Abstract
Background
Therapeutic hypothermia (TH) is the standard of care for neonates with hypoxic-ischemic encephalopathy but is not fully protective in the clinical setting. Hypoxia-ischemia (HI) may cause white matter injury (WMI), leading to neurological and cognitive dysfunction.
Methods
P9 mice were subjected to HI as previously described. Pups underwent 3.5 hours of systemic hypothermia or normothermia. Cresyl violet and Perl’s iron stain for histopathological scoring of brain sections was completed blindly on all brains. Immunocytochemical (ICC) staining for myelin basic protein (MBP), microglia (Iba1), and astrocytes (GFAP) was performed on adjacent sections. Volumetric measurements of MBP coverage were used for quantitative analysis of white matter.
Results
TH provided neuroprotection by injury scoring for the entire group (n=44) (p<0.0002). ICC analysis of a subset of brains showed that the lateral caudate was protected from WMI (p<0.05). Analysis revealed decreased GFAP and Iba1 staining in hippocampal regions, mostly CA2/CA3. GFAP and Iba1directly correlated with injury scores of normothermic brains.
Conclusions
TH reduced injury and qualitative data suggest that hippocampus and lateral caudate are protected from HI. Mildly injured brains may better show the benefits of TH. Overall these data indicate regional differences in WMI susceptibility and inflammation in a P9 murine HI model.
Graphical Abstract
Introduction
Neonatal hypoxic-ischemia (HI) treated with hypothermia has become a standard of care for infants. Hypoxic-ischemia encephalopathy (HIE) occurs in 3–5 per 1,000 live births and produces a high morbidity rate, severe/long-term neurological and cognitive deficits with high outcomes in cerebral palsy, epilepsy and mental retardation (1). However, no therapy completely protects against neonatal HIE, therefore opportunities exist to continue to explore additional agents (2). Therapeutic hypothermia (TH) has been shown to decrease the mortality rate and display neuroprotection in infants with neonatal HIE (3–5). There have been many mechanisms implicated in the effects of hypothermic therapy which include suppression of apoptosis, decreased inflammation, reduction in excitatory amino acids (EAA), decreased reactive oxygen species (ROS), and a reduced cerebral metabolic rate (6).
A previous study using P7 rats investigating the use of hypothermia and erythropoietin suggested that slightly older animals modeled equivalent to the full-term newborn brain may be necessary to observe the benefits of adjuvant therapy (7). Additionally, there has been evidence that TH displays neuroprotection for specific regions of the brain, such as the hippocampus and thalamus (8). Interestingly, the results of some studies show that mildly injured brains may receive the benefits of TH relating to white matter injury (WMI) and overall neuroprotection, whereas severely injured brains do not. This trend of insufficient benefit from TH for severely injured brains is consistent with findings in other animal models (9,10,11).
The presence of white matter (WM) and appropriate axon myelination are essential to normal brain connectivity and development in the newborn infant (12). It has been found that insufficient myelination leads to neurological & cognitive dysfunction (13,14). Little investigation has been done on the effects of hypothermia on WMI. There is evidence showing mild hypoxemia in neonatal mice causes WM hypomyelination and WMI (15). Rodent studies of HI have shown that the developmental stage of the brain, preterm vs. term, at the time of injury has a major role in the underlying WMI, in part due to maturation-dependent oligodendrocyte arrest (16).
TH neuroprotection from WMI in a full-term murine model for HI has described the evolution of brain injury (17). A previous TH study in sheep displayed neuroprotection from WMI (18). The hypothesis tested is that TH will reduce WMI in P9 neonatal mice subjected to a HI insult through reduction of the glial/inflammatory response. The hypothesis is addressed by evaluating the extent of these processes by volumetric analysis and immunocytochemical assessments.
Methods
Hypoxic-Ischemia
P9 male and female CD1 mice were subjected to focal ischemia with global hypoxia as previously described in the Rice-Vannucci model (19). All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at UCSF, in accordance with NIH guidelines for the Care and Use of Laboratory Animals. Ligation of the left common carotid artery (LCCA) was carried out. Mice were anesthetized with 3% isoflurane, balance oxygen. A midline incision of the neck was done to dissect and ligate the LCCA via electrical coagulation. Pups were returned to the dam for 1 hour following the procedure. Hypoxia was induced by placing pups in chambers submerged in a 37°C water bath into which an atmosphere of 10% oxygen, 90% nitrogen was introduced via inlet and outlet tubing (maintained by flow meter). Pups were removed from chambers after 50 minutes and returned to the dam for 1 hour.
Hypothermia
The pups were then equally separated into chambers maintained at either 30°C (n=30) or 36.5 °C (n=24), both chambers were open to room air, for 3.5 hours of either HT or NT. One sentinel cooled mouse was monitored with a temperature probe. All pups also had their temperature monitored with an infrared thermometer (Supplemental Figure S1). Following cooling, the temperature in the chambers of the HT pups was gradually increased to 37°C over 30 min.
Histology
For histopathological examination, pups were sacrificed at P14. A lethal dose of pentobarbital was used for anesthesia prior to transcardiac perfusion with ice-cold 4% paraformaldehyde in 0.1 M PBS. Brains were removed and post-fixed overnight in the same solution for 4 hours, then equilibrated in 30% sucrose in 0.1 M PBS. A majority of the brains (n=40) were cut on a Vibratome and sequential sections (50 um) were collected for Nissl (cresyl violet) and Perl’s iron stains. The remaining brains (n=14, 3 sham brains not analyzed) were frozen, cut on a cryostat (Leica Microsystems, Wetzlar, Germany) and 20-μm coronal sections were collected on Superfrost slides and stored at −80°C. Following sectioning, samples were stained with cresyl violet (Nissl stain) and dehydrated in graded ethanol solutions, cleared in Citrisolv (Fisher Scientific, Hampton, NH) and cover-slipped in Permount (Fisher Scientific).
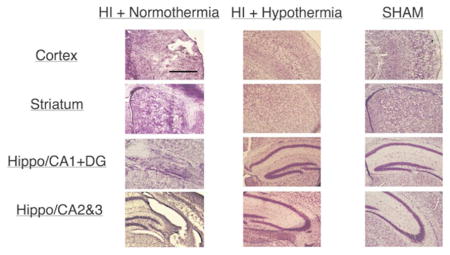
Both cresyl violet and Perl’s iron stained sections were used for histopathological scoring of brain sections, which was completed blindly. Sections were assessed rostral to caudal. Regions scored included: anterior cortex, middle cortex, posterior cortex, caudate/striatum, hippocampus CA1, CA2, and CA3, and the dentate gyrus. Scores were assessed for each region as follows: 0 = no injury, 1 = minimal cell loss manifested by scattered shrunken neurons and glia with small patches of iron deposition, 2 = moderate cell loss with infarction in a columnar distribution in the cortex with concomitant gliosis or shrunken hippocampus with cell loss throughout Sommer’s sector with corresponding iron deposition, and 3 = severe cell loss and gliosis with cystic infarction, for a total score of 0–24. Injury scoring 5 days after HI was completed on P9 mice treated with either normothermia or therapeutic hypothermia (TH) by using cresyl-violet and Perl’s iron-stained adjacent sections (Supplemental Figure S2). The specimens that were used only for immunocytochemistry (ICC) staining were also scored.
Immunofluorescence & Image Capturing
In order to identify and quantify different cell types, 2–4 sections per region were blocked with 25% goat serum in PBST (0.1% Triton-X-100) + 5% BSA followed by quadruple staining with primary antibodies. Primary antibodies were diluted in blocking solution as follows: Iba1 (microglia) at [1:200] (Wako Chemicals, Richmond, VA); Myelin Basic Protein (MBP) at [1:1000] (Abcam Cambridge, MA); Glia fibrillary acidic protein (GFAP) at [1:2000] (Thermo Fisher Scientific, Rockford, IL). Slides were incubated with primary antibodies overnight at 4°C followed by washing and incubation with species-specific secondary antibodies were applied at a dilution of [1:200]: Alexa Fluor 568, 488, 647 and 350 (all Life Technologies Eugene, OR). A final washing was done and then the slides were mounted with a coverslip using ProLong® Gold antifade mountant (Life Technologies).
Image capturing and analysis were completed blinded using Volocity Software, a confocal-like laser-free technology. For data analysis, z-stacks of stained sections were captured at 5μm intervals at 10x (Zeiss Axiovert 100 equipped with Openlab Software, Improvision, Coventry UK). Captured images focused anatomically on the lateral caudate (which included a section of corpus callosum), medial caudate, hippocampus CA1 with dentate gyrus, and hippocampal regions CA2 and CA3. Volume coverage was analyzed for white matter (MBP), astroglia (GFAP) and microglia (Iba1) using an automated protocol for a signal intensity threshold (1.5–2.0 SD background in channels) for the caudate (position 336–353) and hippocampus (position 235–270) according to the Allen Brain Atlas of P14 brain strain C57BL/6J.
Statistical Analysis
Injury scores between normothermic and hypothermic groups were compared by Mann-Whitney test. Analysis was performed with Prism 6.0 (GraphPad Software, La Jolla, CA).
Regional data for the summation of volumes of MBP, GFAP, and Iba1 was done and normalized using the ratio of intact ipsilateral tissue to intact contralateral hemisphere tissue. Data comparing HI treated with normothermia and HI treated with hypothermia was conducted using an unpaired t-test to determine statistical significance.
Data was compared according to degree of injury (Mild = 0–8; Moderate = 8–16; Severe =16–24). The raw volumetric data of MBP, GFAP and Iba1 was plotted against injury score of normothermic and hypothermic brain’s caudate and hippocampal regions. Data collected from the medial and lateral caudate were combined, and the data from the hippocampus DG, CA1, CA2 and CA3 were combined. Correlation coefficients were calculated using the Pearson correlation coefficients test to determine the relationship between injury score with inflammation and white matter injury.
Results
Histopathological Analysis
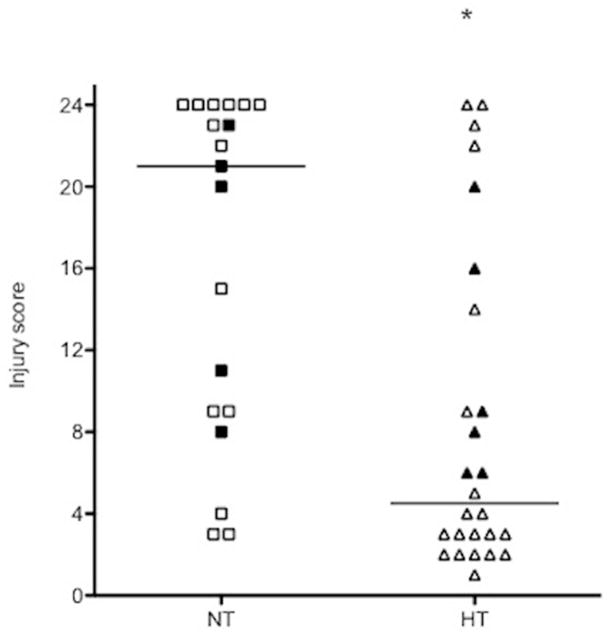
Analysis of all available brain samples showed that TH provides neuroprotection, as determined by injury scoring (Figure 1). The protection was seen in all regions analyzed: cortex (p=0.0002); hippocampus (p=0.0006); and caudate (p=0.01). There were no sex differences in injury severity: NT male vs. NT female (n=10 each), p=0.16; HT male (n=13) vs. HT female (n=12), p=1.0. There was also no difference in incidence of mortality between groups: 4 NT and 5 HT died, p=1.0 by Fisher’s exact test.
Figure 1.
Histopathological analysis injury score of brain samples. Filled data points indicate samples that were also used for ICC analysis. Cresyl violet staining of ipsilateral hemisphere for histopathological scoring of brain sections completed blindly of all samples. Sections were scored rostral to caudal at: anterior cortex, middle cortex, posterior cortex, caudate/striatum, hippocampus CA1, CA2, and CA3, and the dentate gyrus. Scoring was performed following protocol (Ferriero et. al., 1995): 0 = no injury; 1 = minimal cell loss, scattered shrunken neurons and glia; 2 = moderate cell loss; 3 = severe cell loss and gliosis with cystic infarction. Samples were compared according to treatment group: normothermia and hypothermia.
Volumetric Immunocytochemistry Analysis
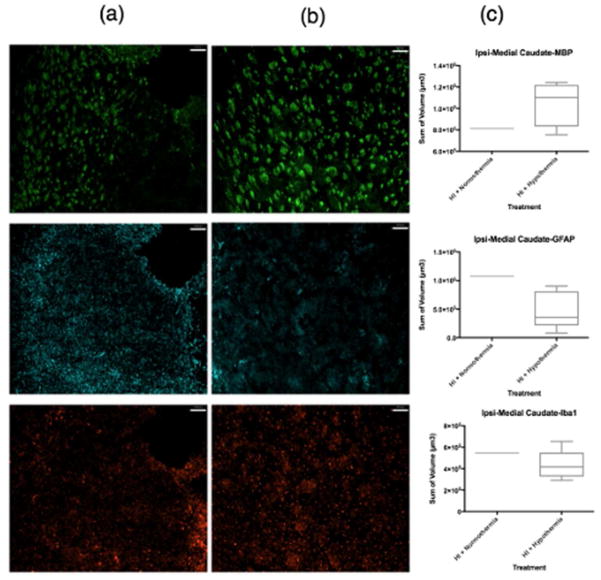
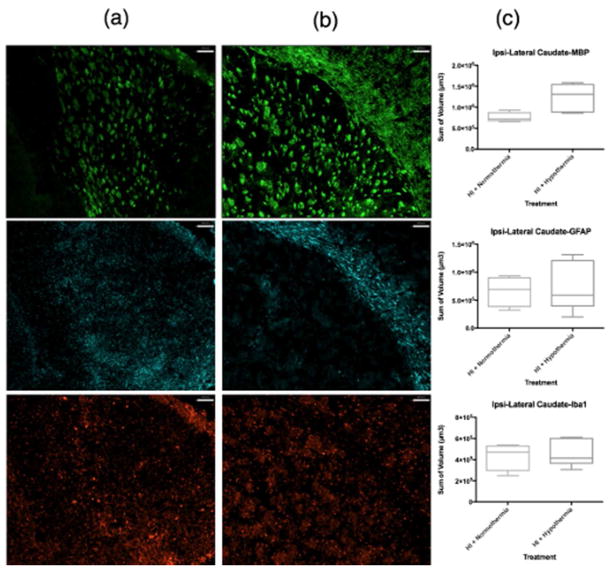
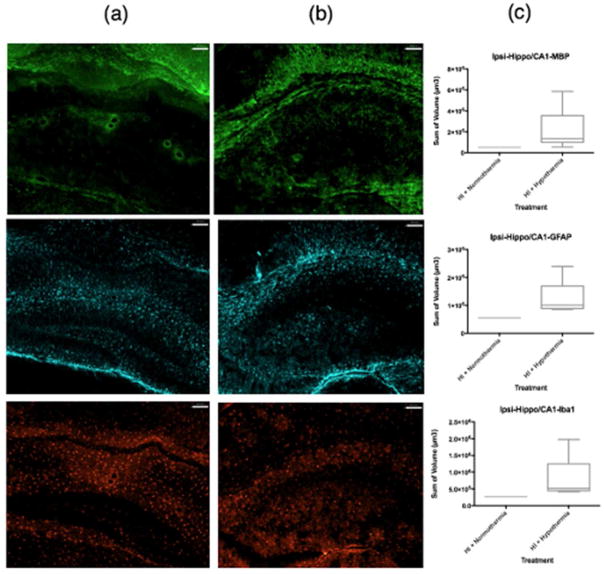
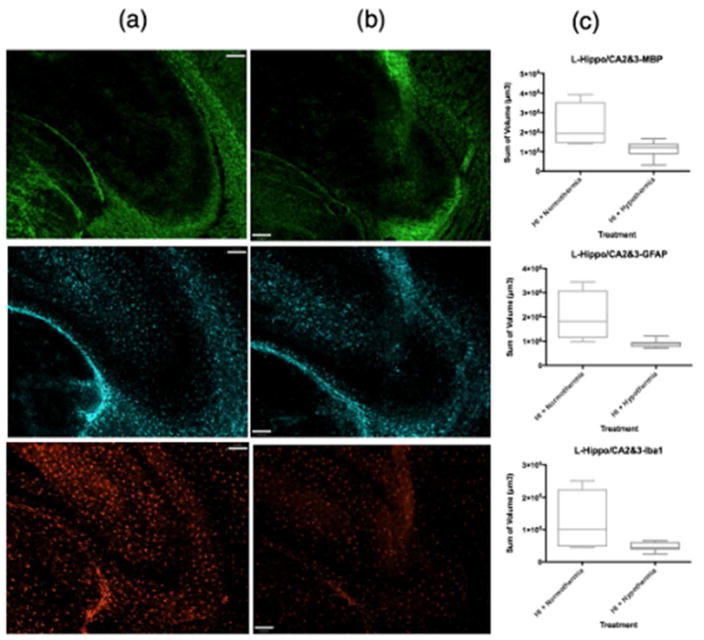
ICC volumetric analysis showed that compared to normothermic brains (Figure 2a, Figure 3a), WMI was less in the ipsilateral caudate following TH (Figure 2b, Figure 3b). The lateral caudate was significantly protected from WMI (p<0.05) with TH (Figure 3c) but there were no significant differences in GFAP or Iba1 volume. Volumetric analysis of the medial caudate showed that TH provides a wide range of protection from WMI, and although significance could not be determined from quantitative imaging data, smaller MBP volumes suggest that TH plays a role in protection from WMI in the medial caudate (Figure 2c). Further, the medial caudate qualitative data of GFAP and Iba1 (Figure 2a, b, c), also shows that TH provides observable differences in imaging data compared to normothermia. Data collected from the hippocampal region CA1 and dentate gyrus showed no differences in MBP, GFAP or Iba1 volumes between normothermic and TH-treated groups (Figure 4c). However, qualitative imaging data again suggests that TH may play a role in preservation of WM and an overall decrease in features of inflammation (Figure 4a, b). Analysis of ICC sections showed decreased GFAP and Iba1 staining in the ipsilateral hippocampal regions, mostly CA2/CA3 treated with TH compared to normothermia (Figure 5a, b, c). However, the MBP staining did not show that the WM is protected in this region.
Figure 2.
MBP, GFAP, Iba1 coverage of medial caudate. MBP (green), GFAP (far red), Iba1 (red). Column (a) contains samples treated with HI + normothermia. Column (b) contains samples treated with HI + hypothermia. (c) Volumetric measurements normalized using the ratio of intact ipsilateral tissue to intact contralateral hemisphere tissue. Data comparing HI treated with normothermia and HI treated with hypothermia using a box-and-whisker graph. The horizontal line indicates the median score. Magnification is 10 X Scale bar = 100 um
Figure 3.
MBP, GFAP, Iba1 coverage of lateral caudate with corpus callosum. MBP (green), GFAP (far red), Iba1 (red). Column (a) contains samples treated with HI + normothermia. Column (b) contains samples treated with HI + hypothermia. (c) Volumetric measurements normalized using the ratio of intact ipsilateral tissue to intact contralateral hemisphere tissue. Data comparing HI treated with normothermia and HI treated with hypothermia using a box-and-whisker graph. The horizontal line indicates the median score. Magnification is 10X. Scale bar = 100 um.
Figure 4.
MBP, GFAP, Iba1 coverage of hippocampus CA1 with dentate gyrus. MBP (green), GFAP (far red), Iba1 (red). Column (a) contains samples treated with HI + normothermia. Column (b) contains samples treated with HI + hypothermia. (c) Volumetric measurements normalized using the ratio of intact ipsilateral tissue to intact contralateral hemisphere tissue. Data comparing HI treated with normothermia and HI treated with hypothermia using a box-and-whisker graph. The horizontal line indicates the median score. Magnification is 10X. Scale bar = 100 um.
Figure 5.
MBP, GFAP, Iba1 coverage of hippocampal regions CA2 and CA3. MBP (green), GFAP (far red), Iba1 (red). Column (a) contains samples treated with HI + normothermia. Column (b) contains samples treated with HI + hypothermia. (c) Volumetric measurements normalized using the ratio of intact ipsilateral tissue to intact contralateral hemisphere tissue. Data comparing HI treated with normothermia and HI treated with hypothermia using a box-and-whisker graph. The horizontal line indicates the median score. Magnification is 10X. Scale bar =100 um
Relationship between Injury, WMI and Inflammation
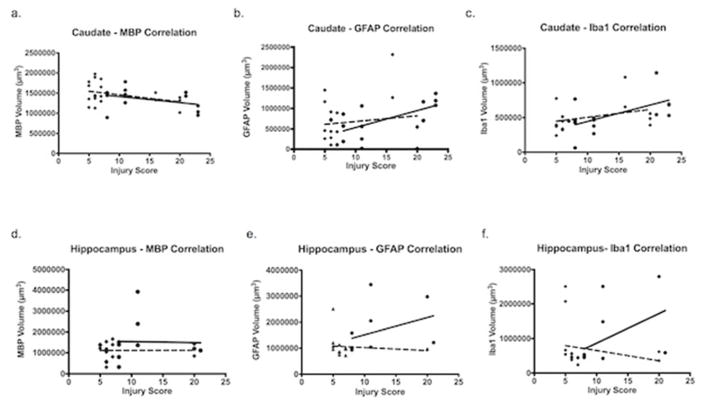
White matter and injury score correlations in TH-treated brains are suggestive of some WMI protection in the caudate (Figure 6a) when the injury score is mild (r = −0.9077) compared to normothermic treated (r = −0.4668). Similarly, the caudate displayed correlation between injury score and inflammation in normothermic brains (Figure 6b, c) for GFAP (r = 0.9128) and Iba1 (r = 0.7524). Caudate hypothermia-treated correlation coefficients of GFAP (r = 0.2448) and Iba1 (r = 0.4958) also displayed a positive correlation with injury score, but not as strong as the normothermic treated. The hippocampus (Figure 6d) shows a negligible difference in the correlation between WMI and injury score of normothermia and hypothermia treated brains.
Figure 6.
Correlation coefficients between injury score and volumetric raw data measurements. Normothermic (circles); hypothermic (squares). The relationship between the raw volumetric data of: MBP, GFAP and Iba1; and injury was used to determine a correlation coefficient. Caudate and hippocampal regions were evaluated. (a) MBP volumetric measurements of caudate regions compared to injury score. Normothermic r = −0.4668 and hypothermic r = −0.9077. (b) GFAP volumetric measurements of caudate regions compared to injury score. Normothermic r = 0.9128 and hypothermic r = 0.2448. (c) Iba1 volumetric measurements of caudate regions compared to injury score. Normothermic r = 0.7524 and hypothermic r = 0.4958. (d) MBP volumetric measurements of hippocampal regions compared to injury score. Normothermic r = −0.3066 and hypothermic r = −0.08531. (e) GFAP volumetric measurements of hippocampal regions compared to injury score. Normothermic r = 0.4062 and hypothermic r = −0.2900. (f) Iba1 volumetric measurements of hippocampal regions compared to injury score. Normothermic r = 0.4475 and hypothermic r = −0.3770.
Normothermic brains displayed a strong positive correlation between injury score and inflammation in the hippocampal region (Figure 6e, f) when compared to TH-treated mice. GFAP assessed in the normothermic treated brains showed a positive correlation (r = 0.4062), compared to TH treatment, which showed a negative correlation (r = −0.2900). Iba1 coverage assessed in the normothermic treated brains showed a positive correlation (r = 0.4475) compared to the TH-treated which showed a negative correlation (r = −0.3770). In normothermic brains the degree of inflammation directly correlated with the injury score of the normothermic brains. In comparison, TH-treatment displayed protection from inflammation in the hippocampal region according to the negative correlation coefficients (Figure 6e, f).
Discussion
This study establishes that TH in a full-term murine HI model provides overall brain protection and specifically WM protection in specific regions. The study also shows a direct correlation between the degree of HI injury and glial response. Additionally, we demonstrate that TH neuroprotection is region dependent and provides protection from WMI in mildly injured brains.
WM is essential to brain connectivity and proper maturation in the neonatal brain (12). In the rodent brain, WM maturation peaks during the second–third weeks of life, thus, distorted myelination or altered composition and function of the WM after HI in P9 mice would adversely affect repair (13, 14). HI causes significant damage to the developing brain, including WMI. Damage and developmental impairment of WM can result in devastating outcomes such as cerebral palsy, long-term cognitive impairment, and other deficits (1). Our data in the lateral caudate region (which included a portion of corpus callosum, a region containing abundant WM) showed that TH provides protection from WMI. This data is consistent with previous studies that show that TH reduces WMI in mice and sheep (17, 18). However, in the caudate region, analysis of MBP presence in relation to the degree of injury also shows that TH preserves WM from mild injury but not from severe injury.
The hippocampus is selectively vulnerable over the cortex and striatum in the HI murine model used here, and there is no significant WM protection in most hippocampal regions, but qualitative data suggest protection in penumbral regions of the hippocampus (CA2/3). The severe injury seen in this region may be resistant to the benefits of TH in this model (20, 21). These data are consistent with previous studies that demonstrated that TH-mediated attenuation of caspase-dependent pathway activation after HI in neonatal mice is region-specific (8) and that TH was neuroprotective in the HI-induced penumbra, but not in the core, and that protective effect was associated with a TH-induced increase of antioxidant enzymes SOD-1 and GPx, leaving several inflammatory mediators unchanged, including IL1β and MMP-9 (22). Another possibility is that the inflammatory response is strongest in the hippocampus, as evident from the Iba1 and GFAP coverage of microglia and astrocytes respectively, thus preferentially affecting oligodendrocytes in that region (23). Also, the hippocampus is distinct from other parenchymal regions due to its proximity to the cerebrovascular spaces and likely exposure to leukocytes in such spaces. This region has the most disturbed blood-brain barrier in the model we are using (24).
Hypothermic protection is a complex process and there is no consensus on the best protocol to achieve beneficial effect. Several groups showed that hypothermia induced immediately after HI in P7 rats (body temperature 30°C Vs. 36°C for 10 h) provided protection and inhibition of caspase activation, thereby preventing apoptotic cell death (25). Even delayed cooling of P7 rats (rectal temperature 37°C Vs. 30°C for 26 h) starting 2 h after HI not only significantly reduced the final size of infarction (hippocampus and, to a lesser extent, cortex) 6 weeks after the insult, but led to long-lasting behavioral improvement throughout brain maturation despite the severity of injury (26). TH during the HI insult significantly reduced spatial learning deficits (27). On the other hand, many studies have shown that by itself hypothermia did not protect against HI unless it was combined with adjunctive therapies, such as topiramate (28) or phenobarbital (29). Furthermore, addition of agents such as bumetanide (a NKCC1 transporter inhibitor) augmented the neuroprotective efficacy of phenobarbital combined with hypothermia (30), demonstrating that limiting multiple pathophysiological mechanisms may be needed to provide benefits. Yet, combination of Epo with TH showed no benefit over Epo alone after HI (7). Studies in larger animals (31) have also proven that TH can provide neuroprotection after HI, but, like in humans, that the severity of injury and timing, depth and duration of hypothermia are major variables. In sheep, significant protection was achieved by initiation of hypothermia at early timepoints, but protection is lost with delay for more than 8 hours, and prolongation of TH was not beneficial (32).
Currently, TH is the standard care for the treatment of HIE in neonates but protection of term infants is incomplete (33–36). This study shows that in a full-term murine model, TH has a significant effect in reducing the injury of mice susceptible to HI, supportive of current studies. However, the severity of injury from HI still cannot be predicted, and the variability in the degree of injury has a relationship with the protective effects of TH. Sex stratification did not show differences in our study, yet a previous study in P10 mice with HI showed that post-TH male mice showed neuroprotection, while the females had variable degrees of injury and protective effects could not be observed (17).
One limitation of this study is a variable degree of injury in individual pups. Variability in injury has been a consistent limitation noted in various murine HI studies, with severe/extensive injury providing the greatest challenge to access TH protection (4, 8, 17). Severe injury in some pups precluded us from systematically quantifying the data. The degree of damage caused by HI, along with the heterogeneity of the neuroanatomy studied, provides challenges in analysis of these regions. Imaging modalities such as MRI has been suggested, and may provide an opportunity to better characterize and stratify the variability of injury.
HI initiates an inflammatory response, which potentiates and exacerbates neural damage (37). The presence of astrocytosis, as seen by GFAP immunolabeling, and microgliosis, as seen by Iba1 immunolabeling, are common findings in HI injury and support the role of neuroinflammation (38, 39). Our correlation analysis of the relationship between injury score and the inflammatory markers show significant regional inflammation protection by TH, even in the hippocampus in severely injured brains. These findings provide an opportunity for further investigation of therapy to prevent injury from HI, as inflammatory mediators have been suggested to play a role in injury due to HI in the neonatal brain (40). TH with adjunctive use of anti-inflammatory mediators could possibly provide significant protection from HI injury. A recent review showed that numerous immunomodulatory therapies attenuating neuroinflammation show effective results in experimental models (41), supporting the use of these immunomodulating therapies with TH in a clinical setting of neonatal HI to prevent further neuronal injury.
Our data on effects of TH on microglial and astroglial coverage support a relationship between the glial coverage and injury, as we observed TH-induced protection in regions with reduced glial coverage and not in regions with unaffected glial coverage. It was long believed that glial cell activation associated with HI is necessarily injurious and that TH is protective by limiting or even aborting microglial accumulation. More recent studies in P7 rats and P9 mice subjected to focal stroke have demonstrated that microglial in fact contribute to endogenous protective mechanisms (42, 43) and that the microglial phenotypes, rather than microglial number, determine destructive or protective features of these cells. Combining melatonin with TH after HI in a piglet model of perinatal asphyxia protected grey and WM, but did not change microglial numbers, but affected the expression of cytotoxic molecules in microglia (44).
Conclusion
Our study shows that TH displays a significant effect in reducing the injury and inflammation in a P9 murine HI model. Protection from WMI is evident, with mildly injured brains showing the greatest benefit and overall more influential neuroprotection. Qualitative data suggest that even the penumbral hippocampal region may be protected from HI with TH. Overall these data indicate regional differences in WMI susceptibility and inflammation in a P9 murine HI model. Inability to score highly injured brains quantitatively and a wide-range of injury was a constraint. Standardization of HI injury would provide a greater opportunity to investigate the full-effects of TH regarding WMI and inflammation.
Supplementary Material
Acknowledgments
Statement of financial support: EK was supported by the APS-SPR student research program. Work supported by NS 33997
EK sponsored by the American Pediatric Society & Society for Pediatric Research Summer Medical Student Program. DMF supported by NIH 33997.
Footnotes
Disclosures: the authors have nothing to disclose
References
- 1.Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database of Systematic Reviews. 2013 doi: 10.1002/14651858.CD003311.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gonzalez FF, Ferriero DM. Therapeutics for neonatal brain injury. Pharmacology & Therapeutics. 2008;120:43–53. doi: 10.1016/j.pharmthera.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Azzopardi DV, Strohm B, Edwards AD, et al. Moderate Hypothermia to Treat Perinatal Asphyxial Encephalopathy. New England Journal of Medicine. 2009;361:1349–58. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]
- 4.Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicenter randomised trial. Lancet. 2005;365:663–70. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 5.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–84. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 6.Drury PP, Gunn ER, Bennet L, Gunn AJ. Mechanisms of Hypothermic Neuroprotection. Clinics in Perinatology. 2014;41:161–75. doi: 10.1016/j.clp.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Fang AY, Gonzalez FF, Sheldon RA, Ferriero DM. Effects of combination therapy using hypothermia and erythropoietin in a rat model of neonatal hypoxia–ischemia. Pediatric research. 2013;73:12–7. doi: 10.1038/pr.2012.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlsson Y, Wang X, Schwendimann L, et al. Combined effect of hypothermia and caspase-2 gene deficiency on neonatal hypoxic-ischemic brain injury. Pediatr Res. 2012;71:566–72. doi: 10.1038/pr.2012.15. [DOI] [PubMed] [Google Scholar]
- 9.Sabir H, Scull-Brown E, Liu X, Thoresen M. Immediate Hypothermia Is Not Neuroprotective After Severe Hypoxia-Ischemia and Is Deleterious When Delayed by 12 Hours in Neonatal Rats. Stroke. 2012;43:3364–70. doi: 10.1161/STROKEAHA.112.674481. [DOI] [PubMed] [Google Scholar]
- 10.Williams CE, Gunn AJ, Mallard C, Gluckman PD. Outcome after ischemia in the developing sheep brain: An electroencephalographic and histological study. Annals of Neurology. 1992;31:14–21. doi: 10.1002/ana.410310104. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Silverstein FS, Skoff R, Barks JDE. Hypoxic-Ischemic Oligodendroglial Injury in Neonatal Rat Brain. Pediatr Res. 2002;51:25–33. doi: 10.1203/00006450-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Nave K-A, Werner HB. Myelination of the Nervous System: Mechanisms and Functions. Annual Review of Cell and Developmental Biology. 2014;30:503–33. doi: 10.1146/annurev-cellbio-100913-013101. [DOI] [PubMed] [Google Scholar]
- 13.McQuillen PS, Ferriero DM. Selective vulnerability in the developing central nervous system. Pediatric Neurology. 2004;30:227–35. doi: 10.1016/j.pediatrneurol.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Back SA, Gan X, Li Y, Rosenberg PA, Volpe JJ. Maturation-Dependent Vulnerability of Oligodendrocytes to Oxidative Stress-Induced Death Caused by Glutathione Depletion. The Journal of Neuroscience. 1998;18:6241–53. doi: 10.1523/JNEUROSCI.18-16-06241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juliano C, Sosunov S, Niatsetskaya Z, et al. Mild intermittent hypoxemia in neonatal mice causes permanent neurofunctional deficit and white matter hypomyelination. Exp Neurol. 2015;264:33–42. doi: 10.1016/j.expneurol.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Back SA, Riddle A, McClure MM. Maturation-Dependent Vulnerability of Perinatal White Matter in Premature Birth. Stroke. 2007;38:724–30. doi: 10.1161/01.STR.0000254729.27386.05. [DOI] [PubMed] [Google Scholar]
- 17.Burnsed JC, Chavez-Valdez R, Hossain MS, et al. Hypoxia-Ischemia and Therapeutic Hypothermia in the Neonatal Mouse Brain – A Longitudinal Study. PLOS ONE. 2015;10:e0118889. doi: 10.1371/journal.pone.0118889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roelfsema V, Bennet L, George S, et al. Window of Opportunity of Cerebral Hypothermia for Postischemic White Matter Injury in the Near-Term Fetal Sheep. Journal of Cerebral Blood Flow & Metabolism. 2004;24:877–86. doi: 10.1097/01.WCB.0000123904.17746.92. [DOI] [PubMed] [Google Scholar]
- 19.Rice JE, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Annals of Neurology. 1981;9:131–41. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- 20.Sheldon RA, Sedik C, Ferriero DM. Strain-related brain injury in neonatal mice subjected to hypoxia–ischemia. Brain Research. 1998;810:114–22. doi: 10.1016/s0006-8993(98)00892-0. [DOI] [PubMed] [Google Scholar]
- 21.Sheldon RA, Hall JJ, Noble LJ, Ferriero DM. Delayed cell death in neonatal mouse hippocampus from hypoxia-ischemia is neither apoptotic nor necrotic. Neuroscience Letters. 2001;304:165–8. doi: 10.1016/s0304-3940(01)01788-8. [DOI] [PubMed] [Google Scholar]
- 22.Chevin M, Guiraut C, Maurice-Gelinas C, Deslauriers J, Grignon S, Sébire G. Neuroprotective effects of hypothermia in inflammatory-sensitized hypoxic-ischemic encephalopathy. International Journal of Developmental Neuroscience. 2016;55:1–8. doi: 10.1016/j.ijdevneu.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Graf AE, Haines KM, Pierson CR, et al. Perinatal inflammation results in decreased oligodendrocyte numbers in adulthood. Life Sci. 2014;94:164–71. doi: 10.1016/j.lfs.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ek CJ, D’Angelo B, Lehner C, Nathanielsz P, Li C, Mallard C. Expression of tight junction proteins and transporters for xenobiotic metabolism at the blood–CSF barrier during development in the nonhuman primate (P. hamadryas) Reproductive Toxicology. 2015;56:32–44. doi: 10.1016/j.reprotox.2015.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu C, Wang X, Cheng X, et al. Post-ischemic hypothermia-induced tissue protection and diminished apoptosis after neonatal cerebral hypoxia–ischemia. Brain Research. 2004;996:67–75. doi: 10.1016/j.brainres.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 26.Wagner BP, Nedelcu J, Martin E. Delayed Postischemic Hypothermia Improves Long-Term Behavioral Outcome after Cerebral Hypoxia-Ischemia in Neonatal Rats. Pediatr Res. 2002;51:354–60. doi: 10.1203/00006450-200203000-00015. [DOI] [PubMed] [Google Scholar]
- 27.Mishima K, Ikeda T, Yoshikawa T, et al. Effects of hypothermia and hyperthermia on attentional and spatial learning deficits following neonatal hypoxia-ischemic insult in rats. Behavioural Brain Research. 2004;151:209–17. doi: 10.1016/j.bbr.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Barks JD, Xu G, Silverstein FS. Topiramate Extends the Therapeutic Window for Hypothermia-Mediated Neuroprotection After Stroke in Neonatal Rats. Stroke. 2004;35:1460–5. doi: 10.1161/01.STR.0000128029.50221.fa. [DOI] [PubMed] [Google Scholar]
- 29.Barks JD, Liu Y-Q, Shangguan Y, Silverstein FS. Phenobarbital Augments Hypothermic Neuroprotection. Pediatr Res. 2010;67:532–7. doi: 10.1203/PDR.0b013e3181d4ff4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Shangguan Y, Barks JDE, Silverstein FS. Bumetanide augments the neuroprotective efficacy of phenobarbital plus hypothermia in a neonatal hypoxia-ischemia model. Pediatr Res. 2012;71:559–65. doi: 10.1038/pr.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gunn AJ, Bennet L, Gunning MI, Gluckman PD, Gunn TR. Cerebral Hypothermia Is Not Neuroprotective When Started after Postischemic Seizures in Fetal Sheep. Pediatr Res. 1999;46:274–80. doi: 10.1203/00006450-199909000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Davidson JO, Yuill CA, Zhang FG, Wassink G, Bennet L, Gunn AJ. Extending the duration of hypothermia does not further improve white matter protection after ischemia in term-equivalent fetal sheep. Scientific Reports. 2016;6:25178. doi: 10.1038/srep25178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kattwinkel J, Perlman JM, Aziz K, et al. Neonatal Resuscitation: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Pediatrics. 2010;126:e1400–e13. doi: 10.1542/peds.2010-2972E. [DOI] [PubMed] [Google Scholar]
- 34.Perlman JM, Wyllie J, Kattwinkel J, et al. Part 11: Neonatal Resuscitation. 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. 2010;122:S516–S38. doi: 10.1161/CIRCULATIONAHA.110.971127. [DOI] [PubMed] [Google Scholar]
- 35.Rutherford M, Ramenghi LA, Edwards AD, et al. Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic–ischaemic encephalopathy: a nested substudy of a randomised controlled trial. The Lancet Neurology. 2010;9:39–45. doi: 10.1016/S1474-4422(09)70295-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shankaran S, Barnes PD, Hintz SR, et al. Brain injury following trial of hypothermia for neonatal hypoxic–ischaemic encephalopathy. Archives of Disease in Childhood - Fetal and Neonatal Edition. 2012;97:F398–F404. doi: 10.1136/archdischild-2011-301524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nathan C. Points of control in inflammation. Nature. 2002;420:846–52. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 38.Middeldorp J, Hol EM. GFAP in health and disease. Progress in Neurobiology. 2011;93:421–43. doi: 10.1016/j.pneurobio.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 39.McRae A, Gilland E, Bona E, Hagberg H. Microglia activation after neonatal hypoxic-ischemia. Developmental Brain Research. 1995;84:245–52. doi: 10.1016/0165-3806(94)00177-2. [DOI] [PubMed] [Google Scholar]
- 40.Saliba E, Henrot A. Inflammatory Mediators and Neonatal Brain Damage. Neonatology. 2001;79:224–7. doi: 10.1159/000047096. [DOI] [PubMed] [Google Scholar]
- 41.Hagberg H, Mallard C, Ferriero DM, et al. The role of inflammation in perinatal brain injury. Nature reviews Neurology. 2015;11:192–208. doi: 10.1038/nrneurol.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernández-López D, Faustino J, Klibanov AL, et al. Microglial Cells Prevent Hemorrhage in Neonatal Focal Arterial Stroke. The Journal of Neuroscience. 2016;36:2881–93. doi: 10.1523/JNEUROSCI.0140-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chip S, Fernández-López D, Li F, Faustino J, Derugin N, Vexler ZS. Genetic deletion of galectin-3 enhances neuroinflammation, affects microglial activation and contributes to sub-chronic injury in experimental neonatal focal stroke. Brain, Behavior, and Immunity. 2016 doi: 10.1016/j.bbi.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robertson NJ, Faulkner S, Fleiss B, et al. Melatonin augments hypothermic neuroprotection in a perinatal asphyxia model. Brain. 2013;136:90–105. doi: 10.1093/brain/aws285. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.