Abstract
Hepatitis C virus (HCV) mediated chronic liver disease is a serious health problem around the world, and often causes fibrosis/cirrhosis, and hepatocellular carcinoma (HCC). The mechanism of liver disease progression during HCV infection is still unclear, although inflammation is believed to be an important player in disease pathogenesis. We previously reported that macrophages including Kupffer cells exposed to HCV induce proinflammatory cytokines. These secreted cytokines may activate hepatic stellate cells (HSCs) towards fibrosis. In this study, we examined cross-talk between macrophages and HSCs following HCV infection. Primary human HSCs and immortalized HSCs (LX2 cells) were incubated with conditioned medium (CM) derived from HCV exposed human macrophages. The expression of inflammasome and fibrosis related genes in these cells were examined and demonstrated an increased expression of inflammatory (NLRP3, IL-1β, IL-6 and CCL5) and profibrogenic (TGFβ1, COL4A1, MMP2 and α-SMA) markers. Further investigation suggested that CCL5, secreted from HCV exposed macrophages, activates inflammasome and fibrosis makers in HSCs, and neutralizing antibody to CCL5 inhibited activation.
Conclusion
Together, our results demonstrate that human macrophages exposed to HCV induce CCL5 secretion, which plays a significant role in hepatic inflammation and fibrosis.
Keywords: Hepatitis C Virus, Hepatic Stellate Cells, Macrophages, CCL5
Introduction
The World Health Organization (WHO) estimates approximately 150 million people have chronic HCV infection in the worldwide (1). A significant number of a chronically infected people will develop a potentially life-threatening problem, such as liver fibrosis, cirrhosis and hepatocellular carcinoma (HCC) (2). Approximately 700,000 people die each year from HCV-related liver diseases (3). Advanced hepatic fibrosis including cirrhosis is the primary lesions in most cases of HCC (4). Sustained inflammatory stimuli cause activation of hepatic stellate cells, leading to the storage of considerable extracellular matrixes in the liver, leading to fibrosis (5). Therefore, understanding the mechanisms of fibrosis following HCV infection is important for prevention and control of liver disease progression.
Different cell types in the liver make a network and relate to hepatic fibrogenesis regulation (6). We and others have shown that macrophages/Kupffer cells (KCs), exposed to HCV, induce IL-1β/IL-18 expression (7–10). However, the mechanism by which HCV induces proinflammatory cytokines in macrophages and its consequence on other liver cell types, such as HSCs, is poorly understood. KCs are resident macrophages of the liver and are the largest population of innate immune cells. Liver damage, including an induction of fibrosis, may at least in part, attribute to cytokines produced by KCs. The onset of fibrosis reflects complicated interplays among them, and the cells secrete cytokines and chemokines. In this study, we examined the relationship between CM from HCV exposed macrophages and HSCs. We observed that CM from HCV exposed macrophages induces inflammasome and fibrosis maker genes. Further study suggested that cysteine-cysteine chemokine ligand 5 (CCL5) is one of players for hepatic stellate cell inflammation and activation.
Experimental Procedures
CELLS AND VIRUSES
Human monocytic THP-1 cells were cultured in RPMI 1640 medium (Sigma-Aldrich, St. Louis) containing L-glutamine, 2-mercaptoethanol (50 μM), 25 mM HEPES, and 10% FBS, and maintained at 37°C in a 5% CO2 atmosphere. For macrophage differentiation, THP-1 cells were seeded at a density of 1 × 105 cells/ml and treated with phorbol 12-myristate 13-acetate (PMA) (100 ng/ml) at 37°C overnight. The cells were then kept in fresh complete medium for 2 days before exposure to virus. Unless mentioned otherwise, we used PMA-differentiated THP-1 cells throughout our studies as described previously (7). Human Kupffer cells with 98% purity (ThermoFisher Scientific) were procured and maintained in DMEM supplemented with 10% human AB serum and 1% antibiotics. Immortalized human hepatic stellate cell line, LX2, was obtained from Dr. Scott Friedmann (Mount Sinai School of Medicine, New York, USA). The cells were cultured in DMEM supplemented with 2% fetal bovine serum and penicillin-streptomycin at 37°C in 5% CO2. Primary human hepatic stellate cells were procured from ScienCell and maintained in Stellate Cell Medium (ScienCell) and cultured in poly-L-lysine-coated flasks.
For infection, cells were incubated with HCV genotype 2a (clone JFH1) (multiplicity of infection of 0.1) in a minimum volume of medium. PMA-treated THP-1 cells and Kupffer cells were incubated with HCV for 4 h. After incubation, cells were washed with medium without serum for 3 times and maintained for 24 h in respective maintenance medium. Culture supernatants were collected and subjected to centrifugation at 10,000xg for 10 minutes at 4°C, and kept frozen at −80°C until use (HCV-MΦ-CM/HCV-Kupffer cell-CM). Culture supernatant from mock-infected THP1 macrophages or Kupffer cells served as a control conditioned medium (Control CM). LX2 cells or primary human hepatic stellate cells were incubated with conditioned medium from control or HCV infected macrophages at indicated time points. Cells were lysed and processed for either RNA isolation or protein lysate preparation.
RNA ISOLATION AND QUANTITATIVE REVERSE TRANSCRIPTION-PCR (qRT-PCR)
Total RNA was isolated by using a TRIZOL reagent (Life Technologies). RNA was quantified by using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific). cDNA was synthesized by using random hexamers and a Superscript III reverse transcriptase kit (Invitrogen, CA). Real-time PCR was performed with cDNA for quantitation using TaqMan gene expression PCR master mix and 6-carboxyfluorescein (FAM)-MGB probes for NLRP3 (assay ID, Hs00918082_m1), IL-1β (assay ID, Hs01555410_m1), ACTA2 (assay ID, Hs00426835_g1), TIMP1 (assay ID, Hs00171558_m1), COL4A1 (assay ID,Hs00266237_m1), TGFβ1 (assay ID, Hs00998133_m1), IL-6 (assay ID, Hs00985639_m1), CCL5 (assay ID, Hs00982282_m1), TNF-α (assay ID, Hs01113624_g1), MMP2 (assay ID, Hs01548727_m1). A FAM-MGB probe for 18S rRNA (assay ID, Hs03928985_g1) was used as an endogenous control. The reactions were performed at 50°C for 2 min and 95°C for 10 min, cycled at 95°C for 15 s and 60°C for 1 min for 40 cycles, and held at 25°C for 2 min in a 7500 real-time PCR system (Applied Biosystems). The relative gene expression levels were normalized to the 18S rRNA level by using the 2−ΔΔCT formula (ΔΔCT = ΔCT sample − ΔCT untreated control).
WESTERN BLOT ANALYSIS
Control or HCV-MΦ-CM treated LX2 cells were lysed by using an SDS-PAGE sample loading buffer. The proteins were subjected to electrophoresis on a polyacrylamide gel and transferred onto a nitrocellulose membrane. The membrane was probed with specific antibody. Proteins were detected by using a chemiluminescent ECL Western blot substrate (Pierce, IL). Membranes were reprobed with antibody to GAPDH (Cell Signaling) as an internal control for normalization of protein load. All Western blot experiments were performed at least three times for reproducibility. ImageJ software (NIH) was used for densitometric scanning of Western blot images. The membrane was probed with antibodies to Phospho-NF-κB p65 (Ser536) (93H1), NF-κB p65 (D14E12), phospho- IκBα (14D4), IκBα (L35A5), phosphor-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) (D13.14.4E), p44/42 MAP Kinase (L34F12), GAPDH (Cell Signaling) and α-Actin (1A4): Sc-32251 (Santacruz).
LUCIFERASE ASSAY
LX2 cells (3×104 per well) were seeded and transfected with pNF-κB Luc reporter plasmid (a minimal promoter with three NF-κB binding sites linked with luciferase reporter gene) (11) using jetPRIME trasnsfection reagent (Polyplus). After 24 h of transfection, cells were treated with control or HCV-MΦ-CM for another 24 h. Cells were harvested in 1X passive lysis buffer (Promega) and luciferase activity was determined using GloMax (Promega).
INFLAMMATION ANTIBODY ARRAY
Conditioned medium from control or HCV infected macrophages was analyzed for the presence of 40 human cytokine proteins using an inflammation membrane antibody array (Abcam; ab134003) following manufacturer’s instructions. The intensity of each cytokine or chemokine spot was determined by ImageJ software and represented as relative expression compared to control CM.
NEUTRALIZATION ASSAY
Neutralizing antibody to IL-1β, TNF-α, CCL5 or normal goat IgG control was procured (R&D Systems, Minneapolis, MN), and used (2 μg/ml) of neutralization assay. Antibodies were incubated with CM for 1 h at 37 °C, before addition on LX2 cells.
STATISTICAL ANALYSIS
The results are presented as means ± standard deviations. Data were analyzed by Student’s t test with a two-tailed distribution. A P value of <0.05 was considered statistically significant.
Results
CONDITIONED MEDIUM FROM HCV EXPOSED MACROPHAGES PROMOTE PRO-INFLAMMATORY MARKER GENE EXPRESSION IN HUMAN HEPATIC STELLATE CELLS
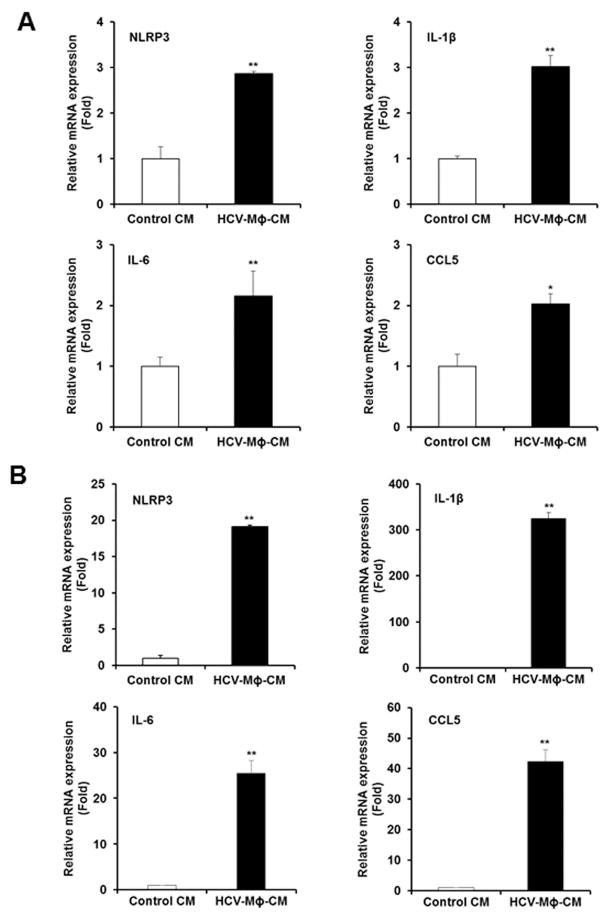
LX2 cells were incubated for 48 h with CM from THP1-macrophages incubated with HCV (HCV-MΦ-CM) and analyzed for changes in the expression levels of various known proinflammatory cytokine genes as compared to CM from THP1-macrophages (Control CM). Our results suggested that enhanced expression (2–3 fold) of NLR Family Pyrin Domain Containing 3 (NLRP3), IL-1β, IL-6 and CCL5 genes in LX2 cells (Fig. 1, panel A). Next, we verified the expression of these genes in primary HSCs. We observed a significant higher level (>20 fold) expression of these proinflammatory cytokine genes in primary HSCs following incubation with CM from macrophages (Fig. 1, panel B). Interestingly, the expression level of IL-1β was highest (>300 fold) as compared to control. NLRP3 inflammasome plays an important role in inflammation and fibrosis during NASH and ASH development (12). However, specific contribution of persistent NLRP3 inflammasome activation in hepatic stellate cells during HCV infection remains to understand.
Figure 1. Conditioned medium from HCV exposed macrophages activates proinflammatory molecules in human hepatic stellate cells.
Panel A. LX2 cells were incubated with CM from mock (control CM) or HCV exposed macrophages CM (HCV-MΦ-CM) for 48 h. Expression of inflammasome markers, NLRP3, IL-1β, IL-6 and CCL5 genes, were examined by qRT-PCR. 18S RNA was used as an internal control. Panel B. Human primary hepatic stellate cells were similarly treated with control CM or HCV-MΦ-CM and inflammasome markers were examined. Values represent from three independent experiments ±SD. Statistical significance was analyzed using the two-tailed Student’s t test: *P < 0.05, **P<0.01.
FIBROGENIC MAKERS ARE ELEVATED IN HEPATIC STELLATE CELLS FOLLOWING EXPOSURE OF CONDITIONED MEDIUM FROM HCV INCUBATED MACROPHAGES
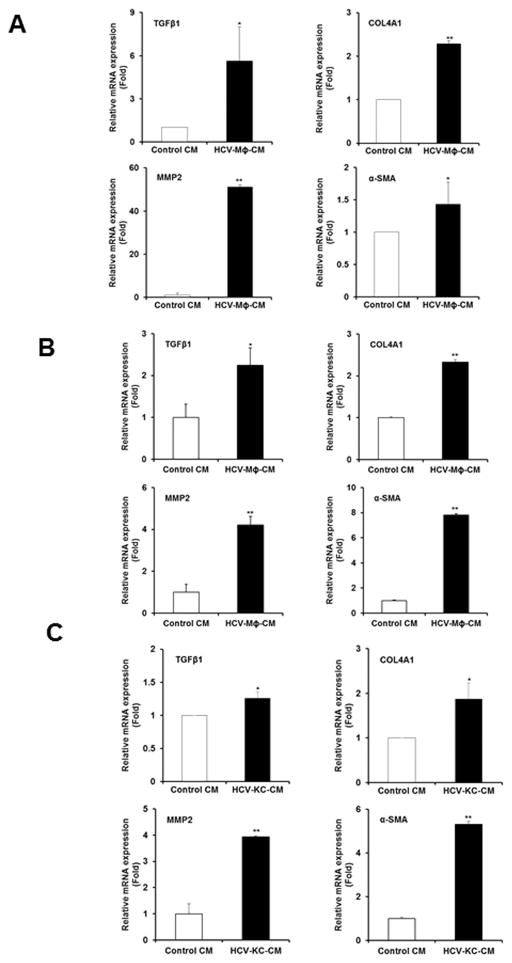
We examined for modulation of fibrosis markers in LX2 cells incubated with HCV-MΦ-CM as compared to Control CM. Our results demonstrated an activation of fibrosis markers in HCV- MΦ-CM incubated LX2 cells (Fig. 2, panel A). The results suggested higher TGFβ1 (~6 fold), COL4A1 (>2 fold), MMP-2 (>50 fold), and α-SMA (~1.5 fold). Similarly, we examined for changes in primary human hepatic stellate cells and observed a significant upregulation of profibrogenic markers TGFβ1 (>2.5 fold), COL4A1 (>2.5 fold), MMP2 (>4 fold) and α-SMA (>8 fold) in primary HSCs incubated with HCV-MΦ-CM as compared to control CM (Fig. 2, panel B). Other collagen markers such as COL1A1 and COL1A2 are also increased in primary HSCs incubated with HCV-MΦ-CM as compared to control CM (data not shown). In order to determine the modulation of hepatic stellate cells by liver resident macrophages, we incubated Kupffer cells (KCs) with HCV. Primary HSCs were exposed to the CM from control or HCV exposed KCs. Our results demonstrated a significant upregulation of profibrogenic markers, TGFβ1 (<1.5 fold), COL4A1 (~2 fold), MMP2 (>4 fold), and α-SMA (>5 fold) in primary HSCs exposed to HCV Kupffer cell conditioned medium as compared to control CM (Fig. 2, panel C). Together, our results indicated that soluble mediators from HCV exposed macrophages when exposed to hepatic stellate cells, exerts proinflammatory and profibrogenic effects on HSCs. Interestingly, the effect was higher in generation of MMP2 and α-SMA as compared to the other two cytokines (COL4A1 and TGFβ1).
Figure 2. Activation of fibrogenic molecules in human hepatic stellate cells following exposure of conditioned medium from HCV exposed macrophages.
Panel A. LX2 cells were incubated with control CM or HCV-MΦ-CM for 48 h. Total RNA was prepared and expression status of TGFβ1, COL4A1, MMP2 and, α-SMA genes were examined by qRT-PCR. 18S RNA was used as an internal control. Panel B. Human primary hepatic stellate cells were similarly treated with control CM or HCV-MΦ-CM and expression of fibrogenic activators were examined as described above. Panel C. Human primary hepatic stellate cells were incubated with control CM or HCV exposed Kupffer cells CM (HCV-KC-CM). Expression of the fibrogenic activators were similarly measured as described above. Values represent from three independent experiments ±SD. Statistical significance was analyzed using the two-tailed Student’s t test: *P < 0.05, **P<0.01.
CONDITIONED MEDIUM FROM HCV EXPOSED MACROPHAGES ACTIVATES NF-κB IN LX2 CELLS
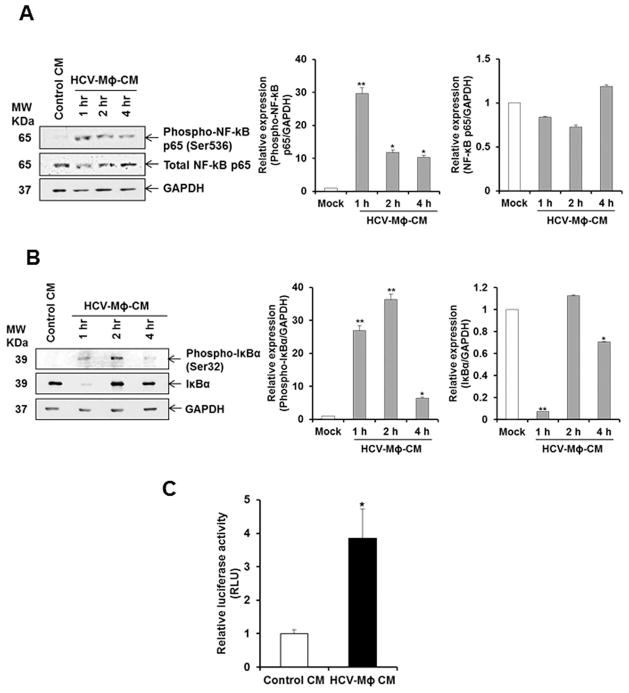
Several signaling pathways regulate the expression of proinflammatory cytokines, of which the NF-kB pathway is the most important pathway (13). To understand the intracellular mechanisms responsible for HSC modulation by HCV-MΦ-CM, NF-kB signaling pathway was examined. We observed an increase of phosphorylated NF-kB in LX2 cells following incubation with CM from HCV exposed macrophages (Fig. 3, panel A). In the canonical pathway, NF-κB/Rel proteins are bound and inhibited by IκB proteins. Proinflammatory cytokines, LPS, growth factors, and antigen receptors activate an IKK complex (IKKβ, IKKα, and NEMO), which phosphorylates IκB proteins. Phosphorylation of IκB leads to its ubiquitination and proteasomal degradation, freeing NF-κB/Rel complexes (14). We therefore examined the phospho-IκBα and IκBα expression in LX2 cells. We observed upregulation of phospho-IκBα and significant reduction in total IκBα level (Fig. 3, panel B). The degradation of IκBα with concomitant increase in phosphorylated NF-kB p65 in LX2 cells suggested the activation of this pathway. We also examined the NF-κB dependent luciferase reporter activity in LX2 cells in presence of control CM or HCV-MΦ-CM. There was a significant induction of NF-κB dependent luciferase activity in LX2 cells incubated with HCV-MΦ-CM as compared to Control CM (Fig. 3, panel C). Together, our results demonstrated that the NF-kB pathway is active in HSCs in response to CM from HCV exposed macrophages.
Figure 3. Conditioned medium from HCV exposed macrophages induces NF-κB in LX2 cells.
Panel A. LX2 cells were incubated with control CM or HCV-MΦ-CM, and cell lysates were prepared as indicated time points. Phospho-NF-κB and total NF-κB was detected by western blot analysis using specific antibodies. The blot was reprobed with antibody to GAPDH for loading control. Densitometric scanning result is presented in the right. Panel B. LX2 cell lysates from the above experiment was analyzed for expression of phospho-IκBα and IκBα expression by Western blot using specific antibody. Densitometric scanning result is shown on the right. Panel C. LX2 cells were transfected with a luciferase reporter construct with NF-κB responsive elements and treated with control CM or HCV-MΦ-CM. Luciferase activity is presented as mean of three independent experiments. Statistical significance was analyzed using the two-tailed Student’s t test: *P < 0.05, **P<0.01.
IDENTIFICATION OF FIBROGENIC ACTIVATOR FROM CONDITIONED MEDIUM OF HCV EXPOSED MACROPHAGES
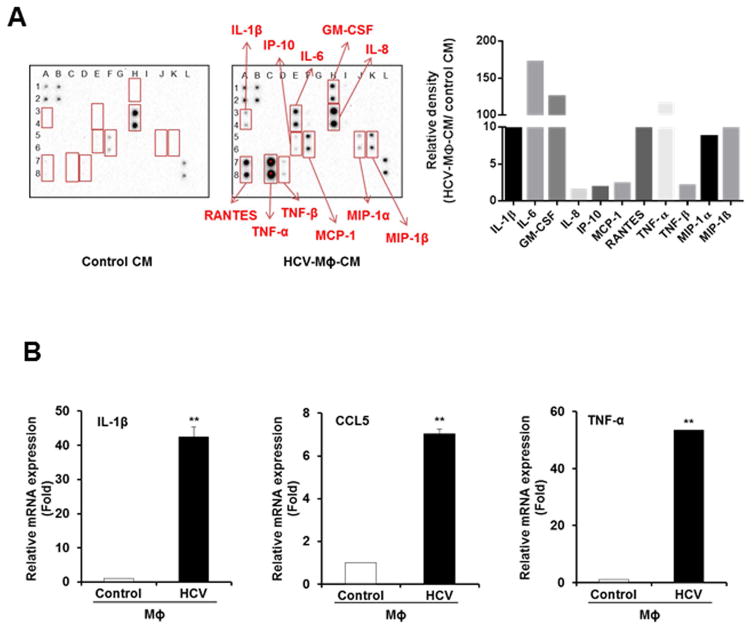
In order to identify soluble mediator in conditioned medium from HCV exposed macrophages, we performed a human inflammation array using control and CM from HCV exposed macrophages. We observed a significant induction of proinflammatory cytokines and chemokines (Fig. 4). IL-1β, IL-6, GM-CSF, IL-8, IP-10, MCP-1, RANTES (CCL5), TNF-α, TNF-β, MIP-1α and MIP-1β levels were significantly higher in CM from HCV exposed macrophages as compared to control CM (Fig. 4, panel A). Based on the information in the literature as well as our previous results, we focused initially on IL-1β and TNF-α, and CCL5 to examine their role in hepatic fibrosis. In HCV infected patients, serum IL-1β and TNF-α, and CCL5 levels were higher as compared to healthy individuals (18–20). We confirmed the expression of these molecules in HCV exposed macrophages at mRNA level (Fig. 4, panel B).
Figure 4. Identification of soluble factor secreted from HCV exposed macrophages.
Panel A. Human inflammation antibody array was screened using HCV-MΦ-CM and normalized with control CM. Semi-quantitation of the inflammatory molecules is shown in the right. Panel B. Expression of IL-1β, TNF-α and CCL5 was examined in HCV exposed macrophages by qRT-PCR, and normalized with 18S RNA. Values represent three independent experiments ±SD. Statistical significance was analyzed using the two-tailed Student’s t test: **P<0.01.
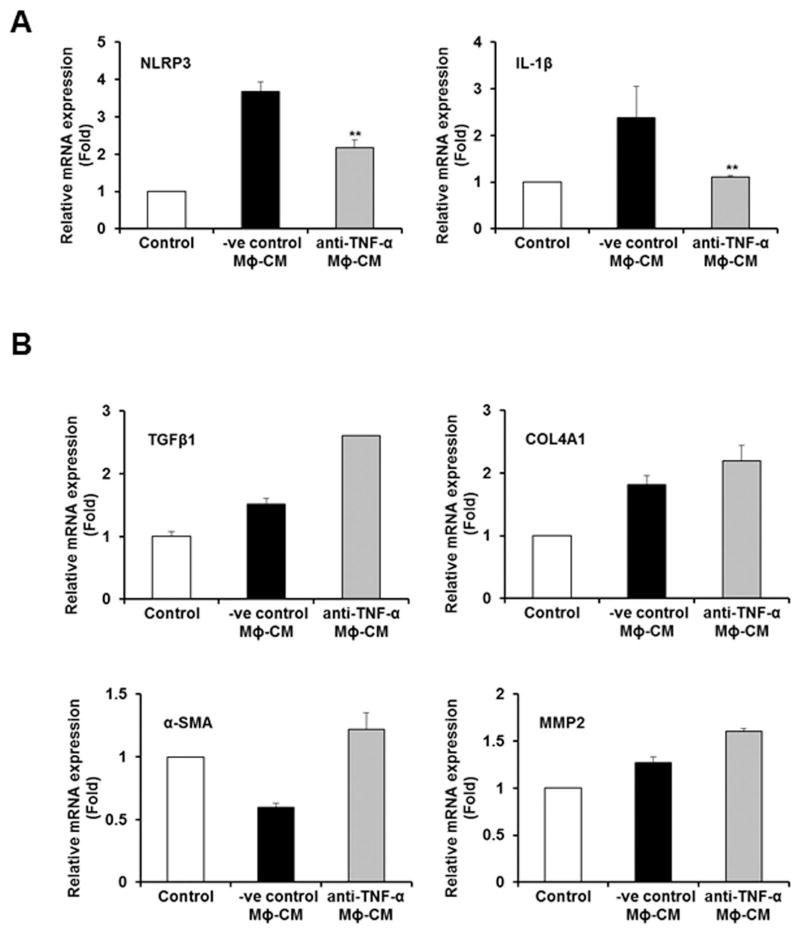
Several studies indicated that IL-1β contributes to the development of NASH and fibrosis (15). We observed higher level expression of IL-1β in HSCs after incubation with CM from HCV exposed macrophages (Fig. 1). We next incubated CM from HCV exposed macrophages with different doses of IL-1β neutralizing antibody and further incubated with HSCs. We did not observe blockade in inflammasome or fibrosis marker gene expression (data not shown). TNF-α has also been implicated to promote HSC survival and proliferation (15–17). We incubated different concentrations of TNF-α neutralizing antibody with CM from HCV exposed macrophages, following exposure to HSCs. The CM from HCV exposed macrophages treated with different concentrations of negative control antibody (−ve cont Ab) was used in parallel as control. Interestingly, we observed a significant reduction in expression of NLRP3 and IL-1β genes after TNF-α neutralization (Fig. 5, panel A) but not the fibrosis marker genes (Fig. 5, panel B), suggesting that TNF-α mediated induction of inflammasome pathway in HSCs.
Figure 5. TNF-α secreted from HCV exposed macrophages induces inflammasome, but not fibrosis markers.
Panel A. LX2 cells were incubated with HCV-MΦ-CM and TNF-α neutralizing antibody or negative control antibody (−ve cont Ab). Total RNA was isolated and qRT-PCR was performed to examine the expression of NLRP3 and IL-1β as described in figure 1. Panel B. LX2 cells were incubated with HCV-MΦ-CM treated with negative control or neutralizing antibody of TNF-α. Total RNA was isolated and qRT-PCR was performed for expression of profibrogenic markers as described in Figure 1.
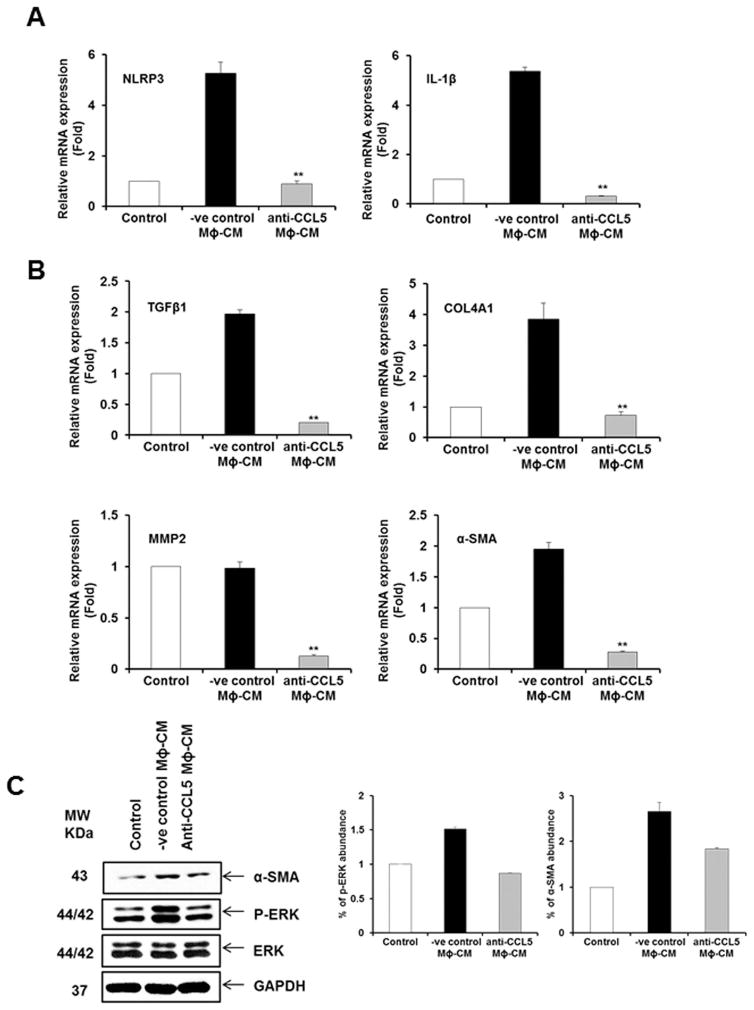
CCL5 SECRETED FROM HCV EXPOSED MACROPHAGES INDUCES INFLAMMASOME AND FIBROSIS MARKERS IN HSCs
CCL5 is upregulated in patients with advanced fibrosis or cirrhosis, and correlated with higher inflammation scores and greater liver damage during chronic HBV or HCV infection (18–21). CCL5 plays a role in hepatic activation in mice when treated with carbon tetrachloride for reactive phenomenon of the liver functions (15). Our inflammation array data demonstrated HCV induced CCL5 secretion in macrophages. To examine the intercellular communication between macrophages and HSCs during HCV infection, we evaluated the role of CCL5 by neutralization assay. The negative control antibody (−ve cont Ab) was used in parallel as control. When LX2 cells were incubated with CCL5 neutralized CM from HCV exposed macrophages, we observed a significant downregulation of inflammatory (NLRP3 and IL-1β) and profibrogenic (TGFβ1, COL4A1, MMP2 and α-SMA) genes compared to the negative control antibody (Fig. 6, panels A and B). We also observed CCL5 mediated α-SMA expression is reduced when neutralizing antibody to CCL5 was used (Fig. 6, panel C). CCL5 induces HSC activation through phosphorylation of ERK (22) and phosphorylation of AKT (23). We observed upregulation of ERK phosphorylation compared to negative control, and phosphorylation of ERK was downregulated in HSCs when HCV-MΦ-CM was incubated with anti-CCL5 neutralizing antibody (Fig. 6, panel C). However, we did not observe a modulation of Akt signaling pathway (data not shown).
Figure 6. CCL5 from CM of HCV exposed macrophages induces inflammasome and profibrogenic markers.
Panel A. LX2 cells were incubated with HCV-MΦ-CM and CCL5 neutralizing antibody or negative control antibody (−ve cont Ab). Total RNA was isolated and qRT-PCR was performed to examine the expression of NLRP3 and IL-1β as described in figure 1. Panel B. LX2 cells were incubated with HCV-MΦ-CM and CCL5 neutralizing antibody or −ve cont Ab. Total RNA was isolated and qRT-PCR was performed for expression fibrogenic markers. Panel C. Cell lysates from above experiment were analyzed for expression of phosphor-ERK, total ERK and α-SMA by Western blot using specific antibodies. The blot was reprobed with antibody to GAPDH for loading control. Results from Densitometric scanning are presented in the right.
Discussion
We have demonstrated that CM from HCV exposed macrophages activates human HSCs. We have also shown that HCV induces secretion of CCL5 from macrophages, which in turn, activates inflammatory molecules and fibrosis markers in HSCs. This indirect pathway is thought to be mediated by HSC activation via Kupffer-cell-derived CCL5 following HCV exposure. Chronic HCV infection promotes secretion of cytokines and chemokines by modulating promoter activity in hepatic cell lines (23), and by circulatory and resident liver macrophages through the NF-κB signaling pathway (7). In diseased liver, most extracellular matrix (ECM) components are produced by HSCs (24), and accumulation of these ECM causes liver fibrosis. Cytokines and chemokines stimulate key biological processes in HSCs, such as activation, proliferation, and migration (22). We have shown recently that exosomes carrying miR-19a from HCV infected hepatocytes induces HSC fibrosis markers by modulating the SOCS-STAT3 axis (25). We also examined whether residual HCV from macrophage CM is activating HSC. However, we could not detect HCV RNA in HSCs incubated with HCV-MΦ-CM. Therefore, chance of residual HCV in activating HSCs is unlikely. Treatment of chronic HCV infection with direct-acting antiviral agents (DAA) is associated with high rates of sustained virologic response (>90%). DAAs can reduce the viral load, and may thus be effective in reverting liver pathogenesis at an early stage of disease. We postulate that HCV infection activates HSCs using multiple pathways, resulting in the development and progression of fibrosis.
Resident macrophages are major regulators of inflammation and fibrogenesis by regulating the cross-talk between HSCs and immune system cells to achieve a cellular response (22, 23). Macrophages produce cytokines and chemokines that directly activate fibroblasts and recruit inflammatory cells (24). We previously demonstrated that HCV exposure to macrophages, including Kupffer cells, induces inflammasome formation (7). In this study, we observed a significant induction of proinflammatory cytokines and chemokines. Although TNF-α enhances inflammasome markers, it did not exert an effect on activation of fibrosis marker genes. Interestingly, IL-1β did not play a role in activation of HSCs in our experimental system.
CCL5 is secreted by macrophages through activation of the Nod-like receptor proteins Nod1 and Nod2, and the NF-κB signaling is a key molecule in Nod-dependent stimulation of the CCL5 promoter (26). We previously reported that HCV induces mature IL-1β/IL-18 in cell-free supernatants via the NF-κB signaling pathway in macrophages (24). Therefore, it is possible that HCV exposed macrophages secrete CCL5 through the NF-κB signaling pathway. Clinically, CCL5 is up-regulated in serum and livers of HCV infected individuals (23). The CCL5 mRNA level correlates to with HCV RNA load and histological activity index, including score of fibrosis, intralobular degeneration and focal necrosis, periportal +/− bridging necrosis and portal inflammation. CCL5 and its receptors, CCR5 and CCR1, are important players in liver fibrosis in carbon tetrachloride mice model (27, 28), and HSCs proliferation and migration in primary human HSCs (17). Our data demonstrate that HSCs incubated with CCL5 neutralized CM of HCV exposed macrophages display a significant downregulation of inflammatory and profibrotic marker genes as compared to negative control. Our study further indicated ERK phosphorylation was up-regulated in HSCs incubated with CM from HCV exposed macrophages. Other inflammatory molecules may also have role in activation of fibrosis and will be investigated in future.
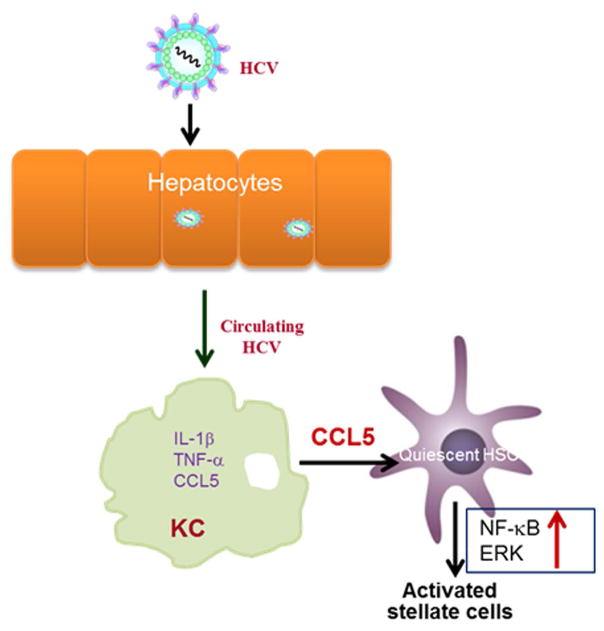
HCV induces CCL5 secretion in human macrophages, which plays a role in hepatic inflammation and fibrosis. Interestingly, our data suggested that HCV-MΦ-CM lead to significant reduction in IκBα level in LX2 cells. The degradation of IκBα with concomitant increase in phosphorylated NF-kB p65 in LX2 cells, suggesting involvement of NF-κB pathway. We also observed a significant induction of NF-κB dependent luciferase activity in LX2 cells in presence of HCV-MΦ-CM. NF-κB has been implicated in activation of cytokines (29, 30). In HIV and HCV co-infection model, HIV and HCV cooperatively promote hepatic fibrogenesis via NF-κB dependent pathway (31). Based on our results, we propose a mechanism (Fig. 7) that suggests the onset of fibrosis during HCV infection. NF-κB plays an important role in the regulation of proinflammatory cytokines and we observed that NF-κB signaling is activated for IL-1β/IL-18 transcription in HCV-exposed macrophages (7). NF-κB signaling is also activated in HSCs treated with HCV-MΦ-CM, but not fibrosis markers. On the other hand, CCL5 is known to activate fibrosis through ERK pathway (22). We demonstrated that CCL5 indeed activates fibrosis markers via ERK pathway and is reduced following the use of neutralizing antibody to CCL5. Thus, NF-κB and ERK signaling pathways are likely to be independently involved in HSC activation. Understanding the in-depth mechanism will help in development of future therapeutic strategies against HCV-associated fibrosis.
Figure 7.
Schematic diagram showing cross-talk among HCV infected hepatocytes, macrophages and hepatic stellate cells.
Acknowledgments
Financial Support: This work was supported by research grant R01 DK081817 from the National Institutes of Health and Saint Louis University Liver Center Seed Grants.
We thank Scott Friedman for providing LX2 cells.
References
“Author names in bold designate shared co-first authorship”
- 1.The World Health Organization (WHO) Hepatitis C Fact sheet. Updated July 2016. [Google Scholar]
- 2.Ibrahim MK, Salum GM, Bader El Din NG, Dawood RM, Barakat A, Khairy A, et al. Transcriptional Dysregulation of Upstream Signaling of IFN Pathway in Chronic HCV Type 4 Induced Liver Fibrosis. PLoS One. 2016;11:e0154512. doi: 10.1371/journal.pone.0154512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Y, El-Serag HB, Jiao L, Lee J, Moore D, Franco LM, et al. WNT Signaling Pathway Gene Polymorphisms and Risk of Hepatic Fibrosis and Inflammation in HCV-Infected Patients. PLoS One. 2013;8:e84407. doi: 10.1371/journal.pone.0084407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sporea I, Sirli R, Bota S, Fierbinteanu-Braticevici C, Petrisor A, Badea R, et al. Is ARFI elastography reliable for predicting fibrosis severity in chronic HCV hepatitis? World J Radiol. 2011;3:188–193. doi: 10.4329/wjr.v3.i7.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo J, Friedman SL. Hepatic fibrogenesis. Semin Liver Dis. 2007;27:413–26. doi: 10.1055/s-2007-991517. [DOI] [PubMed] [Google Scholar]
- 7.Shrivastava S, Mukherjee A, Ray R, Ray RB. Hepatitis C Virus Induces Interleukin-1β (IL-1β)/IL-18 in Circulatory and Resident Liver Macrophages. J Virol. 2013;87:12284–90. doi: 10.1128/JVI.01962-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Negash AA, Ramos HJ, Crochet N, Lau DT, Doehle B, Papic N, et al. IL-1β production through the NLRP3 inflammasome by hepatic macrophages links hepatitis C virus infection with liver inflammation and disease. PLoS Pathog. 2013;9:e1003330. doi: 10.1371/journal.ppat.1003330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chattergoon MA, Latanich R, Quinn J, Winter ME, Buckheit RW, 3rd, Blankson JN, et al. HIV and HCV Activate the Inflammasome in Monocytes and Macrophages via Endosomal Toll-Like Receptors without Induction of Type 1 Interferon. PLoS Pathog. 2014;10:e1004082. doi: 10.1371/journal.ppat.1004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saha B, Kodys K, Szabo G. Hepatitis C Virus-Induced Monocyte Differentiation Into Polarized M2 Macrophages Promotes Stellate Cell Activation via TGF-β. Cell Mol Gastroenterol Hepatol. 2016;2:302–316. doi: 10.1016/j.jcmgh.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghosh AK, Steele R, Ray RB. c-myc Promoter-binding Protein 1 (MBP-1) Regulates Prostate Cancer Cell Growth by Inhibiting MAPK Pathway. J Biol Chem. 2005;280:14325–30. doi: 10.1074/jbc.M413313200. [DOI] [PubMed] [Google Scholar]
- 12.Wree A, Eguchi A, McGeough MD, Pena CA, Johnson CD, Canbay A, et al. NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology. 2014;59:898–910. doi: 10.1002/hep.26592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thirunavukkarasu C, Watkins SC, Gandhi CR. Mechanisms of endotoxin-induced NO, IL-6, and TNF-alpha production in activated rat hepatic stellate cells: role of p38 MAPK. Hepatology. 2006;44:389–98. doi: 10.1002/hep.21254. [DOI] [PubMed] [Google Scholar]
- 14.Ghosh S, Hayden MS. New regulators of NF-kappaB in inflammation. Nat Rev Immunol. 2008;11:837–48. doi: 10.1038/nri2423. [DOI] [PubMed] [Google Scholar]
- 15.Seki E, Schwabe RF. Hepatic inflammation and fibrosis: functional links and key pathways. Hepatology. 2015;61:1066–79. doi: 10.1002/hep.27332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tarrats N, Moles A, Morales A, García-Ruiz C, Fernández-Checa JC, Marí M. Critical role of tumor necrosis factor receptor 1, but not 2, in hepatic stellate cell proliferation, extracellular matrix remodeling, and liver fibrogenesis. Hepatology. 2011;54:319–27. doi: 10.1002/hep.24388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siegmund SV, Schlosser M, Schildberg FA, Seki E, De Minicis S, Uchinami H, et al. Serum Amyloid A Induces Inflammation, Proliferation and Cell Death in Activated Hepatic Stellate Cells. PLoS One. 2016;11:e0150893. doi: 10.1371/journal.pone.0150893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nischalke HD, Nattermann J, Fischer HP, Sauerbruch T, Spengler U, Dumoulin FL. Semiquantitative analysis of intrahepatic CC-chemokine mRNas in chronic hepatitis C. Mediators Inflamm. 2004;13:357–9. doi: 10.1155/S0962935104000523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Apolinario A, Majano PL, Alvarez-Pérez E, Saez A, Lozano C, Vargas J, et al. Increased expression of T cell chemokines and their receptors in chronic hepatitis C: relationship with the histological activity of liver disease. Am J Gastroenterol. 2002;97:2861–70. doi: 10.1111/j.1572-0241.2002.07054.x. [DOI] [PubMed] [Google Scholar]
- 20.Kusano F, Tanaka Y, Marumo F, Sato C. Expression of C-C chemokines is associated with portal and periportal inflammation in the liver of patients with chronic hepatitis C. Lab Invest. 2000;80:415–22. doi: 10.1038/labinvest.3780046. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Wu Y, Zheng X, Cong J, Liu Y, Li J, et al. Cytoplasm-Translocated Ku70/80 Complex Sensing of HBV DNA Induces Hepatitis-Associated Chemokine Secretion. Front Immunol. 2016;7:569. doi: 10.3389/fimmu.2016.00569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwabe RF, Bataller R, Brenner DA. Human hepatic stellate cells express CCR5 and RANTES to induce proliferation and migration. Am J Physiol Gastrointest Liver Physiol. 2003;285:G949–58. doi: 10.1152/ajpgi.00215.2003. [DOI] [PubMed] [Google Scholar]
- 23.Marra F, Tacke F. Roles for chemokines in liver disease. Gastroenterology. 2014;147:577–594. doi: 10.1053/j.gastro.2014.06.043. [DOI] [PubMed] [Google Scholar]
- 24.Karthikeyan S, Potter JJ, Geschwind JF, Sur S, Hamilton JP, Vogelstein B, et al. Deregulation of energy metabolism promotes antifibrotic effects in human hepatic stellate cells and prevents liver fibrosis in a mouse model. Biochem Biophys Res Commun. 2016;469:463–9. doi: 10.1016/j.bbrc.2015.10.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Devhare PB, Sasaki R, Shrivastava S, Di Bisceglie AM, Ray R, Ray RB. Exosome mediated intercellular communication between hepatitis C virus infected hepatocytes and hepatic stellate cells. J Virol. 2017:02225–16. doi: 10.1128/JVI.02225-16. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Werts C, le Bourhis L, Liu J, Magalhaes JG, Carneiro LA, Fritz JH, et al. Nod1 and Nod2 induce CCL5/RANTES through the NF-kappaB pathway. Eur J Immunol. 2007;37:2499–508. doi: 10.1002/eji.200737069. [DOI] [PubMed] [Google Scholar]
- 27.Berres ML, Koenen RR, Rueland A, Zaldivar MM, Heinrichs D, Sahin H, et al. Antagonism of the chemokine Ccl5 ameliorates experimental liver fibrosis in mice. J Clin Invest. 2010;120:4129–40. doi: 10.1172/JCI41732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stock MK, Hammerich L, do ONT, Berres ML, Alsamman M, Heinrichs D, et al. Met-CCL5 modifies monocyte subpopulations during liver fibrosis regression. Int J Clin Exp Pathol. 2013;6:678–85. [PMC free article] [PubMed] [Google Scholar]
- 29.Liu M, Xu Y, Han X, Yin L, Xu L, Qi Y, et al. Dioscin alleviates alcoholic liver fibrosis by attenuating hepatic stellate cell activation via the TLR4/MyD88/NF-κB signaling pathway. Sci Rep. 2015;5:18038. doi: 10.1038/srep18038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang H, Sun Q, Xu T, Hong L, Fu R, Wu J, et al. Resveratrol attenuates the progress of liver fibrosis via the Akt/nuclear factor-κB pathways. Mol Med Rep. 2016;13:224–30. doi: 10.3892/mmr.2015.4497. [DOI] [PubMed] [Google Scholar]
- 31.Lin W, Wu G, Li S, Weinberg EM, Kumthip K, Peng LF, et al. HIV and HCV cooperatively promote hepatic fibrogenesis via induction of reactive oxygen species and NFkappaB. J Biol Chem. 2011;286:2665–74. doi: 10.1074/jbc.M110.168286. [DOI] [PMC free article] [PubMed] [Google Scholar]