Abstract
Background
Ayahuasca is a hallucinogenic plant preparation which usually contains the vine Banisteriopsis caapi and the shrub Psychotria viridis. This tea originates from the Amazon Basin where it is used in religious ceremonies. Because interest in these religious groups spreading as well as awareness of use of ayahuasca for therapeutic and recreational purposes, its use is increasing. Banisteriopsis caapi is rich in β-carbolines, especially harmine, tetrahydroharmine and harmaline, which have monoamine oxidase inhibiting (MAOI) activity. Psychotria viridis contains the 5HT2A/2C/1A receptor agonist hallucinogen N,N-dimethyltryptamine (DMT). Usual desired effects include hallucination, dissociation, mood alteration and perception change. Undesired findings previously reported are nausea, vomiting, hypertension, and tachycardia.
Methods
All human exposure calls reported to the American Association of Poison Controls Centers' (AAPCC) National Poison Data System (NPDS) between September 1, 2005 and September 1, 2015 were reviewed. Cases were filtered for specific plant derived ayahuasca-related product codes. Abstracted data included the following: case age and gender, exposure reason, exposure route, clinical manifestations, treatments given, medical outcomes and fatality.
Results
Five hundred and thirty-eight exposures to ayahuasca botanical products were reported. The majority of the calls to poison control centers came from healthcare facilities (83%). The most common route of exposure was ingestion. Most cases were men (437, 81%, 95% CI 77.7% - 84.3%). The median age was 21 (IQR 18-29). Most exposures were acute. Three hundred thirty-seven (63%) were reported to have a major or moderate clinical effect. The most common clinical manifestations reported were hallucinations (35%), tachycardia (34%), agitation (34%), hypertension (16%), mydriasis (13%) and vomiting (6%). Benzodiazepines were commonly given (30%). There were 28 cases in the series who required endotracheal intubation (5%). Four cases were reported to have had a cardiac arrest and 7 a respiratory arrest. Twelve cases had a seizure. Reports of exposures called to poison centers appeared to increase during this period based on annual estimates. Three fatalities were reported.
Conclusions
Ayahuasca use appears to be rising in the United States based on calls to poison control centers. While most use is reported to be safe and well tolerated, with possible beneficial effects, serious and life threatening adverse manifestations are possible. Most of the exposures reported to poison control centers were young people, more likely to be men and already in a healthcare facility. Further research, which includes comprehensive drug testing, will be needed to better identify the risks and effects of ayahuasca use.
Keywords: Ayahuasca, Banisteriopsis, Dimethyltryptamine
Background
Ayahuasca is a hallucinogenic plant preparation originally used by people indigenous to South America, including the Quechua. A loose translation of the Quechua term ayahuasca is “rope of the souls.” This substance, often consumed as a tea, originates from the Amazon Basin where it is used in religious ceremonies for many centuries. Due to interest in these religious groups, as well as use of ayahuasca for therapeutic and recreational purposes, ayahuasca use appears to be increasing. N,N-Dimethyltryptamine (DMT) is explicitly illegal for uses in most countries of the world but continues to be legal in the USA when used for legitimate religious purposes [1]. In general, growing and possessing the plants used are legal and they are readily available over the Internet or by mail order. Some variation of the plants used and brews created is likely.
Ayahuasca usually contains the vine Banisteriopsis caapi and shrub Psychotria viridis [2]. B. caapi is rich in β-carbolines, especially harmine, tetrahydroharmine, and harmaline, which have reversible monoamine oxidase A inhibiting activity [3, 4]. P. viridis contains the 5HT 2A/2C/1A receptor agonist hallucinogen DMT [5, 6]. The MAOI activity of the B. caapi allows DMT to be consumed orally, not metabolized by gut MAO, and penetrate the CNS. Desired effects by those who use ayahuasca include hallucinations, dissociation, mood alterations, and perception changes. Undesired effects include nausea, vomiting, hypertension, and tachycardia.
The purpose of this work was to better understand the use of ayahuasca and the resulting clinical manifestations. A descriptive analysis of calls to US poison centers is provided.
Methods
All human exposure calls reported to the American Association of Poison Control Centers’ (AAPCC) National Poison Data System (NPDS) between September 1, 2005 and September 1, 2015 were retrospectively analyzed. NPDS data, not including case notes, was received for 1890 cases which could possibly have been related to ayahuasca use, but most were linked to non-specific codes. The following product codes were requested: 2330118 (B. caapi), 3633355 (hallucinogenic tryptamines), 4798273 (ayahuasca), and 7238375 (plants-psychoactive, all codes from the National Poison Date System codes from Micromedex ®, Truven Health Analytics, Michigan, USA). Once received, cases were filtered for product codes, resulting in 538 cases most specific for botanically derived ayahuasca (2330118 and 4798273), to eliminate other plants and synthetic tryptamines. Abstracted data included the following: case age and gender, exposure reason, exposure route, clinical effects, treatment given, medical outcomes, and fatality. NPDS major-effect cases exhibited signs or symptoms as a result of the exposure that were life-threatening or resulted in significant residual disability or disfigurement. Moderate-effect cases exhibited signs or symptoms as a result of the exposure that were more pronounced, more prolonged, or more systemic in nature than minor symptoms. Minor-effect cases developed some signs or symptoms as a result of the exposure, but they were minimally bothersome and generally resolved rapidly with no residual disability or disfigurement. Usually, some form of treatment was indicated [7].
All case ages and exposure reasons were included; information and animal calls (no human exposure) were excluded. A master list was generated with unique case numbers assigned to each chart. The collected data were secured in password-protected electronic computer files with limited access. Descriptive statistics were used, other than to report the confidence interval around gender of callers. In this case, a binomial distribution 95% confidence interval was used. This activity was approved by the University of Arizona institutional review board.
Results
During this 10-year period, 538 human exposure cases were reported to NPDS. Most were men (437, 81%, 95% CI 77.7–84.3%) and most were already in a healthcare facility (447, 83%). The median age was 21 years old (IQR 18–29). One hundred and seven cases were under 18 years of age. Ten of these were under 10 years of age. One substance exposure only was reported in 332 cases, two in 118, three in 45, and greater than three in the remainder.
Almost all exposures were acute and intentional. Forty-one cases (7%) were noted to have major clinical manifestations. There were 296 cases reported to have moderate clinical manifestations (55%). The most common clinical effects were hallucinations (190, 35%), agitation (181, 34%), tachycardia (180, 34%), confusion (99, 18%), hypertension (87, 16%), mydriasis (72, 13%), and vomiting (32, 6%). The most severe effects noted were seizures (12, 2%), respiratory arrest (7, 1%), and cardiac arrest (4, 1%). Three fatalities were reported to poison control, all adults, with two cases marked as indirect reports.
Unknown or unreported duration of effect was noted in 171 cases, less than 2 h of manifestations in 27 cases, 2 to 8 h in 144 cases, 8 to 24 h in 105 cases, 24 h to 3 days in 57 cases, more than 3 days to a week in 25 cases, more than a week and less than a month in 6 cases, greater than 1 month in 2 cases, and permanent in 1 case.
Of the 538 exposures, 258 (48%) were treated and released from a healthcare facility. Ninety-two were admitted to a critical care unit (17%). Fifty-eight were admitted to a non-critical care unit (11%). Thirty-three were medically cleared and admitted to a psychiatric unit (6%). The remaining 97 either were lost to follow-up or not reported (18%).
The most common treatments received were intravenous fluids (190, 35%) and benzodiazepines (162, 30%). Intubation was performed on 28 cases (5%).
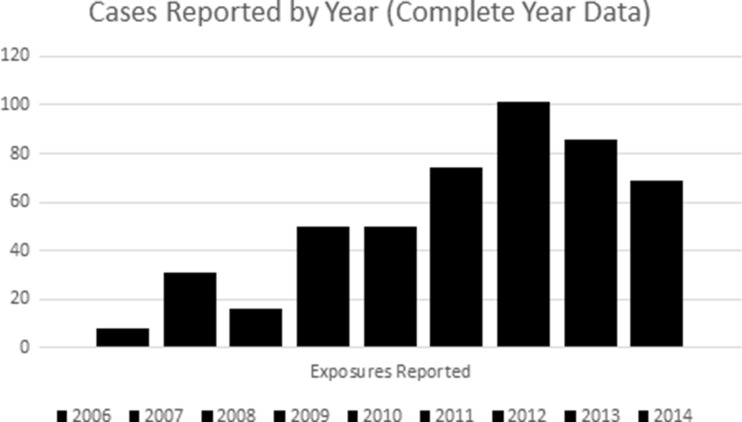
In general, the number of exposures reported to poison centers increased during the review period, with the apparent peak in 2012 (Fig. 1).
Fig. 1.
The number of exposures reported to poison centers increased during the review period, with the apparent peak in 2012
Discussion
It appears that most clinical effects reported to poison control centers (PCC) are common side effects from the use of ayahuasca preparations. These are often tolerated or even part of the ceremony surrounding use. It is unknown if the effects reported in this review represented ADEs due to the concurrent use of other pharmacologically similar medications (e.g., SSRIs). It is also unknown if the reported effects were due to unknown or intentionally increased doses of the constituent plant alkaloids. Most ingestions are probably not reported to PCC, even those with adverse effects, given the uncertainty of their legal status and use in a religious ceremony.
While most patients probably tolerate ayahuasca well based on other literature reports, severe clinical effects can result, with some cases suffering cardiac or respiratory arrest or death. The demographic profiles of cases reported to poison control centers reflect a similarity to those of other psychoactive substance use—predominantly young men [8]. A significant number of reported cases required admission to a hospital facility.
The reported duration of effect in these cases is similar to that reported in the medical literature, with a mode duration of 2 to 8 h, similar to the peak of 90–120 min and resolution at 360 min [2].
One case in the medical literature described the death of a young person with very high levels of some of the psychoactive substances found in ayahuasca, although the psychoactive substances in this case may have been synthetic rather than botanically derived [9, 10]. Many forms of DMT are synthetically derived and may have sympathomimetic activity not usually found in the botanical versions, such as “foxy methoxy” or 5-methoxy-N,N-diisopropyltryptamine. These synthetic agents cause hyperthermia, rhabdomyolysis, seizure, and potentially death [11, 12]. Case reports of adverse outcomes and human deaths may be not from botanical ayahuasca at all [10]. A recent very small open-label trial with six volunteers given a single treatment of plant-derived ayahuasca demonstrated decreased depression scores and a common side effect of vomiting. However, because of the small sample size, no placebo group, and limited monitoring of potential side effects, more research is needed before meaningful conclusions about safety or efficacy can be made [13].
Given the MAOI inhibiting activity, it is possible adverse effects may occur when a person is also on a pharmacological agent such as a serotonin reuptake inhibitor or a tricyclic antidepressant. However, given the local intestinal MAOI-A activity and relatively short duration of ayahuasca, this may not be a serious issue. Unfortunately, poison control center records do not allow analysis of adverse events that occur when a patient is taking a drug that may increase the risk of harm (such as serotonin reuptake inhibitors or tricyclic antidepressants), because they only report other substances known to be taken at the same time.
There were several limitations involved in this work. The retrospective review of calls received by US PCCs introduced selection and reporting bias. It is assumed that not all ayahuasca cases, particularly those with minimal or no clinical effects, were reported to a poison center. Fatalities, if they occur, are not always reported to PCCs. Finally, this review did not have specific drug testing that confirmed use of ayahuasca and ruled out the involvement of other drugs or medications.
Conclusion
Ayahuasca use appears to be rising in the USA based on calls to poison control centers. While most use is reported to be safe and well tolerated, with possible beneficial effects for the treatment of psychiatric illness, serious and life-threatening adverse effects are possible. Further research, which includes comprehensive analytical testing, will enhance the characterization of vulnerable populations or those who would receive benefit from using ayahuasca.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Sources of Funding
No funding was used for this project.
References
- 1.United States Supreme Court case 2005. Accessible at: http://www.supremecourt.gov/opinions/05pdf/04-1084.pdf
- 2.Riba J, Valle M, Urbano G, Yritia M, Morte A, Barbanoj MJ. Human pharmacology of ayahuasca: subjective and cardiovascular effects, monoamine metabolite excretion, and pharmacokinetics. J Pharmacol Exp Ther. 2003;306(1):73–83. doi: 10.1124/jpet.103.049882. [DOI] [PubMed] [Google Scholar]
- 3.Buckholtz NS, Boggan WO. Monoamine oxidase inhibition in brain and liver produced by β-carbolines: structure-activity relationships and substrate specificity. Biochem Pharmacol. 1977;26(21):1991–1996. doi: 10.1016/0006-2952(77)90007-7. [DOI] [PubMed] [Google Scholar]
- 4.Samoylenko V, Rahman MM, Tekwani BL, Tripathi LM, Wang YH, Khan SI, Khan IA, Miller LS, Joshi VC, Muhammad I. Banisteriopsis caapi, a unique combination of MAO inhibitory and antioxidative constituents for the activities relevant to neurodegenerative disorders and Parkinson’s disease. J Ethnopharmacol. 2010;127(2):357–367. doi: 10.1016/j.jep.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pierce PA, Peroutka SJ. Hallucinogenic drug interactions with neurotransmitter receptor binding sites in human cortex. Psychopharmacology. 1989;97(1):118–122. doi: 10.1007/BF00443425. [DOI] [PubMed] [Google Scholar]
- 6.Smith RL, Canton H, Barrett RJ, Sanders-Bush E. Agonist properties of N, N-dimethyltryptamine at serotonin 5-HT 2A and 5-HT 2C receptors. Pharmacol Biochem Behav. 1998;61(3):323–330. doi: 10.1016/S0091-3057(98)00110-5. [DOI] [PubMed] [Google Scholar]
- 7.Mowry JB, Spyker DA, Brooks DE, McMillan N, Schauben JL. 2014 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 32nd annual report. Clin Toxicol. 2015;53(10):962–1147. doi: 10.3109/15563650.2015.1102927. [DOI] [PubMed] [Google Scholar]
- 8.Palamar JJ, Martins SS, Su MK, Ompad DC. Self-reported use of novel psychoactive substances in a US nationally representative survey: prevalence, correlates, and a call for new survey methods to prevent underreporting. Drug Alcohol Depend. 2015;156:112–119. doi: 10.1016/j.drugalcdep.2015.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sklerov J, Levine B, Moore KA, King T, Fowler D. A fatal intoxication following the ingestion of 5-methoxy-N, N-dimethyltryptamine in an ayahuasca preparation. J Anal Toxicol. 2005;29(8):838–841. doi: 10.1093/jat/29.8.838. [DOI] [PubMed] [Google Scholar]
- 10.Callaway JC, Grob CS, McKenna DJ, Nichols DE, Shulgins A, Tupper KW. A demand for clarity regarding a case report on the ingestion of 5-methoxy-N, N-dimethyltryptamine (5-MeO-DMT) in an ayahuasca preparation. J Anal Toxicol. 2006;30(6):406–407. doi: 10.1093/jat/30.6.406. [DOI] [PubMed] [Google Scholar]
- 11.Alatrash G, Majhail NS, Pile JC. Rhabdomyolysis after ingestion of “foxy,” a hallucinogenic tryptamine derivative. In: Mayo clinic proceedings 2006 Apr 30 (Vol. 81, No. 4, pp. 550–551). Elsevier. [DOI] [PubMed]
- 12.Smolinske SC, Rastogi R, Schenkel S. Foxy methoxy: a new drug of abuse. Journal of medical toxicology. 2005;1(1):23–25. doi: 10.1007/BF03160901. [DOI] [PubMed] [Google Scholar]
- 13.Osório FD, Sanches RF, Macedo LR, dos Santos RG, Maia-de-Oliveira JP, Wichert-Ana L, de Araujo DB, Riba J, Crippa JA, Hallak JE. Antidepressant effects of a single dose of ayahuasca in patients with recurrent depression: a preliminary report. Rev Bras Psiquiatr. 2015;37(1):13–20. doi: 10.1590/1516-4446-2014-1496. [DOI] [PubMed] [Google Scholar]