Abstract
Introduction
The objective of this study was to investigate the epidemiology of dietary supplement exposures in the USA.
Methods
A retrospective analysis was conducted of out-of-hospital dietary supplement exposures reported to the National Poison Data System from 2000 through 2012.
Results
There were 274,998 dietary supplement exposures from 2000 through 2012. The annual rate of dietary supplement exposures per 100,000 population increased by 46.1% during 2000–2002, decreased 8.8% during 2002–2005, and then increased again by 49.3% from 2005 to 2012. These trends were influenced by the decrease in ma huang exposures starting in 2002. Miscellaneous dietary supplements accounted for 43.9% of all exposures, followed by botanicals (31.9%), hormonal products (15.1%), and other supplements (5.1%). The majority of dietary supplement exposures (70.0%) occurred among children younger than 6 years old and were acute (94.0%) and unintentional (82.9%). Serious medical outcomes accounted for 4.5% of exposures and most (95.0%) occurred among individuals 6 years and older. Ma huang products, yohimbe, and energy products were the categories associated with the greatest toxicity.
Conclusions
There was an overall increase in the rate of dietary supplement exposures from 2000 through 2012. Although the majority of these exposures did not require treatment at a health care facility or result in serious medical outcomes, exposures to yohimbe and energy products were associated with considerable toxicity. Our results demonstrate the success of the FDA ban on ma huang products and the need for FDA regulation of yohimbe and energy products in the USA.
Keywords: Dietary supplement, Poison control center, Ma huang, Yohimbe, Energy products
Introduction
The United States (US) Dietary Supplement Health and Education Act of 1994 defines dietary supplements as products (excluding tobacco) intended to supplement the diet by increasing total dietary intake and/or providing one or more of the following: vitamins, minerals, herbs, botanicals, or amino acids [1]. The definition also includes concentrates, metabolites, constituents, or extracts of these ingredients. Dietary supplement use in the US has increased over the past several decades, with an estimated 42% of adults using at least one dietary supplement in 1988–1994, compared with a peak of 54% in 2003–2006 [2, 3]. In 2011–2012, an estimated 52% of adults in the US reported having used a dietary supplement within the past 30 days [3]. Consumers often believe that dietary supplements are held to the same safety and efficacy standards as over-the-counter medications; however, dietary supplements are not considered drugs, and are not required to undergo clinical trials or obtain approval from the US Food and Drug Administration (FDA) prior to their sale in the US, unless the product is intended for therapeutic use, such as treating or preventing disease [4–6]. This lack of federal oversight has led to inconsistencies in the quality of dietary supplements, product mislabeling, and contamination with other substances [5, 7].
Previous studies have used the National Poison Data System (NPDS), and its predecessor, the Toxic Exposure Surveillance System (TESS), to describe the characteristics of calls to poison control centers (PCCs) reporting dietary supplement exposures and toxicity [8–12]. Others studies have focused on emergency department visits resulting from adverse effects related to dietary supplement exposures [13]. The objective of this study was to provide an epidemiological description of dietary supplement exposures reported to PCCs in the US over a 13-year period. Unlike prior studies that focused on specific dietary supplements, included small sample sizes, or were conducted more than 10 years ago, the current study uses the NPDS database to investigate all dietary supplement exposures resulting in calls to PCCs in the US from 2000 through 2012 [8–13].
Methods
Dietary supplement exposure data from 2000 through 2012 were obtained from the NPDS, which is maintained by the American Association of Poison Control Centers (AAPCC) and contains reported detailed data from telephone calls about substance exposures received by regional PCCs in the US and its territories [14]. PCC specialists input data from each call into the NPDS database using a coding system and strict quality control protocols. This database can then be queried to obtain data about these reported exposures. For this study, the term “reported exposure” will be referred to simply as “exposure” for brevity.
Case Selection Criteria
All single-substance, dietary supplement exposure cases involving humans were extracted from the NPDS database using the generic codes listed in the Table 5. Cases were excluded if the medical outcome was determined by the PCC specialist to be a “confirmed non-exposure” or “unrelated effect.” Exposures occurring at a health care facility (HCF) were excluded from the analyses, but treatment at a HCF post-exposure was not a cause for exclusion.
Table 5.
AAPCC generic codes for dietary supplements included in study
| Generic code | Dietary supplement |
|---|---|
| Amino acids | |
| 0201083 | Other amino acid dietary supplements |
| Botanicals | |
| 0201088 | Blue cohosh |
| 0201094 | Citrus aurantium (single ingredient) |
| 0201090 | Echinacea |
| 0201089 | Ginkgo biloba |
| 0201091 | Ginseng |
| 0201092 | Kava kava |
| 0201093 | Ma huang/Ephedra (Single Ingredient) |
| 0201100 | Multi-botanicals with Citrus aurantium |
| 0201098 | Multi-botanicals with Ma huang |
| 0201099 | Multi-botanicals without Ma huang or Citrus aurantium |
| 0201095 | St. John’s Wort |
| 0201096 | Valerian |
| 0201097 | Yohimbe |
| 0201101 | Other single ingredient botanicals |
| Cultural medicines | |
| 0201085 | Asian medicines |
| 0201084 | Ayurvedic medicines |
| 0201086 | Hispanic medicines |
| 0201087 | Other cultural medicines |
| Energy products | |
| 0200605 | Energy drinks: caffeine containing (from any source including guarana, kola nut, tea, yerba mate, cocoa, etc.) |
| 0200606 | Energy drinks: caffeine only (without guarana, kola nut, tea, yerba mate, cocoa, etc.) |
| 0200610 | Energy drinks: ethanol and caffeine containing (from any source including guarana, kola nut, tea, yerba mate, cocoa, etc.) |
| 0200611 | Energy drinks: ethanol and caffeine only (without guarana, kola nut, tea, yerba mate, cocoa, etc.) |
| 0200607 | Energy drinks: no caffeine (from any source) |
| 0200608 | Energy drinks: unknown |
| 0200609 | Energy products: other |
| Hormonal products | |
| 0201103 | Androgen or androgen precursor dietary supplements |
| 0201105 | Glandular dietary supplements |
| 0201106 | Melatonin |
| Miscellaneous dietary supplements/herbals/homeopathic | |
| 0201102 | Homeopathic agents |
| 0077922 | Unknown dietary supplements or homeopathic agents |
| Other dietary supplements | |
| 0201107 | Blue-green algae |
| 0201108 | Glucosamine (with or without chondroitin) |
| 0201109 | Other single ingredient non-botanical dietary supplements |
Variables
Data were analyzed by dietary supplement category, exposed person’s age and gender, reason for exposure, chronicity of exposure, route of exposure, exposure site, management site, clinical effect, level of health care received, therapy received, and medical outcome. The dietary supplement categories created by the AAPCC were used in this study and include the following: amino acids, botanicals, cultural medicines, energy products, hormonal products, miscellaneous dietary supplements, and other dietary supplements (Table 5). In the botanicals category, ma huang (single ingredient) and multi-botanicals with ma huang were grouped into ma huang products for sub-analysis. Generic codes for energy products were introduced into the NPDS in mid-2010 and include both energy products and energy drinks. Other variable categories also followed AAPCC definitions [14]. The AAPCC classifies medical outcomes as minor, moderate, or major. “Minor effect” corresponds to minimal symptoms; “moderate effect” signifies more pronounced, prolonged, or systemic symptoms, usually requiring some form of treatment; and “major effect” corresponds to life-threatening symptoms or significant residual disability or disfigurement. In this study, moderate effect, major effect, and death categories were collectively defined as “serious outcomes.” Exposures were grouped by exposed person’s age into <6 years, ≥ 6 years, and unknown age. Reasons for exposure were classified as unintentional, intentional, adverse reaction, and other/unknown.
Statistical Analyses and Ethical Considerations
SPSS 21.0 (IBM Corp., Armonk, NY) and SAS 9.3 (SAS Institute Inc., Cary, NC) software were used for statistical analyses, and the descriptive statistics reported in this article include all dietary supplements. Because population data on the use of dietary supplements were not available, exposure rates were calculated using the US Census Bureau July 1st intercensal and postcensal population estimates for 2000–2012 [15]. Based on the scatterplot of the annual exposure rate (Fig. 2), there were changes in the directionality of the overall rate and the rates associated with ma huang and homeopathic agents. Therefore, piecewise linear regression models were used to analyze these trends. For each piecewise linear regression model, exposure rate was the response variable and year of exposure, parametrized by the time period segments, were the predictors. To clearly describe the trend without the effects of the FDA’s 2004 ban on ma huang, a separate trend analysis was performed without ma huang included. Simple linear regression models were used to assess the trends (while excluding ma huang) in Fig. 3. The model assumptions were assessed, and all models met the requirements. A t test was used to test the slope of the regression line to determine if it was significantly different from zero. The estimated annual rate of change from the regression model, denoted by “m,” is reported along with the associated p value. Statistical significance was determined at alpha = 0.05. Person-identifying information was removed from NPDS data by the AAPCC before being made available to the investigators. This study was determined to be exempt by the Institutional Review Board at our institution.
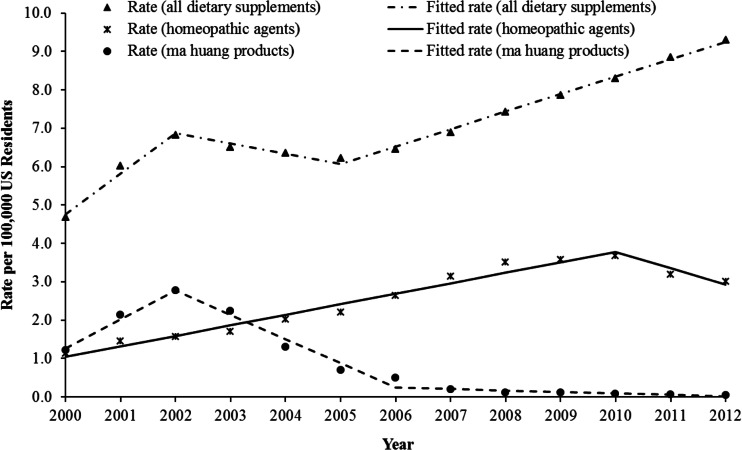
Fig. 2.
Annual rates of exposure for all dietary supplements, ma huang products, and homeopathic agents, National Poison Data System 2000–2012
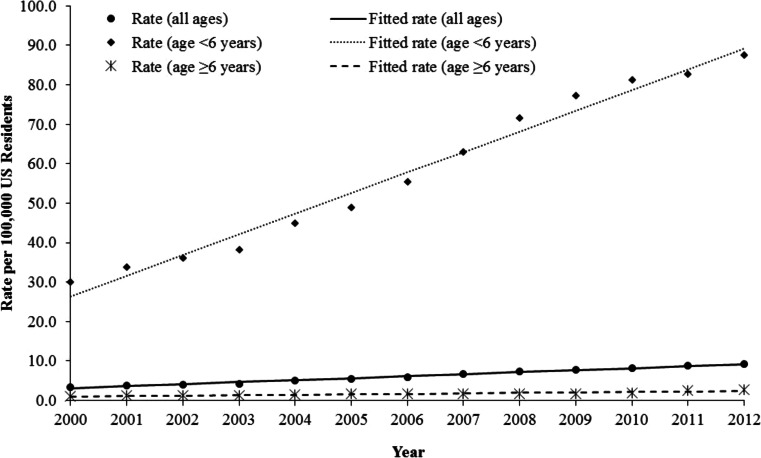
Fig. 3.
Annual rates of dietary supplement exposures (excluding ma huang products) by age group, National Poison Data System 2000–2012
Results
General Characteristics
From 2000 through 2012, there were 274,998 dietary supplement exposures that met study criteria reported to the NPDS, averaging 21,154 exposures annually or 7.05 exposures per 100,000 population. The majority of dietary supplement exposures (70.0%) occurred among individuals younger than 6 years old (Fig. 1). Females accounted for 49.4% of dietary supplement exposures among individuals younger than 6 years and 58.0% of exposures among those 6 years and older (Table 1). The majority of dietary supplement exposures overall and among individuals younger than 6 years old were unintentional (82.9 and 99.4%, respectively). Among those 6 years and older, the majority of exposures were intentional (33.4%) or adverse reactions (21.2%). Overall, most exposures were acute (94.0%) and occurred at the person’s own residence or another person’s residence (97.3%). In this study, the most common routes of exposure were ingestion only (97.7%), ocular (0.8%), and dermal (0.5%).
Fig. 1.
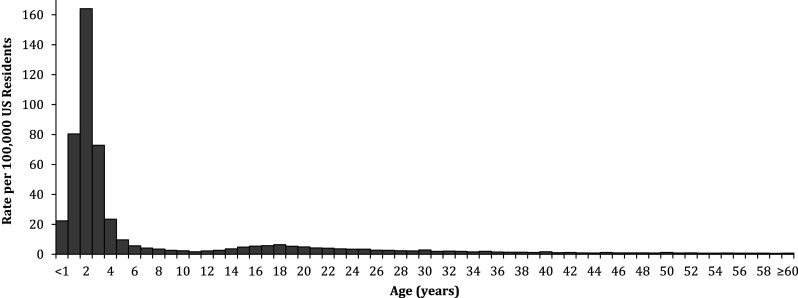
Rate of dietary supplement exposures by age, National Poison Data System 2000–2012
Table 1.
Characteristics of dietary supplement exposures by age group, National Poison Data System 2000–2012
| Characteristics | <6 years (N = 192,583) | ≥6 years (N = 81,165) | Totala (N = 274,998) |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Gender | |||
| Female | 95,150 (49.4) | 47,113 (58.0) | 142,861 (51.9) |
| Male | 97,015 (50.4) | 33,778 (41.6) | 131,230 (47.7) |
| Unknown | 418 (0.2) | 274 (0.3) | 907 (0.3) |
| Reason for exposure | |||
| Unintentional | 191,443 (99.4) | 35,735 (44.0) | 227,886 (82.9) |
| Intentional | 264 (0.1) | 27,090 (33.4) | 27,678 (10.1) |
| Adverse reaction | 661 (0.3) | 17,187 (21.2) | 17,989 (6.5) |
| Other or unknown | 215 (0.1) | 1153 (1.4) | 1445 (0.5) |
| Chronicity | |||
| Acute | 188,927 (98.1) | 68,399 (84.3) | 258,366 (94.0) |
| Acute-on-chronic | 3001 (1.6) | 6489 (8.0) | 9540 (3.5) |
| Chronic | 457 (0.2) | 4949 (6.1) | 5470 (2.0) |
| Unknown | 198 (0.1) | 1328 (1.6) | 1622 (0.6) |
| Exposure site | |||
| Own or other residence | 190,860 (99.1) | 75,488 (93.0) | 267,411 (97.3) |
| Other or unknown | 1723 (0.9) | 5677 (7.0) | 7557 (2.7) |
| Management site | |||
| Managed on-site (non-HCF) | 175,886 (91.3) | 40,966 (50.5) | 217,478 (79.1) |
| Individual already in/enroute to HCF | 10,336 (5.4) | 25,227 (31.1) | 35,776 (13.0) |
| Individual referred by PCC to HCF | 5209 (2.7) | 12,960 (16.0) | 18,464 (6.7) |
| Other unknown | 1152 (0.6) | 2012 (2.5) | 3280 (1.2) |
| HCF level of care | |||
| No HCF treatment received | 177,038 (91.9) | 42,978 (53.0) | 220,758 (80.3) |
| Treated/evaluated and released | 12,671 (6.6) | 20,392 (25.1) | 33,177 (12.1) |
| Admitted to critical care unit | 206 (0.1) | 1938 (2.4) | 2154 (0.8) |
| Admitted to noncritical care unit | 409 (0.2) | 1967 (2.4) | 2383 (0.9) |
| Admitted to psychiatric care unit | 6 (0.0) | 3122 (3.8) | 3139 (1.1) |
| Refused referral/ did not arrive at HCF | 866 (0.4) | 3889 (4.8) | 4856 (1.8) |
| Lost to follow up or left AMA | 1387 (0.7) | 6879 (8.5) | 8531 (3.1) |
| Medical outcome | |||
| Serious outcome | 570 (0.3) | 11,628 (14.3) | 12,243 (4.5) |
| Death | 3 (0.0) | 31 (0.0) | 34 (0.0) |
| Major effect | 37 (0.0) | 586 (0.7) | 626 (0.2) |
| Moderate effect | 530 (0.3) | 11,011 (13.6) | 11,583 (4.2) |
| Minor effect | 7795 (4.0) | 17,774 (21.9) | 25,697 (9.3) |
| No effect | 53,774 (27.9) | 9693 (11.9) | 63,611 (23.1) |
| Not followed/unable to follow | 130,444 (67.7) | 42,070 (51.8) | 173,447 (63.1) |
aTotal includes cases with age unknown (n = 1250). Percentages may not sum to 100.0% due to rounding
PCC Poison Control Center, HCF health care facility, AMA against medical advice
Management Site, Level of Health Care Received, and Medical Outcome
Most (91.3%) exposures among children younger than 6 years old were managed on-site rather than in a HCF, compared with one-half (50.5%) of exposures among older individuals (Table 1). The proportion of exposed individuals who received HCF treatment or admission was almost five times higher among those 6 years or older compared with those younger than 6 years (33.8 and 6.9%, respectively). A small percentage (1.7%) of all dietary supplement exposures involved hospital admission, including 0.8% resulting in admission to a critical care unit. Thirty-four dietary supplement exposures resulted in death. The three dietary supplements associated with the most deaths were other/unknown multi-ingredient supplements (n = 8), ma huang (n = 5), and multi-botanicals without ma huang or Citrus aurantium (n = 4) (Table 2). Overall, 4.5% of exposures resulted in serious medical outcomes, and the majority (95.0%) of these serious outcomes occurred to individuals 6 years and older (Table 1).
Table 2.
Dietary supplement exposures by AAPCC-defined category and description, serious outcomes, and HCF level of care, National Poison Data System 2000–2012
| Dietary supplements | N (%) | Serious outcomes | HCF level of care | |||||
|---|---|---|---|---|---|---|---|---|
| Death | Major | Moderate | Total (%)a | CCU | Non-CCU | Total (%)a | ||
| Amino acids | 4556 (1.7) | 2 | 16 | 155 | 173 (3.8) | 20 | 39 | 59 (1.3) |
| Other amino acids | 4556 (1.7) | 2 | 16 | 155 | 173 (3.8) | 20 | 39 | 59 (1.3) |
| Botanicals | 87,699 (31.9) | 15 | 416 | 8441 | 8872 (10.1) | 1495 | 1485 | 2980 (3.4) |
| Yohimbe | 1818 (0.7) | 1 | 23 | 488 | 512 (28.2) | 58 | 46 | 104 (5.7) |
| Ma huang/Ephedra (single ingredient) | 5995 (2.2) | 5 | 50 | 936 | 991 (16.5) | 196 | 176 | 372 (6.2) |
| Multi-Botanicals with Ma huang | 27,325 (9.9) | 1 | 191 | 4254 | 4446 (16.3) | 821 | 697 | 1518 (5.6) |
| Citrus aurantium (single ingredient) | 75 (0.0) | 11 | 11 (14.7) | 1 | 1 | 2 (2.7) | ||
| Multi-botanicals with Citrus aurantium | 1646 (0.6) | 13 | 191 | 204 (12.4) | 36 | 34 | 70 (4.3) | |
| Kava kava | 815 (0.3) | 4 | 75 | 79 (9.7) | 16 | 22 | 38 (4.7) | |
| Multi-botanicals without Ma huang or Citrus aurantium | 20,351 (7.4) | 4 | 74 | 1616 | 1694 (8.3) | 212 | 277 | 489 (2.4) |
| Valerian | 1687 (0.6) | 5 | 92 | 97 (5.7) | 28 | 36 | 64 (3.8) | |
| Ginseng | 1934 (0.7) | 1 | 5 | 96 | 102 (5.3) | 13 | 14 | 27 (1.4) |
| Other single ingredient botanicals | 18,897 (6.9) | 3 | 42 | 569 | 614 (3.2) | 93 | 144 | 237 (1.3) |
| Ginkgo biloba | 1392 (0.5) | 4 | 38 | 42 (3.0) | 5 | 13 | 18 (1.3) | |
| St. John’s Wort | 1974 (0.7) | 3 | 44 | 47 (2.4) | 15 | 18 | 33 (1.7) | |
| Echinacea | 3782 (1.4) | 2 | 31 | 33 (0.9) | 1 | 7 | 8 (0.2) | |
| Cultural medicines | 1605 (0.6) | 2 | 21 | 131 | 154 (9.6) | 54 | 65 | 119 (7.4) |
| Other cultural medicines | 328 (0.1) | 5 | 38 | 43 (13.1) | 14 | 24 | 38 (11.6) | |
| Hispanic medicines | 93 (0.0) | 11 | 11 (11.8) | 6 | 6 (6.5) | |||
| Asian medicines | 1099 (0.4) | 2 | 16 | 75 | 93 (8.5) | 39 | 33 | 72 (6.6) |
| Ayurvedic medicines | 85 (0.0) | 7 | 7 (8.2) | 1 | 2 | 3 (3.5) | ||
| Energy products | 5103 (1.9) | 1 | 24 | 527 | 552 (10.8) | 49 | 69 | 118 (2.3) |
| Energy drinks: ethanol and caffeine only (1) | 7 (0.0) | 0 | 0 | 2 | 2 (28.6) | 0 | 1 | 1 (14.3) |
| Energy drinks: ethanol and caffeine containing (2) | 279 (0.1) | 0 | 9 | 65 | 74 (26.5) | 18 | 14 | 32 (11.5) |
| Energy products: other | 522 (0.2) | 0 | 3 | 77 | 80 (15.3) | 6 | 17 | 23 (4.4) |
| Energy drinks: caffeine containing (2) | 1349 (0.5) | 1 | 3 | 149 | 153 (11.3) | 9 | 13 | 22 (1.6) |
| Energy drinks: unknown | 808 (0.3) | 0 | 3 | 84 | 87 (10.8) | 6 | 11 | 17 (2.1) |
| Energy drinks: caffeine only (1) | 2098 (0.8) | 0 | 6 | 150 | 156 (7.4) | 10 | 13 | 23 (1.1) |
| Hormonal products | 41,440 (15.1) | 1 | 9 | 259 | 269 (0.6) | 104 | 163 | 267 (0.6) |
| Androgen/androgen precursor supplements | 1239 (0.5) | 1 | 6 | 66 | 73 (5.9) | 9 | 13 | 22 (1.8) |
| Glandular supplements | 476 (0.2) | 0 | 0 | 6 | 6 (1.3) | 3 | 2 | 5 (1.1) |
| Melatonin | 39,725 (14.4) | 0 | 3 | 187 | 190 (0.5) | 92 | 148 | 240 (0.6) |
| Miscellaneous supplements | 120,610 (43.9) | 11 | 123 | 1851 | 1985 (1.6) | 401 | 506 | 907 (0.8) |
| Other/unknown multi-ingredient supplements | 21,612 (7.9) | 8 | 93 | 1386 | 1487 (6.9) | 272 | 324 | 596 (2.8) |
| Homeopathic agents | 98,998 (36.0) | 3 | 30 | 465 | 498 (0.5) | 129 | 182 | 311 (0.3) |
| Other supplements | 13,985 (5.1) | 2 | 17 | 219 | 238 (1.7) | 31 | 56 | 87 (0.6) |
| Blue-green algae | 1186 (0.4) | 1 | 2 | 42 | 45 (3.8) | 6 | 9 | 15 (1.3) |
| Other single ingredient non-botanical supplements | 6590 (2.4) | 1 | 12 | 138 | 151 (2.3) | 21 | 40 | 61 (0.9) |
| Glucosamine | 6209 (2.3) | 0 | 3 | 39 | 42 (0.7) | 4 | 7 | 11 (0.2) |
The numbers in italics are the values for the major categories of dietary supplements. The number that are not in italics are the values for the subcategories, or descriptions, within the major categories
(1) Without Guarana, Kola Nut, Tea, Yerba Mate, Cocoa, etc.; (2) from any source including Guarana, Kola Nut, Tea, Yerba Mate, Cocoa, etc.; (3) from any source
CCU critical care unit
aRow percentages
Clinical Effects and Therapies
The clinical effects resulting most frequently from dietary supplement exposures were tachycardia (4.0%, n = 10,890), vomiting (3.5%, n = 9669), nausea (2.9%, n = 8058), irritability (2.9%, n = 7849), drowsiness (2.3%, n = 6290), and dizziness (1.4%, n = 3873). More than one-half of dietary supplement exposures did not require therapy (56.5%, n = 155,339), but some required one (32.7%, n = 89,942), two (9.8%, n = 26,956), or three or more (1.0%, n = 2761) therapies. Decontamination was the most frequently administered therapy (36.7%, n = 100,865).
Dietary Supplement Characteristics
Miscellaneous supplements accounted for 43.9% of all dietary supplement exposures, followed by botanicals (31.9%), hormonal products (15.1%), and other supplements (5.1%) (Table 3). Amino acids, cultural medicines, and energy products each accounted for less than 2% of total dietary supplement exposures. The dietary supplement categories with the highest proportion of serious medical outcomes were energy products (10.8%), botanicals (10.8%), and cultural medicines (9.6%). Within the botanical category, yohimbe accounted for the largest proportion of serious medical outcomes (28.2%), followed by ma huang (16.5%), and multi-botanicals with ma huang (16.3%). Due to the relatively small number of exposures in the energy products category, it was not possible to determine which product subcategories were most commonly associated with serious medical outcomes.
Table 3.
Dietary supplement exposures by AAPCC-defined category and description and by age group, National Poison Data System 2000–2012
| Dietary supplements | <6 years | ≥6 years | Totala |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Amino acids | 2778 (1.4) | 1745 (2.1) | 4556 (1.7) |
| Other amino acid dietary supplements | 2778 (1.4) | 1745 (2.1) | 4556 (1.7) |
| Botanicals | 43,392 (22.5) | 43,741 (53.9) | 87,699 (31.9) |
| Multi-botanicals with Ma huang | 10,233 (5.3) | 16,910 (20.8) | 27,325 (9.9) |
| Multi-botanicals without Ma huang or Citrus aurantium | 11,082 (5.8) | 9191 (11.3) | 20,351 (7.4) |
| Other single ingredient botanicals | 11,561 (6.0) | 7186 (8.9) | 18,897 (6.9) |
| Ma huang | 2333 (1.2) | 3616 (4.5) | 5995 (2.2) |
| Echinacea | 2932 (1.5) | 837 (1.0) | 3782 (1.4) |
| St. John’s Wort | 1197 (0.6) | 760 (0.9) | 1974 (0.7) |
| Ginseng | 1119 (0.6) | 800 (1.0) | 1934 (0.7) |
| Yohimbe | 387 (0.2) | 1422 (1.8) | 1818 (0.7) |
| Valerian | 613 (0.3) | 1055 (1.3) | 1687 (0.6) |
| Multi-botanicals with Citrus aurantium | 716 (0.4) | 915 (1.1) | 1646 (0.6) |
| Ginkgo biloba | 905 (0.5) | 482 (0.6) | 1392 (0.5) |
| Kava kava | 280 (0.1) | 520 (0.6) | 815 (0.3) |
| Citrus aurantium | 30 (0.0) | 44 (0.1) | 75 (0.0) |
| Blue cohosh | 4 (0.0) | 3 (0.0) | 8 (0.0) |
| Cultural medicines | 774 (0.4) | 821 (1.0) | 1605 (0.6) |
| Asian medicines | 529 (0.3) | 563 (0.7) | 1099 (0.4) |
| Other cultural medicines | 150 (0.1) | 176 (0.2) | 328 (0.1) |
| Hispanic medicines | 52 (0.0) | 41 (0.1) | 93 (0.0) |
| Ayurvedic medicines | 43 (0.0) | 41 (0.1) | 85 (0.0) |
| Energy products | 2279 (1.2) | 2792 (3.4) | 5103 (1.9) |
| Energy drinks (caffeine only) | 1162 (0.6) | 921 (1.1) | 2098 (0.8) |
| Energy drinks (caffeine containing) | 585 (0.3) | 759 (0.9) | 1349 (0.5) |
| Energy drinks (unknown) | 290 (0.2) | 511 (0.6) | 808 (0.3) |
| Energy products (other) | 190 (0.1) | 328 (0.4) | 522 (0.2) |
| Energy drinks (ethanol and caffeine containing) | 32 (0.0) | 246 (0.3) | 279 (0.1) |
| Energy drinks (no caffeine) | 19 (0.0) | 21 (0.0) | 40 (0.0) |
| Energy drinks (ethanol and caffeine only) | 1 (0.0) | 6 (0.0) | 7 (0.0) |
| Hormonal products | 28,777 (14.9) | 12,495 (15.4) | 41,440 (15.1) |
| Melatonin | 27,616 (14.3) | 11,946 (14.7) | 39,725 (14.4) |
| Androgens or androgen precursors | 790 (0.4) | 446 (0.5) | 1239 (0.5) |
| Glandular dietary supplements | 371 (0.2) | 103 (0.1) | 476 (0.2) |
| Miscellaneous dietary supplements | 104,937 (54.5) | 15,310 (18.9) | 120,610 (43.9) |
| Homeopathic agents | 91,781 (47.7) | 6983 (8.6) | 98,998 (36.0) |
| Other/unknown multi-ingredient supplements | 13,156 (6.8) | 8327 (10.3) | 21,612 (7.9) |
| Other dietary supplements | 9646 (5.0) | 4261 (5.2) | 13,985 (5.1) |
| Other single ingredient non-botanicals | 4751 (2.5) | 1810 (2.2) | 6590 (2.4) |
| Glucosamine | 4482 (2.3) | 1692 (2.1) | 6209 (2.3) |
| Blue-green algae | 413 (0.2) | 759 (0.9) | 1186 (0.4) |
| Total | 192,583 (100.0) | 81,165 (100.0) | 274,998 (100.0) |
The numbers in italics are the values for the major categories of dietary supplements. The number that are not in italics are the values for the subcategories, or descriptions, within the major categories
aTotal includes cases with age unknown (n = 1250). Percentages may not sum to 100.0% due to rounding
Yohimbe, ma huang products, and energy product exposures are examined in greater detail (Table 4) and are discussed individually below. Homeopathic agents are also highlighted because of their association with more exposures than any other individual dietary supplement category (n = 98,998, 36.0%).
Table 4.
Characteristics of exposures to yohimbe, energy products, and ma huang products, National Poison Data System 2000–2012
| Characteristics | Yohimbe (n = 1818) | Energy products (n = 5103) | Ma huang productsa (n = 33,320) |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Gender | |||
| Female | 396 (21.8) | 2023 (39.6) | 19,146 (57.5) |
| Male | 1417 (77.9) | 3065 (60.1) | 14,073 (42.2) |
| Unknown | 5 (0.3) | 15 (0.3) | 101 (0.3) |
| Age group | |||
| Younger than 6 years | 387 (21.3) | 2279 (44.7) | 12,566 (37.7) |
| 6 years or older | 1422 (78.2) | 2792 (54.7) | 20,526 (61.6) |
| Reason for exposure | |||
| Unintentional | 769 (42.3) | 3147 (61.7) | 18,072 (54.2) |
| Intentional | 324 (17.8) | 1180 (23.1) | 10,388 (31.2) |
| Adverse reaction | 701 (38.6) | 729 (14.3) | 4557 (13.7) |
| Other or unknown | 24 (1.3) | 47 (0.9) | 303 (0.9) |
| Chronicity | |||
| Acute | 1691 (93.0) | 4894 (95.9) | 29,890 (89.7) |
| Acute-on-chronic | 45 (2.5) | 91 (1.8) | 1533 (4.6) |
| Chronic | 54 (3.0) | 83 (1.6) | 1411 (4.2) |
| Unknown | 28 (1.5) | 35 (0.7) | 486 (1.5) |
| Exposure site | |||
| Own residence | 1702 (93.6) | 4401 (86.2) | 30,466 (91.4) |
| Other residence | 44 (2.4) | 157 (3.1) | 850 (2.6) |
| Other or unknown | 72 (4.0) | 543 (10.7) | 2004 (6.0) |
| Management site | |||
| Managed on-site (non HCF) | 655 (36.0) | 3416 (66.9) | 14,404 (43.2) |
| Individual already in/enroute to HCF | 776 (42.7) | 828 (16.2) | 11,333 (34.0) |
| Individual referred by PCC to HCF | 360 (19.8) | 663 (13.0) | 6991 (21.0) |
| Other or unknown | 27 (1.5) | 196 (3.8) | 592 (1.8) |
| HCF level of care | |||
| No HCF treatment received | 682 (37.5) | 3612 (70.8) | 14,996 (45.0) |
| Treated/evaluated and released | 720 (39.6) | 847 (16.6) | 11,017 (33.1) |
| Admitted to critical care unit | 58 (3.2) | 49 (1.0) | 1017 (3.1) |
| Admitted to noncritical care unit | 46 (2.5) | 69 (1.4) | 873 (2.6) |
| Admitted to psychiatric unit | 10 (0.6) | 12 (0.2) | 1046 (3.1) |
| Individual refused referral/did not arrive at HCF | 114 (6.3) | 207 (4.1) | 1825 (5.5) |
| Individual lost to follow up or left AMA | 188 (10.3) | 307 (6.0) | 2546 (7.6) |
| Medical outcome | |||
| Death | 1 (0.1) | 1 (0.0) | 6 (0.0) |
| Major effect | 23 (1.3) | 24 (0.5) | 241 (0.7) |
| Moderate effect | 488 (26.8) | 527 (10.3) | 5190 (15.6) |
| Minor effect | 342 (18.8) | 1046 (20.5) | 7157 (21.5) |
| No effect | 267 (14.7) | 898 (17.6) | 8140 (24.4) |
| Not followed/unable to follow | 697 (38.3) | 2607 (51.1) | 12,586 (37.8) |
PCC poison control center, HCF health care facility, AMA against medical advice
aSingle ingredient and multi-botanicals with ma huang. Percentages may not sum to 100.0% due to rounding
Yohimbe
Most yohimbe exposures occurred among males (77.9%) and individuals 6 years and older (78.2%) (Table 4). Among reported yohimbe exposures, 3.2% were admitted to a critical care unit for treatment. Yohimbe resulted in major effects in 1.3% of exposures and moderate effects in 26.8%. One death attributable to yohimbe exposure was reported.
Ma Huang Products
More than one-half of ma huang exposures were unintentional (54.2%) and occurred among females (57.5%) and individuals 6 years and older (61.6%) (Table 4). Three percent (3.1%) of individuals with a ma huang product exposure were admitted to a critical care unit, and there were six deaths attributed to ma huang product exposure reported during the study period. Of these fatalities, four occurred prior to 2004, one was reported in 2004, and one occurred in 2007.
Energy Products
Most energy product exposures occurred among males (60.1%) and were unintentional (61.7%) (Table 4). Individuals younger than 6 years old accounted for 44.7% of energy product exposures. Of all reported energy product exposures, 1.0% were admitted to a critical care unit. Energy products were associated with one death from 2010 through 2012.
Homeopathic Agents
Most homeopathic agent exposures occurred among children younger than 6 years old (92.7%, Table 3) and were unintentional (97.2%, n = 96,245). Nearly all homeopathic agent exposures were managed on-site without treatment in a HCF (93.8%, n = 92,900). Homeopathic agents accounted for 36.0% of dietary supplement exposures, but only 0.5% of homeopathic agent exposures experienced serious medical outcomes and 0.3% were admitted. Three deaths were attributed to homeopathic agent exposures (Table 2).
Trends
The annual rate of exposure per 100,000 population for all dietary supplements increased by 46.1% (m = 1.05, p < 0.001) from 2000 to 2002, followed by a decrease of 8.8% (m = −0.27, p < 0.001) from 2002 to 2005, before increasing again by 49.3% (m = 0.45, p < 0.001) from 2005 to 2012 (Fig. 2). These trends were influenced by the decrease in ma huang exposures starting in 2002. Although the annual rate of exposure to ma huang products increased by 129.3% (m = 0.75, p < 0.001) from 2000 to 2002, the rate decreased by 81.2% (m = −0.63, p < 0.001) during 2002 to 2006, and then continued to decline at a much slower rate (m = −0.04, p = 0.136) from 2006 through 2012. The annual rate of exposure to homeopathic agents per 100,000 population increased 227.1% (m = 0.27, p < 0.001) from 2000 through 2010, followed by a decrease of 18.3% (m = −0.43, p = 0.001) from 2010 through 2012.
After excluding ma huang products, the annual rate of exposure to dietary supplements per 100,000 population increased by 167.2% (m = 0.51, p < 0.001) from 3.46 in 2000 to 9.25 in 2012 (Fig. 3). From 2000 through 2012, the annual rate of dietary supplement exposures (excluding ma huang products) increased by 192.1% (m = 5.24, p < 0.001) among individuals younger than 6 years old and increased by 148.2% (m = 0.12, p < 0.001) among individuals 6 years and older.
Discussion
Negative outcomes resulting from dietary supplement exposures have become an important public health problem due to widespread dietary supplement use and inadequate regulation of their availability, quality, and safety [2, 7, 9, 10, 12, 16]. This study defines the epidemiologic characteristics of these exposures. While self-reported dietary supplement use among adults in the US remained generally steady at 49 to 54%, the rate of dietary supplement exposures reported to PCCs increased significantly from 2000 to 2002, followed by a decrease from 2002 to 2005, and then increased from 2005 to 2012 [3]. At least part of the decrease from 2002 through 2005 can be attributed to the 81.2% decrease in the rate of ma huang exposures associated with the activities leading up to the FDA’s 2004 ban on ma huang.
The majority of dietary supplement exposures occurred among children younger than 6 years old, which is consistent with previously published findings [8, 17]. As in previous studies, most of these exposures did not require treatment in a health care facility or result in long-term effects [10]. In this study, serious medical outcomes occurred more frequently among individuals 6 years and older. The less severe outcomes observed for exposures among young children may be a result of the higher proportion of unintentional and acute ingestions in this subgroup and more consistent reporting to PCCs of even minimal ingestions among young children [17]. In contrast, intentional and non-acute exposures may be more likely to go unreported among the older age group. Also, reported exposures among children younger than 6 years old were less often attributed to the dietary supplement categories that most frequently resulted in serious medical outcomes, compared with older individuals. Given that a greater proportion of the serious exposures occurred among the 6 years and older age group, future prevention efforts should include appropriate attention to this population. In addition, a few categories of dietary supplements, specifically yohimbe, products containing ma huang, cultural medicines, and energy products, were associated with higher rates of serious outcomes and merit special focus.
Yohimbe
Yohimbe is a botanical supplement used for a number of purposes, including to enhance male sexual performance [18]. Yohimbe is known to be associated with significant morbidity and mortality and has been reported to cause tachycardia, dysrhythmia, renal failure, seizure, and myocardial infarction [12, 19]. Our study found that almost 30% of reported yohimbe exposures resulted in moderate or major effects; 3.2% resulted in critical care unit admission, and there was one associated death. The potential toxicity of yohimbe exposures suggest the need for increased review and potential rule making by the FDA.
Ma Huang Products
Ma huang, also known as ephedra, is a botanical stimulant used to boost energy, improve alertness, lose weight, and improve athletic performance [12]. Ma huang is known to increase blood pressure and has been associated with myocardial infarction and stroke [20]. Calls to PCCs for ma huang product exposures decreased significantly during the study period. Although ma huang exposure rates began to decline prior to the FDA’s 2004 ban of the substance, the agency’s action was likely responsible for the continued and dramatic decrease in exposures [20]. In the current study, only one of the six ma huang-related deaths occurred after 2004. The decrease in ma huang product exposure calls to PCCs over the past decade provides support for using the NPDS not only to monitor trends in toxic exposures but also to gauge the availability and usage of substances nationally. The continued decline in ma huang-related exposures provides evidence of the effectiveness of FDA regulatory actions, and this strategy should be considered for other high-risk substances.
Energy Products
Energy drinks, advertised to increase energy and mental performance, contain one or more psychoactive ingredients, usually caffeine, but sometimes taurine, guarana, ginseng, Ginkgo biloba, l-carnitine, milk thistle, B vitamins, or other substances [21]. Energy products have been associated with dysrhythmias, seizure, and tachypnea, among other clinical effects [11]. In this study, 1.0% of exposures resulted in admission to a critical care unit and one exposure resulted in death. Many energy product exposures were unintentional and occurred among young children. These findings corroborate the need for improved energy product regulation, child-resistant packaging, and caregiver education [11, 22].
Homeopathic Agents
Homeopathic agents accounted for 36% of all exposures and 97% of those exposed were among children younger than 6 years old. Homeopathic agents are frequently used to treat conditions such as respiratory problems, eczema, pain, colic, migraines, attention-deficit hyperactivity disorder, and asthma among children [23–28]. The rate of exposure to homeopathic agents increased by more than 200% from 2000 to 2010 and then decreased by 18% from 2010 to 2012. The reasons for this observed decrease are uncertain. Reported exposures were largely benign and usually managed on-site; however, there were three deaths associated with homeopathic agents in this study.
Study Limitations
This study has several limitations. NPDS data are based on self-reported information provided by callers, which cannot be completely verified by PCCs or the AAPCC. Because reporting to the NPDS is voluntary, this study underestimates the number of dietary supplement exposures in the US. Exposures to some dietary supplements may be more likely to be reported to the NPDS than others. Dietary supplement exposures among women and young children may be reported to PCCs more frequently than those among men and older children or adults [8, 10]. This potential reporting bias should be considered when interpreting results. This study may also underestimate dietary supplement toxicity because 3.7% of medical outcomes were potentially toxic but could not be followed. Reported exposures do not necessarily represent a poisoning or overdose. In addition, calls to PCCs usually involve acute exposures; therefore, NPDS data likely do not reflect the true prevalence of chronic toxicity from dietary supplements. Finally, fatalities are reviewed in the NPDS using a three-tiered process (often including review of autopsy results) and substances are classified regarding their relative contribution to fatality, including undoubtedly related, probably related, contributory, and not related; however, this study does not fully elaborate on the classification of the reported deaths. Despite these limitations, NPDS data are entered by highly qualified poison experts using strict quality controls and case follow-up protocols. The NPDS offers an inclusive and detailed database for investigating dietary supplement exposures in the US.
Conclusions
There was an overall increase in the rate of dietary supplement exposures reported to US PCCs from 2000 through 2012. Although the majority of dietary supplement exposures did not require treatment at a HCF or result in serious medical outcomes, two categories of dietary supplements were notable for their adverse consequences: yohimbe and energy products. Our results demonstrate the need for FDA regulation of yohimbe and energy products in the US as was done successfully with ma huang products in 2004.
Abbreviations
- AAPCC
American Association of Poison Control Centers
- AMA
Against medical advice
- CCU
Critical care unit
- FDA
Food and Drug Administration
- HCF
Health care facility
- NPDS
National Poison Data System
- PCC
Poison control center
- TESS
Toxic Exposure Surveillance System
- US
United States
Appendix
Compliance with Ethical Standards
Conflicts of Interest
The authors have no conflicts of interest or financial disclosures relevant to this study to disclose.
Sources of Funding
None.
Financial Disclosure
The authors have no financial disclosures relevant to this study.
References
- 1.U.S. Food and Drug Administration. Dietary Supplement Health and Education Act of 1994. https://ods.od.nih.gov/About/DSHEA_Wording.aspx. Accessed December 29, 2016.
- 2.Gahche J, Bailey R, Burt V, Hughes J, Yetley E, Dwyer J, Picciano MF, McDowell M, Sempos C. Dietary supplement use among U.S. adults has increased since NHANES III (1988-1994). NCHS Data Brief 2011(61):1–8. [PubMed]
- 3.Kantor ED, Rehm CD, Du M, White E, Giovannucci EL. Trends in dietary supplement use among US adults from 1999-2012. JAMA. 2016;316(14):1464–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dangerous supplements: Still at large. Consumer Reports. April 24, 2004. http://consumersunion.org/pub/0504%20DietarySup.pdf. Accessed January 11, 2017. [PubMed]
- 5.Sadovsky R, Collins N, Tighe AP, Brunton SA, Safeer R. Patient use of dietary supplements: a clinician's perspective. Curr Med Res Opin. 2008;24(4):1209–16. [DOI] [PubMed] [Google Scholar]
- 6.U.S. Food and Drug Administration. Guidance for clinical investigators, sponsors, and IRBs: Investigational new drug applications (INDs)--Determining whether human research studies can be conducted without an IND. 2013. http://www.fda.gov/downloads/drugs/guidances/ucm229175.pdf. Accessed December 29, 2016.
- 7.Zelig R, Rigassio RD. Understanding the properties of common dietary supplements: clinical implications for healthcare practitioners. Nutr Clin Pract. 2012;27(6):767–76. [DOI] [PubMed] [Google Scholar]
- 8.Gryzlak BM, Wallace RB, Zimmerman MB, Nisly NL. National surveillance of herbal dietary supplement exposures: the poison control center experience. Pharmacoepidemiol Drug Saf. 2007;16(9):947–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haller C, Kearney T, Bent S, Ko R, Benowitz N, Olson K. Dietary supplement adverse events: report of a one-year poison center surveillance project. J Med Toxicol. 2008;4(2):84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palmer ME, Haller C, McKinney PE, Klein-Schwartz W, Tschirgi A, Smolinske SC, et al. Adverse events associated with dietary supplements: an observational study. Lancet. 2003;361(9352):101–6. [DOI] [PubMed] [Google Scholar]
- 11.Seifert SM, Seifert SA, Schaechter JL, Bronstein AC, Benson BE, Hershorin ER, et al. An analysis of energy-drink toxicity in the National Poison Data System. Clin Toxicol (Phila). 2013;51(7):566–74. [DOI] [PubMed] [Google Scholar]
- 12.Woolf AD, Watson WA, Smolinske S, Litovitz T. The severity of toxic reactions to ephedra: comparisons to other botanical products and national trends from 1993-2002. Clin Toxicol (Phila). 2005;43(5):347–55. [DOI] [PubMed] [Google Scholar]
- 13.Geller AI, Shehab N, Weidle NJ, Lovegrove MC, Wolpert BJ, Timbo BB, et al. Emergency department visits for adverse events related to dietary supplements. N Engl J Med. 2015;373(16):1531–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mowry JB, Spyker DA, Cantilena LR Jr, Bailey JE, Ford M. 2012 annual report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 30th annual report. Clin Toxicol (Phila). 2013;51(10):949–1229. [DOI] [PubMed] [Google Scholar]
- 15.U.S. Census Bureau. Intercensal estimates of the resident population by single year of age, sex, race, and Hispanic origin for the United States: April 1, 2000-July 1, 2010 2012. http://www.census.gov/data/tables/time-series/demo/popest/intercensal-2000-2010-national.html. Accessed January 3, 2017. [Google Scholar]
- 16.Kinariwala N. Tighter regulation needed for dietary supplements in USA. Lancet. 2003;361(9368):1566. [DOI] [PubMed] [Google Scholar]
- 17.Polivka BJ, Elliott MB, Wolowich WR. Comparison of poison exposure data: NHIS and TESS data. J Toxicol Clin Toxicol. 2002;40(7):839–45. [DOI] [PubMed] [Google Scholar]
- 18.Bucci LR. Selected herbals and human exercise performance. Am J Clin Nutr. 2000;72(2 Suppl):624S–36S. [DOI] [PubMed] [Google Scholar]
- 19.WebMD. Find a vitamin or supplement: Yohimbe. http://www.webmd.com/vitamins-supplements/ingredientmono-759-yohimbe.aspx?activeingredientid=759&. Accessed December 29, 2016.
- 20.U.S. Food and Drug Administration. FDA issues regulation prohibiting sale of dietary supplements containing ephedrine alkaloids and reiterates its advice that consumers stop using these products. 2004. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2004/ucm108242.htm. Accessed December 29, 2016.
- 21.Wolk BJ, Ganetsky M, Babu KM. Toxicity of energy drinks. Curr Opin Pediatr. 2012;24(2):243–51. [DOI] [PubMed] [Google Scholar]
- 22.Sepkowitz KA. Energy drinks and caffeine-related adverse effects. JAMA. 2013;309(3):243–4. [DOI] [PubMed] [Google Scholar]
- 23.Danno K, Colas A, Masson JL, Bordet MF. Homeopathic treatment of migraine in children: results of a prospective, multicenter, observational study. J Altern Complement Med. 2013;19(2):119–23. [DOI] [PubMed] [Google Scholar]
- 24.Pellow J, Solomon EM, Barnard CN. Complementary and alternative medical therapies for children with attention-deficit/hyperactivity disorder (ADHD). Altern Med Rev. 2011;16(4):323–37. [PubMed] [Google Scholar]
- 25.Torres-Llenza V, Bhogal S, Davis M, Ducharme F. Use of complementary and alternative medicine in children with asthma. Can Respir J. 2010;17(4):183–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pitetti R, Singh S, Hornyak D, Garcia SE, Herr S. Complementary and alternative medicine use in children. Pediatr Emerg Care. 2001;17(3):165–9. [DOI] [PubMed] [Google Scholar]
- 27.Silverberg JI, Lee-Wong M, Silverberg NB. Complementary and alternative medicines and childhood eczema: a US population-based study. Dermatitis. 2014;25(5):246–54. [DOI] [PubMed] [Google Scholar]
- 28.Beer AM, Burlaka I, Buskin S, Kamenov B, Pettenazzo A, Popova D, Riveros Huckstadt MP, Sakalinskas V, Oberbaum M. Usage and Attitudes Towards Natural Remedies and Homeopathy in General Pediatrics: A Cross-Country Overview. Glob Pediatr Health. 2016;3:2333794X15625409. [DOI] [PMC free article] [PubMed]