral compression fractures as evident in our patients. The nine adults with low BMD (26%) in our study were mostly young patients (median age 29.8 years; Supplemental Table S2 (online)) in whom multifactorial etiologies may have contributed to the increased prevalence of low BMD.
The prevalence of low BMD HAZ in children with FA was 33%. This is contrary to the previous report of normal BMD in children with FA after correction for height age [10]. However, the children in our study were slightly older (13–18y) and two of the three with low BMD HAZ had undergone HSCT. Glucocorticoid therapy, hypogonadism and growth hormone deficiency may be other contributory factors in FA. We previously showed a high prevalence of primary ovarian insufficiency (POI) in young adult women with FA [16]. In the current study, premature ovarian failure/hypogonadism appears to be the predominant underlying cause of low BMD in adult patients with FA. Five of 15 adults with FA (33%) had low BMD. They were all females and had primary ovarian insufficiency; only one had undergone HSCT 16 years previously.
Glucocorticoid therapy, iron overload and hypogonadism were the likely risk factors for low BMD in DBA. The child with DBA with low femoral neck BMD HAZ was on glucocorticoid therapy and had iron overload while the two adults with DBA with low BMD had hypogonadism secondary to iron overload; one was also on glucocorticoid. No risk factor could be identified in the child with SDS who had low femoral neck BMD but normal spine BMD HAZ.
Thirty-two percent of the children with DC had low BMD HAZ. Glucocorticoid use and iron overload were identified as risk factors (Supplemental Table S1 (online)). A male child with DC in our study had multiple long bone fractures reported with minimal trauma but had completely normal BMD measurements. Additional BMD measurement in the lateral distal femur may be considered in such patients since conventional DXA scans alone may be insufficient to predict fracture risk [17]. One adult patient with DC had received chelation for iron overload and no apparent cause could be identified in the other adult patient with DC and low BMD.
Iron overload may be a predominant factor causing low BMD, especially in DBA and DC. While the molecular mechanisms of iron overload resulting in osteoporosis are not well understood, it appears to decrease bone formation and affect the microarchitecture by increasing apoptosis of osteoblasts and impeding their recruitment and function [18]. HSCT was associated with significantly lower BMD measures in the femoral neck but not the lumbar spine in our study. Further studies are warranted to determine whether HSCT differentially affects BMD at different sites, as well as to study the change in BMD seen over time after HSCT. Glucocorticoids have long been known to decrease bone formation by reduced osteoblast formation, increased apoptosis of osteoblasts and osteocytes, with reduced bone turnover from decreased osteoclastogenesis [19]. Several patients with low BMD in this study had history of glucocorticoid use but the small number of total patients precluded further analysis.
We studied the correlation of BMD Z-scores at spine and femoral neck and found that the measures were strongly correlated with height in adults. This raises the possibility of whether adult patients with IBMFS with short stature, particularly those with FA or SDS who have a low height Z-score, may be incorrectly diagnosed with low BMD. In the multivariate regression analysis, height did not predict BMD Z-score at the spine but did predict femoral neck BMD Z-scores (p=0.015). Volumetric BMaD measures have been proposed for adjustment of stature in adults. Indeed, we noted that BMaD measures did not correlate with height. But the use of BMaD measurements may not add to the management of these patients at this time due to insufficient normative data published on BMaD, and the inability to predict fracture risk. The impact of height on BMD measurements in adults should, however, raise caution in interpretation. Further assessment of BMD Z-scores in larger populations of IBMFS patients and correlation of diagnosis and measures with fracture risk is warranted.
Management of low BMD should begin with recognition and treatment of the risk factors causing secondary osteopenia such as hypogonadism and iron overload. Treatment of osteoporosis should be undertaken in consultation with an endocrinologist. Bisphosphonates are the typical first line of therapy.
The strength of our study is that it provides the first comprehensive assessment and comparison of BMD in children and adults with the four major IBMFS and addresses the associated risk factors for low BMD. The limitations of our study include the lack of data on cumulative glucocorticoid or androgen doses, and small sample size which may have contributed to the lack of differences in BMD related to steroid therapy and iron overload. We were unable to distinguish patients with adequately corrected hypogonadism or to quantify the duration of untreated hypogonadism that may be specifically causative of low BMD. Despite these limitations, our data contribute to the understanding of BMD distribution in patients with IBMFS, the correlation of measures at different body sites, as well as the different factors that may be associated with low BMD.
In summary, our study demonstrates a high prevalence of low BMD in patients with IBMFS. Low BMD in adults with FA was mainly associated with hypogonadism. Early detection of hypogonadism and hormonal supplementation may facilitate adequate bone acquisition in these patients. Iron overload and chronic glucocorticoid therapy are other important causes of low BMD in IBMFS, specifically in patients with DC and DBA. We show a high prevalence of low BMD in children as well. While low BMD at the femoral neck may suggest a difference in skeletal maturity, low BMD at the lumbar spine may result from iron overload and/or chronic glucocorticoid therapy, which can lead to vertebral compression fractures. Prompt recognition and treatment of low BMD and the associated risk factors are important in the clinical management of these individuals. Future studies are needed to explore the fracture risk in patients with IBMFS and low BMD, as well as, the contribution of HSCT and glucocorticoid therapy to the risk of low BMD and osteoporosis in these patients.
Supplementary Material
Acknowledgments
STATEMENT OF FINANCIAL SUPPORT
This research was supported in part by the Intramural Research Programs of the National Cancer Institute and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, of the National Institutes of Health, and by contract HHSN261201100018C with Westat.
We thank Lisa Leathwood, RN, Maureen Risch, RN, and Ann Carr, MS, CGC, and other members of the Westat Inherited Bone Marrow Failure Syndromes team for their extensive assistance. We are grateful to the patients and their families for their valuable contributions.
Footnotes
DISCLOSURE STATEMENT
The authors have no conflicts of interest to disclose
References
- 1.Shimamura A, Alter BP. Pathophysiology and management of inherited bone marrow failure syndromes. Blood Rev. 2010;24:101–22. doi: 10.1016/j.blre.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: Pathophysiology and therapy. Osteoporos Int. 2007;18:1319–28. doi: 10.1007/s00198-007-0394-0. [DOI] [PubMed] [Google Scholar]
- 3.Kim MK, Lee JW, Baek KH, et al. Endocrinopathies in transfusion-associated iron overload. Clin Endocrinol (Oxf) 2013;78:271–7. doi: 10.1111/j.1365-2265.2012.04495.x. [DOI] [PubMed] [Google Scholar]
- 4.Giri N, Batista DL, Alter BP, Stratakis CA. Endocrine abnormalities in patients with fanconi anemia. J Clin Endocrinol Metab. 2007;92:2624–31. doi: 10.1210/jc.2007-0135. [DOI] [PubMed] [Google Scholar]
- 5.McClune BL, Majhail NS. Osteoporosis after stem cell transplantation. Curr Osteoporos Rep. 2013;11:305–10. doi: 10.1007/s11914-013-0180-1. [DOI] [PubMed] [Google Scholar]
- 6.Shepherd JA, Baim S, Bilezikian JP, Schousboe JT. Executive summary of the 2013 international society for clinical densitometry position development conference on body composition. J Clin Densitom. 2013;16:489–95. doi: 10.1016/j.jocd.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Crabtree NJ, Arabi A, Bachrach LK, et al. Dual-energy X-ray absorptiometry interpretation and reporting in children and adolescents: The revised 2013 ISCD pediatric official positions. J Clin Densitom. 2014;17:225–42. doi: 10.1016/j.jocd.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Carter DR, Bouxsein ML, Marcus R. New approaches for interpreting projected bone densitometry data. J Bone Miner Res. 1992;7:137–45. doi: 10.1002/jbmr.5650070204. [DOI] [PubMed] [Google Scholar]
- 9.Melton LJ, 3rd, Atkinson EJ, O’Connor MK, O’Fallon WM, Riggs BL. Bone density and fracture risk in men. J Bone Miner Res. 1998;13:1915–23. doi: 10.1359/jbmr.1998.13.12.1915. [DOI] [PubMed] [Google Scholar]
- 10.Rose SR, Rutter MM, Mueller R, et al. Bone mineral density is normal in children with fanconi anemia. Pediatr Blood Cancer. 2011;57:1034–8. doi: 10.1002/pbc.22956. [DOI] [PubMed] [Google Scholar]
- 11.Toiviainen-Salo S, Mayranpaa MK, Durie PR, et al. Shwachman-diamond syndrome is associated with low-turnover osteoporosis. Bone. 2007;41:965–72. doi: 10.1016/j.bone.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 12.Dokal I. Dyskeratosis congenita in all its forms. Br J Haematol. 2000;110:768–79. doi: 10.1046/j.1365-2141.2000.02109.x. [DOI] [PubMed] [Google Scholar]
- 13.Alter BP, Giri N, Savage SA, et al. Malignancies and survival patterns in the National Cancer Institute inherited bone marrow failure syndromes cohort study. Br J Haematol. 2010;150:179–88. doi: 10.1111/j.1365-2141.2010.08212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC growth charts for the United States: Methods and development. National Center for Health Statistics. Vital Health Stat 11. 2002;246:1–190. [PubMed] [Google Scholar]
- 15.Zemel BS, Kalkwarf HJ, Gilsanz V, et al. Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: Results of the bone mineral density in childhood study. J Clin Endocrinol Metab. 2011;96:3160–9. doi: 10.1210/jc.2011-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sklavos M, Giri N, Stratton P, Alter BP, Pinto LA. Anti-Müllerian Hormone Deficiency in Females with Fanconi Anemia. Clin Endocrinol Metab. 2014;99:1608–14. doi: 10.1210/jc.2013-3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zemel BS, Stallings VA, Leonard MB, et al. Revised Pediatric Reference Data for the Lateral Distal Femur measured by the Hologic Discovery/Delphi Dual-Energy X-Ray Absorptiometry. Journal of Clinical Densitometry: Assessment of Skeletal Health. 2009;12:207–18. doi: 10.1016/j.jocd.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doyard M, Chappard D, Leroyer P, Roth MP, Loreal O, Guggenbuhl P. Decreased Bone Formation Explains Osteoporosis in a Genetic Mouse Model of Hemochromatosis. PLoS One. 2016;11:e0148292. doi: 10.1371/journal.pone.0148292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Invest. 1998;102:274–82. doi: 10.1172/JCI2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.