Abstract
Noroviruses (NoVs) are important human pathogens associated with foodborne and waterborne gastroenteritis. These viruses are genetically highly heterogeneous, with more than forty genotypes within three genogroups (GI, GII, and GIV) identified in humans. However, the vast majority of human infections are associated with variants of a unique genotype, GII.4. Aside from these NoV strains of epidemiological relevance, NoV strains of genogroup GIV (Alphatron-like) are reported in a sporadic fashion and their overall prevalence in the community is unknown and this likely reflects the lack of specific diagnostic tools. We analyzed raw sewages collected from 32 wastewater treatment plants distributed throughout Italy (307 samples) and stool specimens collected from hospitalized patients with clinical signs of diarrhea of unknown etiology (285 samples). By using specific qualitative and quantitative RT-PCR assays, 21.8 % of the sewage samples and 3.2 % of the stool specimens tested positive for GIV NoVs. The number of genome copies in fecal samples ranged from 5.08 × 104 to 1.73× 106/g of feces. Sequence analysis showed limited genetic variability in human GIV viruses. The presence of GIV NoV both in sewage and in clinical samples confirms that not only GI and GII NoVs but also GIV strains are circulating in humans. Monitoring of GIV NoV is recommended in order to understand the dynamics of circulation in human populations, environmental contamination, and potential health risks.
Keywords: Norovirus genogroup IV, Nested PCR, Real-time PCR, Sewage, Stool samples
Introduction
Noroviruses (NoVs) are small, round non-enveloped viruses with an RNA genome within the family Caliciviridae. NoVs are important enteric pathogens of humans, frequently transmitted by contaminated water or food (Matthews et al. 2012; Patel et al. 2009). Within the genus NoV, six distinct genogroups (GI to GVI), further classified into more than 40 genotypes, have been identified, with GI, GII, and GIV being associated with human infections (Mesquita et al. 2010; Zheng et al. 2006). Punctate mutations and recombination contribute to the broad heterogeneity of NoVs and trigger the periodical emergence of new epidemic strains. In spite of this massive genetic heterogeneity, the vast majority of human NoV infections are associated with GII viruses (Matthews et al. 2012). The widespread circulation of the more common human NoV GI and GII variants in clinical settings as well as in raw and treated sewage has been extensively documented (Glass et al. 2009; Haramoto et al. 2006; Iwai et al. 2009, 2008a, b; La Rosa et al. 2010, b; Nordgren et al. 2008; Patel et al. 2009). In contrast, a few studies document the detection in either clinical or environmental samples of GIV NoVs (Alphatron-like) (Fankhauser et al. 2002; Iritani et al. 2002; Kitajima et al. 2009, 2011; La Rosa et al. 2008; 2010a, 2012; Lindell et al. 2005). GIV NoVs were first detected by Vinje and Koopmans in stool samples from sporadic cases of gastroenteritis (Vinje and Koopmans 2000). Later on, they were occasionally detected in the course of routine NoV screenings (Fankhauser et al. 2002; Iritani et al. 2002; Lindell et al. 2005). Interestingly, Alphatron-like viruses have also been detected in domestic and captive carnivores (Di Martino et al. 2009; Martella et al. 2007; Martella et al. 2008; Pinto et al. 2012). Unlike genogroups I and II, for which thousands of sequences are available in the public sequence databases, less than 70 partial sequences of GIV viruses are present in GenBank. The full-length genomes of three GIV strains have been published: a GIV.2 feline virus associated with an outbreak of enteritis in kittens in the USA (Pinto et al. 2012) and two GIV.1 human strains identified in Australia (Eden et al. 2012) and in China (unpublished manuscript).
In previous studies, our group detected NoV GIV in sewage samples collected from wastewater treatment plants (WTPs), in clinical samples from pediatric patients, and in edible bivalves, thus gathering firm evidence for the circulation of GIV NoVs in Italy (La Rosa et al. 2008, 2010a, 2012). In a recent paper, Kitajima and coworkers, using quantitative methods, detected GIV NoV in wastewaters and river waters in Japan (Kitajima et al. 2009, 2011) at high prevalence and with high viral loads (up to 104 copies/l). GIV NoVs have also been detected in wastewaters in Luxembourg (Kremer et al. 2011; Skraber et al. 2011). These studies confirm that not only GI and GII NoVs but also GIV NoV strains circulate worldwide, highlighting the relevance of investigations on this pathogen.
In the present study we coupled an environmental investigation with a clinical epidemiological survey in order to further explore the ecology and genetic diversity of GIV NoVs in the Italian population. For this purpose, we expanded an existing WTP-based environmental network (La Rosa et al. 2010a) so as to have a monitoring point (at least one WTP) in each Italian region (for a total of 32 WTPs in 20 regions). In addition, stool samples were obtained and analyzed from patients with watery diarrhea of unknown etiology hospitalized at the A. Gemelli Hospital of Rome (Catholic University Medical School of Rome, Italy).
Materials and Methods
Environmental Samples
Samples of raw sewage (a total of 307 grab samples) were collected at 32 WTPs throughout Italy (all 20 regions). Figure 1 is a geographic information system (GIS) map of the WTPs under study. Further details (European Environment Agency WTP code, Population Equivalents served by each WTP, and number of positive samples/WTP) are shown in Table 1.
Fig. 1.
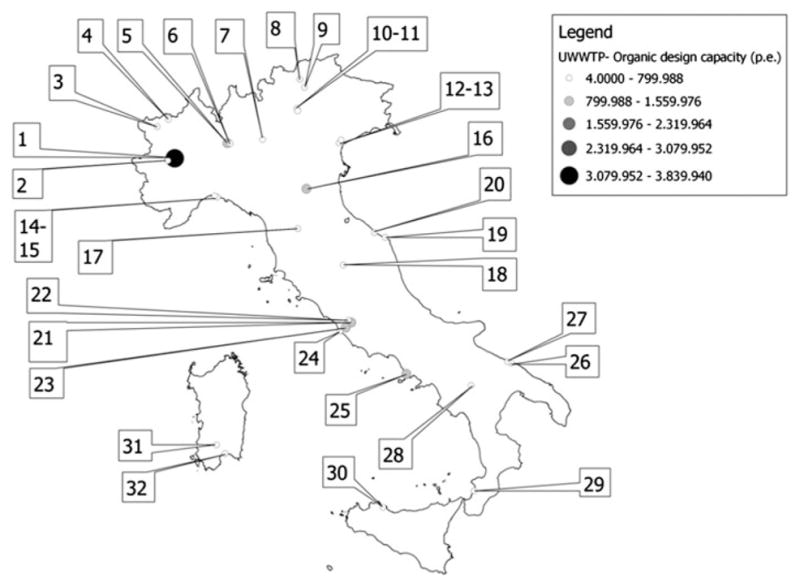
GIS map of the WTPs under study. The geographic information system map shows the localization of the 32 WTPs under study. Further details (European Environment Agency WTP code, Population Equivalents served by each WTP, and number of positive samples/WTP) are given in Table 1. The map was created using Quantum GIS (QGIS) version 1.8.0-Lisboa (www.qgis.org), an open source geographic information system licensed under the GNU General Public Licence
Table 1.
List of WTPs under study
| ID | ID code of European Environment Agency (EEA) | City | Population equivalent served by WTPs | Number of GIV-positive samples |
|---|---|---|---|---|
| 1 | IT01000000000043 | Torino | 3.8E+06 | 2 |
| 2 | IT01000000000078 | Collegno | 2.7E+05 | 0 |
| 3 | IT02Q90000000583 | Aosta | 1.5E+05 | 1 |
| 4 | IT02Q90000000592 | Valtournanche | 4.0E+04 | 1 |
| 5 | IT03160128000456 | Milano | 1.3E+06 | 2 |
| 6 | IT03160128000464 | Milano | 5.7E+05 | 2 |
| 7 | IT03160129000531 | Brescia | 2.5E+05 | 4 |
| 8 | IT21000000000008 | Merano | 3.6E+05 | 1 |
| 9 | IT21000000000013 | Bolzano | 3.7E+05 | 1 |
| 10 | IT220000000064 | Trento | 1.2E+05 | 5 |
| 11 | IT220000000065 | Trento | 1.0E+05 | 1 |
| 12 | IT05000000000213 | Venezia | 4.0E+05 | 4 |
| 13 | IT05000000000212 | Venezia | 1.1E+05 | 2 |
| 14 | IT070000000034 | Genova | 2.5E+05 | 0 |
| 15 | IT070000000046 | Genova | 1.3E+05 | 3 |
| 16 | IT08000000000007 | Bologna | 9.0E+05 | 3 |
| 17 | IT090000000080 | Firenze | 6.0E+05 | 9 |
| 18 | IT100000000050 | Perugia | 9.0E+04 | 0 |
| 19 | IT11000000000089 | Ancona | 1.0E+05 | 2 |
| 20 | IT11000000000123 | Senigallia | 1.0E+05 | 4 |
| 21 | IT12000000000304 | Roma | 9.2E+05 | 5 |
| 22 | IT12000000000311 | Roma | 7.8E+05 | 2 |
| 23 | IT12000000000317 | Roma | 1.2E+06 | 3 |
| 24 | IT12000000000321 | Roma | 3.5E+05 | 2 |
| 25 | IT15Q90000003543 | Napoli | 1.2E+06 | 2 |
| 26 | IT160000000065 | Bari | 3.9E+05 | 0 |
| 27 | IT160000000066 | Bari | 2.4E+05 | 0 |
| 28 | IT170000000143 | Potenza | 1.6E+05 | 1 |
| 29 | IT18Q90000003087 | Reggio Calabria | 2.6E+05 | 0 |
| 30 | IT19Q90000002303 | Palermo | 4.4E+05 | 2 |
| 31 | IT20000000000120 | Cagliari | 2.3E+05 | 2 |
| 32 | IT20000000000135 | Cagliari | 5.6E+05 | 1 |
Starting from May 2011, and over a 1-year period, samples were collected at either 1- or 2-month intervals, in collaboration with the regional environmental agencies (ARPAs). Upon arrival, untreated wastewater samples were divided into 2 × 40 ml aliquots and stored at −20 °C before use. One aliquot was seeded with a known amount of murine NoV (MNV1), added to the samples as a sample process control (Diez-Valcarce et al. 2011; D’Agostino et al. 2011), in order to calculate viral recovery efficiency (by quantitative PCR) and check for potential inhibitors (by qualitative PCR). The PCR primers used in the study are shown in Table 2. An aliquot of 20 ml was treated with 2 ml of 2.5 M glycine pH 9.5 and incubated in ice for 30′; the solution was then treated with 2.2 ml chloroform and centrifuged at 5,000 rpm for 10′. Viral nucleic acids were extracted from 10 ml of chloroform-treated samples using the NucliSENS easyMAG (BioMerieux, Marcy l’Etoile, France) semi-automated nucleic acid extraction system with magnetic silica, according to the manufacturer’s instructions. After nucleic acid extraction, eluates (100 μl each) were frozen at −70 °C until analyzed.
Table 2.
PCR and primers used in this study
| Primer ID | Sequence (5′ > 3′) | PCR ID | Product length (bp) | Organism | References |
|---|---|---|---|---|---|
| 1803-f | TTT GGA ACA ATG GAT GCT GA | 748 | 746 | Murine NoV (first cycle) | This study |
| 1804-r | AGT CGA CCA TCC GGT AGA TG | ||||
| 1801-f | AAT TGA CCC CTG GAT CTT CC | 747 | 352 | Murine NoV (nested cycle) | |
| 1802-r | GAC TCG ACG CAC ATC AAG AA | ||||
| 1798-f | GGC TGG GTT GGG AAC ATG | 746 | 84 | Murine NoV (real-time) | This study |
| 1799-r | TGG TAC AAG GGC AAC AAC CA | ||||
| 1800-f | CTG GTC CTC GCC GGC AAT GC (5′FAM 3′TAMRA) | ||||
| 1531-f | GCA CTC GGC ATC ATG ACA AAA TTC A | 612 | 995 | GIV NoV (first cycle) | La Rosa et al. (2008) |
| 1532-r | GTT TGG GTC CCA ATT CCA A | ||||
| 1698-f | GTA CTG GAC CAA GGG CCC GA | 686 | 323 | GIV NoV (nested cycle) | This study |
| 1699-r | GAG GTT GCC CGC ACC ATC CG | ||||
| 1773-f (MON 4F) | TTT GAG TCY ATG TAC AAG TGG ATG C | 756 | 133 | GIV NoV (real-time) | Trujillo et al. (2006), this study |
| 1790a-r | GTT GCC CGC ACC ATC CGY AG | ||||
| 1775-f (RING 4) | TGG GAG GGG GAT CGC GAT CT (5′FAM-3′BHQ) |
Replaces the original MON4R (Trujillo et al. 2006)
Clinical Samples
Stool specimens (285 samples, ~100 mg in weight) were collected by the A. Gemelli Hospital (Catholic University Medical School of Rome, Italy), from patients with symptoms of diarrhea, hospitalized between 2011 and 2012. Total nucleic acid was extracted using the automated platform NucliSENS EasyMAG (BioMerieux, Marcy l’Etoile, France), according to the manufacturer’s instructions. Eluates (100 μl each) were frozen at −70 °C until analyzed.
PCR, Sequencing, and Phylogenetic Analysis
We used a conventional RT-PCR with a nested strategy for the screening of both sewage and stool samples. In the first-round amplification, previously published primers were used that target the ORF1/ORF2 region (PCR 612, 995 bp, of which 716 in the ORF1 and 234 in the ORF2) (La Rosa et al. 2010a). For the second-round PCR we designed a new assay (PCR 686, 323 bp), taking into consideration all the GIV sequences available in GenBank. The amplicon spans positions 4799-5102 of the genome of a GIV.1 NoV strain (accession JQ613567) and covers 288 bp in ORF1 and 34 bp in ORF2. The primer pair was selected among several primer pairs after testing with various positive and negative controls, to assess the sensitivity and specificity (data not shown). In order to assess the sensitivity, recombinant plasmids containing a fragment of the ORF1/ORF2 region of GIV NoVs detected in our previous studies were used as positive controls (La Rosa et al. 2008, 2012). In order to evaluate the specificity and to rule out possible cross-reactions, a panel of NoV genotypes of genogroup GI (GI.2, GI.4, GI.7, GI.8) and GII (GII.1, GII.2, GII.3, GII.4, GII.6, G2II.7, GIIb/GII.13), kindly provided by the ISGEV (Italian Study Group for Enteric Viruses), was used as negative control. Both the first- and second-round PCRs were specific for GIV NoVs as they did not amplify non-GIV.1 NoVs, with the only exception of the GIIb/GII.13 strain, which yielded an aspecific amplicon in the second-round of PCR.
Two microliters of the extracted RNA and 22 pmol of each primer were used in a final mixture of 50 μl using the One-Step RT-PCR Kit (Bioline). After the first round of PCR amplification (35 cycles), one tenth of the volume of the PCR product obtained was used for the second PCR assay (30 cycles). Because of the extreme sensitivity of PCR, we paid particular attention to the prevention of contamination: separate rooms were used for the preparation or mixing of reagents, sample processing, and gel electrophoresis; reagents and samples were stored in separate rooms; and the equipment used in each room was not used in other areas. Negative PCR mixture controls and extraction controls were systematically used.
PCR amplicons were directly sequenced with a capillary automatic sequencer (ABI PRISM 310 Genetic Analyzer; Applied Biosystems).
Bioinformatic analysis was performed as follows: the raw forward and reverse ABI files were aligned and assembled into a single consensus sequence using MEGA5 software. All sequences were submitted to BLAST analysis for genotyping at http://blast.ncbi.nlm.nih.gov/Blast.cgi. Molecular phylogeny was also performed using MEGA5. The best fit model of nucleotide substitution was selected (Kimura-2parameter +I) from among 24 models available in the MEGA5 software, based on the minimum Akaike Information Criterion (AIC) value. The reliability of the phylogenetic tree was determined by bootstrap re-sampling of 1,000 replicates. For genetic distance calculations and pairwise distance comparisons, Kimura’s two-parameter model was used, integrated into the MEGA software.
Real-Time PCR
Positive stool samples were further analyzed in order to obtain quantitative data on the viral loads. The Real Time TaqMan assay is a modified protocol of the original assay developed by Trujillo et al. (2006) at the Centers for Disease Control and Prevention. The assay is able to amplify a 98-bp segment of the ORF1/ORF2 junction of NoV genome. The assay was modified to increase its specificity and sensitivity by designing a new reverse primer in a region located after the original primer, based on a multi-alignment study of the target region in all published human NoVs. The new assay amplifies a 133-bp-long fragment and was validated using the positive and negative controls described above, before screening the clinical samples. All PCRs, primers and probes used in this study are shown in Table 1. The recombinant plasmid (pCR4TOPO vector) used to construct the standard curve for the absolute quantification of GIV NoV contains a 998-bp fragment targeting the ORF1/ORF2 region of a GIV NoV (sample 980) detected in a sewage sample in Italy, in 2008 (La Rosa et al. 2008). The plasmidic DNA was quantified by optical density using the ND-1000 instrument (Nanodrop, Wilmington, DE, USA). DNA concentrations were then converted into copy numbers using the following equation: [GC/ml = C/MW * 6.02 × 1023], where C represents DNA concentration (C = g/ml) and MW is molecular weight. The standard curve was constructed on the basis of five serial dilutions of the plasmid. The absolute quantification was achieved by comparing sample quantification cycle (Cq) values to the standard curve. A no-template control was used in every assay to rule out the possibility of contamination. The real-time PCRs were carried out in a Bio-Rad Miniopticon RealTime PCR System on a triplicate set using 5 μl of the extracted genome. The reaction mixture was initially incubated at 42 °C for 45 min to transcribe the RNA and then heated at 95 °C for 10 min. Activation was followed by a 40-cycle, two-step process, each cycle consisting of denaturation at 95 °C for 15 s and annealing or extension at 60 °C for 1 min. The reactions were carried out in disposable optical 96-well PCR plates in a 25 μl mixture using SensiMix One-Step kit (Bioline). Run acceptability was defined as a correlation coefficient (R2) > 0.98 and a slope between −3.6 and −3.1. Quantification data were analyzed with CFX Manager software, and exported into a Microsoft Excel file for subsequent statistical analysis.
Results
A total of 307 sewage samples and 285 stool specimens were tested for GIV NoVs by the newly designed nested PCR assay in the ORF1/ORF2 region. Of these, 67 (21.8 %, see Table 1) and 9 (3.2 %) were positive for GIV NoV, respectively. Extraction efficiency, calculated on randomly selected samples, showed an average murine norovirus recovery exceeding 35 %. Inhibition in negative samples was ruled out using the sample process control (positive PCR signal for MNV1, random sampling). All the PCR amplicons were sequenced and submitted to Genbank under accession numbers HG004588–HG004608.
Of the 307 wastewater samples, 71 were collected in spring (March, April, and May), 85 in summer (June, July, and August), 70 in autumn (September, October, and November), and 81 in winter (December, January, and February). Overall, 10/71 (14 %) of samples collected in spring were found to be positive, 14/85 (16.5 %) in summer, 24/70 (34.3 %) in autumn, and 19/81 (23.5 % in winter).
The collection of clinical stool samples was not performed regularly during the year, but concentrated in March–June 2011 and May–June 2012.
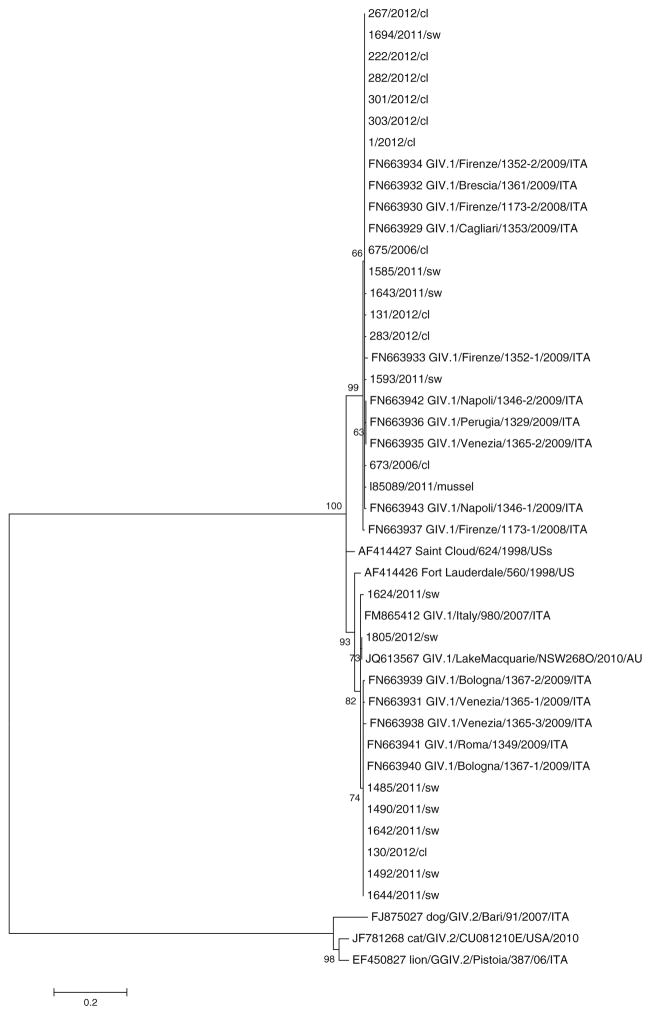
The results of the phylogenetic study performed on the sequences obtained in the ORF1/ORF2 region are presented in Fig. 2. All the sequences generated in this study grouped with the GIV.1 human cluster, segregating away from the GIV.2 animal cluster (dog, cat, and lion). The first cluster further subdivided into two subclusters (mean distance between group = 0.082 number of base substitutions per site). One lineage included the vast majority of the sewage (60/67) and clinical (8/9) samples detected in this study, along with most of the sequences detected in our previous studies, i.e., 10 sewage sequences, one mussel sequence, and two sequences obtained from children with GE. The other lineage included seven sewage samples and one clinical sample from the present study, six sewage samples detected previously in Italy, the prototype strain Fort Lauderdale, and the recently identified Lake Macquarie strain from Australia.
Fig. 2.
Phylogenetic tree displaying the genetic relationships between GIV NoVs. The tree includes a total of 102 sequences. Sequences from the present study are shown in bold (ID number, followed by the year of identification, and by the suffix “sw” for sewage samples and “cl” for clinical samples). To simplify the tree, a single sample (ID 1694) was used to represent 57 of our samples the sequences of which were found to be identical. The tree also includes all animal (GIV.2) and human (GIV.1) sequences available in GenBank for the genomic region under study. The animal prototypes reported in the phylogenetic tree are: EF450827 (lion/GGIV.2/Pistoia/06/ITA), JF781268 (CAT/giv.2/CU081E/USA/2010), and FJ875027 (dog/GIV.2/Bari/91/ITA). The human prototypes include AF414426 (Fort Lauderdale/560/1998/US), AF414427 (Saint Cloud/624/1998/US), and the strain JQ613567 recently completely sequenced (Lake Macquarie/NSW2680/2010/AU). We also included sequences identified by our group in the previous studies from sewage samples (FN663936, FN663935, FN663942, FN663932, FN663934, FN663930, FN663933, FN663929, FN663937, FN663943, and 980/2007/ITA), clinical samples (673/2006/cl and 675/2006/cl), and one mussel sample (I85089/2011)
By real-time PCR, the viral load of GIV NoVs was determined in all the stool samples that tested positive by nested PCR. The number of genome copies (GC) ranged from 5.08 × 104 to 1.73 × 106/g of feces.
Discussion
Noroviruses may be shed at high titers in the feces and are often found at high concentrations in sewage (Rodríguez-Lázaro et al. 2012). Several studies have investigated the presence of GI and GII NoVs in urban sewage (Haramoto et al. 2006; Iwai et al. 2009; La Rosa et al. 2010b; Nordgren et al. 2008). GIV NoVs have also been the object of investigation in a few environmental studies, revealing that Alphatron-like NoVs frequently contaminate sewage water (Kitajima et al. 2009; Kitajima et al. 2010, 2011; La Rosa et al. 2008, 2010a). Since most molecular tools for human NoVs have been optimized for the detection of GI and GII NoV, the limited literature on GIV NoVs is not surprising. In the present study we coupled an environmental epidemiological study with a clinical epidemiological survey, in order to gain insights into the ecology and genetic diversity of GIV NoV strains in the Italian population. Indeed, the association between the presence of pathogens in water environments and in human populations is a key issue in understanding waterborne epidemics and potential health risks to humans.
Monitoring sewer systems is a useful approach for the study of the prevalence and epidemiology of enteric pathogens (Sinclair et al. 2008; La Rosa and Muscillo 2013), as this may reflect the actual level of viral activity in the population more accurately than surveillance of reported cases, which usually represent only a small proportion of the total cases. Sewage-based studies on GIV NoV have been conducted previously (Kitajima et al. 2009, 2011; La Rosa et al. 2008, 2010a), but this is the first large-scale study, in which 32 WTPs were enrolled and a total of 307 sewage samples were analyzed. A molecular epidemiological investigation of 11 wastewater treatment plants (122 grab samples) throughout Italy conducted by our group (La Rosa et al. 2010a) revealed 10 positive samples. The percentage of positive samples in the present study was 2.5 times higher than that in our previous investigation (21.8 vs 8 %). This could be accounted for by the lower sensitivity of the primer pair used in the 2010 study, which was designed on the basis of a smaller set of sequences. Also, the “long template PCR” method employed in the previous study (1,526 bp-long amplicons for the first-round and 995 bp-long amplicons for the second-round reaction) is expected to have a lower sensitivity, as it requires the RNA to retain its integrity. In the present study, a short-template assay (323 bp in the second-round reaction) was designed on the basis of a larger set of GenBank sequences. With this optimized conventional assay, the percentage of positive samples was similar to the prevalence values found by Kitajima et al. (2011), in a smaller WTP-based study (Kitajima et al. 2011). The newly designed assay proved to be a useful tool for the detection of GIV strains from both clinical specimens and environmental samples. DNA sequencing confirmed all positive samples.
The year-long monitoring of sewer systems allowed us to study the seasonality of GIV NoVs. It is well known that although NoV infection can occur at any time of year, the incidence tends to increase in the winter season. In this study, GIV NoVs were detected in sewage samples throughout the year, with an autumn/winter peak. This seasonal pattern is similar to that displayed by GI and GII NoVs, generally referred to as “winter vomiting disease viruses.” It should be noted, however, that the virus was present in a significant percentage of samples collected in spring and summer (14–16.5 %).
As for the geographic distribution, GIV NoVs were detected in 27/32 of the analyzed WTPs (Table 1) and were distributed across the entire country. A detailed comparative analysis of the distribution of positive samples across WTPs was not feasible, however, since the number of collected wastewater samples varied greatly from one WTP to another. This was due to differences in compliance between WTPs and to the fact that some plants were enrolled relatively late in the study.
Hospital-based surveillance identified GIV NoVs in 3 % of hospitalized patients. This epidemiological study suffers from a number of limitations. First, specimens were collected from patients with clinical signs of diarrhea of unknown etiology. Of the nine patients positive for GIV NoV, only two patients had been hospitalized with GE, while the others were hospitalized for other reasons (transplant patients, endocarditis or other cardiac problems, pneumonia, Crohn syndrome, and regional enteritis). We are, therefore, unable to speculate on the role of the virus in GE. Conceivably, this virus, which seems to circulate widely in the population (as reflected in wastewaters), rarely affects people with healthy immune systems, but causes opportunistic infections in susceptible patients. Obviously, further investigations are needed to either confirm or refute this hypothesis.
Another limitation of the clinical study relies in the collection of the fecal samples, which was not performed regularly during the year, but concentrated in March–June 2011 and May–June 2012. This precluded the possibility of conducting a paired comparison between the clinical and environmental samples to investigate the observed temporal patterns.
We quantified viral loads in clinical samples, using a modified protocol of the original assay developed by Trujillo et al. (2006). For the absolute quantification of viruses in feces, we used a DNA-based calibration curve (recombinant plasmid) rather than an in vitro transcribed RNA. The advantage of using an in vitro transcribed RNA as template is that it involves cDNA synthesis and, therefore, takes into account the efficiency of the reverse transcription reaction. A recent study by Bowers and Dhar, however, indicates that if the standard curve is generated with over 100 template copies, the nature of the template has no effect on the standard curve. In such cases, plasmid DNA, which is cheap and relatively easy to produce in large quantity, should be preferred (Bowers and Dhar 2011). Moreover, DNA has the advantage of being more stable than RNA and thus has a longer shelf-life. The viral loads in stool specimens ranged from 5.08 × 104 to 1.73 × 106/genomic copies/g of feces (with higher titers in the two patients with GE), values that are lower than those found usually in the stools of patients with GI and GII NoV infections (up to 109–1012 genomic copies/g) (Ajami et al. 2010; Chan et al. 2006; Teunis et al. 2008). A preliminary attempt to quantify GIV NoV in a small number of positive environmental samples was unsuccessful, in that no amplification curves were obtained. This may be attributed to either low viral concentrations or the presence of PCR inhibitors in the water samples tested. The nested PCR used for the qualitative tests, on the other hand, mitigates the effect of inhibitory substances on the reaction. This is because the inhibition of nucleic acid amplification is often partial. The first step can thus produce a small amount of template that may not be visible by gel electrophoresis, but can nonetheless be successfully amplified in a second round of PCR. Moreover, performing the second round of PCR on an aliquot of the product obtained in the first cycle of amplification increases the sensitivity of the standard RT-PCR.
Molecular and phylogenetic analysis showed moderate genetic variability in GIV viruses, with the vast majority of sequences detected in the present study belonging to a lineage which also included environmental and clinical sequences generated in the previous investigations by our group. It seems, therefore, that human GIV NoV, with only one genotype identified so far, exhibits less genetic variability than GI and GII.
In conclusion, our findings revealed unexpectedly high rates of detection of GIV NoV in sewage samples (21.8 %), demonstrating firmly that not only GI and GII but also GIV strains circulate widely in the Italian population. Further studies will be needed to allow an accurate interpretation of the presence of GIV NoV in clinical samples, and to shed light on both the dynamics of circulation in human populations and the significance of environmental contamination in terms of potential health risks.
Our goals for the future are to determine the occurrence and quantity of GIV NoVs in different water environments, following the route of contamination from raw sewage through the treated effluent to the superficial waters receiving wastewater discharges, in order to shed light on the fate of these viruses in water environments and their potential for waterborne transmission.
Acknowledgments
This study was financed by the joint Italian–American Project “Assessing the Impact of GIV Norovirus on Human Health: a Molecular Epidemiological Investigation on Environmental and Clinical Samples as a Basis for the Design of Novel Diagnostic Tools for an Emerging Pathogen,” and partially by the Grant “Calicivirus nei carnivori e nell’uomo: caratterizzazione molecolare, epidemiologia, implicazioni zoonosiche”—PRIN 2008. We thank Professor Herbert W. Virgin, Washington University (St. Louis, Missouri, United States) for providing the murine NoV stain used as sample process control. We gratefully acknowledge for wastewater sample collection: 1–2. E. Lorenzi, L. Meucci, M. Deceglie (SMAT Spa, Torino), E. Garrou, M. Morello, G. Mantovani (ARPA Piemonte, Torino); 3–4. G. Manassero (Arpa Valle d’Aosta), A. Martello (Corpo Forestale Valdostano); 5. W. Bodini, C. Amadasi (Vettabbia Spa, Milano); 6. L. Boscolo (Amiacque Spa, Milano); 7. M. Tomasoni, D. Monteverdi (A2A Spa, Brescia); 8–9. M. Poli, M. Dekas (Eco Center Spa AG, Bolzano), W. Strobl, E. Scarperi (APPA, Bolzano); 10–11. L. Bruni, G. Gatti, L.Tomasi, G. Cimadon (APPA,Trento); 12–13. P. Parati, E. Dell’Andrea, G. Gambillara (Arpa Veneto, Venezia); 14–15. S. Gaiter, L. Sola (ARPA Liguria, Genova); 16. F.Cornia, M.A. Corvaglia, P. Albertelli (ARPA Emilia Romagna, Bologna); 17. A. Gambaccioni, M. Razzolini (Publiacqua S.p.A. Firenze); 18. E. Renna, G. Saltalamacchia, M. Lucarini (ARPA Umbria, Perugia); 19–20. C. Mengarelli, Trimboli (ARPA Marche, Ancona); 21–24. C. Gala, R. Tomassini (Arpalazio, Roma), G. Ranalletta (ACEA ATO2 S.p.A., Roma); 25. E. Rufolo, R. Martino (ARPA Campania, Sezione provinciale di Napoli); 26–27. G. Assennato, G. Blonda, (Regione Puglia, Direzione Scientifica), V. Perrino, M. Mariani (ARPA Puglia, Bari); 28. R. Vita, R. Masotti, R. Martoccia (ARPA Basilicata, Potenza); 29. F. Pedulla, G. Belmusto (ARPA Calabria, Reggio Calabria); 30. L. Librici G. Abbate (ARPA Sicilia, Palermo); 31–32. M. G. Mulas, G. Campus (Regione Sardegna), A.M. Mereu, M. Secci (ARPA Sardegna, Cagliari).
Footnotes
Conflict of interest
There is no conflict of interest for all the authors. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the 1975 Helsinki Declaration, as revised in 2008. Informed consent was obtained from all patients included in the study.
Contributor Information
M. Muscillo, Department of Environment and Primary Prevention, Istituto Superiore di Sanità, Viale Regina Elena 299, 00161 Rome, Italy
M. Fratini, Department of Environment and Primary Prevention, Istituto Superiore di Sanità, Viale Regina Elena 299, 00161 Rome, Italy
R. Graffeo, Policlinico A. Gemelli, Istituto di Microbiologia, Università Cattolica del Sacro Cuore, Rome, Italy
M. Sanguinetti, Policlinico A. Gemelli, Istituto di Microbiologia, Università Cattolica del Sacro Cuore, Rome, Italy
V. Martella, Dipartimento di Sanità Pubblica e Zootecnia, Università degli Studi Aldo Moro, Bari, Italy
K. Y. Green, Laboratory of Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, DHHS, Bethesda, MD, USA
S. Della Libera, Department of Environment and Primary Prevention, Istituto Superiore di Sanità, Viale Regina Elena 299, 00161 Rome, Italy.
Giuseppina La Rosa, Department of Environment and Primary Prevention, Istituto Superiore di Sanità, Viale Regina Elena 299, 00161 Rome, Italy.
References
- Ajami N, Koo H, Darkoh C, Atmar RL, Okhuysen PC, Jiang ZD, et al. Characterization of norovirus-associated traveler’s diarrhea. Clinical Infectious Diseases. 2010;51:123–130. doi: 10.1086/653530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers RM, Dhar AK. Effect of template on generating a standard curve for absolute quantification of an RNA virus by real-time reverse transcriptase-polymerase chain reaction. Molecular and Cellular Probes. 2011;25:60–64. doi: 10.1016/j.mcp.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Chan MC, Sung JJ, Lam RK, Chan PK, Lee NL, Lai RW, et al. Fecal viral load and norovirus-associated gastroenteritis. Emerging Infectious Diseases. 2006;12:1278–1280. doi: 10.3201/eid1208.060081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Agostino M, Cook N, Rodríguez-Lázaro D, Rutjes S. Nucleic acid amplification-based methods for detection of enteric viruses: Definition of controls and interpretation of results. Food and Environmental Virology. 2011;3:55–60. [Google Scholar]
- Di Martino B, Marsilio F, Di Profio F, Lorusso E, Friedrich KG, Buonavoglia C, et al. Detection of antibodies against genogroup GIV norovirus in carnivores. Clinical and Vaccine Immunology. 2009;17(1):180–182. doi: 10.1128/CVI.00312-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez-Valcarce M, Cook N, Hernández M, Rodríguez-Lázaro D. Analytical application of a sample process control in detection of foodborne viruses. Food Analytical Methods. 2011;4:614–618. [Google Scholar]
- Eden JS, Lim KL, White PA. Complete genome of the human norovirus GIV.1 strain lake Macquarie virus. Journal of Virology. 2012;86:10251–10252. doi: 10.1128/JVI.01604-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fankhauser RL, Monroe SS, Noel JS, Humphrey CD, Bresee JS, Parashar UD, et al. Epidemiologic and molecular trends of “Norwalk-like viruses” associated with outbreaks of gastroenteritis in the United States. Journal of Infectious Diseases. 2002;186:1–7. doi: 10.1086/341085. [DOI] [PubMed] [Google Scholar]
- Glass RI, Parashar UD, Estes MK. Norovirus gastroenteritis. New England Journal of Medicine. 2009;361:1776–1785. doi: 10.1056/NEJMra0804575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haramoto E, Katayama H, Oguma K, Yamashita H, Tajima A, Nakajima H, et al. Seasonal profiles of human noroviruses and indicator bacteria in a wastewater treatment plant in Tokyo, Japan. Water Science and Technology. 2006;54:301–308. doi: 10.2166/wst.2006.888. [DOI] [PubMed] [Google Scholar]
- Iritani N, Seto Y, Kubo H, Haruki K, Ayata M, Ogura H. Prevalence of “Norwalk-like virus” infections in outbreaks of acute nonbacterial gastroenteritis observed during the 1999–2000 season in Osaka City, Japan. Journal of Medical Virology. 2002;66:131–138. doi: 10.1002/jmv.2121. [DOI] [PubMed] [Google Scholar]
- Iwai M, Hasegawa S, Obara M, Nakamura K, Horimoto E, Takizawa T, et al. Continuous presence of noroviruses and sapoviruses in raw sewage reflects infections among inhabitants of Toyama, Japan (2006 to 2008) Applied and Environment Microbiology. 2009;75:1264–1270. doi: 10.1128/AEM.01166-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima M, Haramoto E, Phanuwan C, Katayama H, Ohgaki S. Detection of genogroup IV norovirus in wastewater and river water in Japan. Letters in Applied Microbiology. 2009;49:655–658. doi: 10.1111/j.1472-765X.2009.02718.x. [DOI] [PubMed] [Google Scholar]
- Kitajima M, Oka T, Haramoto E, Phanuwan C, Takeda N, Katayama K, et al. Genetic diversity of genogroup IV noroviruses in wastewater in Japan. Letters in Applied Microbiology. 2011;52:181–184. doi: 10.1111/j.1472-765X.2010.02980.x. [DOI] [PubMed] [Google Scholar]
- Kitajima M, Oka T, Haramoto E, Takeda N, Katayama K, Katayama H. Seasonal distribution and genetic diversity of genogroups I, II, and IV noroviruses in the Tamagawa River, Japan. Environmental Science and Technology. 2010;44:7116–7122. doi: 10.1021/es100346a. [DOI] [PubMed] [Google Scholar]
- Kremer JR, Langlet J, Skraber S, Weicherding P, Weber B, Cauchie HM, et al. Genetic diversity of noroviruses from outbreaks, sporadic cases and wastewater in Luxembourg 2008–2009. Clinical Microbiology & Infection. 2011;17:1173–1176. doi: 10.1111/j.1469-0691.2010.03407.x. [DOI] [PubMed] [Google Scholar]
- Kroneman A, Harris J, Vennema H, Duizer E, van Duynhoven Y, Gray J, et al. Data quality of 5 years of central norovirus outbreak reporting in the European Network for food-borne viruses. Journal of Public Health (Oxford) 2008a;30:82–90. doi: 10.1093/pubmed/fdm080. [DOI] [PubMed] [Google Scholar]
- Kroneman A, Verhoef L, Harris J, Vennema H, Duizer E, van Duynhoven Y, et al. Analysis of integrated virological and epidemiological reports of norovirus outbreaks collected within the foodborne viruses in Europe Network from 1 July 2001 to 30 June 2006. Journal of Clinical Microbiology. 2008b;46:2959–2965. doi: 10.1128/JCM.00499-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G, Fratini M, Spuri-Vennarucci V, Guercio A, Purpari G, Muscillo M. GIV Noroviruses and other enteric viruses in bivalves: A preliminary study. New Microbiologica. 2012;35(1):27–34. [PubMed] [Google Scholar]
- La Rosa G, Iaconelli M, Pourshaban M, Fratini M, Muscillo M. Molecular detection and genetic diversity of norovirus genogroup IV: A yearlong monitoring of sewage throughout Italy. Archives of Virology. 2010a;155:589–593. doi: 10.1007/s00705-010-0619-y. [DOI] [PubMed] [Google Scholar]
- La Rosa G, Iaconelli M, Pourshaban M, Muscillo M. Detection and molecular characterization of noroviruses from five sewage treatment plants in central Italy. Water Research. 2010b;44:1777–1784. doi: 10.1016/j.watres.2009.11.055. [DOI] [PubMed] [Google Scholar]
- La Rosa G, Pourshaban M, Iaconelli M, Muscillo M. Quantitative real-time PCR of enteric viruses in influent and effluent samples from wastewater treatment plants in Italy. Annali dell Istituto Superiore di Sanita. 2010c;46:266–273. doi: 10.4415/ANN_10_03_07. [DOI] [PubMed] [Google Scholar]
- La Rosa G, Muscillo M. Molecular detection of viruses in water and sewage. In: Cook N, editor. Viruses in food and water: Risks, surveillance and control. Woodhead Publishing Series in Food Science; 2013. pp. 97–126. Technology and Nutrition No. 249. [Google Scholar]
- La Rosa G, Pourshaban M, Iaconelli M, Muscillo M. Detection of genogroup IV noroviruses in environmental and clinical samples and partial sequencing through rapid amplification of cDNA ends. Archives of Virology. 2008;153:2077–2083. doi: 10.1007/s00705-008-0241-4. [DOI] [PubMed] [Google Scholar]
- Lindell AT, Grillner L, Svensson L, Wirgart BZ. Molecular epidemiology of norovirus infections in Stockholm, Sweden, during the years 2000 to 2003: Association of the GGIIb genetic cluster with infection in children. Journal of Clinical Microbiology. 2005;43:1086–1092. doi: 10.1128/JCM.43.3.1086-1092.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martella V, Campolo M, Lorusso E, Cavicchio P, Camero M, Bellacicco AL, et al. Norovirus in captive lion cub (Panthera leo) Emerging Infectious Diseases. 2007;13:1071–1073. doi: 10.3201/eid1307.070268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martella V, Lorusso E, Decaro N, Elia G, Radogna A, D’Abramo M, et al. Detection and molecular characterization of a canine norovirus. Emerging Infectious Diseases. 2008;14:1306–1308. doi: 10.3201/eid1408.080062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews JE, Dickey BW, Miller RD, Felzer JR, Dawson BP, Lee AS, et al. The epidemiology of published norovirus outbreaks: A review of risk factors associated with attack rate and genogroup. Epidemiology and Infection. 2012;140:1161–1172. doi: 10.1017/S0950268812000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesquita JR, Barclay L, Nascimento MS, Vinje J. Novel norovirus in dogs with diarrhea. Emerging Infectious Diseases. 2010;16:980–982. doi: 10.3201/eid1606.091861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordgren J, Matussek A, Mattsson A, Svensson A, Lindgren P. Prevalence of norovirus and factors influencing virus concentrations during one-year in a fullscale wastewater treatment plant. Water Research. 2008;43(4):1117–1125. doi: 10.1016/j.watres.2008.11.053. [DOI] [PubMed] [Google Scholar]
- Patel MM, Hall AJ, Vinje J, Parashar UD. Noroviruses: A comprehensive review. Journal of Clinical Virology. 2009;44:1–8. doi: 10.1016/j.jcv.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Pinto P, Wang Q, Chen N, Dubovi EJ, Daniels JB, Millward LM, et al. Discovery and genomic characterization of noroviruses from a gastroenteritis outbreak in domestic cats in the US. PLoS One. 2012;7:e32739. doi: 10.1371/journal.pone.0032739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Lázaro D, Cook N, Ruggeri FM, Sellwood J, Nasser A, Nascimento MS, et al. Virus hazards from food and the environment. FEMS Microbiology Reviews. 2012;36:786–814. doi: 10.1111/j.1574-6976.2011.00306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair RG, Choi CY, Riley MR, Gerba CP. Pathogen surveillance through monitoring of sewer systems. Advances in Applied Microbiology. 2008;65:249–269. doi: 10.1016/S0065-2164(08)00609-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skraber S, Langlet J, Kremer JR, Mossong J, De LS, Even J, et al. Concentration and diversity of noroviruses detected in Luxembourg wastewaters in 2008–2009. Applied and Environment Microbiology. 2011;77:5566–5568. doi: 10.1128/AEM.00632-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teunis PF, Moe CL, Liu P, Miller SE, Lindesmith L, Baric RS, et al. Norwalk virus: How infectious is it? Journal of Medical Virology. 2008;80:1468–1476. doi: 10.1002/jmv.21237. [DOI] [PubMed] [Google Scholar]
- Trujillo AA, McCaustland KA, Zheng DP, Hadley LA, Vaughn G, Adams SM, et al. Use of TaqMan real-time reverse transcription-PCR for rapid detection, quantification, and typing of Norovirus. Journal of Clinical Microbiology. 2006;44:1405–1412. doi: 10.1128/JCM.44.4.1405-1412.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinje J, Koopmans MP. Simultaneous detection and genotyping of “Norwalk-like viruses” by oligonucleotide array in a reverse line blot hybridization format. Journal of Clinical Microbiology. 2000;38:2595–2601. doi: 10.1128/jcm.38.7.2595-2601.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng DP, Ando T, Fankhauser RL, Beard RS, Glass RI, Monroe SS. Norovirus classification and proposed strain nomenclature. Virology. 2006;346:312–323. doi: 10.1016/j.virol.2005.11.015. [DOI] [PubMed] [Google Scholar]