Abstract
Introduction
Monensin is a veterinary antibiotic with a narrow therapeutic window that has led to lethal intoxication in many animal species. Only two prior cases of human toxicity have been reported, both fatal. We present the first case of survival from severe toxicity following monensin ingestion.
Case
A 58-year-old man presented with 8 days of vomiting and abdominal pain. Due to delusions of central nervous system toxoplasmosis, he ingested 300 mg of monensin. His laboratory studies revealed severe rhabdomyolysis without renal dysfunction. Total creatine kinase (CK) peaked above 100,000 U/L. His CK decreased to 5192 U/L after 15 days of aggressive hydration and sodium bicarbonate therapy. His ejection fraction on echocardiogram decreased from 69 to 56%.
Discussion
Reports on acute clinical effects after human exposure to monensin are limited. Ingestion is known to cause skeletal and cardiac muscle rhabdomyolysis and necrosis. Animal studies demonstrate that monensin’s toxicity is due to increases in intracellular sodium concentrations and Ca2+ release. To date, no effective antidotal treatment has been described.
Conclusions
Monensin is a veterinary medication not approved for human use by the US Food and Drug Administration. Though poorly studied in humans, this case demonstrates the severe harm that may occur following ingestion.
Keywords: Rhabdomyolysis, Monensin ingestion, Human, Monensin toxicity
Background
Monensin is a highly selective sodium ionophore veterinary antibiotic used as a food additive and for prevention of coccidiosis infections in poultry and cattle. Due to its narrow therapeutic window, multiple outbreaks of lethal intoxication have been reported in many animal species [1, 2]. While only two prior cases of human toxicity have been reported, both were fatal exposures [3, 4]. We present the first reported human case of survival after severe toxicity following monensin ingestion.
Case
A 58-year-old man presented to the emergency department for evaluation of vomiting and abdominal pain. Due to delusions of central nervous system toxoplasmosis, the patient purchased monensin online from a foreign distributor in an attempt to self-treat. Nine days prior to presentation, he ingested an estimated 100 mg of monensin, followed by an additional 200 mg the following day. Within hours after the second dose, the patient developed vomiting and abdominal pain and, over several days, began to experience significant leg pain, generalized weakness, and decreased urine output.
His physical exam was significant for dry mucous membranes, mild tachycardia, mild tachypnea, and diffuse tenderness to palpation of his abdomen and lower extremities. Significant laboratory values, which included a CK on presentation of 77,010 U/L, are noted in Table 1. Due to his severe rhabdomyolysis and concern for ongoing monensin toxicity, he was admitted to the intensive care unit.
Table 1.
Laboratory values
| Initial (8 days post ingestion) | Peak | Discharge | |
|---|---|---|---|
| Creatine kinase (U/L) | 77,010 | >100,000 | 5192 |
| AST (U/L) | 1224 | 2542 | 174 |
| ALT (U/L) | 93 | 303 | 134 |
| Serum creatinine (mg/dL) | 0.82 | 0.88 | 0.44 |
| Troponin-I (ng/mL) | <0.03 | 0.16 | 0.05 |
| Monensin (ppm) | 0 (9 days post ingestion) |
An echocardiogram on admission showed an ejection fraction of 69% and no wall motion abnormalities. Medical toxicology was consulted and stated concern for potential development of rhabdomyolysis-induced renal failure and cardiac necrosis leading to heart failure based on descriptions of animal and human toxicity in the literature. Over the next 8 days, he received continuous intravenous fluids and a bicarbonate infusion for urine alkalization. He remained hemodynamically stable.
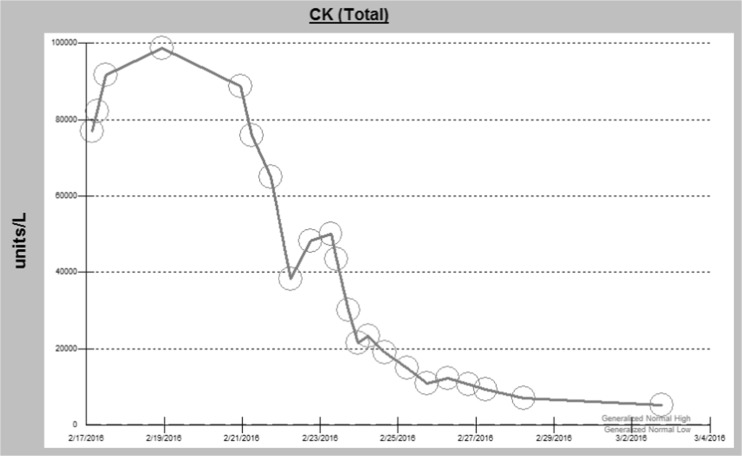
His CK peaked at over 100,000 U/L and remained elevated at 5192 U/L several days prior to discharge. The trend of his CK levels is shown in Fig. 1. Despite severe rhabdomyolysis, there was no evidence of acute kidney injury. His discharge creatinine was 0.44 mg/dL, compared to his previous baseline of 0.9 mg/dL and was felt to be secondary to muscle wasting as a result of this intoxication. A repeat echocardiogram 1 week after admission was within normal limits with an ejection fraction of 56%.
Fig. 1.
CK trend during hospitalization. Note: normal range 62–325 units/L. Maximum lab value >100,000
Serum sodium remained normal throughout his admission, ranging from 135 to 143 mEq/L, while his calcium level was consistently low, between 7.4 and 8.3 mg/dL with a serum albumin of 2.7 g/dL. A serum monensin concentration sent on hospital day two was undetectable.
The patient’s hospitalization was prolonged at 21 days, mostly due to difficulty in obtaining placement at a skilled nursing facility for his severe debilitation. When seen for follow-up by his primary care provider 2 months after discharge, the patient was doing well physically although he had ongoing delusions about having toxoplasmosis. Verbal informed consent and permission was obtained from this patient during his hospitalization for publication of this case report.
Discussion
Reports on clinical effects after human exposure to monensin are limited. Monensin has a narrow therapeutic window and animal toxicity is most commonly secondary to errors in dosing or mixing in feed. Similar to lethal ingestions in animals, human ingestions of monensin have been shown in two prior reports to cause severe skeletal and cardiac muscle rhabdomyolysis and necrosis, resulting in death.
In vitro studies have shown monensin increases intracellular sodium concentrations by two mechanisms. First, the monensin ionophore complexes with sodium and nonselectively transfers through myocyte lipid membranes [4]. Secondly, monensin upregulates the Na+/H+ antiporter, thereby increasing intracellular sodium and pH. Increased intracellular Na+ stimulates the Na+/Ca2+ antiporter to extrude Na+ and influx Ca2+. This influx of Ca2+ causes Ca2+-mediated Ca2+ release. Additionally, increased intracellular pH leads to further intracellular Ca2+ release. This significant increase in intracellular Ca2+ levels overwhelm normal mitochondrial sequestration capacity, leading to activation of phospholipases and proteolytic enzymes, resulting in myocyte necrosis [5].
Animal studies propose the initial elevations of Na+ in muscle lead to increases in muscle Ca2+ in a concentration-dependent manner causing reduced muscle contractile and metabolic function in addition to a loss of CK [6]. Skeletal and cardiac myocytes are thought to suffer the most damage from monensin due to the negative impact on oxidative phosphorylation and Ca2+ metabolism. Damage in other cells is more likely related to cellular swelling from the increased intracellular Na+ and less so from changes in Ca2+ metabolism [1].
Signs of monensin toxicity include skeletal muscle weakness, tachycardia, dyspnea, myoglobinuria, cardiac failure, and acute renal failure. In horses administered monensin, the first signs of toxicity are anorexia and diarrhea which develop within 24 h [7]. The lethal dosages in animals vary widely from 2 to 3 mg/kg in horses, to 26 mg/kg in cattle, to 200 mg/kg in chickens [8]. The fatal dosage is not known in humans. However, in previously described reports of lethal human toxicity, one patient ingested 500 mg and the other an unknown amount. Our patient ingested an estimated 300 mg or 4.6 mg/kg.
The serum monensin concentration for this patient was measured via electrospray ionization liquid chromatography tandem mass spectrometry and was undetectable (limit of detection 0.5 parts per billion). A study investigating the bioavailability and tissue deposition of monensin in poultry identified an elimination half-life of 1.3 to 5.5 h [9]. Monensin’s pharmacokinetics in humans are unknown; however, it is plausible the monensin ingested by our patient completely metabolized by the time of presentation, 8 days after the last ingested dose. Although monensin was not detected at that time, the history and clinical course support monensin as the cause of his toxicity.
In prior reported cases of human toxicity, rhabdomyolysis occurred approximately 5 days after ingestion and death followed within 11 days. On autopsy, these patients were found to have findings consistent with skeletal rhabdomyolysis, acute renal tubular damage, and microscopic evidence of early myocardial damage [3, 4]. Although there are a few discrepancies between the histories in these two previously described cases, the laboratory data are strikingly similar and in fact may describe the same patient.
Horses poisoned with monensin show elevations of cardiac troponin I (cTnI). In one study, cTnI concentrations were elevated within 24–72 h, with the greatest elevations seen in those horses that died [7]. Our patient developed a more delayed increase in his cTnI seen first on hospital day 6. The patient had no evidence of cardiac or hemodynamic instability during the hospitalization and an echocardiogram prior to discharge showed normal wall motion and an ejection fraction of 56%. The elevated ejection fraction on admission likely represented a hyper-dynamic heart in the setting of volume depletion.
To date, no effective antidotal treatment has been described. Selenium and vitamin E have been proposed to decrease monensin toxicity by stabilizing myocyte membranes. In one study, six pigs were given selenium-vitamin E and 1 day later given 50 mg/kg of monensin. The selenium-vitamin E group had a higher rate of survival and lower CK values compared with six pigs given a placebo [10].
Calcium modulators, such as diltiazem, have been evaluated for their potential role in monensin toxicity, however were found to potentiate monensin toxicity [11]. The results of these studies suggest that multiple mechanisms, beyond calcium modulation, play important roles in monensin toxicity. None of the described treatments were used for our patient due to his late presentation and the suspected lack of efficacy given his advanced course.
Conclusions
We describe the first reported human case of survival from severe rhabdomyolysis following monensin ingestion. Monensin is a veterinary medication with a narrow therapeutic window and is not FDA approved for human use. Monensin intoxication can lead to severe rhabdomyolysis, renal failure, cardiac failure, and death. There is currently no validated targeted treatment or antidote. Though poorly described in humans, this case demonstrates the severe harm that may occur following monensin ingestion.
Compliance with Ethical Standards
Consent for publication of this case was obtained and provided to the journal in accordance with JMT policy.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Sources of Funding
None
References
- 1.Jones A. Monensin toxicosis in 2 sheep flocks. Can Vet J. 2001;42:135–136. [PMC free article] [PubMed] [Google Scholar]
- 2.Schweitzer D, Kimberling C, Spraker T, Sterner FE, McChesney AE. Accidental monensin sodium intoxication of feedlot cattle. J Am Vet Med Assoc [Internet]. 1984;184:1273–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/6735846. [PubMed]
- 3.Caldeira C, Neves WS, Cury PM, Serrano P, Baptista MA, Burdmann EA. Rhabdomyolysis, acute renal failure, and death after monensin ingestion. Am J Kidney Dis [Internet]. 2001;38:1108–12. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11684567. [DOI] [PubMed]
- 4.Kouyoumdjian JA, Da Penha Ananias Morita M, Sato AK, Pissolatti AF. Fatal rhabdomyolysis after acute sodium monensin (Rumensin®) toxicity: case report. Arq Neuropsiquiatr. 2001;59:596–598. doi: 10.1590/S0004-282X2001000400022. [DOI] [PubMed] [Google Scholar]
- 5.DuBourdieu DJ, Shier WT. Sodium- and calcium-dependent steps in the mechanism of neonatal rat cardiac myocyte killing by ionophores. II. The calcium-carrying lonophore, A23187. Toxicol Appl Pharmacol. 1992;116:47–56. doi: 10.1016/0041-008X(92)90143-G. [DOI] [PubMed] [Google Scholar]
- 6.Sandercock DA, Mitchell MA. The role of sodium ions in the pathogenesis of skeletal muscle damage in broiler chickens. Poult Sci. 2004;83:701–706. doi: 10.1093/ps/83.4.701. [DOI] [PubMed] [Google Scholar]
- 7.Divers TJ, Kraus MS, Jesty SA, Miller AD, Mohammed HO, Gelzer ARM, et al. Clinical findings and serum cardiac troponin I concentrations in horses after intragastric administration of sodium monensin. J Vet Diagn Investig [Internet]. 2009;21:338–43. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19407085. [DOI] [PubMed]
- 8.Todd GC, Novilla MN, Howard LC. Comparative toxicology of monensin sodium in laboratory animals. J Anim Sci. 1984;58:1512–1517. doi: 10.2527/jas1984.5861512x. [DOI] [PubMed] [Google Scholar]
- 9.Henri J, Maurice R, Postollec G, Dubreil-Cheneau E, Roudaut B, Laurentie M, et al. Comparison of the oral bioavailability and tissue disposition of monensin and salinomycin in chickens and turkeys. J Vet Pharmacol Ther. 2012;35:73–81. doi: 10.1111/j.1365-2885.2011.01285.x. [DOI] [PubMed] [Google Scholar]
- 10.Van Vleet J, Amstutz H, Weirich W, et al. Acute monensin toxicosis in swine: effect of graded doses of monensin and protection of swine by pretreatment with selenium-vitamin E. Am J Vet Res. 1983;44:1460–1468. [PubMed] [Google Scholar]
- 11.Mitema E, Sangiah S, Martin T. Effects of some calcium modulators on monensin toxicity. Vet Hum Toxicol. 1988;30(5):409–413. [PubMed] [Google Scholar]