Abstract
Background
Frailty is associated with increased morbidity and mortality in older persons. We sought to characterize the associations between the frailty syndrome and long-term risk of sepsis in a large cohort of community-dwelling adults.
Methods
We analyzed data on 30 239 community-dwelling adult participants in the REasons for Geographic and Racial Differences in Stroke (REGARDS) cohort. We defined frailty as the presence of at least 2 frailty indicators (weakness, exhaustion, and low physical activity). We defined sepsis as hospitalization for a serious infection with ≥2 system inflammatory response syndrome criteria, identified for the period 2003–2012. We determined the associations between frailty and risk of first sepsis and sepsis 30-day case fatality.
Results
Among REGARDS participants, frailty was present in 6018 (19.9%). Over the 10-year observation period, there were 1529 first-sepsis hospitalizations. Frailty was associated with increased risk of sepsis (adjusted hazard ratio [HR] 1.44; 95% CI: 1.26 to 1.64). The total number of frailty indicators was associated with increased risk of sepsis (P trend <.001). Among first-sepsis hospitalizations, frailty was associated with increased sepsis 30-day case fatality (adjusted OR 1.62; 95% CI: 1.06 to 2.50).
Conclusions
In the REGARDS cohort, frailty was associated with increased long-term risk of sepsis and sepsis 30-day case fatality.
Keywords: sepsis, frailty, infection, epidemiology
Introduction
Sepsis is the clinical syndrome of life-threatening organ dysfunction caused by dysregulated host response to infection.1 Sepsis may lead to systemic vasodilation, organ injury, shock, and death. Sepsis is a major public health problem associated with over 750 000 hospitalizations, 570 000 Emergency Department visits, and 200 000 deaths annually in the United States (US).2,3 Current sepsis scientific and clinical initiatives focus on the detection and treatment of acute sepsis. Relatively little attention has been focused on the identification the precursors for developing sepsis. With an understanding of the risk factors for acute sepsis, it may be possible to identify the person most vulnerable to the condition, offering opportunities to reduce the risk or impact of sepsis episodes.
Frailty is a state of increased vulnerability to stressors resulting from age-related decline in reserve and function across multiple physiologic systems.4 Frailty is a widespread health problem, with prevalence estimates of 13% to 50% among community-dwelling adults.5–11 Frailty has been associated with a host of health risks including increased hip fracture, disability, hospitalization, and death.12 There are several plausible associations between frailty and sepsis risk. Frailty and sepsis are both associated with advanced age and chronic medical conditions.13 Decline in physical activity is also associated with both frailty and sepsis.14 Biomarkers of cellular aging and inflammation such as high-sensitive C-reactive protein, interleukin-6 (IL-6), intercellular adhesion molecule-1 (ICAM-1), and E-selectin have been associated with both frailty and sepsis.15,16 Frailty is also associated with suppression of immune function.17
The REasons for Geographic and Racial Differences in Stroke (REGARDS) study is one of the nation’s largest population-based cohorts of community-dwelling adults. We sought to determine the associations between frailty and long-term risk of community-acquired sepsis. We also sought to examine the association between frailty and sepsis case fatality.
Methods
Study Design
We analyzed data from the population-based REGARDS cohort. The Institutional Review Board of the University of Alabama at Birmingham approved the study.
Data Source
REGARDS is one of the largest ongoing national longitudinal cohorts of community-dwelling adults in the United States.18 Designed to evaluate the origins for racial and geographic differences in stroke mortality, REGARDS includes 30 239 participants aged ≥45 years. The cohort is 45% male, 41% black race, and 69% >60 years old. REGARDS recruited participants between January 2003 and October 2007, collecting detailed information about participant demographics, health behaviors, chronic medical conditions, physical status diet, and medications.18 The study also collected blood and urine samples. In 6-month intervals, REGARDS contacted the participants by telephone to identify any hospitalizations experienced by the participant. REGARDS also reviewed death certificates, medical records, and interviewed proxies to ascertain causes of the death.
Identification of Frailty
Numerous criteria have been used to define frailty.4,19–21 Garcia-Garcia et al defined a frailty trait scale incorporating seven dimensions (energy balance nutrition, physical activity, nervous system, vascular system, strength, endurance, and gait speed).19 The Cardiovascular Health Study defined frailty using five components (shrinking, weakness, exhaustion, low activity, and slowness).4 Johansen et al defined frailty as the presence of two of three factors: (1) weakness, (2) exhaustion, and (3) low physical activity.20
Using these prior studies as a conceptual framework, we focused on a definition of frailty syndrome that could be identified by data collected on REGARDS participants. Based upon participant responses to the 12-Item Short Form Health Survey (SF-12), we defined weakness as a Physical Composite Score of <75.12,21 We defined exhaustion as responses of “a little of the time” or “none of the time” to the question “How much of the time during the past 4 weeks did you have energy?” We defined low physical activity as responses of “almost never” or “never exercising enough to work up a sweat” to the question “How many times per week do you engage in intense physical activity, enough to build up a sweat?”12,21 Following Johansen et al, we defined frailty as the presence of two of these three indicators.20
Identification of Sepsis Events
The primary outcomes were (1) first hospitalization for a sepsis event and (2) fatality within 30 days after a first sepsis event. Using international consensus definitions, we defined sepsis events as hospital admission for a serious infection with the presence of at least two systemic inflammatory response syndrome (SIRS) criteria, including heart rate >90 beats/min, fever (temperature >38.3°C or <36°C), tachypnea (>20 breaths/min) or PCO2 <32 mmHg, and leukocytosis (white blood cells >12 000 or <4000 cells/mm3 or >10% band forms).22 We used vital signs and laboratory test results for the initial 28 h of hospitalization. Because of our focus on “community-acquired” (vs “hospital-acquired”) sepsis, we did not include sepsis developing at later points during hospitalization. We did not include organ dysfunction in the definition of sepsis. Among entire REGARDS cohort (n = 30,238), initial review of 1349 hospital records indicated excellent interrater agreement for presence of serious infection (κ = .92) and the presence of sepsis (κ = .90) upon hospital presentation.
We defined fatal sepsis as either death during a hospitalization for sepsis or death within 30 days of discharge for a hospitalization for sepsis. We included hospitalization events reported from January 1, 2003, through December 31, 2012.
Participant Characteristics
Participant demographics included age, race, sex, income, education, and geographic location. Health behaviors included tobacco and alcohol use. We defined alcohol use as moderate (1 drink per day for women or 2 drinks per day for men) and heavy alcohol use (>1 drink per day for women and >2 drinks per day for men), per the National Institute on Alcohol Abuse and Alcoholism classification.23 Participant’s chronic medical conditions included atrial fibrillation, chronic lung disease, chronic kidney disease, coronary artery disease, deep vein thrombosis, diabetes, dyslipidemia, hypertension, obesity, peripheral artery disease, and stroke. Deep vein thrombosis, peripheral artery disease, and stroke history were based upon self-reports. (Detailed definitions are provided in Appendix A.)
We included the biomarkers serum high-sensitivity C-reactive protein (hsCRP) and urinary albumin-to-creatinine ratio in this study.12 We dichotomized both biomarkers as abnormal or normal within statistical models. Consistent with prior REGARDS studies, we defined hsCRP >3.0 mg/dL as abnormal.15,24 We defined albumin-to-creatinine ratio (ACR) ≥30 mg/g as abnormal.
Hospital course variables included infection type, Sequential Organ Failure Assessment (SOFA) for respiratory, renal hepatic, cardiovascular, hematologic, and neurologic systems, Mortality in Emergency Department Sepsis (MEDS) score, intensive care unit (ICU) admission, and hospital mortality.
Data Analysis
We compared baseline demographics, health behaviors, chronic medical conditions, biomarkers, infection types, and hospital characteristics between frail and non-frail individuals using a chi-square test for categorical characteristics, analysis of variance (ANOVA) for continuous variables, and Kruskal-Wallis test for nonparametric continuous variables (eg, SOFA score).
To estimate the relative risk of first-sepsis events by frailty status, we fit a series of Cox proportional hazards models with time to first sepsis as the primary endpoint. We censored individuals at the time of their event, death, or end of follow-up (December 31, 2012). We sequentially adjusted the estimates for participant sociodemographics, health behaviors, chronic medical conditions, and biomarkers. We also examined models for each individual frailty indicator as well the number of frailty components. We examined [frailty × age], [frailty × sex], and [frailty × race] multiplicative interactions.
To compare differences in case fatality between frail and non-frail individuals, we used multivariable logistic regression. Because of the limited number of fatal sepsis events (n = 172), we limited risk adjustment to variables with a P < .2 on univariate analysis. The final multivariable model included adjustment for age, sex, obesity, dyslipidemia, coronary artery disease, chronic kidney disease, hsCRP >30 mg/dL, ACR >30 mg/g, and total SOFA score.
We performed all statistical analyses with Stata 14.0 (Stata, Inc., College Station, Texas).
Results
Among 30 239 REGARDS participants, 8914 (29.4%) reported weakness, 4205 (13.9%) reported exhaustion, and 10 240 (33.9%) reported low physical activity. Defined as the presence of at least 2 of these indicators (weakness, exhaustion, low physical activity), frailty was present in 6018 (19.9%) of REGARDS participants. Among the 29 192 with complete information, the number of frailty indicators per individual was as follows: 0 indicator—14 064 (48.2%), 1 indicator—9110 (31.2%), 2 indicators—4405 (15.1%), 3 indicators—1613 (5.5%).
Frailty was more common among female and black participants as well as those with lower education and income (Table 1). Frailty was more common among tobacco users but less common among alcohol users. Chronic medical conditions and elevated hsCRP and ACR were more common among frail participants.
Table 1.
Baseline Characteristics of REGARDS Participants, Stratified by Frailty Status.a
| Variable | Frail (n = 6018) | Non-Frail (n = 23 174) | P Valueb |
|---|---|---|---|
| Age, mean (SD) | 66.2 (10.0) | 64.3 (9.2) | <.001 |
| Sex (%) | <.001 | ||
| Male | 2024 (33.6) | 11 072 (47.8) | |
| Female | 3994 (66.4) | 12 102 (52.2) | |
| Race (%) | <.001 | ||
| White | 3205 (53.3) | 13 917 (60.1) | |
| Black | 2813 (46.7) | 9257 (40.0) | |
| Education (%) | <.001 | ||
| ≤High school | 1182 (19.7) | 2374 (10.2) | |
| High school graduate | 1857 (30.9) | 5676 (24.5) | |
| Some college | 1633 (27.1) | 6212 (26.8) | |
| College graduate or higher | 1335 (22.2) | 8902 (38.4) | |
| Missing N (%) | 10 (0.2) | 10 (0.04) | |
| Income (%) | <.001 | ||
| ≤$20 000 | 1878 (31.2) | 3368 (14.5) | |
| $20 000 to $34 000 | 1607 (26.7) | 5442 (23.5) | |
| $35 000 to $74 000 | 1324 (22.0) | 7409 (32.0) | |
| $75 000 and above | 406 (6.8) | 4282 (18.5) | |
| Unknown | 803 (13.3) | 2673 (11.5) | |
| Geographic region (%) | <.001 | ||
| Non-stroke belt or buckle | 2498 (41.5) | 10 528 (45.4) | |
| Stroke belt | 2209 (36.7) | 7902 (34.1) | |
| Stroke buckle | 1312 (21.8) | 4744 (20.5) | |
| Missing N (%) | |||
| Tobacco use (%) | <.001 | ||
| Never | 2404 (40.0) | 10 790 (46.6) | |
| Past | 2414 (40.1) | 9238 (40.0) | |
| Current | 1175 (19.5) | 3065 (13.2) | |
| Missing N (%) | 25 (0.4) | 81 (0.4) | |
| Alcohol use (%) | <.001 | ||
| None | 4370 (72.6) | 13 497 (58.2) | |
| Moderate | 1366 (22.7) | 8268 (35.7) | |
| Heavy | 159 (2.6) | 990 (4.3) | |
| Missing N (%) | 123 (2.0) | 419 (1.8) | |
| Chronic medical conditions (%) | |||
| Atrial fibrillation | 836 (13.9) | 1635 (7.1) | <.001 |
| Chronic lung disease | 924 (15.4) | 1751 (7.6) | <.001 |
| Chronic kidney disease | 1086 (18.1) | 2040 (8.8) | <.001 |
| Coronary artery disease | 1590 (26.4) | 3503 (15.1) | <.001 |
| Deep vein thrombosis | 493 (8.2) | 1019 (4.4) | <.001 |
| Diabetes | 2080 (34.6) | 4436 (19.3) | <.001 |
| Dyslipidemia | 3749 (62.3) | 12 906 (55.7) | <.001 |
| Hypertension | 4356 (72.4) | 12 856 (55.5) | <.001 |
| Obesity | 4129 (68.6) | 11 474 (49.5) | <.001 |
| Peripheral artery disease | 250 (4.2) | 386 (1.7) | <.001 |
| Stroke | 788 (13.1) | 1054 (4.6) | <.001 |
| Biomarkers | |||
| hsCRP >3.0 mg/dL | 3032 (50.4) | 8051 (34.7) | <.001 |
| ACR >30 mg/g | 1236 (20.5) | 2937 (12.7) | <.001 |
Abbreviations: hsCRP, high-sensitivity C-reactive protein
Column totals and percentages do not always equal to 100% due to missing data.
Significance by chi-square test for categorical variables and t test for continuous variables.
Over the 10-year observation period, there were 1529 first-sepsis hospitalizations. Among frail individuals, first-sepsis hospitalizations were more likely to be due to lung and kidney infections (Table 2). Compared with non-frail individuals, frail sepsis participants exhibited higher rates of intensive care unit admission and 30-day case fatality. Sequential Organ Failure Assessment and MEDS scores and hospital fatality were similar between frail and non-frail sepsis participants.
Table 2.
Infection Types, Hospital Characteristics, and Outcomes Among 1479 First-Sepsis Hospitalizations, Stratified by Frailty Status.
| Variable | Frail (N = 509) | Non-Frail (N = 970) | P Valuea |
|---|---|---|---|
| Infection type—n (%) | |||
| Lung | 268 (52.7) | 449 (46.3) | .001 |
| Kidney | 97 (19.1) | 153 (15.8) | |
| Abdominal | 54 (10.6) | 169 (17.4) | |
| Skin | 25 (6.9) | 82 (8.5) | |
| Sepsis | 38 (7.5) | 62 (6.4) | |
| Other | 17 (3.3) | 55 (5.7) | |
| Hospital characteristics | |||
| SOFA Score—median (IQR) | 2 (1–3) | 1 (0–3) | <.001 |
| MEDS Score | 11 (8–14) | 9 (6–13) | .008 |
| ICU Admission—n (%) | 358 (76.3) | 688 (81.4) | .03 |
| Outcomes | |||
| Hospital death | 53 (10.4) | 80 (8.3) | .17 |
| 30-Day case fatality | 74 (14.5) | 98 (10.1) | .01 |
Abbreviations: ICU, intensive care unit; MEDS, Mortality in Emergency Department Sepsis Score; SOFA, Sequential Organ Failure Assessment Score.
Significance determined using chi-square Wilcoxon Rank-Sum or chi-square test.
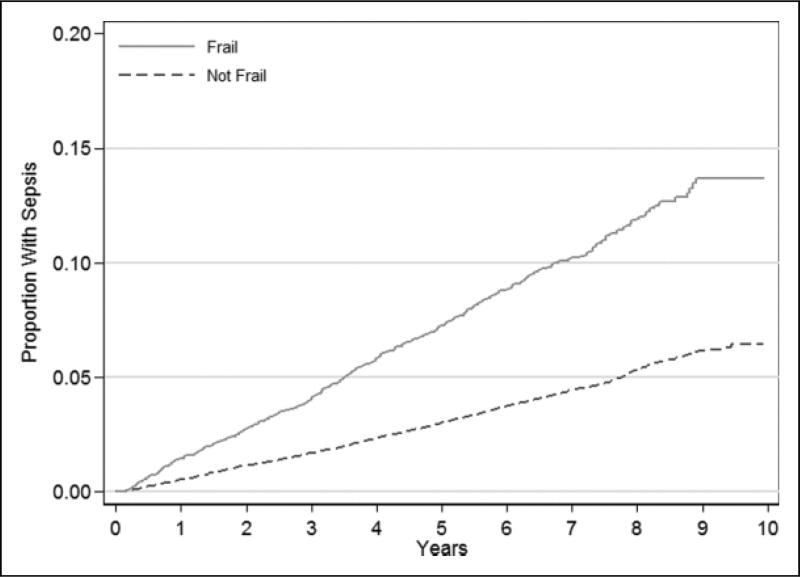
First-sepsis incidence was higher for frail participants (Table 3 and Figure 1). After adjustment for confounders, first-sepsis events were independently associated with frailty (adjusted HR 1.44; 95% CI: 1.26 to 1.64). The frailty indicators, weakness and low physical activity, were each independently associated with risk of sepsis (Table 4). While there was a relationship between exhaustion and sepsis, this association did not reach statistical significance. The total number of frailty indicators was associated with increased risk of sepsis (test of trend P < .001) (Table 4).
Table 3.
| Frailty Status | No. Sepsis Events |
Incidence per 1000 Person-Years (95% CI) |
Crude HR (95% CI) |
Model 1 HR (95% CI) |
Model 2 HR (95% CI) |
Model 3 HR (95% CI) |
Model 4 HR (95% CI) |
|---|---|---|---|---|---|---|---|
| Frail | 509 (8.5) | 15.5 (14.2–16.9) | 2.39 (2.14–2.66) | 2.16 (1.93–2.41) | 2.05 (1.83–2.30) | 1.52 (1.34–1.72) | 1.44 (1.26–1.64) |
| Non-Frail | 970 (4.2) | 6.6 (6.2–7.0) | Reference | Reference | Reference | Reference | Reference |
Estimated from Cox Proportional Hazard models.
Crude: unadjusted association; Model 1: adjusted for sex, race, age decile, geographic region, education level, and income. Model 2: additionally adjusted for tobacco and alcohol use. Model 3: additionally adjusted for baseline chronic medical conditions. Model 4: additionally adjusted for biomarkers (hsCRP and ACR).
Figure 1.
Kaplan-Meier graph for first sepsis events, stratified by frailty status.
Table 4.
Associations Between Frailty Indicators and First Sepsis Events.a
| Frailty Indicators | No. Events (%)b | Incidence Per 1000 Person-Years (95% CI) | Crude HR (95% CI) | Adjusted HR (95% CI) |
|---|---|---|---|---|
| Weakness | ||||
| Weak | 684 (7.7) | 13.6 (12.6–14.7) | 1.92 (1.72–2.15) | 1.37 (1.20–1.56) |
| Not weak | 820 (3.9) | 6.2 (5.8–6.6) | Reference | Reference |
| Exhaustion | ||||
| Exhausted | 333 (7.9) | 14.3 (12.9–16.0) | 1.41 (1.23–1.61) | 1.15 (0.99–1.35) |
| Not exhausted | 1193 (4.6) | 7.4 (7.0–7.8) | Reference | Reference |
| Physical activity | ||||
| Low | 659 (6.4) | 11.0 (10.2–11.9) | 1.36 (1.23–1.52) | 1.15 (1.01–1.29) |
| Normal | 845 (4.3) | 6.9 (6.4–7.3) | Reference | Reference |
| Number of frailty indicators | ||||
| 0 | 512 (3.6) | 5.6 (5.2–6.1) | Reference | Referenceb |
| 1 | 458 (5.0) | 8.1 (7.4–8.9) | 1.46 (1.29–1.65) | 1.23 (1.07–1.42)b |
| 2 | 341 (7.7) | 13.9 (12.5–15.4) | 2.51 (2.20–2.88) | 1.54 (1.30–1.81)b |
| 3 | 168 (10.4) | 20.2 (17.4–23.5) | 3.68 (3.09–4.38) | 1.78 (1.43–2.22)b |
Estimated from Cox Proportional Hazards Model. Multivariable model adjusted for sex, age, and geographic region, education level, tobacco, alcohol use, baseline chronic medical conditions, and biomarkers.
Test of trend P < .001.
While the [frailty × age] and [frailty × sex] multiplicative interactions were not statistically significant (P = .32 and .56, respectively), the [frailty × race] multiplicative interaction was statistically significant (P = .02). We therefore examined separate models stratified by race. Frailty was significantly associated with risk of sepsis among whites (adjusted HR 1.58; 95% CI: 1.35 to 1.86). However, frailty was not associated with risk of sepsis among blacks (adjusted HR 1.17; 95% CI: 0.92 to 1.48).
Among first-sepsis events, the frailty was associated with increased sepsis in 30-day case fatality (Table 5). The number of frailty indicators was independently associated with increased sepsis 30-day case fatality (P trend = .03).
Table 5.
Associations Between Frailty and Sepsis 30-day Case Fatality.a
| Frailty Status | No. Death Events (%) | Crude OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|---|
| Frail | 74 (14.5) | 1.51 (1.10–2.09) | 1.62 (1.06–2.50) |
| Non-frail | 98 (10.1) | Reference | Reference |
| Number of frailty indicators | |||
| 0 | Reference | Reference | |
| 1 | 1.18 (0.78–1.80) | 1.05 (0.61–1.80)b | |
| 2 | 1.62 (1.06–2.48) | 1.53 (0.88–2.66)b | |
| 3 | 1.69 (1.01–2.83) | 2.03 (1.02–4.06)b | |
Estimated from logistic regression models. Based Upon n = 1529 first-sepsis events. Multivariable models adjusted for age, sex, obesity, dyslipidemia, coronary artery disease, chronic kidney disease, hsCRP >30 mg/dL, ACR >30 mg/g, and Total SOFA Score.
Test of P trend = .03.
Discussion
In this study of community-dwelling adults in the REGARDS cohort, we found the frailty was independently associated with sepsis risk. Among first-sepsis hospitalizations, the presence of all three frailty indicators was associated with increased odds of sepsis 30-day case fatality. These results suggest that frailty may represent a novel risk factor for sepsis independent of participant sociodemographics, health behaviors, or comorbid burden.
Frailty has been highlighted as risk factor for numerous medical conditions such as cardiovascular disease, congestive heart failure, chronic kidney disease, and even hip fractures.12,25–29 There are plausible connections between frailty and sepsis. For example, pathophysiologic abnormalities such as chronic inflammation, dysfunctional epithelium, and impaired immune response are common in both frailty and sepsis.15–17,30,31 Exhaustion may be due to neuroendocrine dysfunction as a result of cell senescence or physical inactivity.32 Regular physical activity has been shown to modulate inflammatory response; we have previously shown that decreased physical activity is associated with increased sepsis risk.14 We note that the observed independent associations between frailty and first-sepsis events persisted even after adjustment for range of confounders, suggesting that frailty is not merely an indicator of comorbid burden. Further studies are needed to confirm these hypotheses.
Several important observations of this analysis are worthy of comment. We observed that the risk of sepsis was related to the number of frailty indicators. These observations suggest that the frailty indicators may influence sepsis risk in an additive fashion. Also, while weakness, exhaustion, and low physical activity were more common among blacks than white, the number of frailty indicators was independently associated with increased sepsis risk in whites but not in blacks. In prior studies, we found that black REGARDS participants were less likely than whites to experience infection and sepsis events, which may have been due to a slightly higher comorbidity burden among whites than black participants in REGARDS.23,33–35 We also observed that only individuals with all three frailty indicators were at increased odds of 28-day death after a sepsis hospitalization. These intriguing findings merit further examination to better elucidate the biologic pathways linking frailty to sepsis susceptibility and survival.24
These findings have 2 important implications for sepsis care. First, among patients hospitalized for sepsis, the presence of frailty may be viewed as a risk factor for death. Since frail individuals may be less able to tolerate the systemic inflammation and organ dysfunction of sepsis, these individuals may merit more proactive and aggressive sepsis care. Second, frailty may potentially play a role in community sepsis prevention. Current clinical and scientific initiatives focus on the early identification and treatment of acute sepsis; clinicians do not customarily conceptualize sepsis as a preventable condition.36 However, we have previously identified that individual health characteristics such as sociodemographics, health behaviors, chronic medical conditions, and biomarkers are able to predict 10-year risk of sepsis hospitalization in community-dwelling adults.37 Thus, one might consider frailty as an additional indicator of long-term sepsis risk. Aggressive preventive interventions such as vaccination and early antibiotic initiation for infections may be warranted for frail individuals. Of note, frailty was present in 20% of the REGARDS participants, underscoring that frailty is common among community-dwelling adults.38
This analysis contains certain limitations. We limited the analysis to 3 indicators of frailty; we did not assess other frailty measures. REGARDS did not assess other measures of frailty, such as muscle mass. REGARDS is not a surveillance study, and thus complete ascertainment of all sepsis events is unlikely. By design, the REGARDS cohort includes only African Americans and whites, and thus these results may not generalize to other ethnic groups. Although we adjusted for confounders, the associations between frailty and sepsis risk and case fatality may have been influenced by other variables such as access to health care. Both recall and information biases are limitations of this study, as the 3 studied indicators of frailty were subjective measures reported by participants. Future studies using objective measures could attenuate frailty misclassification.
In conclusion, in the REGARDS cohort, frailty was associated with increased long-term risk of sepsis and sepsis 30-day case fatality. Frailty may identify individuals at heightened risk of sepsis and sepsis death.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org and http://www.regardssepsis.org.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by award R01-NR012726 from the National Institute for Nursing Research, UL1-RR025777 from the National Center for Research Resources, as well as by grants from the Center for Clinical and Translational Science and the Lister Hill Center for Health Policy of the University of Alabama at Birmingham. The parent REGARDS study was supported by cooperative agreement U01-NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies. Representatives of the funding agencies have been involved in the review of the manuscript but not directly involved in the collection, management, analysis, or interpretation of the data. Mr. Donnelly was supported by grant T32-HS013852 from the Agency for Healthcare Research and Quality, Rockville, MD, USA. Mr. Moore received grant support from R25-CA47888 from the National Cancer Institute.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Dr. Safford reports the following potential conflicts of interest: Amgen—salary support to study patterns of statin use in Medicare and other large databases; diaDexus—salary support for a research grant on lipids and CAD outcomes; diaDexus—consulting to help with FDA application; NIH, AHRQ—salary support for research grants.
Appendix A
Detailed Definitions for Sociodemographics, Health Behaviors, Chronic Medical Conditions, and Biomarkers
| Variable | Definition |
|---|---|
| Sociodemographics | |
| Age | Age in years, dichotomized to ≥75 years vs <75 years |
| Gender | Male, female |
| Race | African American, white |
| Education | Participant reported: |
| Less than high school | |
| High school graduate | |
| Some college | |
| College or higher | |
| Missing | |
| Income | Participant reported: |
| <$20k | |
| $20k to $34k | |
| $35k to $74k | |
| ≥$75k | |
| Missing (not reported) | |
| Geographic region | Participant residence: |
| Stroke buckle (coastal plains of North Carolina, South Carolina, and Georgia) | |
| Stroke belt (remainder of North Carolina, South Carolina and Georgia, plus Tennessee, Mississippi, Alabama, Louisiana, and Arkansas) | |
| Non-belt/buckle (other states) | |
| Health behaviors | |
| Smoking status | Participant reported: |
| Current | |
| Past | |
| Never | |
| Alcohol use | Participant reported: |
| None | |
| Moderate (up to 1 drink per day for women or 2 drinks per day for men) | |
| Heavy (>1 drink per day for women and >2 drinks per day for men)39 | |
| Chronic medical conditions | |
| Atrial fibrillation | Participant reported history of atrial fibrillation |
| Chronic lung disease | Participant use of pulmonary medications (β agonists, leukotriene inhibitors, inhaled corticosteroids, combination inhalers, ipratropium, cromolyn, aminophylline, and theophylline) as a surrogate for chronic lung disease |
| Coronary artery disease | Participant reported history of myocardial infarction, coronary artery bypass grafting, or cardiac angioplasty or stenting, or baseline electrocardiographic evidence of myocardial infarction |
| Diabetes | Fasting glucose ≥ 126 mg/L (or a glucose ≥200 mg/L for those not fasting) or participant-reported use of insulin or oral hypoglycemic agents |
| Deep vein thrombosis | Participant reported history of deep vein thrombosis |
| Dyslipidemia | Low-density lipoprotein cholesterol >130 mg/dL or participant reported usage of lipid-lowering medications |
| Hypertension | Systolic blood pressure ≥ 140 mm Hg, diastolic blood pressure ≥90 mm Hg, or participant reported antihypertensive agent usage |
| Obesity | {Waist circumference [>102 cm for males or >88 cm for females]} or {body mass index ≥30 kg/m}. |
| Peripheral artery disease | Participant reported history of lower extremity arterial bypass or leg amputation |
| Stroke | Participant reported history of stroke or transient ischemic attack |
| Biomarkers | |
| Estimated glomerular filtration rate (eGFR) | Abnormal defined as eGFR <60 mL/min/1.73m2 |
| Assay by colorimetric reflectance spectrophotometry (Ortho Vitros Clinical Chemistry System 950IRC, Johnson & Johnson Clinical Diagnostics, Raritan, New Jersey). eGFR based upon CKD-Epi equation40 | |
| Albumin-to-creatinine ratio (ACR) | Abnormal defined as ACR ≥30 mcg/mg |
| Albumin assay by nephelometry (BN ProSpec Nephelometer, Dade Behring, Siemens Healthcare, Deerfield, Illinois). Urinary creatinine assay determined by rate blanked Jaffe´ procedure (Modular-P analyzer, Roche/ Hitachi, Roche Diagnostics, Indianapolis, Indiana). | |
| High-sensitivity C-reactive protein (hsCRP) | Abnormal defined as hsCRP >3.0 mg/dL |
| Assay by particle-enhanced immunonephelometry (N High-sensitivity CRP, Siemens AG, Munich, Germany). | |
Footnotes
Author Contributions
MM, JXM, JPD, MMS, and HEW conceived the study. HEW and MMS oversaw data collection. MM, JXM, JPD, and HEW conducted the analysis. MM and JXM drafted the manuscript, and all authors contributed to its critical review. HEW assumes overall responsibility for the manuscript.
Declaration of Conflicting Interests
Ms. Mahalingam, Mr. Moore, Mr. Donnelly, and Dr. Wang do not report any related conflict of interest.
References
- 1.Singer M, Deutschman CS, Seymour CW, et al. The Third International consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Wang HE1, Shapiro NI, Angus DC, et al. National estimates of severe sepsis in United States emergency departments. Crit Care Med. 2007 Aug;35(8):1928–1936. doi: 10.1097/01.CCM.0000277043.85378.C1. [DOI] [PubMed] [Google Scholar]
- 4.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 5.Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60(8):1487–1492. doi: 10.1111/j.1532-5415.2012.04054.x. [DOI] [PubMed] [Google Scholar]
- 6.Biritwum RB, Minicuci N, Yawson AE, et al. Prevalence of and factors associated with frailty and disability in older adults from China, Ghana, India, Mexico, Russia and South Africa. Maturitas. 2016;91:8–18. doi: 10.1016/j.maturitas.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 7.Gale CR, Cooper C, Sayer AA. Prevalence of frailty and disability: findings from the English longitudinal study of ageing. Age Ageing. 2015;44(1):162–165. doi: 10.1093/ageing/afu148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Juarez-Cedillo T, Basurto-Acevedo L, Vega-Garcia S, et al. Prevalence of anemia and its impact on the state of frailty in elderly people living in the community: SADEM study. Annals Hematol. 2014;93(12):2057–2062. doi: 10.1007/s00277-014-2155-4. [DOI] [PubMed] [Google Scholar]
- 9.Zheng Z, Guan S, Ding H, et al. Prevalence and incidence of frailty in community-dwelling older people: Beijing longitudinal study of aging II. J Am Geriatr Soc. 2016;64(6):1281–1286. doi: 10.1111/jgs.14135. [DOI] [PubMed] [Google Scholar]
- 10.Noguchi N, Blyth FM, Waite LM, et al. Prevalence of the geriatric syndromes and frailty in older men living in the community: the concord health and ageing in men project. Australas J Ageing. 2016;35(4):255–261. doi: 10.1111/ajag.12310. [DOI] [PubMed] [Google Scholar]
- 11.Choi J, Ahn A, Kim S, Won CW. Global prevalence of physical frailty by fried’s criteria in community-dwelling elderly with national population-based surveys. J Am Med Dir Assoc. 2015;16(7):548–550. doi: 10.1016/j.jamda.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Woods NF, LaCroix AZ, Gray SL, et al. Frailty: emergence and consequences in women aged 65 and older in the women’s health initiative observational study. J Am Geriatr Soc. 2005;53(8):1321–1330. doi: 10.1111/j.1532-5415.2005.53405.x. [DOI] [PubMed] [Google Scholar]
- 13.Wang HE, Shapiro NI, Griffin R, Safford MM, Judd S, Howard G. Chronic medical conditions and risk of sepsis. PLoS One. 2012;7(10):e48307. doi: 10.1371/journal.pone.0048307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang HE, Baddley J, Griffin RL, et al. Physical inactivity and long-term rates of community-acquired sepsis. Prev Med. 2014;65:58–64. doi: 10.1016/j.ypmed.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang H, Shapiro N, Safford MM, et al. High-sensitivity C-reactive protein and risk of sepsis. PLoS One. 2013;8(7):e69232. doi: 10.1371/journal.pone.0069232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang HE, Shapiro NI, Griffin R, Safford MM, Judd S, Howard G. Inflammatory and endothelial activation biomarkers and risk of sepsis: a nested case-control study. J Crit Care. 2012;28(5):549–555. doi: 10.1016/j.jcrc.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yao X, Li H, Leng SX. Inflammation and immune system alterations in frailty. Clin Geriatr Med. 2011;27(1):79–87. doi: 10.1016/j.cger.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howard VJ, Cushman M, Pulley L, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25(3):135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Garcia FJ, Carcaillon L, Fernandez-Tresguerres J, et al. A new operational definition of frailty: the Frailty Trait Scale. J Am Med Dir Assoc. 2014;15(5):371. doi: 10.1016/j.jamda.2014.01.004. e7-371 e13. [DOI] [PubMed] [Google Scholar]
- 20.Johansen KL, Dalrymple LS, Delgado C, et al. Comparison of self-report-based and physical performance-based frailty definitions among patients receiving maintenance hemodialysis. Am J Kidney Dis. 2014;64(4):600–607. doi: 10.1053/j.ajkd.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnsen KL, Chertow GM, Jin C, Kutner NG. Significance of frailty among dialysis patients. J Am Soc Nephrol. 2007;18(11):2960–2967. doi: 10.1681/ASN.2007020221. [DOI] [PubMed] [Google Scholar]
- 22.Mayr FB, Yende S, Angus DC. Epidemiology of severe sepsis. Virulence. 2014;5(1):4–11. doi: 10.4161/viru.27372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willenbring ML, Massey SH, Gardner MB. Helping patients who drink too much: an evidence-based guide for primary care physicians. Am Fam Physician. 2009;80(1):44–50. [PubMed] [Google Scholar]
- 24.Moore JX, Donnelly JP, Griffin R, et al. Black-white racial disparities in sepsis: a prospective analysis of the REasons for geographic and racial differences in stroke (REGARDS) cohort. Crit Care. 2015;19:279. doi: 10.1186/s13054-015-0992-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan H, Kalogeropoulos AP, Georgiopoulou VV, et al. Frailty and risk for heart failure in older adults: the health, aging, and body composition study. Am Heart J. 2013;166(5):887–894. doi: 10.1016/j.ahj.2013.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jha SR, Ha HS, Hickman LD, et al. Frailty in advanced heart failure: a systematic review. Heart Fail Rev. 2015;20(5):553–560. doi: 10.1007/s10741-015-9493-8. [DOI] [PubMed] [Google Scholar]
- 27.Baptista G, Dupuy AM, Jaussent A, et al. Low-grade chronic inflammation and superoxide anion production by NADPH oxidase are the main determinants of physical frailty in older adults. Free Radic Res. 2012;46(9):1108–1114. doi: 10.3109/10715762.2012.692784. [DOI] [PubMed] [Google Scholar]
- 28.McAdams-DeMarco MA, Law A, Salter ML, et al. Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J Am Geriatr Soc. 2013;61(6):896–901. doi: 10.1111/jgs.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newman AB, Gottdiener JS, McBurnie MA, et al. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. 2001;56(3):M158–M166. doi: 10.1093/gerona/56.3.m158. [DOI] [PubMed] [Google Scholar]
- 30.Remick DG. Pathophysiology of sepsis. Am J Pathol. 2007;170(5):1435–1444. doi: 10.2353/ajpath.2007.060872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu Y, Tan CT, Nyunt MS, et al. Inflammatory and immune markers associated with physical frailty syndrome: findings from Singapore longitudinal aging studies. Oncotarget. 2016;7(20):28783–28795. doi: 10.18632/oncotarget.8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lang PO, Mitchell WA, Lapenna A, Pitts D, Aspinall R. Immuno-logical pathogenesis of main age-related diseases and frailty: role of immunosenescence. Euro Geriatr Med. 2010;1:112–121. [Google Scholar]
- 33.Mayr FB, Yende S, Linde-Zwirble WT, et al. Infection rate and acute organ dysfunction risk as explanations for racial differences in severe sepsis. JAMA. 2010;303(24):2495–2503. doi: 10.1001/jama.2010.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Occurrence and outcomes of sepsis: influence of race. Crit Care Med. 2007;35(3):763–768. doi: 10.1097/01.CCM.0000256726.80998.BF. [DOI] [PubMed] [Google Scholar]
- 35.Barnato AE, Alexander SL, Linde-Zwirble WT, Angus DC. Racial variation in the incidence, care, and outcomes of severe sepsis: analysis of population, patient, and hospital characteristics. Am J Respir Crit Care Med. 2008;177(3):279–284. doi: 10.1164/rccm.200703-480OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 37.Wang HE, Donnelly JP, Griffin R, et al. Derivation of novel risk prediction scores for community-acquired sepsis and severe sepsis. Crit Care Med. 2016;44(7):1285–1294. doi: 10.1097/CCM.0000000000001666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kojima G. Prevalence of frailty in nursing homes: a systematic review and meta-analysis. J Am Med Dir Assoc. 2015;16(11):940–945. doi: 10.1016/j.jamda.2015.06.025. [DOI] [PubMed] [Google Scholar]
- 39.National Institute on Alcohol Abuse and Alcoholism. [Accessed February 13, 2012];Helping Patients Who Drink Too Much, a Clinician’s Guide. 2005 http://pubs.niaaa.nih.gov/publications/Practitioner/CliniciansGuide2005/guide.pdf.
- 40.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]