Abstract
Objective
Among African Americans, the APOL1 risk variants have been associated with various types of kidney disease and chronic kidney disease progression. We aimed to determine whether these same risk variants also confer an increased risk for cardiovascular disease (CVD).
Approach and Results
In a cohort of African Americans with hypertension-attributed chronic kidney disease followed for up to 12 years, we used Cox proportional hazards models to estimate the relative hazard of a composite CVD outcome (cardiovascular death or hospitalization for myocardial infarction, cardiac revascularization procedure, heart failure, or stroke) for the APOL1 high- (2 risk variants) versus low-risk (0–1 risk variant) genotypes. We adjusted for age, gender, ancestry, smoking, heart disease history, body mass index, cholesterol, randomized treatment groups, and baseline and longitudinal estimated glomerular filtration rate (eGFR), systolic blood pressure, and proteinuria. Among 693 participants with APOL1 genotyping available (23% high-risk), the high-risk group had lower mean eGFR (44.7 vs. 50.1 ml/min/1.73 m2) and greater proteinuria (median 0.19 vs. 0.06) compared to the low-risk group at baseline. There was no significant association between APOL1 genotypes and the composite CVD outcome in both unadjusted (HR=1.23; 95% CI: 0.83 to 1.81) and fully adjusted (HR=1.16; 95% CI: 0.77 to 1.76) models; however, in using an additive model, APOL1 high-risk variants were associated with increased cardiovascular mortality.
Conclusions
Among African Americans with hypertension-attributed chronic kidney disease, APOL1 risk variants were not associated with an overall risk for CVD, though some signals for cardiovascular mortality were noted.
Keywords: APOL1, Apolipoprotein L1, Cardiovascular Disease, AASK, Coronary Artery Disease, Heart Failure
Subject Codes: Cardiovascular Disease, Nephrology and Kidney, Race and Ethnicity, Genetics
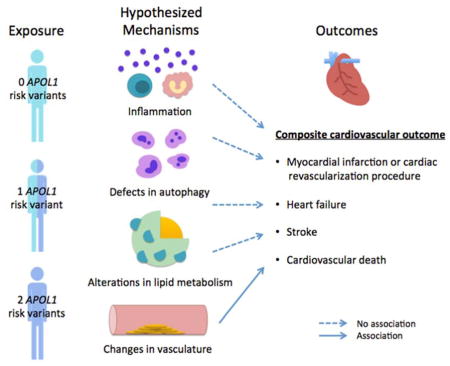
Graphical Abstract
INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of death in patients with chronic kidney disease (CKD), with the risk for CVD mortality increasing incrementally as kidney function declines.1–3 Compared to European Americans, African Americans are at heightened risk for all-cause mortality and CVD associated with CKD.4 While racial disparities in traditional risk factors and quality of care exist, genetic susceptibilities may also contribute.2,4,5
Among African Americans, risk variants in the gene encoding apolipoprotein L1 (APOL1) have been associated with many types of kidney disease.6–10 Parsa and colleagues reported in the African American Study of Kidney Disease and Hypertension (AASK) that individuals with 2 copies of the APOL1 high-risk variants had a nearly 2-fold greater risk of CKD progression compared to individuals with 0 or 1 copy.10 Whether these risk variants also confer an increased risk for CVD is uncertain, as results from the few available studies have been conflicting.11–15 In the Jackson Heart Study (JHS), the Women’s Health Initiative (WHI), and the Cardiovascular Heart Study (CHS), the APOL1 high-risk variants have been associated with an increased risk of adverse cardiovascular events.11,16 Other studies, however, have suggested no association between the risk variants and CVD.11,12,15 Importantly, the majority of participants in these cohorts did not have established CKD. Thus, how APOL1 relates to CVD in the context of reduced kidney function remains unknown.
With up to 12 years of follow-up from the well-characterized population of AASK, we aimed to determine whether the APOL1 high-risk variants were associated with an increased risk for CVD outcomes in African Americans with hypertension-attributed CKD.
MATERIALS AND METHODS
Materials and Methods are available in the online-only Data Supplement.
RESULTS
Baseline Characteristics
Among the 693 AASK participants with APOL1 genotyping available, 33 (21%) individuals with the APOL1 high-risk genotypes (2 risk variants) and 111 (21%) individuals with the low-risk genotypes (0–1 risk variant) experienced the composite CVD outcome, which consisted of cardiovascular death or hospitalization for nonfatal myocardial infarction, cardiac revascularization procedure, heart failure, or stroke, prior to the onset of end-stage renal disease (ESRD). At baseline, individuals with the APOL1 high-risk genotypes were younger (mean age: 51.7 vs. 54.8 years) and had lower estimated glomerular filtration rates (mean eGFR: 44.7 vs. 50.1 ml/min/1.73 m2) and greater proteinuria (median urine protein-to-creatinine ratio: 0.19 vs. 0.06 g/g Cr) compared to those with the low-risk genotypes. History of heart disease was less common in the APOL1 high-risk group compared to the low-risk group (43% vs. 53%, respectively; Table 1).
Table 1.
Baseline characteristics of study population, by APOL1 risk allele status.
| Characteristic | APOL1 Low-Risk (n=533) | APOL1 High-Risk (n=160) | P-value |
|---|---|---|---|
| Age at randomization, years | 54.8 ± 10.1 | 51.7 ± 11.8 | <0.01 |
| Female | 210 (39%) | 69 (43%) | 0.40 |
| European ancestry, % | 17 ± 14 | 16 ± 12 | 0.19 |
| Smoking | 0.73 | ||
| Never | 224 (42%) | 72 (45%) | |
| Current | 150 (28%) | 45 (28%) | |
| Past | 159 (30%) | 43 (27%) | |
| History of heart disease | 281 (53%) | 69 (43%) | 0.03 |
| Body mass index, kg/m2 | 30.9 ± 6.5 | 31.7 ± 7.2 | 0.23 |
| Systolic blood pressure, mmHg | 144 ± 23 | 141 ± 23 | 0.24 |
| Total cholesterol, mg/dL | 211 ± 44 | 213 ± 44 | 0.70 |
| eGFR, ml/min/1.73 m2 | 50.1 ± 14.7 | 44.7 ± 13.5 | <0.01 |
| Urine protein-to-creatinine ratio, g/g Cr | 0.06 (0.03 to 0.22) | 0.19 (0.04 to 0.72) | <0.01 |
| Randomized blood pressure goal | 0.99 | ||
| Low | 270 (51%) | 81 (51%) | |
| Usual | 263 (49%) | 79 (49%) | |
| Randomized blood pressure drug | 0.77 | ||
| Metoprolol | 205 (38%) | 66 (41%) | |
| Ramipril | 224 (42%) | 66 (41%) | |
| Amlodipine | 104 (20%) | 28 (18%) |
Values presented as mean ± standard deviation, median (interquartile range), or n (%).
APOL1 high-risk defined as having 2 risk variants and low-risk defined as having 0–1 risk variant. Abbreviations: eGFR=estimated glomerular filtration rate; Cr=creatinine.
Association between APOL1 Risk Variants and Incident CVD
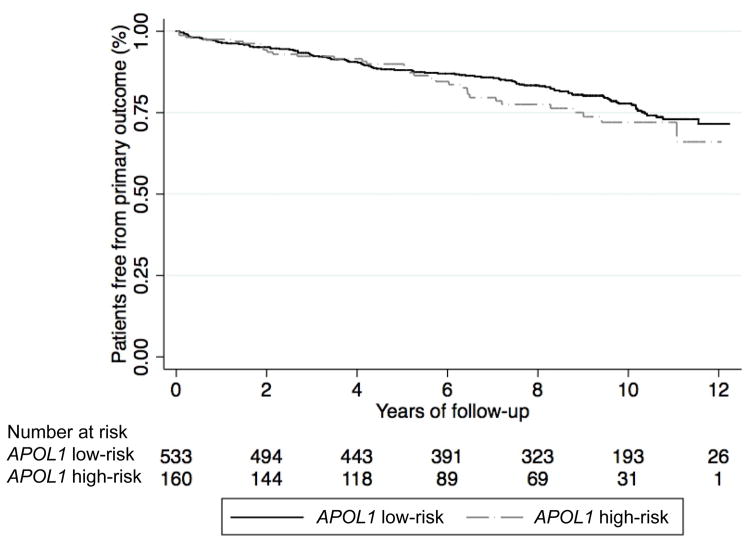
Over a mean follow-up of 7.7 years, the risk of a CVD event was not significantly different in the APOL1 high-risk group compared to the low-risk group (unadjusted hazard ratio [HR]=1.23; 95% confidence interval [CI]: 0.83 to 1.81; p=0.31; Table 2 and Figure 1). After adjusting for sociodemographic and clinical factors, the association between APOL1 risk status and CVD event remained null (Model 4 adjusted HR=1.16; 95% CI: 0.77 to 1.76; p=0.47; Table 2). Similar results were obtained when analyses were repeated using an additive or dominant genetic model (data not shown).
Table 2.
APOL1 risk status and the relative hazard of composite cardiovascular outcomes.
| Model | N | Events | HR APOL1 high- vs. low-risk* | 95% CI | P-value |
|---|---|---|---|---|---|
| Unadjusted | 693 | 144 | 1.23 | (0.83, 1.81) | 0.31 |
| Model 1: Adjusted for age, gender, % European ancestry, and randomized treatment groups | 693 | 144 | 1.25 | (0.84, 1.85) | 0.27 |
| Model 2: Additionally adjusted for baseline smoking, body mass index, total cholesterol, systolic blood pressure, and history of heart disease | 684 | 140 | 1.33 | (0.89, 1.99) | 0.16 |
| Model 3: Additionally adjusted for baseline eGFR and log-transformed proteinuria. | 682 | 139 | 1.23 | (0.81, 1.86) | 0.34 |
| Model 4: Additionally adjusted for longitudinal systolic blood pressure, eGFR, and log-transformed proteinuria as time-varying covariates | 682 | 139 | 1.16 | (0.77, 1.76) | 0.47 |
APOL1 high-risk defined as having 2 risk variants and low-risk defined as having 0–1 risk variant.
Abbreviations: HR=hazard ratio; CI=confidence interval; eGFR=estimated glomerular filtration rate
Figure 1.
Kaplan-Meier survival curves for composite cardiovascular events, by APOL1 risk status.
In sensitivity analyses, we found no significant association between APOL1 risk status and individual components of the composite CVD outcome in fully adjusted recessive genetic models (Supplementary Table I). We did, however, note that increasing numbers of APOL1 risk variants were associated with increased risk of cardiovascular death when using the additive genetic model (Model 4 adjusted HR=1.74; 95% CI: 1.03 to 2.96; p=0.04; Table 3). Other analyses using the additive and dominant genetic models revealed no significant association between APOL1 risk status and secondary CVD outcomes (p>0.05 for each). When considering 2 vs. 0 APOL1 risk variants for the composite CVD outcome (Model 4 adjusted HR=1.53; 95% CI: 0.92 to 2.54; p=0.10) or when using an alternative composite outcome of myocardial infarction, revascularization procedure, and stroke (Model 4 adjusted HR=1.41; 95% CI: 0.85 to 2.36; p=0.19; recessive genetic model), the hazard ratios were slightly higher but still not statistically significant. Finally, we performed analyses taking into account the competing risks of ESRD and death and found that the results for the composite CVD outcome were similar (adjusted sub-HR=1.16; 95% CI: 0.74 to 1.80; p=0.52).
Table 3.
APOL1 risk status and the relative hazard of cardiovascular death.
| Additive Genetic Model | |||
|---|---|---|---|
| Model | HR per APOL1 risk variant | 95% CI | P-value |
| Unadjusted | 1.62 | (0.99, 2.65) | 0.05 |
| Model 1: Adjusted for age, gender, % European ancestry, and randomized treatment groups | 1.74 | (1.05, 2.86) | 0.03 |
| Model 2: Additionally adjusted for baseline smoking, body mass index, total cholesterol, systolic blood pressure, and history of heart disease | 1.87 | (1.11, 3.14) | 0.02 |
| Model 3: Additionally adjusted for baseline eGFR and log-transformed proteinuria. | 1.91 | (1.12, 3.25) | 0.02 |
| Model 4: Additionally adjusted for longitudinal systolic blood pressure, eGFR, and log-transformed proteinuria as time-varying covariates | 1.74 | (1.03, 2.96) | 0.04 |
| Dominant Genetic Model | |||
| Model | HR comparing APOL1 1 or 2 risk variants vs. no risk variant | 95% CI | P-value |
| Unadjusted | 2.35 | (0.97, 5.74) | 0.06 |
| Model 1: Adjusted for age, gender, % European ancestry, and randomized treatment groups | 2.47 | (1.01, 6.05) | 0.05 |
| Model 2: Additionally adjusted for baseline smoking, body mass index, total cholesterol, systolic blood pressure, and history of heart disease | 2.44 | (0.99, 6.01) | 0.05 |
| Model 3: Additionally adjusted for baseline eGFR and log-transformed proteinuria. | 2.45 | (0.99, 6.03) | 0.05 |
| Model 4: Additionally adjusted for longitudinal systolic blood pressure, eGFR, and log-transformed proteinuria as time-varying covariates | 2.35 | (0.94, 5.84) | 0.07 |
Abbreviations: HR=hazard ratio; CI=confidence interval; eGFR=estimated glomerular filtration rate
DISCUSSION
Among African Americans with CKD attributed to hypertension, the APOL1 high-risk genotypes were not associated with an increased risk for CVD events. These results were consistent in crude analysis, analysis adjusted for demographics, traditional risk factors, and kidney function, further adjustment for longitudinal systolic blood pressure, eGFR, and log-transformed proteinuria, and when we accounted for the competing risks of ESRD and death. Furthermore, the APOL1 high-risk genotypes were not associated with any single component of the CVD composite outcome with the exception of cardiovascular death (additive model only). These findings add to the growing body of literature on the APOL1 risk variants and CVD in the general population.11,12,15,16 Consistent with our study, the Systolic Blood Pressure Intervention Trial (SPRINT) and Atherosclerosis Risk in Communities study suggested no association between the APOL1 high-risk variants and prevalent12 or incident CVD,15 respectively. However, in the JHS and the WHI, individuals with two APOL1 risk variants compared to those with no risk variant had a 1.8- to 3.2- fold higher risk of experiencing a major adverse cardiovascular event.11 In the CHS, a cohort of adults aged 65 years and older, the APOL1 high-risk genotypes were associated with an increased risk for incident myocardial infarction but not stroke or congestive heart failure.16
Our study population is unique in that it consisted of individuals with moderate CKD (mean eGFR 45 ml/min/1.73 m2 for APOL1 high-risk and 50 ml/min/1.73 m2 for low-risk groups), whereas the other studies included individuals with mild or no CKD (mean eGFR from 73 to 112 ml/min/1.73 m2).11,12,15,16 Like SPRINT,12 another negative study on the association of APOL1 with CVD, AASK excluded individuals with diabetes. Perhaps, the association between APOL1 risk variants and CVD differs in the context of diabetes. Indeed, in the three cohorts that reported a positive association between the APOL1 high-risk variants and CVD, approximately one quarter of participants had a history of diabetes.11,16 Our study has frequent assessments of kidney function, allowing for the investigation of mediation by change in kidney function; in contrast, the analyses in SPRINT were limited to prevalent, self-reported CVD.12
In support of our findings, associations between APOL1 risk variants with subclinical CVD have been largely negative. While apolipoprotein L1’s role in trafficking of high-density lipoprotein (HDL)17 and expression within endothelial and smooth muscle cells could suggest a role in CVD,18,19 Ito et al. reported an association between the APOL1 risk variants and lower Agatston scores (as a measure of coronary artery calcium) in the JHS.11 Similarly, the APOL1 risk variants were associated with less carotid and coronary artery calcified plaques in the African American Diabetes Heart Study (AA-DHS).13 Freedman et al. reported that the APOL1 risk variants were associated with less white matter lesion volume (a marker of severe cerebral small vessel disease) in the AA-DHS Memory IN Diabetes (MIND).14
We did find an association between the number of APOL1 risk variants and an increased risk for cardiovascular death; however, this was only when using the additive genetic model. These results should be interpreted with caution, as they may be spurious in the context of multiple testing and few events (n=31) for this secondary outcome. To our knowledge, only one other study has examined the association between APOL1 risk variants and cardiovascular mortality. In the CHS, risk for cardiovascular mortality was not significantly greater among individuals with 2 APOL1 risk variants compared to individuals with one or no variants, though a modest increase for total and noncardiovascular mortality was noted.16 Other studies have reported either improved survival13,20 or no difference in risk for all-cause mortality15 associated with the APOL1 high-risk variants.
Our study has several strengths. First, we utilized a well-characterized cohort of African Americans in which data were collected prospectively with an active follow-up process. Second, all CVD events were centrally adjudicated by trained clinicians using a common protocol.21–23 Third, given the known associations between the APOL1 risk variants and kidney disease, the results of our study are applicable to a large proportion of patients seen in clinical practice who are already at increased risk for CVD due to their underlying CKD. Finally, our findings were robust, with similar conclusions obtained when using additive or dominant genetic models and after accounting for competing risks (ESRD and death).
Limitations include a relatively small number of outcome events, which may have limited our power to detect associations between the APOL1 risk variants and CVD. Indeed, with only 144 cases of the composite CVD outcome, we had 80% power to detect a minimum hazard ratio of 1.85 for the APOL1 high-risk compared to the low-risk genotypes (recessive genetic model). Still, this hazard ratio is within the range of what was reported in the JHS and WHI.11 Our sensitivity analyses examining secondary endpoints were likely underpowered, particularly for cardiovascular death, which had the fewest number of events (minimum detectable hazard ratio of 3.74 for 80% power). Furthermore, study participants were not followed for CVD events once they developed ESRD. There may have been additional cases of CVD that were not captured, however, the pathophysiology of CVD events post-ESRD (i.e. more sudden cardiac death) likely differs from that of pre-ESRD CVD events.24
In conclusion, we did not detect an association of APOL1 high-risk variants with a composite CVD outcome in African Americans with CKD attributed to hypertension. Sensitivity analyses, however, suggested an increased risk of cardiovascular death associated with increasing number of APOL1 risk variants. Our results are consistent with some, but not all, prior studies. In view of the common association of CVD with kidney disease, additional research is warranted to better understand whether the APOL1 risk variants are associated with CVD outcomes, and if so, the pathophysiological basis for an association.
Supplementary Material
HIGHLIGHTS.
Prior studies have demonstrated that carrying two risk variants in the gene encoding apolipoprotein L1 (APOL1) are associated with an increased risk of kidney disease among African Americans. Whether these same risk variants are also associated with an increased risk of cardiovascular disease is less clear.
In a cohort of African Americans with hypertension-attributed chronic kidney disease, we report that the risk of experiencing a composite cardiovascular outcome (cardiovascular death or hospitalization for myocardial infarction, cardiac revascularization procedure, heart failure, or stroke) did not differ significantly by APOL1 risk status.
In sensitivity analyses using an additive model, we found that with each additional number of APOL1 risk variants, the risk of cardiovascular mortality incrementally increased.
Acknowledgments
Portions of this work have been presented at the 2016 American Society of Nephrology Kidney Week in Chicago, IL. We thank the participants of the AASK trial.
SOURCES OF SUPPORT: TKC is funded by the Extramural Grant Program (EGP) by Satellite Healthcare, a not-for-profit renal care provider. LJA is supported by NIH/NIDDK grant 1R01DK108803. MEG is supported by NIH/NIDDK grant 1R01DK108803. AT is supported by NIH/NIDDK grants 1R01DK108803 and 1R21DK112087. MME is supported by NIH/NIDDK grant 1R01DK103574. The AASK trial and cohort were supported by institutional grants from the NIH/NIDDK (M01 RR-00080, M01 RR-00071, M0100032, P20-RR11145, M01 RR00827, M01 RR00052, 2P20 RR11104, RR029887, DK 2818-02, DK057867, and DK048689), and the following pharmaceutical companies (King Pharmaceuticals, Pfizer, AstraZeneca, GlaxoSmithKline, Forest Laboratories, Pharmacia, and Upjohn). This project has been funded in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN26120080001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
This Research was supported [in part] by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Abbreviations and Acronyms
Abbreviations
- CVD
cardiovascular disease
- eGFR
estimated glomerular filtration rate
- CKD
chronic kidney disease
- APOL1
gene encoding apolipoprotein L1
- ESRD
end-stage renal disease
- HR
hazard ratio
- CI
confidence interval
Acronyms
- AASK
African American Study of Kidney Disease and Hypertension
- JHS
Jackson Heart Study
- WHI
Women’s Health Initiative
- CHS
Cardiovascular Heart Study
- SPRINT
Systolic Blood Pressure Intervention Trial
- AA-DHS
African American Diabetes Heart Study
Footnotes
DISCLOSURES: TKC previously owned stock in Pfizer Pharmaceuticals. The other authors have nothing to declare.
References
- 1.van der Velde M, Matsushita K, Coresh J, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79:1341–1352. doi: 10.1038/ki.2010.536. [DOI] [PubMed] [Google Scholar]
- 2.Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, Matsushita K, Wen CP. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382:339–352. doi: 10.1016/S0140-6736(13)60595-4. [DOI] [PubMed] [Google Scholar]
- 3.Chronic Kidney Disease Prognosis C. Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, Salem DN, Levey AS, Sarnak MJ. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol. 2004;15:1307–1315. doi: 10.1097/01.asn.0000123691.46138.e2. [DOI] [PubMed] [Google Scholar]
- 5.Elliott MK, McCaughan JA, Fogarty DG. Do patients with chronic kidney disease get optimal cardiovascular risk reduction? Curr Opin Nephrol Hypertens. 2014;23:267–274. doi: 10.1097/01.mnh.0000444913.78536.b1. [DOI] [PubMed] [Google Scholar]
- 6.Genovese G, Friedman DJ, Ross MD, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329:841–845. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tzur S, Rosset S, Shemer R, Yudkovsky G, Selig S, Tarekegn A, Bekele E, Bradman N, Wasser WG, Behar DM, Skorecki K. Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet. 2010;128:345–350. doi: 10.1007/s00439-010-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kopp JB, Nelson GW, Sampath K, et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol. 2011;22:2129–2137. doi: 10.1681/ASN.2011040388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lipkowitz MS, Freedman BI, Langefeld CD, et al. Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int. 2013;83:114–120. doi: 10.1038/ki.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parsa A, Kao WH, Xie D, et al. APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med. 2013;369:2183–2196. doi: 10.1056/NEJMoa1310345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito K, Bick AG, Flannick J, et al. Increased burden of cardiovascular disease in carriers of APOL1 genetic variants. Circ Res. 2014;114:845–850. doi: 10.1161/CIRCRESAHA.114.302347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Langefeld CD, Divers J, Pajewski NM, Hawfield AT, Reboussin DM, Bild DE, Kaysen GA, Kimmel PL, Raj DS, Ricardo AC, Wright JT, Jr, Sedor JR, Rocco MV, Freedman BI. Apolipoprotein L1 gene variants associate with prevalent kidney but not prevalent cardiovascular disease in the Systolic Blood Pressure Intervention Trial. Kidney Int. 2015;87:169–175. doi: 10.1038/ki.2014.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freedman BI, Langefeld CD, Lu L, Palmer ND, Carrie Smith S, Bagwell BM, Hicks PJ, Xu J, Wagenknecht LE, Raffield LM, Register TC, Jeffrey Carr J, Bowden DW, Divers J. APOL1 associations with nephropathy, atherosclerosis, and all-cause mortality in African Americans with type 2 diabetes. Kidney Int. 2015;87:176–181. doi: 10.1038/ki.2014.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freedman BI, Gadegbeku CA, Bryan RN, et al. APOL1 renal-risk variants associate with reduced cerebral white matter lesion volume and increased gray matter volume. Kidney Int. 2016;90:440–449. doi: 10.1016/j.kint.2016.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grams ME, Rebholz CM, Chen Y, Rawlings AM, Estrella MM, Selvin E, Appel LJ, Tin A, Coresh J. Race, APOL1 Risk, and eGFR Decline in the General Population. J Am Soc Nephrol. 2016;27:2842–2850. doi: 10.1681/ASN.2015070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mukamal KJ, Tremaglio J, Friedman DJ, Ix JH, Kuller LH, Tracy RP, Pollak MR. APOL1 Genotype, Kidney and Cardiovascular Disease, and Death in Older Adults. Arterioscler Thromb Vasc Biol. 2016;36:398–403. doi: 10.1161/ATVBAHA.115.305970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duchateau PN, Pullinger CR, Orellana RE, Kunitake ST, Naya-Vigne J, O’Connor PM, Malloy MJ, Kane JP. Apolipoprotein L, a new human high density lipoprotein apolipoprotein expressed by the pancreas. Identification, cloning, characterization, and plasma distribution of apolipoprotein L. J Biol Chem. 1997;272:25576–25582. doi: 10.1074/jbc.272.41.25576. [DOI] [PubMed] [Google Scholar]
- 18.Madhavan SM, O’Toole JF, Konieczkowski M, Ganesan S, Bruggeman LA, Sedor JR. APOL1 localization in normal kidney and nondiabetic kidney disease. J Am Soc Nephrol. 2011;22:2119–2128. doi: 10.1681/ASN.2011010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma L, Shelness GS, Snipes JA, et al. Localization of APOL1 protein and mRNA in the human kidney: nondiseased tissue, primary cells, and immortalized cell lines. J Am Soc Nephrol. 2015;26:339–348. doi: 10.1681/ASN.2013091017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma L, Langefeld CD, Comeau ME, Bonomo JA, Rocco MV, Burkart JM, Divers J, Palmer ND, Hicks PJ, Bowden DW, Lea JP, Krisher JO, Clay MJ, Freedman BI. APOL1 renal-risk genotypes associate with longer hemodialysis survival in prevalent nondiabetic African American patients with end-stage renal disease. Kidney Int. 2016;90:389–395. doi: 10.1016/j.kint.2016.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Appel LJ, Middleton J, Miller ER, 3rd, et al. The rationale and design of the AASK cohort study. J Am Soc Nephrol. 2003;14:S166–172. doi: 10.1097/01.asn.0000070081.15137.c0. [DOI] [PubMed] [Google Scholar]
- 22.Norris K, Bourgoigne J, Gassman J, et al. Cardiovascular outcomes in the African American Study of Kidney Disease and Hypertension (AASK) Trial. Am J Kidney Dis. 2006;48:739–751. doi: 10.1053/j.ajkd.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Alves TP, Wang X, Wright JT, Jr, Appel LJ, Greene T, Norris K, Lewis J, Group ACR. Rate of ESRD exceeds mortality among African Americans with hypertensive nephrosclerosis. J Am Soc Nephrol. 2010;21:1361–1369. doi: 10.1681/ASN.2009060654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whitman IR, Feldman HI, Deo R. CKD and sudden cardiac death: epidemiology, mechanisms, and therapeutic approaches. J Am Soc Nephrol. 2012;23:1929–1939. doi: 10.1681/ASN.2012010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.