Abstract
Objective
To examine associations between short-term exposure to ambient air pollution and circulating biomarkers of systemic inflammation in participants from the Framingham Offspring and Third Generation cohorts in the Greater Boston area.
Approach and Results
We included 3,996 non-current smoking participants (mean age 53.6 years, 54% women) who lived within 50 km from a central air pollution monitoring site in Boston, Massachusetts, and calculated the 1- to 7-day moving averages of fine particulate matter (PM2.5), black carbon (BC), sulfate, nitrogen oxides (NOx), and ozone prior to the examination visits. We used linear mixed effects models for C-reactive protein (CRP) and tumor necrosis factor receptor 2 (TNFR2) which were measured up to twice for each participant; we used linear regression models for interleukin-6, fibrinogen, and tumor necrosis factor α (TNFα) which were measured once. We adjusted for demographics, socioeconomic position, lifestyle, time, and weather. The 3- to 7-day moving averages of PM2.5 and sulfate were positively associated with CRP concentrations. A 5 μg/m3 higher 5-day moving average PM2.5 was associated with 4.2% (95% CI: 0.8, 7.6) higher circulating CRP. Positive associations were also observed for NOx with interleukin-6, and for BC, sulfate, and ozone with TNFR2. However, BC, sulfate, and NOx were negatively associated with fibrinogen, and sulfate was negatively associated with TNFα.
Conclusions
Higher short-term exposure to relatively low levels of ambient air pollution was associated with higher levels of CRP, interleukin-6, and TNFR2, but not fibrinogen or TNFα, in individuals residing in the Greater Boston area.
Keywords: Air Pollution, Inflammation, Epidemiology, Biomarkers
Subject Terms: Epidemiology, Inflammation, Risk factor
Introduction
Air pollution-induced systemic inflammation is hypothesized to be one of the underlying mechanisms linking ambient air pollution exposure to the risk of cardiovascular disease.1, 2 Elevated short-term exposure to ambient fine particulate matter (PM2.5, diameter<2.5 μm) and gaseous pollutants, such as ozone (O3), have been associated with higher levels of biomarkers of systemic inflammation in controlled animal studies.3–5 However, the majority of human studies that have explored these associations had relatively small sample sizes or had participants with conditions that may predispose them to the health effects of air pollution.6–16 Moreover, results from larger-scale epidemiological studies on the associations between air pollution and inflammatory response have yielded mixed results.17–23 For example, in the Multi-Ethnic Study of Atherosclerosis with over 6,000 participants, exposure to higher levels of PM2.5 on the day of the blood draw was only weakly associated with higher blood levels of C-reactive protein (CRP), however, the association was not observed for circulating interleukin-6 or fibrinogen.22 In the Tel-Aviv Medical Center Inflammation Survey, Steinvil et al. found negative associations of sulfur dioxide and nitrogen dioxide with fibrinogen across multiple lags only among male participants (N=2,203), and the associations for CRP were generally null.17 In contrast, Bind et al. reported positive associations of 28-day moving averages of PM2.5, BC, and particle number with circulating concentrations of CRP in 1,112 participants (3,615 visits in total) in the Normative Aging Study.24
Studies conducted in the Greater Boston area have reported positive associations between short-term exposure to ambient air pollutants and acute cardiovascular events,24–26 and our group previously has observed positive associations between short-term exposure to higher levels of ambient air pollution and biomarkers of oxidative stress (myeloperoxidase and 8-epi-prostaglandin F2α) among participants from the Framingham Heart Study.27 However, the associations for circulating biomarkers of systemic inflammation among the participants have not been studied.
We therefore, examined the associations of short-term exposure to ambient air pollutants, measured at central and local air pollution monitors, with circulating concentrations of several biomarkers of systemic inflammation, including CRP, fibrinogen, interleukin-6, tumor necrosis factor α (TNFα), and TNF receptor 2 (TNFR2) among participants from the Framingham Offspring and Third Generation cohorts.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
The mean age of our study sample was 53.6 years old (standard deviation: 14.2) and 54% of the observations were in women (Table 1). The characteristics of measured biomarkers are shown in Table 1. The average levels of 1-day moving average PM2.5 was 9.7 μg/m3 (Table 2). As expected, BC and SO42− were highly correlated with PM2.5 (r=0.73 and 0.82, respectively), and O3 was negatively correlated with NOx (Table 2). Descriptive statistics and correlation matrix of the 1- to 7-day moving averages of the air pollutants are shown in Supplemental Table I and II. The distributions of measured air pollutants are shown in Supplemental Figure I and the distributions of measured inflammatory biomarkers are shown in Supplemental Figure II.
Table 1.
Characteristics of the 6,814 observations from 3,996 participants in the Framingham Offspring cohort examination 7 (1998–2001), examination 8 (2005–2008), Third Generation cohort examination 1 (2002–2005), and examination 2 (2008–2011).
| Characteristics | Offspring cohort* (N=1,999) | Third Generation cohort* (N=1,997) | Overall* (N=3,996) |
|---|---|---|---|
| Number of observations | 3,396 | 3,418 | 6,814 |
| Age, years | 64.2 [9.8] | 43.1 [9.3] | 53.6 [14.2] |
| Women | 1,823 (54%) | 1,825 (53%) | 3,648 (54%) |
| Body mass index, kg/m2 | 28.5 [5.4] | 27.6 [5.7] | 28.1 [5.6] |
| Alcohol, drinks/week | 4.3 [6.9] | 4.4 [6.2] | 4.3 [6.5] |
| Former Smoker | 1,938 (57%) | 1,114 (33%) | 3,052 (45%) |
| Education† | |||
| High school or less | 1,217 (36%) | 496 (15%) | 1,713 (25%) |
| Some college | 1,046 (31%) | 1,059 (31%) | 2,105 (31%) |
| College graduate | 1,103 (32%) | 1,858 (54%) | 2,961 (43%) |
| Antihypertensive medication use | 1,602 (47%) | 507 (15%) | 2,109 (31%) |
| Statins use | 1,083 (32%) | 386 (11%) | 1,469 (22%) |
| Cardiovascular disease | 504 (15%) | 66 (2%) | 570 (8%) |
| Diabetes | 534 (16%) | 143 (4%) | 677 (10%) |
|
| |||
| CRPǂ, mg/l | 2.0 [2.2] | 1.2 [1.5] | 1.6 [1.8] |
| Fibrinogenǂ, mg/100 ml | 372 [72] | 331 [66] | 351 [72] |
| Interleukin-6ǂ, pg/ml | 2.0 [1.5] | 1.4 [0.9] | 1.6 [1.2] |
| TNFαǂ, pg/ml | 1.3 [0.6] | 1.3 [0.6] | |
| TNFR2ǂ, pg/ml | 2289 [812] | 2,180 [524] | 2,250 [720] |
Abbreviation: SD, standard deviation; CRP, C-reactive protein; TNFR2, tumor necrosis factor receptor 2; TNFα: tumor necrosis factor α.
Mean [SD] or N (%).
There were 35 observations where educational attainment information were missing.
Geometric mean [standard deviation of the geometric mean].
Table 2.
Characteristics of air pollutants 1 day prior to the examination date in the study sample.
| Pollutant | Number of observations | Mean (SD) | Interquartile Range | Spearman Correlation Coefficients
|
|||
|---|---|---|---|---|---|---|---|
| BC | SO42− | NOx | O3 | ||||
| PM2.5, μg/m3 | 6,800 | 9.7 (5.8) | 5.7 | 0.73 | 0.82 | 0.43 | 0.02 |
| BC, μg/m3 | 6,793 | 0.8 (0.4) | 0.5 | 0.55 | 0.58 | −0.25 | |
| SO42−, μg/m3 | 5,921 | 2.9 (2.4) | 2.2 | 0.32 | 0.12 | ||
| NOx, ppb | 6,510 | 36.5 (20.0) | 19.0 | −0.54 | |||
| O3, ppb | 6,805 | 23.7 (10.9) | 14.4 | ||||
Abbreviation: SD, standard deviation; PM2.5, fine particulate matter; BC, black carbon; SO42−, sulfate; NOx, nitrogen oxides; O3, ozone.
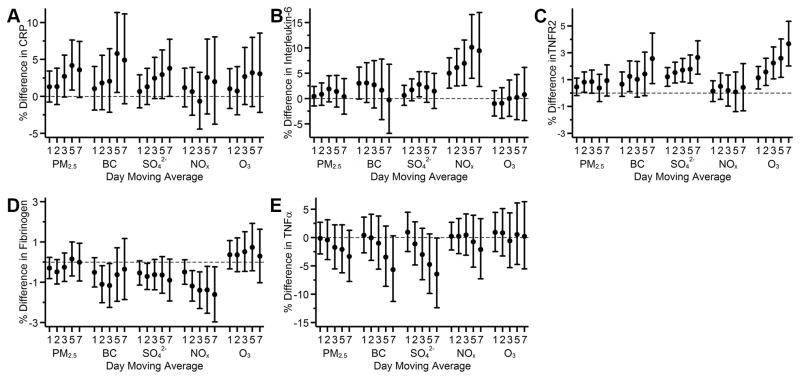
Higher levels of 3- to 7-day moving averages of PM2.5 and SO42− were associated with higher CRP levels (Figure 1A): a 5 μg/m3 higher 5-day moving average PM2.5 was associated with 4.2% (95% CI: 0.8, 7.6) higher circulating CRP, and a 2 μg/m3 higher 5-day moving average SO42− was associated with 2.9% (95% CI: −0.3, 6.3) higher circulating CRP (Figure 1A). A 0.5 μg/m3 higher 5-day moving average BC was associated with 5.8% (95% CI: 0.5, 11.4) higher CRP, however, the associations with other moving averages were generally null. NOx was consistently and positively associated with interleukin-6 across multiple moving averages (Figure 1B); and BC, SO42−, and O3 were positively associated with TNFR2 (Figure 1C). We unexpectedly observed a pattern of negative associations of BC, SO42−, and NOx with fibrinogen (Figure 1D), and of 5- and 7-day moving averages of SO42− with TNFα (Figure 1E). Overall, the magnitude of the associations appeared larger at longer moving averages, and associations otherwise were generally null.
Figure 1.
Associations of 1- to 7-day moving averages of air pollutants with A) loge C-reactive protein (CRP); B) loge interleukin-6; C) loge tumor necrosis factor receptor 2 (TNFR2); D) loge fibrinogen; and E) loge TNFα among participants from the Framingham Offspring cohort examination 7 (1998–2001), examination 8 (2005–2008), Third Generation cohort examination 1 (2002–2005), and examination 2 (2008–2011). Models were adjusted for centered age, (centered age)2, sex, body mass index, smoking status, pack years, alcohol intake, educational attainment, census tract median household income, date of examination visit, sine and cosine season, day of week, temperature, and relative humidity. An exam identifier was added for CRP, interleukin-6, TNFR2, and fibrinogen. Results were scaled to 5 μg/m3 for fine particulate matter (PM2.5), 0.5 μg/m3 for black carbon (BC), 2 μg/m3 for sulfate (SO42−), 20 ppb for nitrogen oxides (NOx), and 10 ppb for ozone (O3). Error bars indicate the 95% confidence intervals.
In sensitivity analyses, we examined the associations under the following conditions separately and together, our results were not materially changed: excluding observations that had a daily average PM2.5 concentration>35 μg/m3 in any one of the 7 days prior to the examination date, including current smokers in the analyses, restricting study participants to those who lived within 40 km from the central monitoring site, restricting analyses to the same participants across multiple moving averages for each pollutant, and adjusting for additional individual- and area-level socioeconomic position variables and clinical factors. The results are shown in Supplemental Figure III–VII.
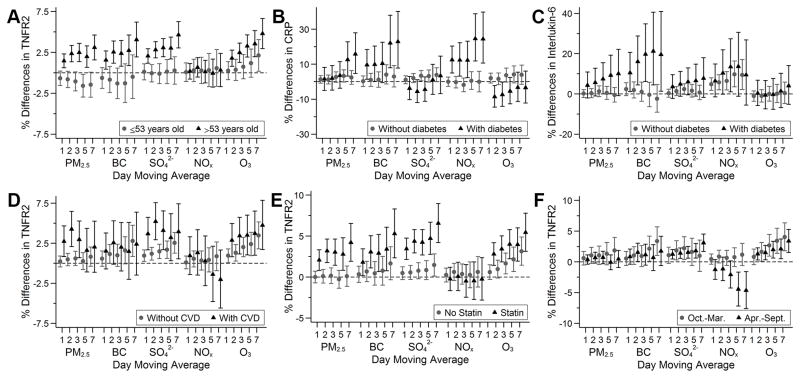
The association between PM2.5, BC, and SO42− and TNFR2 was stronger among participants older than 53 years old than those who were younger (Figure 2A). Among participants with diabetes, we found a pattern of stronger associations between BC and NOx and CRP, and between BC and interleukin-6 across multiple moving averages compared with participants without diabetes (Figure 2B and 2C). Additionally, associations between longer moving averages of PM2.5 and CRP were stronger among participants with diabetes than those without (Figure 2B). Furthermore, we observed some evidence of stronger associations of air pollutants with TNFR2 among participants with cardiovascular disease or those who were using statins (Figure 2D and 2E). Although there was no overall association between NOx and TNFR2, there was an apparent negative association in the warm seasons (April–September) with longer moving averages (Figure 2F). Associations otherwise did not differ by median age, sex, educational attainment, diabetes status, cardiovascular disease status, statins use, anti-hypertensives use, or season (Supplemental Figure VIII–XV). We separately examined the associations between air pollutants and the biomarkers among participants from each cohort and have included the results as Supplemental Figure XVI and XVII.
Figure 2.
Associations of 1- to 7-day moving averages of air pollutants with TNFR2 stratified by median age (A); with C-reactive protein (CRP) and interleukin-6 stratified by diabetes status (B and C); and with TNFR2 stratified by season (D) among participants from the Framingham Offspring cohort examination 7 (1998–2001), examination 8 (2005–2008), Third Generation cohort examination 1 (2002–2005), and examination 2 (2008–2011). Models were adjusted for centered age, (centered age)2, sex, body mass index, smoking status, pack years, alcohol intake, educational attainment, census tract median household income, date of examination visit, sine and cosine season, day of week, temperature, and relative humidity. An exam identifier was added for CRP, interleukin-6, TNFR2, and fibrinogen. Results were scaled to 5 μg/m3 for fine particulate matter (PM2.5), 0.5 μg/m3 for black carbon (BC), 2 μg/m3 for sulfate (SO42−), 20 ppb for nitrogen oxides (NOx), and 10 ppb for ozone (O3). Error bars indicate the 95% confidence intervals.
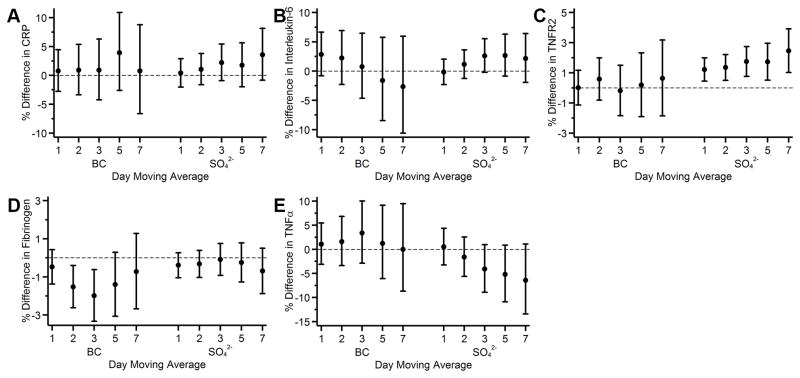
Because BC and SO42− were moderately correlated (r=0.55), we conducted a sensitivity analysis where we put both pollutants (of the same day moving average) in the same model: the associations for both BC and SO42− were attenuated, however, SO42− was still positively associated with TNFR2 and negatively associated with TNFα, and BC was still negatively associated with fibrinogen (Figure 3C, 3D, and 3E).
Figure 3.
Associations of 1- to 7-day moving averages of black carbon (BC) and sulfate (SO42−) with A) loge C-reactive protein (CRP); B) loge interleukin-6; C) loge tumor necrosis factor receptor 2 (TNFR2); D) loge fibrinogen; and E) loge TNFα among participants from the Framingham Offspring cohort examination 7 (1998–2001), examination 8 (2005–2008), Third Generation cohort examination 1 (2002–2005), and examination 2 (2008–2011). Models were adjusted for same-day moving averages of BC and SO42−. Covariates included in the models were centered age, (centered age)2, sex, body mass index, smoking status, pack years, alcohol intake, educational attainment, census tract median household income, date of examination visit, sine and cosine season, day of week, temperature, and relative humidity. An exam identifier was added for TNFR2 and fibrinogen. Results were scaled to 5 μg/m3 for fine particulate matter (PM2.5), 0.5 μg/m3 for BC, 2 μg/m3 for SO42−, 20 ppb for nitrogen oxides (NOx), and 10 ppb for ozone (O3). Error bars indicate the 95% confidence intervals.
Discussion
Among participants from the community-based Framingham Offspring and Third Generation cohorts living in the greater Boston area, we observed positive associations of PM2.5 and SO42− with blood levels of CRP, of NOx with serum interleukin-6, and of BC, SO42− and O3 with plasma TNFR2 concentrations. We also observed negative associations between BC, SO42−, and NOx with plasma fibrinogen and between SO42− and TNFα. Stronger associations were mostly found with longer moving averages. We further showed that the observed associations for CRP were stronger among participants who had diabetes mellitus, and the observed associations for TNFR2 were stronger among those who were older than 53 years at the time of their examination visits.
Acute inflammation may initiate increased secretion of pro-inflammatory cytokines such as interleukin-6 and TNFα, and further stimulate and mediate hepatic production of acute phase proteins, such as CRP and fibrinogen.28, 29 The levels of these biomarkers would increase within a few days after initiation of inflammatory response.29, 30 Results from studies in vivo suggested a downmodulating role of TNFR2 in TNFα-induced inflammatory responses.31–34 Moreover, TNF receptor plays an important role in mediating O3-induced pulmonary inflammation and hyperreactivity.35 Our work in the Framingham Offspring cohort showed that higher short-term exposures to ambient air pollutants measured at the central site was associated with higher levels of oxidative stress biomarkers.27 Thus, it is reasonable to expect that higher levels of air pollution may also be associated with higher levels of inflammatory response among the participants. The magnitude of the associations in our findings were rather small. Thus, the interpretation of this likely transient change may not be suitable for clinical interpretation. From a physiological perspective, however, this transient but small difference may still contribute to the associations between air pollution and acute cardiovascular events, as air pollution-induced oxidative stress and inflammation was considered as one of the underlying mechanisms.
In the current study, we observed positive associations of PM2.5, BC, and SO42− with serum CRP at longer moving averages of these pollutants. Past studies reported mixed associations between acute exposures to ambient air pollutants and CRP, interleukin-6, TNFα, or TNFR2.8–13, 22, 23 In the Multi-Ethnic Study of Atherosclerosis, higher levels of PM2.5 on the day of blood draw was associated with higher CRP concentrations (1% difference in CRP for each 5 μg/m3 difference in PM2.5, 95% CI: 0–3%), but this positive association was not found with longer moving averages.22, 23 The discrepancy may be due to different population characteristics or different compositions of ambient PM2.5. We did not find positive associations for TNFα, however, O3 was positively associated with TNFR2. It is possible that higher levels of TNFR2 masked the associations for TNFα. However, given the observational design of our study, we cannot rule out residual confounding or unmeasured confounding.
The associations of PM2.5 with fibrinogen in our current study were generally null, but we observed negative associations of BC, SO42−, and NOx with fibrinogen. Prior studies have not found consistent associations between ambient air pollutants and fibrinogen.6, 7, 13–15, 17–22 For example, in the Normative Aging Study, a cohort of elderly men, short-term exposure to BC and NOx were associated with higher blood levels of fibrinogen.6 Among 2,086 women, Green et al. reported null associations between 1-day average PM2.5 and O3 with plasma fibrinogen.19 In another study conducted in Italy with 1,218 healthy participants, the 7-day moving average of O3 was negatively associated with plasma fibrinogen.21 The reasons for the observed negative associations between BC, SO42−, and NOx and fibrinogen were unclear and we do not have a plausible explanation; it could be a chance finding, however, we cannot rule out residual confounding or unmeasured confounding.
We observed consistently stronger associations of PM2.5, BC, and SO42− with TNFR2 among older participants. Older populations are generally considered particularly susceptible to the adverse health effects of air pollution on cardiovascular disease morbidity and mortality.36–38 Although air pollution-induced inflammation may be one of the underlying pathways linking air pollution exposure to acute cardiovascular events, few studies have examined whether age modifies the association between air pollution and inflammation, and have had mixed results.13, 23 Our findings of stronger associations of PM2.5, BC, and SO42− with TNFR2 among older participants adds some supportive new evidence to the literature. Our findings further suggest that the relatively higher prevalence of cardiovascular disease, diabetes, and indication for medication use may contribute to the susceptibility. Sustained baseline inflammation may induce endothelial dysfunction and dysregulation of cytokine secretion, which may exaggerate inflammatory response among participants with diabetes.10 Similar to our previous report of stronger associations between higher levels of ambient air pollution and biomarkers of oxidative stress in the Framingham Heart Study participants who had diabetes,27 we observed some evidence of a larger magnitude of the associations of PM2.5, BC, and NOx with CRP, and of BC with interleukin-6 among participants with diabetes than those without. The wide 95% CIs indicate a loss of statistical power that may be due to the relatively low prevalence of diabetes in our study sample. Lastly, the observed differing associations between air pollutants and TNFR2 between the Offspring and Third Generation cohorts were likely driven by the uneven distribution of age and clinical factors such as cardiovascular disease and indication for statins use.
In our study region, ambient air pollution is from both regional emissions and local sources such as traffic and residential heating.39 In this region, BC and NOx were viewed as correlates of local traffic, and SO42− is primarily transported from coal-fired power plants and to a lesser extent is generated from diesel exhaust.40 In our sensitivity analysis, adjusting for both BC and SO42− in the models led to attenuated associations but the positive associations between SO42− and TNFR2 and the negative associations between SO42− and TNFα remained, suggesting a possibly stronger role of transported air pollutants in these associations.
Our study has several limitations that should be noted. The exposures were measured at central air pollution monitoring stations and were assigned to each participant. This may induce potential exposure measurement error, likely non-differential, which may decrease our statistical power and attenuate our results. Previous studies in the Boston region compared air pollutants measured by personal monitor and the central site, and showed moderate correlations between PM2.5 and SO42− measured at this central site and personal exposure levels (slope for PM2.5 was 0.3 in winter and 0.8–0.9 in summer; slope for SO42− was 0.4–0.6 in winter and 0.7 in summer),41, 42 which provided support and rationale for the exposure assignment. Additionally, most of the short-term variability in exposure within our region is related to temporal (day to day) variability, rather than spatial variability.43 Moreover, the levels of air pollutants were related to the date that participants came for their examination appointment. Since participants scheduled their appointments months earlier, it is unlikely that the choice of date would be related to the air pollution levels before the pre-scheduled appointment. Thus, we expect the exposure measurement error due to assignment to be non-differential, leading to attenuated point estimates and wider confidence intervals. The study participants were predominantly middle-aged and older adults of European ancestry, which limits the generalizability of our results to populations of different ethnicities or age groups. Last, we cannot exclude the possibility of residual confounding, unmeasured confounding, thus, the observed associations should not be used to infer causality.
There are also several strengths worth noting. First, we had a relatively large study sample from two community-based cohorts with standardized protocols for physical examinations and high-quality biomarker assessments. Second, we adjusted for demographic characteristics, lifestyle, individual- and area-level of socioeconomic position, meteorology, and time in our analyses. Third, assessments of air pollutants and biomarkers were performed separately in our study (blinded to the results of each other), and the participants scheduled their examination visit months in advance.
In conclusion, our findings suggest that in a region in compliance with current air quality standards, elevated exposure to ambient air pollutants over a few days was associated with higher levels of biomarkers of systemic inflammation. Together with our previous work on biomarkers of oxidative stress, we have provided suggestive evidence for potential pathways that may partly explain the link between short-term air pollution exposures and acute cardiovascular events in our study region.
Supplementary Material
Highlights.
Air pollution-induced systemic inflammation may partially explain the link between short-term exposure to higher levels of air pollution and acute cardiovascular events, however, results from prior large-scale epidemiological studies on the associations between short-term exposure to air pollution and systemic inflammation are mixed.
We found positive associations of short-term exposure to fine particulate matter and sulfate with C-reactive protein, of nitrogen oxides with interleukin-6, and of black carbon, sulfate, and ozone with tumor necrosis factor receptor 2 in a large sample of generally healthy participants in a region with relatively low levels of air pollution.
We unexpectedly observed negative associations of black carbon, sulfate, and nitrogen oxides with fibrinogen.
Older people and individuals with type 2 diabetes might be more susceptible to a pro-inflammatory response to high air pollution exposure.
Acknowledgments
We thank the Framingham Offspring and Third Generation Study Participants.
Sources of Funding
This publication was made possible by USEPA grant numbers RD-834798 and RD-835872. Its contents are solely the responsibility of the grantee and do not necessarily represent the official views of the USEPA. Further, USEPA does not endorse the purchase of any commercial products or services mentioned in the publication. This work was further supported by the National Heart, Lung, and Blood Institute (NHLBI) contracts and grants HHSN268201500001I, N01-HC 25195, 1RO1HL64753, R01HL076784, 1R01AG028321, 1R01HL128914, 1P50HL120163, and T32HL007575, and National Institute of Environmental Health Sciences (NIEHS) grants P01ES09825, K23ES026204, R00ES022243, and P30ES000002.
Nonstandard Abbreviations and Acronyms
- PM2.5
Fine particulate matter
- BC
Black carbon
- SO42−
Sulfate
- NOx
Nitrogen oxides
- O3
Ozone
- CRP
C-reactive protein
- TNFα
Tumor necrosis factor α
- TNFR2
Tumor necrosis factor receptor 2
Footnotes
Disclosures
WL, KSD, MBR, PLL, JDS, BAC, PK, DRG, JFK, RSV, EJB, and MAM declare no conflicts of interest. EHW received non-financial support from Servier.
References
- 1.Brook RD, Rajagopalan S, Pope CA, 3rd, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the american heart association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 2.Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, Luepker R, Mittleman M, Samet J, Smith SC, Jr, Tager I Expert Panel on Population, Prevention Science of the American Heart Association. Air pollution and cardiovascular disease: A statement for healthcare professionals from the expert panel on population and prevention science of the american heart association. Circulation. 2004;109:2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- 3.Xu X, Jiang SY, Wang TY, Bai Y, Zhong M, Wang A, Lippmann M, Chen LC, Rajagopalan S, Sun Q. Inflammatory response to fine particulate air pollution exposure: Neutrophil versus monocyte. PLoS One. 2013;8:e71414. doi: 10.1371/journal.pone.0071414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun L, Liu C, Xu X, et al. Ambient fine particulate matter and ozone exposures induce inflammation in epicardial and perirenal adipose tissues in rats fed a high fructose diet. Part Fibre Toxicol. 2013;10:43. doi: 10.1186/1743-8977-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson DW, Aung HH, Lame MW, Plummer L, Pinkerton KE, Ham W, Kleeman M, Norris JW, Tablin F. Exposure of mice to concentrated ambient particulate matter results in platelet and systemic cytokine activation. Inhal Toxicol. 2010;22:267–276. doi: 10.3109/08958370903278069. [DOI] [PubMed] [Google Scholar]
- 6.Bind MA, Baccarelli A, Zanobetti A, Tarantini L, Suh H, Vokonas P, Schwartz J. Air pollution and markers of coagulation, inflammation, and endothelial function: Associations and epigene-environment interactions in an elderly cohort. Epidemiology. 2012;23:332–340. doi: 10.1097/EDE.0b013e31824523f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeka A, Sullivan JR, Vokonas PS, Sparrow D, Schwartz J. Inflammatory markers and particulate air pollution: Characterizing the pathway to disease. Int J Epidemiol. 2006;35:1347–1354. doi: 10.1093/ije/dyl132. [DOI] [PubMed] [Google Scholar]
- 8.Fang SC, Mehta AJ, Alexeeff SE, Gryparis A, Coull B, Vokonas P, Christiani DC, Schwartz J. Residential black carbon exposure and circulating markers of systemic inflammation in elderly males: The normative aging study. Environ Health Perspect. 2012;120:674–680. doi: 10.1289/ehp.1103982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson AM, Zanobetti A, Silverman F, Schwartz J, Coull B, Urch B, Speck M, Brook JR, Manno M, Gold DR. Baseline repeated measures from controlled human exposure studies: Associations between ambient air pollution exposure and the systemic inflammatory biomarkers il-6 and fibrinogen. Environ Health Perspect. 2010;118:120–124. doi: 10.1289/ehp.0900550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubowsky SD, Suh H, Schwartz J, Coull BA, Gold DR. Diabetes, obesity, and hypertension may enhance associations between air pollution and markers of systemic inflammation. Environ Health Perspect. 2006;114:992–998. doi: 10.1289/ehp.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delfino RJ, Staimer N, Tjoa T, Gillen DL, Polidori A, Arhami M, Kleinman MT, Vaziri ND, Longhurst J, Sioutas C. Air pollution exposures and circulating biomarkers of effect in a susceptible population: Clues to potential causal component mixtures and mechanisms. Environ Health Perspect. 2009;117:1232–1238. doi: 10.1289/ehp.0800194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delfino RJ, Staimer N, Tjoa T, Polidori A, Arhami M, Gillen DL, Kleinman MT, Vaziri ND, Longhurst J, Zaldivar F, Sioutas C. Circulating biomarkers of inflammation, antioxidant activity, and platelet activation are associated with primary combustion aerosols in subjects with coronary artery disease. Environ Health Perspect. 2008;116:898–906. doi: 10.1289/ehp.11189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruckerl R, Hampel R, Breitner S, et al. Associations between ambient air pollution and blood markers of inflammation and coagulation/fibrinolysis in susceptible populations. Environ Int. 2014;70:32–49. doi: 10.1016/j.envint.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 14.Ruckerl R, Greven S, Ljungman P, et al. Air pollution and inflammation (interleukin-6, c-reactive protein, fibrinogen) in myocardial infarction survivors. Environ Health Perspect. 2007;115:1072–1080. doi: 10.1289/ehp.10021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghio AJ, Hall A, Bassett MA, Cascio WE, Devlin RB. Exposure to concentrated ambient air particles alters hematologic indices in humans. Inhal Toxicol. 2003;15:1465–1478. doi: 10.1080/08958370390249111. [DOI] [PubMed] [Google Scholar]
- 16.Williams L, Ulrich CM, Larson T, Wener MH, Wood B, Chen-Levy Z, Campbell PT, Potter J, McTiernan A, Roos AJ. Fine particulate matter (pm2.5) air pollution and immune status among women in the seattle area. Arch Environ Occup Health. 2011;66:155–165. doi: 10.1080/19338244.2010.539636. [DOI] [PubMed] [Google Scholar]
- 17.Steinvil A, Kordova-Biezuner L, Shapira I, Berliner S, Rogowski O. Short-term exposure to air pollution and inflammation-sensitive biomarkers. Environ Res. 2008;106:51–61. doi: 10.1016/j.envres.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Liao D, Heiss G, Chinchilli VM, Duan Y, Folsom AR, Lin HM, Salomaa V. Association of criteria pollutants with plasma hemostatic/inflammatory markers: A population-based study. J Expo Anal Environ Epidemiol. 2005;15:319–328. doi: 10.1038/sj.jea.7500408. [DOI] [PubMed] [Google Scholar]
- 19.Green R, Broadwin R, Malig B, Basu R, Gold EB, Qi L, Sternfeld B, Bromberger JT, Greendale GA, Kravitz HM, Tomey K, Matthews K, Derby CA, Jackson EA, Ostro B. Long- and short-term exposure to air pollution and inflammatory/hemostatic markers in midlife women. Epidemiology. 2016;27:211–220. doi: 10.1097/EDE.0000000000000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panasevich S, Leander K, Rosenlund M, Ljungman P, Bellander T, de Faire U, Pershagen G, Nyberg F. Associations of long- and short-term air pollution exposure with markers of inflammation and coagulation in a population sample. Occup Environ Med. 2009;66:747–753. doi: 10.1136/oem.2008.043471. [DOI] [PubMed] [Google Scholar]
- 21.Baccarelli A, Zanobetti A, Martinelli I, Grillo P, Hou L, Giacomini S, Bonzini M, Lanzani G, Mannucci PM, Bertazzi PA, Schwartz J. Effects of exposure to air pollution on blood coagulation. J Thromb Haemost. 2007;5:252–260. doi: 10.1111/j.1538-7836.2007.02300.x. [DOI] [PubMed] [Google Scholar]
- 22.Hajat A, Allison M, Diez-Roux AV, Jenny NS, Jorgensen NW, Szpiro AA, Vedal S, Kaufman JD. Long-term exposure to air pollution and markers of inflammation, coagulation, and endothelial activation: A repeat-measures analysis in the multi-ethnic study of atherosclerosis (mesa) Epidemiology. 2015 doi: 10.1097/EDE.0000000000000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diez Roux AV, Auchincloss AH, Astor B, Barr RG, Cushman M, Dvonch T, Jacobs DR, Jr, Kaufman J, Lin X, Samson P. Recent exposure to particulate matter and c-reactive protein concentration in the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2006;164:437–448. doi: 10.1093/aje/kwj186. [DOI] [PubMed] [Google Scholar]
- 24.Wellenius GA, Burger MR, Coull BA, Schwartz J, Suh HH, Koutrakis P, Schlaug G, Gold DR, Mittleman MA. Ambient air pollution and the risk of acute ischemic stroke. Arch Intern Med. 2012;172:229–234. doi: 10.1001/archinternmed.2011.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Link MS, Luttmann-Gibson H, Schwartz J, Mittleman MA, Wessler B, Gold DR, Dockery DW, Laden F. Acute exposure to air pollution triggers atrial fibrillation. J Am Coll Cardiol. 2013;62:816–825. doi: 10.1016/j.jacc.2013.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peters A, Dockery DW, Muller JE, Mittleman MA. Increased particulate air pollution and the triggering of myocardial infarction. Circulation. 2001;103:2810–2815. doi: 10.1161/01.cir.103.23.2810. [DOI] [PubMed] [Google Scholar]
- 27.Li W, Wilker EH, Dorans KS, Rice MB, Schwartz J, Coull BA, Koutrakis P, Gold DR, Keaney JF, Jr, Lin H, Vasan RS, Benjamin EJ, Mittleman MA. Short-term exposure to air pollution and biomarkers of oxidative stress: The framingham heart study. J Am Heart Assoc. 2016:5. doi: 10.1161/JAHA.115.002742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stoner L, Lucero AA, Palmer BR, Jones LM, Young JM, Faulkner J. Inflammatory biomarkers for predicting cardiovascular disease. Clin Biochem. 2013;46:1353–1371. doi: 10.1016/j.clinbiochem.2013.05.070. [DOI] [PubMed] [Google Scholar]
- 29.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 30.Oda S, Hirasawa H, Shiga H, Nakanishi K, Matsuda K, Nakamua M. Sequential measurement of il-6 blood levels in patients with systemic inflammatory response syndrome (sirs)/sepsis. Cytokine. 2005;29:169–175. doi: 10.1016/j.cyto.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 31.Peschon JJ, Torrance DS, Stocking KL, Glaccum MB, Otten C, Willis CR, Charrier K, Morrissey PJ, Ware CB, Mohler KM. Tnf receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J Immunol. 1998;160:943–952. [PubMed] [Google Scholar]
- 32.MacEwan DJ. Tnf ligands and receptors--a matter of life and death. Br J Pharmacol. 2002;135:855–875. doi: 10.1038/sj.bjp.0704549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hehlgans T, Pfeffer K. The intriguing biology of the tumour necrosis factor/tumour necrosis factor receptor superfamily: Players, rules and the games. Immunology. 2005;115:1–20. doi: 10.1111/j.1365-2567.2005.02143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chandrasekharan UM, Siemionow M, Unsal M, Yang L, Poptic E, Bohn J, Ozer K, Zhou Z, Howe PH, Penn M, DiCorleto PE. Tumor necrosis factor alpha (tnf-alpha) receptor-ii is required for tnf-alpha-induced leukocyte-endothelial interaction in vivo. Blood. 2007;109:1938–1944. doi: 10.1182/blood-2006-05-020875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cho HY, Zhang LY, Kleeberger SR. Ozone-induced lung inflammation and hyperreactivity are mediated via tumor necrosis factor-alpha receptors. Am J Physiol Lung Cell Mol Physiol. 2001;280:L537–546. doi: 10.1152/ajplung.2001.280.3.L537. [DOI] [PubMed] [Google Scholar]
- 36.Pope CA., 3rd Epidemiology of fine particulate air pollution and human health: Biologic mechanisms and who’s at risk? Environmental health perspectives. 2000;108(Suppl 4):713–723. doi: 10.1289/ehp.108-1637679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sacks JD, Stanek LW, Luben TJ, Johns DO, Buckley BJ, Brown JS, Ross M. Particulate matter-induced health effects: Who is susceptible? Environmental health perspectives. 2011;119:446–454. doi: 10.1289/ehp.1002255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bell ML, Zanobetti A, Dominici F. Evidence on vulnerability and susceptibility to health risks associated with short-term exposure to particulate matter: A systematic review and meta-analysis. Am J Epidemiol. 2013;178:865–876. doi: 10.1093/aje/kwt090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Masri S, Kang CM, Koutrakis P. Composition and sources of fine and coarse particles collected during 2002–2010 in boston, ma. J Air Waste Manag Assoc. 2015;65:287–297. doi: 10.1080/10962247.2014.982307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee HJ, Kang CM, Coull BA, Bell ML, Koutrakis P. Assessment of primary and secondary ambient particle trends using satellite aerosol optical depth and ground speciation data in the new england region, united states. Environ Res. 2014;133:103–110. doi: 10.1016/j.envres.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sarnat JA, Brown KW, Schwartz J, Coull BA, Koutrakis P. Ambient gas concentrations and personal particulate matter exposures: Implications for studying the health effects of particles. Epidemiology. 2005;16:385–395. doi: 10.1097/01.ede.0000155505.04775.33. [DOI] [PubMed] [Google Scholar]
- 42.Brown KW, Sarnat JA, Suh HH, Coull BA, Spengler JD, Koutrakis P. Ambient site, home outdoor and home indoor particulate concentrations as proxies of personal exposures. Journal of environmental monitoring : JEM. 2008;10:1041–1051. doi: 10.1039/b805991h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee HJ, Gent JF, Leaderer BP, Koutrakis P. Spatial and temporal variability of fine particle composition and source types in five cities of connecticut and massachusetts. Sci Total Environ. 2011;409:2133–2142. doi: 10.1016/j.scitotenv.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.