Abstract
The Toxicology Investigators Consortium (ToxIC) Case Registry was established by the American College of Medical Toxicology in 2010. The Registry contains data from participating sites with the agreement that all bedside medical toxicology consultations will be entered. Currently, 83% of accredited medical toxicology fellowship programs in the USA participate. The Registry continues to grow each year, and as of 31 December 2016, a new milestone was reached, with more than 50,000 cases reported since its inception. The objective of this seventh annual report is to summarize the Registry’s 2016 data and activity with its additional 8529 cases. Cases were identified for inclusion in this report by a query of the ToxIC database for any case entered from 1 January to 31 December 2016. Detailed data was collected from these cases and aggregated to provide information which includes the following: demographics (age, gender, race, ethnicity, HIV status), reason for medical toxicology evaluation (intentional pharmaceutical exposure, envenomation, withdrawal from a substance), agent and agent class, clinical signs and symptoms (vital sign abnormalities, organ system dysfunction), treatments and antidotes administered, fatality and life support withdrawal data. Fifty percent of cases involved females, and adults aged 19–65 were the most commonly reported. There were 86 patients (1.0%) with HIV-positive status known. Non-opioid analgesics were the most commonly reported agent class, with acetaminophen the most common agent reported. There were 126 fatalities reported in 2016 (1.5% of cases). Major trends in demographics and exposure characteristics remained similar overall with past years’ reports. While treatment interventions were commonly required, fatalities were rare.
Electronic supplementary material
The online version of this article (doi:10.1007/s13181-017-0627-3) contains supplementary material, which is available to authorized users.
Keywords: Poisonings, Overdose, Epidemiology, Medical toxicology
Introduction
The Toxicology Investigators Consortium (ToxIC) was created by the American College of Medical Toxicology (ACMT) in 2010 as a tool for clinical toxicology research and toxicosurveillance [1]. Unlike other poisoning databases, ToxIC is a prospective case registry based on medical toxicologists’ experience performing consultations in both inpatient and ambulatory settings. Cases where a formal consultation was not done, such as where advice was given over the telephone, are not included in the database.
The ToxIC Registry is unique in several important ways. Because all information was entered by treating medical toxicologists, the toxicological data reflects the best professional judgment of skilled clinicians. Much of the information on the database is not available from other sources and includes not only medical data but also demographics including race and ethnicity and HIV status.
In 2016, there was a 2.0% decrease in sites included in the ToxIC Registry, with four sites added and five withdrawn or dropped from participation because they withdrew or failed to meet quality standards set by the Registry. Two of the international sites were not included in 2016 due to withdrawal or lack of case entry. At the end of 2016, there were 46 sites, comprising 79 facilities with active case entry. Currently, 83% of active accredited medical toxicology fellowship programs in the USA participate in the ToxIC Registry. The objective of this report is to summarize the Registry’s 2016 data and activity. Cases entered in 2016 are described in this seventh annual report.
Since its inception, several supplemental or subregistries have been created within ToxIC. These are designed to collect more detailed information in specific areas. Our subregistries studying caustic ingestions and prescription opioid abuse were closed at the end of 2016; data from these subregistries are now being analyzed. Subregistries on lipid resuscitation therapy, North American snake bites, and extracorporeal substance removal continued to collect data in 2016 and are continuing into 2017. Three additional subregistries were approved and developed in 2016 and became live on January 1, 2017; these include subregistries on plant, mushroom and herbal toxicity; pediatric opioid exposures; and pediatric marijuana exposures.
In 2016, 14 abstracts and five manuscripts using ToxIC Registry data were published and are listed on http://www.ToxICRegistry.org (Farrugia et al. [2]; Beauchamp et al. [3]; Judge et al. [4]; Riederer et al. [5]; Wang et al. [6]).
In 2016, ToxIC was supported by a continuation of a grant from the US National Institutes of Health on cardiovascular drug toxicity, a new contract with the US Food and Drug Administration, and the continuation of unrestricted grant support from BTG International, which was used to support the North American Snakebite Registry.
Methods
A detailed description of the creation and design of the ToxIC Registry has been previously reported by Wax et al. [1]. To be part of the consortium, all medical toxicologists at participating institutions agree to enter data into the ToxIC Registry on all medical toxicology consultations performed. Cases are entered on a password-protected encrypted online data collection form. The site is maintained by ACMT with oversight by the ToxIC Leadership Group. The Registry is compliant with the Health Insurance Portability and Accountability Act (HIPAA) and does not collect any protected health information or otherwise identifying fields. Registry participation is pursuant to the participating institution’s Institutional Review Board (IRB) approval and compliant with their policies and procedures. The Registry has also been independently reviewed by the Western IRB and determined not to meet the threshold of human subject research under federal regulation 45 CFR 46 and associated guidance.
Data collected on each case include presenting signs and symptoms, clinical course, treatments, limited patient demographics, outcome, laboratory values, and circumstances of and reasons for toxicological exposure. The term “consultation” is used in this report to describe any in-person encounter with a medical toxicologist in which a formal evaluation was conducted and placed in the medical record. Such encounters may include admission to a medical toxicology in-patient service, or evaluation by a medical toxicologist as a consulting physician in an emergency department, inpatient unit, or outpatient clinic. The online data collection form is formatted to ensure data entry remains organized and searchable. Free text entry fields allow providers to provide further detail or supplementary information. As part of the Registry’s toxicosurveillance mission, one component of the standard data form is a sentinel detection field that signals novel or unusual cases. Analysis of novel exposure surveillance data in the Registry has begun, with results to be published in a separate report.
For this report, a search of the database was performed to identify cases recorded from January 1, 2016, through December 31, 2016. Additional data from the subregistries will be published separately. This descriptive report summarizes case demographics, source and location of consultation, and reasons for encounter, and provides case frequencies by individual agent class and treatment provided.
In the tables describing individual agents or agent classes, unless otherwise indicated, values with fewer than five occurrences were not listed as separate items but are further grouped as “miscellaneous.” Percentages noted in tables for individual agents represent their relative proportion within their respective agent class.
For clinical signs or symptoms, the tables provide the percentage of individual signs or symptoms relative to the total number of Registry cases in 2016. Signs and symptoms include the presence or absence of a toxidrome, vital sign abnormalities, and a variety of organ system-based derangements which may arise from a toxic exposure. For each subheading in the data collection instrument, investigators are required to either select an abnormality, or “None,” to improve the accuracy of data collection. In the detailed treatment tables, percentages for each treatment modality represent the relative frequency among all treatments rendered.
Results
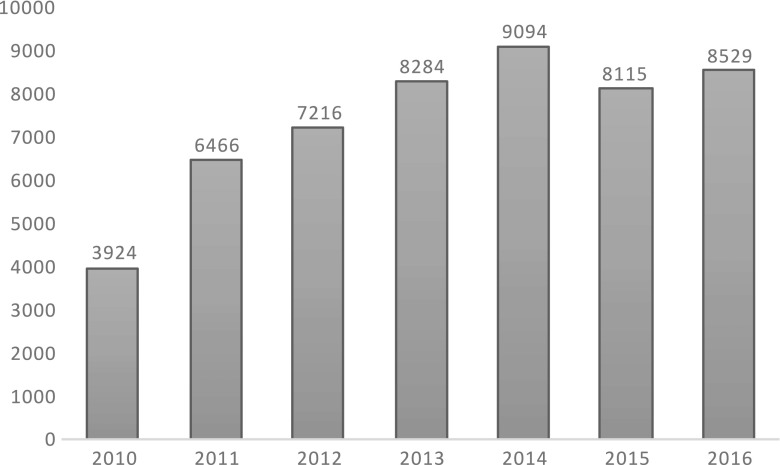
A total of 8529 cases were entered in the ToxIC Registry in 2016, up slightly from 8115 in 2015 (Fig. 1) [2]. Table 1 lists the individual sites entering cases in 2016.
Fig. 1.
Case numbers
Table 1.
Participating institutions providing cases to ToxIC in 2016
| Arizona | |
| Phoenix | |
| Banner- University Medical Center Phoenix | |
| Phoenix Children’s Hospital | |
| California | |
| Fresno | |
| UCSF Fresno Medical Center | |
| Loma Linda | |
| Loma Linda University Medical Center | |
| Los Angeles | |
| University of Southern California Verdugo Hills | |
| San Diego | |
| Rady Children’s Hospital | |
| San Francisco | |
| San Francisco General Hospital | |
| Colorado | |
| Denver | |
| Children’s Hospital Colorado | |
| Denver Health Medical Center | |
| Porter and Littleton Adventist Hospital | |
| Swedish Medical Center | |
| University of Colorado Medical Center | |
| Connecticut | |
| Hartford | |
| Hartford Hospital | |
| Georgia | |
| Atlanta | |
| Children’s Healthcare of Atlanta Egelston | |
| Children’s Healthcare of Atlanta Hughes Spalding | |
| Emory University Hospital | |
| Emory University Hospital Midtown | |
| Grady Health System | |
| Grady Memorial Hospital | |
| Illinois | |
| Chicago | |
| Cook County Hospital | |
| Evanston | |
| Evanston North Shore University Health System | |
| Indiana | |
| Indianapolis | |
| IU-Eskenazi Hospital | |
| IU-Indiana University Hospital | |
| IU-Methodist Hospital-Indianapolis | |
| IU-Riley Hospital for Children | |
| IU Wishard Memorial Hospital | |
| Massachusetts | |
| Boston | |
| Beth Israel Boston | |
| Children’s Hospital Boston | |
| Worcester | |
| University of Massachusetts Memorial Medical Center | |
| Michigan | |
| Grand Rapids | |
| Spectrum Health Hospitals | |
| Minnesota | |
| St. Paul | |
| Regions Hospital | |
| Missouri | |
| Kansas City | |
| Children’s Mercy Hospitals & Clinics | |
| St. Louis | |
| Washington University School of Medicine | |
| Nebraska | |
| Omaha | |
| University of Nebraska Medical Center | |
| New Mexico | |
| Albuquerque | |
| University of New Mexico | |
| New Jersey | |
| Morristown | |
| Morristown Medical Center | |
| New Brunswick | |
| Robert Wood Johnson University Hospital | |
| Newark | |
| NJMS/Rutgers | |
| New York | |
| Albany | |
| Albany Medical Center | |
| Manhasset | |
| Long Island Jewish | |
| North Shore University Hospital | |
| New York | |
| Bellevue Medical Center | |
| Mount Sinai Hospital | |
| NYU Langone Medical Center | |
| Staten Island | |
| Staten Island University Hospital | |
| Rochester | |
| Highland Hospital | |
| Strong Memorial Hospital | |
| Syracuse | |
| Upstate Medical University-Downtown Campus | |
| North Carolina | |
| Charlotte | |
| Carolinas Medical Center | |
| Greenville | |
| Vidant Medical Center | |
| Oregon | |
| Portland | |
| Doernbecher Children’s Hospital | |
| Oregon Health & Science University Hospital | |
| Pennsylvania | |
| Harrisburg | |
| PinnacleHealth-Community General Osteopathic | |
| PinnacleHealth-Harrisburg Hospital | |
| PinnacleHealth-West Shore | |
| Lehigh Valley | |
| Lehigh Valley Hospital Cedar Crest | |
| Lehigh Valley Hospital Muhlenberg | |
| Philadelphia | |
| Hahnemann University Hospital | |
| Mercy Fitzgerald Hospital | |
| Mercy Hospital of Philadelphia | |
| St. Christopher’s Hospital for Children | |
| Pittsburgh | |
| UPMC Children’s Hospital of Pittsburgh | |
| UPMC Magee Women’s Hospital | |
| UPMC Mercy Hospital | |
| UPMC Presbyterian/Shadyside | |
| Texas | |
| Dallas | |
| Children’s Medical Center Dallas | |
| Parkland Memorial Hospital | |
| University of Texas Southwestern Clinic | |
| William P Clements University Hospital | |
| Houston | |
| Ben Taub General Hospital | |
| Texas Children’s Hospital | |
| San Antonio | |
| San Antonio Military Medical Center | |
| Utah | |
| Salt Lake City | |
| Primary Children’s Hospital | |
| University of Utah Hospital | |
| Virginia | |
| Charlottesville | |
| University of Virginia Health Systems | |
| Richmond | |
| Virginia Commonwealth University Medical Center | |
| Wisconsin | |
| Milwaukee | |
| Froedtert Memorial Lutheran Hospital | |
| Israel | |
| Haifa | |
| Rambam Health Care Campus |
Demographics
Selected demographics are summarized in Tables 2, 3, 4, 5, 6, 7, and 8. In 2016, male (50.0%) and female (50.0%) cases were distributed evenly and 0.6% were pregnant. Adults age 19–65 years comprised the majority (67.0%) of cases, with children age 13–18 years the next most common (16.8%). There were a total of 2280 (26.7%) pediatric cases (0–18 years) overall. The most commonly reported race categories were Caucasian (58.1%) and black/African (14.1%), with 18.0% unknown/uncertain. Nine hundred fifty-one cases (11.2%) reported Hispanic ethnicity, while 19.1% reported ethnicity as unknown. Race and ethnicity are not self-reported in the Registry, and limitations in these data points continue to be addressed with ongoing quality improvement measures.
Table 2.
ToxIC 2016 case demographics—age and gender
| N (%) | |
|---|---|
| Gender | |
| Male | 4267 (50.0) |
| Female | 4262 (50.0) |
| Pregnant | 47 (0.6) |
| Age (years) | |
| <2 | 270 (3.2) |
| 2–6 | 358 (4.2) |
| 7–12 | 215 (2.5) |
| 13–18 | 1437 (16.8) |
| 19–65 | 5714 (67.0) |
| 66–89 | 477 (5.6) |
| >89 | 21 (0.2) |
| Unknown | 29 (0.4) |
| Total | 8529 (100) |
Table 3.
ToxIC 2016 case demographics—race and Hispanic ethnicity
| N (%) | |
|---|---|
| Race | |
| Caucasian | 4953 (58.1) |
| Unknown/uncertain | 1537 (18.0) |
| Black/African | 1201 (14.1) |
| Other | 466 (5.5) |
| Asian | 169 (2.0) |
| American Indian/Alaska Native | 76 (0.9) |
| Mixed | 108 (1.3) |
| Native Hawaiian or Pacific Islander | 18 (0.2) |
| Australian aboriginal | 1 (0.0) |
| Total | 8529 (100) |
| Hispanic ethnicitya | |
| Hispanic | 951 (11.2) |
| Non-Hispanic | 5949 (69.8) |
| Unknown | 1629 (19.1) |
| Total | 8529 (100) |
aHispanic ethnicity as indicated exclusive of race
Table 4.
ToxIC 2016 case demographics—HIV-positive patients
| N (%) | |
|---|---|
| Reason for encounter | |
| Intentional pharmaceutical | 42 (49.0) |
| Attempt at self-harm | 27 (31.4) |
| Misuse/abuse | 8 (9.3) |
| Therapeutic use | 3 (3.5) |
| Unknown | 4 (4.7) |
| Intentional non-pharmaceutical | 24 (28.0) |
| Attempt at self-harm | 1 (1.1) |
| Misuse/abuse | 19 (22.1) |
| Unknown | 3 (3.5) |
| Use for therapeutic intent | 1 (1.1) |
| Unintentional pharmaceutical | 1 (1.1) |
| Unintentional non-pharmaceutical | 4 (5.0) |
| Race | |
| American Indian/Alaskan Native | 1 (1.1) |
| Black/African | 29 (33.7) |
| Caucasian | 36 (42.0) |
| Other | 9 (10.5) |
| Unknown/uncertain | 11 (12.8) |
| Ethnicity | |
| Hispanic | 10 (11.6) |
| Not Hispanic | 62 (72.1) |
| Unknown | 14 (16.3) |
| Total HIV-positive patients | 86 (100) |
Table 5.
ToxIC 2016 case referral sources by inpatient/outpatient status
| N (%) | |
|---|---|
| Emergency department (ED) or inpatient (IP)a | |
| ED | 4772 (59.8) |
| Admitting service | 2085 (26.1) |
| Outside hospital transfer | 729 (9.1) |
| Request from another hospital service (not ED) | 362 (4.5) |
| Poison Center | 27 (0.3) |
| Primary care provider or other outpatient treating physician | 5 (0.1) |
| Self-referral | 5 (0.1) |
| ED/IP total | 7985 (100) |
| Outpatient (OP)/clinic/office consultationb | |
| Primary care provider or other OP physician | 231 (42.5) |
| Self-referral | 211 (38.8) |
| Poison Center | 42 (7.7) |
| Employer/independent medical eval | 39 (7.2) |
| ED | 15 (2.8) |
| Admitting service | 3 (0.6) |
| Request from another hospital service (not ED) | 3 (0.6) |
| OP total | 544 (100) |
aPercentage based on the total number of cases (N = 7985) seen by a medical toxicologist as consultant (ED or IP) or as attending (IP)
bPercentage based on the total number of cases (N = 544) seen by a medical toxicologist as outpatient, clinic visit, or office consultation
Table 6.
ToxIC 2016 cases—primary reason for medical toxicology encounter
| N (%) | |
|---|---|
| Intentional exposure—pharmaceutical | 4591 (53.8) |
| Intentional exposure—non-pharmaceutical | 1038 (12.2) |
| Unintentional exposure—pharmaceutical | 659 (7.7) |
| Organ system dysfunction | 309 (3.6) |
| Unintentional exposure—non-pharmaceutical | 296 (3.5) |
| Envenomation—snake | 285 (3.3) |
| Unknown | 275 (3.2) |
| Withdrawal—opioid | 264 (3.1) |
| Interpretation of toxicology data | 177 (2.1) |
| Environmental evaluation | 149 (1.7) |
| Withdrawal—ethanol | 140 (1.6) |
| Occupational evaluation | 121 (1.4) |
| Ethanol abuse | 90 (1.1) |
| Envenomation—spider | 43 (0.5) |
| Withdrawal—sedative/hypnotic | 27 (0.3) |
| Malicious/criminal | 22 (0.3) |
| Withdrawal—other | 13 (0.2) |
| Envenomation—other | 10 (0.1) |
| Envenomation—scorpion | 7 (0.1) |
| Marine/fish poisoning | 7 (0.1) |
| Withdrawal—cocaine/amphetamine | 6 (0.1) |
| Total | 8529 (100) |
Table 7.
ToxIC 2016 cases—detailed reason for encounter, intentional pharmaceutical exposures
| N (%) | |
|---|---|
| Reason for intentional pharmaceutical exposure subgroupa | |
| Attempt at self-harm | 3099 (67.5) |
| Misuse/abuse | 915 (19.9) |
| Therapeutic use | 376 (8.2) |
| Unknown | 202 (4.4) |
| Total | 4592 (100) |
| Attempt at self-harm—suicidal intent subclassificationb | |
| Suicidal intent | 2700 (87.1) |
| No suicidal intent | 115 (3.7) |
| Suicidal intent unknown | 284 (9.2) |
| Total | 3099 (100) |
aPercentage of total number of cases (N = 4592) indicating primary reason for encounter due to intentional pharmaceutical exposure
bPercentage of number of cases (N = 3099) indicating attempt at self-harm
Table 8.
ToxIC 2016 case demographics—race/gender/ethnicity and exposure type
| Intentional pharmaceutical, N (%) | Intentional non-pharmaceutical, N (%) | Unintentional pharmaceutical, N (%) | Unintentional non-pharmaceutical, N (%) | |
|---|---|---|---|---|
| Race | ||||
| American Indian/Alaskan Native | 44 (57.9) | 11 (14.5) | 2 (2.6) | 3 (4.0) |
| Asian | 82 (48.5) | 24 (14.2) | 13 (7.7) | 6 (3.6) |
| Black/African | 598 (49.8) | 186 (15.5) | 112 (9.3) | 67 (5.6) |
| Caucasian | 2765 (55.8) | 576 (11.6) | 358 (7.2) | 130 (2.6) |
| Mixed | 53 (49.1) | 14 (13.0) | 9 (8.3) | 7 (6.5) |
| Native Hawaiian or Pacific Islander | 13 (72.2) | 1 (5.6) | 1 (5.6) | 1 (5.6) |
| Other | 224 (48.1) | 65 (14.0) | 39 (8.8) | 29 (6.2) |
| Unknown/uncertain | 812 (52.8) | 161 (10.5) | 125 (8.1) | 53 (3.5) |
| Ethnicity | ||||
| Hispanic | 485 (51.0) | 119 (12.5) | 71 (7.5) | 60 (6.3) |
| Not Hispanic | 3277 (55.1) | 733 (12.3) | 450 (7.6) | 190 (3.2) |
| Unknown | 829 (50.9) | 186 (11.4) | 138 (8.5) | 46 (2.8) |
| Gender | ||||
| Female | 2714 (63.7) | 289 (6.8) | 323 (7.6) | 117 (2.8) |
| Male | 1877 (44.0) | 749 (17.6) | 336 (7.9) | 179 (4.2) |
Table 4 presents demographic data on the 86 HIV-positive patients (1.0%) reported in the Registry. Table 5 details the referral source of medical toxicology evaluations. Table 6 provides information on the type of exposure which prompted a medical toxicology encounter. More detailed information on the intent surrounding the intentional pharmaceutical exposures is provided in Table 7. Table 8 depicts the frequency of common types of exposures as broken down by race, ethnicity, and gender. In all races, and for both Hispanics, non-Hispanics, and both genders, intentional pharmaceutical exposures were more common than intentional non-pharmaceutical exposures.
Agent Classes
There were 11,352 individual agent entries in 2016 for 8529 reported cases with 2519 (29.5%) cases involving multiple agents. The top agent classes were the same as 2015 [2], with non-opioid analgesics the most common (12.8%), followed by sedative-hypnotics/muscle relaxants (11.8%), antidepressants (11.1%), and opioids (9.8%) (Table 9).
Table 9.
ToxIC 2016—agent classes
| N (%)a | |
|---|---|
| Analgesic | 1453 (12.8) |
| Sedative-hypnotic/muscle relaxant | 1339 (11.8) |
| Antidepressant | 1256 (11.1) |
| Opioid | 1118 (9.8) |
| Sympathomimetic | 728 (6.4) |
| Anticholinergic/antihistamine | 704 (6.2) |
| Ethanol | 694 (6.1) |
| Cardiovascular | 654 (5.8) |
| Antipsychotic | 642 (5.7) |
| Anticonvulsant | 370 (3.3) |
| Envenomation and marine | 317 (2.8) |
| Psychoactive | 296 (2.6) |
| Lithium | 176 (1.6) |
| Diabetic medication | 161 (1.4) |
| Cough and cold products | 145 (1.3) |
| Metals | 145 (1.3) |
| Herbal products/dietary supplements | 144 (1.3) |
| Gases/irritants/vapors/dusts | 125 (1.1) |
| Toxic alcohol | 123 (1.1) |
| Hydrocarbon | 94 (0.8) |
| Caustic | 85 (0.7) |
| Household products | 83 (0.7) |
| Plants and fungi | 80 (0.7) |
| Antimicrobial | 73 (0.6) |
| Other pharmaceutical product | 62 (0.5) |
| Other non-pharmaceutical product | 50 (0.4) |
| Anticoagulant | 42 (0.4) |
| Chemotherapeutic/immunological | 42 (0.4) |
| Insecticide | 38 (0.3) |
| Endocrine | 29 (0.3) |
| Gastrointestinal agents | 20 (0.2) |
| Rodenticide | 17 (0.1) |
| Anesthetic | 12 (0.1) |
| Anti-parkinsonism drugs | 9 (0.1) |
| Amphetamine-like hallucinogen | 6 (0.1) |
| Pulmonary | 6 (0.1) |
| Herbicide | 5 (0.0) |
| WMD/riot agent/radiological | 4 (0.0) |
| Ingested foreign body | 3 (0.0) |
| Fungicide | 2 (0.0) |
| Total | 11,352 (100) |
aPercentages are out of total number of reported agent entries per year; 2519 cases (29.5%) reported multiple agents
Individual Agents by Class
Tables 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, and 26 show frequencies of individual agent entries by class, presented in order of most to least common class. Three agents—ethanol, lithium, and amphetamine-like hallucinogens—are their own classes but are reported with other agent classes (toxic alcohols, anticonvulsants and mood stabilizers, and psychoactives, respectively) for brevity. Tables S1–S18 in the Supplementary Material present frequencies for agent classes with little diversity, few overall cases, or when infrequently reported miscellaneous agents made up a significant portion of entries.
Table 10.
ToxIC 2016 agent entries—analgesics
| N (%) | |
|---|---|
| Acetaminophen | 987 (67.9) |
| Aspirin | 197 (13.6) |
| Ibuprofen | 164 (11.3) |
| Naproxen | 39 (2.7) |
| Salicylic acid | 26 (1.8) |
| Acetylsalicylic acid | 15 (1.0) |
| Diclofenac | 4 (0.3) |
| Miscellaneousa | 22 (1.5) |
| Class total | 1453 (100) |
aIncludes aminophenazone, unspecified analgesic, carprofen, indomethacin, ketorolac, meloxicam, metamizole (dipyrone), methylsalicylate, unspecified NSAID, phenazopyridine, salicylamide, and salsalate
Table 11.
ToxIC 2016 agent entries—sedative-hypnotics/muscle relaxants
| N (%) | |
|---|---|
| Benzodiazepines | 704 (52.6) |
| Alprazolam | 276 (20.6) |
| Clonazepam | 207 (15.5) |
| Lorazepam | 99 (7.4) |
| Diazepam | 55 (4.1) |
| Benzodiazepine unspecified | 36 (2.7) |
| Temazepam | 18 (1.3) |
| Miscellaneousa | 13 (1.0) |
| Muscle relaxants | 260 (19.4) |
| Cyclobenzaprine | 92 (6.9) |
| Baclofen | 89 (6.6) |
| Carisoprodol | 37 (2.8) |
| Tizanidine | 20 (1.5) |
| Methocarbamol | 12 (0.9) |
| Metaxalone | 7 (0.5) |
| Miscellaneousb | 3 (0.2) |
| Other sedatives | 230 (17.2) |
| Gabapentin | 157 (11.7) |
| Buspirone | 29 (2.2) |
| Pregabalin | 18 (1.3) |
| Phenibut | 8 (0.6) |
| Etizolam | 7 (0.5) |
| Sedative-hypnotic/muscle relaxant unspecified | 7 (0.5) |
| Miscellaneousc | 4 (0.3) |
| Non-benzodiazepine agonists (“Z” drugs) | 93 (6.9) |
| Zolpidem | 83 (6.2) |
| Eszopiclone | 8 (0.6) |
| Miscellaneousd | 2 (0.1) |
| Barbiturates | 52 (3.9) |
| Butalbital | 24 (1.8) |
| Phenobarbital | 20 (1.5) |
| Miscellaneouse | 8 (0.6) |
| Class total | 1339 (100) |
aIncludes chlordiazepoxide, clorazepate, midazolam, triazolam, flubromazepam, and oxazepam
bIncludes orphenadrine and atracurium
cIncludes propofol and chlomethiazole
dIncludes eszopiclone and zaleplon
eIncludes butabarbital, barbiturate unspecified, and pentobarbital
Table 12.
ToxIC 2016 agent entries—antidepressants
| N (%) | |
|---|---|
| Other antidepressants | 487 (38.8) |
| Bupropion | 260 (20.7) |
| Trazodone | 153 (12.2) |
| Mirtazapine | 57 (4.5) |
| Vilazodone | 5 (0.4) |
| Miscellaneousa | 12 (1.0) |
| Selective serotonin reuptake inhibitors (SSRIs) | 431 (34.3) |
| Sertraline | 144 (11.5) |
| Fluoxetine | 100 (8.0) |
| Citalopram | 96 (7.6) |
| Escitalopram | 73 (5.8) |
| Paroxetine | 18 (1.4) |
| Tricyclic antidepressants (TCAs) | 190 (15.1) |
| Amitriptyline | 125 (10.0) |
| Doxepin | 42 (3.3) |
| Nortriptyline | 18 (1.4) |
| Miscellaneousb | 5 (0.4) |
| Serotonin-norepinephrine reuptake inhibitors (SNRIs) | 148 (11.8) |
| Venlafaxine | 90 (7.2) |
| Duloxetine | 49 (3.9) |
| Desvenlafaxine | 5 (0.4) |
| Miscellaneousc | 4 (0.3) |
| Class total | 1256 (100) |
aIncludes antidepressant unspecified, phenelzine, vortioxetine, levomilnacipran, tranylcypromine, and nefazodone
bIncludes clomipramine, imipramine, and desipramine
cIncludes fluvoxamine
Table 13.
ToxIC 2016 agent entries—opioids
| N (%) | |
|---|---|
| Heroin | 316 (28.3) |
| Oxycodone | 199 (17.8) |
| Methadone | 99 (8.9) |
| Opioid unspecified | 90 (8.1) |
| Tramadol | 87 (7.8) |
| Hydrocodone | 86 (7.7) |
| Morphine | 57 (5.1) |
| Buprenorphine | 47 (4.2) |
| Fentanyl | 46 (4.1) |
| Codeine | 36 (3.2) |
| Hydromorphone | 19 (1.7) |
| Loperamide | 9 (0.8) |
| Oxymorphone | 7 (0.6) |
| Naltrexone | 5 (0.4) |
| Miscellaneousa | 15 (1.3) |
| Class total | 1118 (100) |
aIncludes 3-methylfentanyl, desomorphine, naloxone, pentazocine, propoxyphene, remifentanil, tapentadol, and U47700 (designer opioid)
Table 14.
ToxIC 2016 agent entries—sympathomimetics
| N (%) | |
|---|---|
| Cocaine | 250 (34.3) |
| Methamphetamine | 187 (25.7) |
| Amphetamine | 90 (12.4) |
| Methylphenidate | 48 (6.6) |
| Dextroamphetamine | 32 (4.4) |
| Methylenedioxy-N-methamphetamine | 31 (4.3) |
| Lisdexamfetamine | 24 (3.3) |
| Phenylephrine | 10 (1.4) |
| Pseudoephedrine | 9 (1.2) |
| Phentermine | 8 (1.1) |
| Cathinone | 6 (0.8) |
| Phentermine | 7 (1.2) |
| Sympathomimetic unspecified | 5 (0.7) |
| Miscellaneousa | 28 (3.8) |
| Class total | 728 (100) |
aIncludes 25I-NBOMe, atomoxetine, clenbuterol, dexmethylphenidate, ephedrine, 2C series drugs, epinephrine, 2,5-dimethoxy-4-bromophenethylamine, 4-fluoroamphetamine, 6-(2-Aminopropyl)benzofuran, butylone, N-ethylhexedrone, norepinephrine, phenylethylamine designer drug unspecified, prolintane, and tetrahydrozoline
Table 15.
ToxIC 2016 agent entries—anticholinergics and antihistamines
| N (%) | |
|---|---|
| Diphenhydramine | 416 (59.1) |
| Hydroxyzine | 105 (14.9) |
| Chlorpheniramine | 34 (4.8) |
| Doxylamine | 32 (4.5) |
| Benztropine | 29 (4.1) |
| Promethazine | 16 (2.3) |
| Loratadine | 15 (2.1) |
| Cetirizine | 9 (1.3) |
| Dicyclomine | 6 (0.9) |
| Antihistamine unspecified | 5 (0.7) |
| Cyproheptadine | 5 (0.7) |
| Miscellaneousa | 32 (4.5) |
| Class total | 704 (100) |
aIncludes anticholinergic unspecified, atropine, belladonna, brompheniramine, cyclopentolate, dimenhydrinate, fexofenadine, glycopyrrolate, hyoscyamine, meclizine, oxybutynin, pyrilamine, scopolamine, trihexyphenidyl, and triprolidine
Table 16.
ToxIC 2016 agent entries— ethanol and toxic alcohols
| N (%) | |
|---|---|
| Ethanola | 694 (100) |
| Non-ethanol alcohols and glycols | |
| Ethylene glycol | 56 (45.5) |
| Isopropanol | 40 (32.5) |
| Methanol | 17 (13.8) |
| Propylene glycol | 4 (3.3) |
| Miscellaneousb | 6 (4.9) |
| Class total | 123 (100) |
aEthanol is considered a separate agent class
bIncludes acetone, denatured alcohol non-ethanol, diethylene glycol, glycol ethers, and toxic alcohol unspecified
Table 17.
ToxIC 2016 agent entries—cardiovascular
| N (%) | |
|---|---|
| Beta blockers | 181 (27.7) |
| Metoprolol | 70 (10.7) |
| Propranolol | 56 (8.6) |
| Atenolol | 24 (3.7) |
| Carvedilol | 16 (2.4) |
| Labetalol | 7 (1.1) |
| Miscellaneousa | 8 (1.2) |
| Sympatholytics | 169 (25.8) |
| Clonidine | 141 (21.6) |
| Guanfacine | 28 (4.3) |
| Calcium channel antagonists | 113 (17.3) |
| Amlodipine | 63 (9.6) |
| Diltiazem | 23 (3.5) |
| Verapamil | 18 (2.8) |
| Nifedipine | 8 (1.2) |
| Miscellaneousb | <5 (<0.8) |
| Angiotensin-converting enzyme (ACE) inhibitors | 52 (8.0) |
| Lisinopril | 47 (7.2) |
| Miscellaneousc | 5 (0.8) |
| Cardiac glycosides | 46 (7.0) |
| Digoxin | 44 (6.7) |
| Digitoxin | 2 (0.3) |
| Other antihypertensives and vasodilators | 32 (4.9) |
| Prazosin | 16 (2.4) |
| Miscellaneousd | 16 (2.4) |
| Antidysrhythmics and other cardiovascular agents | 27 (4.1) |
| Atorvastatin | 8 (1.2) |
| Simvastatin | 6 (1.0) |
| Miscellaneouse | 13 (2.0) |
| Diuretics | 25 (3.8) |
| Hydrochlorothiazide | 16 2.4) |
| Furosemide | 5 (0.8) |
| Miscellaneousf | 4 (0.6) |
| Angiotensin receptor blockers | 9 (1.4) |
| Losartan | 5 (0.8) |
| Miscellaneousg | 4 (0.6) |
| Class total | 654 (100) |
aIncludes nebivolol, bisoprolol, nadolol, and timolol
bIncludes nicardipine
cIncludes benazepril, enalapril, and quinapril
dIncludes hydralazine, nitroprusside, isosorbide, nitroglycerin, terazosin, alkyl nitrite, doxazosin, and minoxidil
eIncludes flecainide, sotalol, amiodarone, midodrine, cardiovascular agent unspecified, quinidine, pravastatin
fIncludes chlorthalidone, acetazolamide, spironolactone
gIncludes valsartan, irbesartan, olmesartan
Table 18.
ToxIC 2016 agent entries—antipsychotics
| N (%) | |
|---|---|
| Quetiapine | 307 (47.8) |
| Olanzapine | 87 (13.6) |
| Aripiprazole | 52 (8.1) |
| Risperidone | 41 (6.4) |
| Haloperidol | 39 (6.1) |
| Clozapine | 26 (4.0) |
| Lurasidone | 20 (3.1) |
| Ziprasidone | 17 (2.6) |
| Chlorpromazine | 15 (2.3) |
| Paliperidone | 9 (1.4) |
| Perphenazine | 7 (1.1) |
| Fluphenazine | 6 (0.9) |
| Miscellaneousa | 16 (2.5) |
| Class total | 642 (100) |
aIncludes antipsychotic unspecified, asenapine, brexpiprazole, loxapine, penfluridol, prochlorperazine, thiothixene, and trifluoperazine
Table 19.
ToxIC 2016 agent entries—anticonvulsants and mood stabilizers, and lithium
| N (%) | |
|---|---|
| Lithiuma | 176 (100) |
| Lamotrigine | 100 (27.0) |
| Valproic acid | 98 (26.5) |
| Phenytoin | 56 (15.1) |
| Carbamazepine | 42 (11.4) |
| Topiramate | 29 (7.8) |
| Oxcarbazepine | 20 (5.4) |
| Levetiracetam | 9 (2.4) |
| Miscellaneousb | 16 (4.3) |
| Class total | 370 (100) |
aLithium is considered a separate agent class
bIncludes anticonvulsant unspecified, divalproex, fosphenytoin, lacosamide, and zonisamide
Table 20.
ToxIC 2016 agent entries—envenomations and marine poisonings
| N (%) | |
|---|---|
| Crotalus spp. | 106 (34.1) |
| Agkistrodon spp. | 96 (30.9) |
| Snake unspecified | 64 (20.6) |
| Loxosceles spp. | 13 (4.2) |
| Latrodectus spp. | 10 (3.2) |
| Centuroides spp. | 5 (1.6) |
| Hymenoptera | 5 (1.6) |
| Spider unspecified | 5 (1.6) |
| Miscellaneousa | 7 (2.3) |
| Class total | 311 (100) |
aIncludes Naja nigricinta, Vipera palaestinae, Micrurus spp., scorpion unspecified, and stingray
Table 21.
ToxIC 2016 agent entries—psychoactives
| N (%) | |
|---|---|
| Cannabinoid synthetic | 95 (31.5) |
| Marijuana | 88 (29.1) |
| LSD | 22 (7.3) |
| Phencyclidine | 20 (6.6) |
| Gamma hydroxybutyrate | 16 (5.3) |
| Cannabinoid non-synthetica | 12 (4.0) |
| Ketamine | 11 (3.6) |
| Nicotine | 9 (3.0) |
| Molly-amphetamine-like hallucinogenb | 6 (2.0) |
| Miscellaneousc | 23 (7.6) |
| Class total | 302 (100) |
aThe cannabinoid nonsynthetic group refers to exposures to unspecified naturally occurring cannabinoids, such as cannabis extracts or cannabidiol
bAmphetamine-like hallucinogens are presented with psychoactives for brevity, though listed as an individual Registry class
cIncludes 1-propionyl-lysergic acid diethylamide (1P-LSD), 3-methoxyphencyclidine, dimethyltryptamine (DMT), donepezil, gamma butyrolactone, hallucinogen unspecified, hallucinogenic amphetamines, methylenedioxymethamphetamine, methylone, pharmaceutical tetrahydrocannabinol (THC), psychoactive unspecified, and varencicline
Table 22.
ToxIC 2016 agent entries—diabetic medications
| N (%) | |
|---|---|
| Metformin | 66 (41.0) |
| Insulin | 40 (24.8) |
| Glipizide | 26 (16.1) |
| Glyburide | 14 (8.7) |
| Glimepiride | 8 (5.0) |
| Miscellaneousa | 7 (4.3) |
| Class total | 161 (100) |
aIncludes empagliflozin, exenatide, gliclazide, and saxagliptin
Table 23.
ToxIC 2016 agent entries—metals
| N (%) | |
|---|---|
| Lead | 44 (30.3) |
| Iron | 30 (20.7) |
| Mercury | 20 (13.8) |
| Chromium | 11 (7.6) |
| Cobalt | 7 (4.8) |
| Arsenic | 5 (3.4) |
| Copper | 5 (3.4) |
| Miscellaneousa | 23 (15.9) |
| Class total | 145 (100) |
aIncludes manganese, metal unspecified, cadmium, silver, titanium, antimony, barium, magnesium, nickel, selenium, thorium, uranium, and zinc sulfate
Table 24.
ToxIC 2016 agent entries—gases, irritants, vapors, and dusts
| N (%) | |
|---|---|
| Carbon monoxide | 54 (43.2) |
| Hydrogen sulfide | 14 (11.2) |
| Cyanide | 11 (8.8) |
| Smoke | 10 (8.0) |
| Chlorine | 5 (4.0) |
| Gas/irritant/vapor/dust unspecified | 5 (4.0) |
| Miscellaneousa | 26 (20.8) |
| Class total | 125 (100) |
aIncludes petroleum vapors, silica, chloramine, diesel exhaust, dust, ethylene oxide, sewer gas, acetonitrile, duster (canned air), ethylene, natural gas, nitric oxide, ozone, plastic fumes, polyurethane vapors, welding fumes, and vaping NOS
Table 25.
ToxIC 2016 agent entries—household products
| N (%) | |
|---|---|
| Sodium hypochlorite ≤6% | 26 (31.3) |
| Cleaning solutions and disinfectants | 17 (20.5) |
| Laundry detergent pod | 11 (13.3) |
| Ammonia ≤10% | 6 (7.2) |
| Paint | 5 (6.0) |
| Miscellaneousa | 18 (21.7) |
| Class total | 83 (100) |
aIncludes deodorants and antiperspirants, dishwasher detergent, dishwasher detergent pod, flower food, hair product, hand sanitizer unspecified, household product unspecified, mouthwash unspecified, perfume, phenylenediamine (hair dye), soaps and detergents, sunscreens, and windshield washer fluid
Table 26.
ToxIC 2016 agent entries—plants and fungi
| N (%) | |
|---|---|
| Mold unspecified | 33 (41.3) |
| Mushroom, other/unknown | 9 (11.3) |
| Mitragyna speciosa (kratom) | 6 (7.5) |
| Mushroom, psilocibin | 4 (5.0) |
| Valerian root | 4 (5.0) |
| Miscellaneousa | 24 (30.0) |
| Class total | 80 (100) |
aIncludes aconitum, Agastache scrophulariifolia (purple giant hyssop), Amanita muscaria, Brugmansia (angels trumpet), Convallaria majalis (lily of the valley), Crataegus (hawthorn), Datura inoxia (moonflower, thornapple), Dieffenbachia, Ganoderma mushroom, kola nut, kombucha tea, Nerium oleander, petasites (butterbur), Phytolacca americana (pokeweed), Piper methysticum (kava), plants and fungi unspecified, poppy seeds (Papaver), Ricinus communis (castor beans), and Sanseviera (Snake plant, Mother in Laws tongue)
Table 10 presents the non-opioid analgesics class. This has been the most frequently reported agent class since 2013 [2, 7, 8]. The most common agent was acetaminophen (67.9%), which was also the most common agent in the Registry overall, involved in 11.6% cases, consistent with previous years.
Table 11 reports the sedative-hypnotics/muscle relaxants class. Benzodiazepines were the most frequently reported (52.6%) with alprazolam (20.6%), clonazepam (15.5%), and lorazepam (7.4%) the top three reported. In 2016, alprazolam eclipsed clonazepam as the most commonly reported benzodiazepine for the first time since the ToxIC Registry began [2, 7–11]. Other commonly reported agents in this class included the muscle relaxants cyclobenzaprine and baclofen, gabapentin (in the “Other sedatives” subclass), and zolpidem (in the “Non-benzodiazepine agonists” subclass). Barbiturates were infrequently mentioned, consistent with prior years.
Table 12 presents the antidepressant class. Bupropion (in the “Other antidepressants” subclass) was the most frequently reported, comprising 20.7% of antidepressant cases and 3.0% of 2016 Registry cases overall, consistent with prior years. The selective serotonin reuptake inhibitors (SSRIs), particularly sertraline and fluoxetine, were the second most common subclass (34.3%), followed by the tricyclic antidepressants (TCAs) (15.1%) (e.g., amitriptyline), and the serotonin-norepinephrine reuptake inhibitors (SNRIs) (e.g., venlafaxine).
Table 13 summarizes opioid frequencies, including naturally derived, synthetic, and semisynthetic agents. As in previous years, heroin was the most commonly reported (28.3%), followed by oxycodone (17.8%) and methadone (8.9%).
Table 14 reports the sympathomimetic class. Cocaine was the most commonly reported (34.3%), followed by methamphetamine (25.7%) and amphetamine (12.4%), consistent with previous years. The pharmaceutical stimulants methylphenidate and lisdexamfetamine together accounted for 9.9% of the class. A number of designer stimulant drugs were reported, with methylenedioxy-N-methamphetamine the most common (4.3%). Less common designer agents, combined in the miscellaneous category, included the 2C series drugs, 25I-NBOMe, and other unspecified phenethylamines.
Table 15 describes the anticholinergic and antihistamine class (Table 15), and the most commonly reported agent was diphenhydramine (59.1%), which was also one of the most commonly reported agents in the Registry overall, involved in 4.9% of cases. Hydroxyzine was the next most common (14.9%), followed by other first-generation antihistamines chlorpheniramine and doxylamine. Second-generation antihistamines were reported less frequently.
Table 16 presents frequencies for ethanol and the toxic alcohols. Ethanol was again the second most commonly reported agent overall in the Registry (N = 694), involved in 8.1% of cases. Among the toxic alcohols, ethylene glycol was the most common (45.5%), followed by isopropanol (32.5%), while methanol was less commonly reported (13.8%).
Table 17 presents the cardiovascular agent class. The 654 cardiovascular agent entries comprised 5.8% of all Registry entries in 2016. In this class, beta adrenergic receptor antagonists (beta-blockers) were the most commonly reported (27.7%), followed by sympatholytics (25.8%), similar to prior years. Metoprolol was the most common beta-blocker. Clonidine (21.6%) was the most common sympatholytic reported with guanfacine also reported but to a much lower extent (4.3%). Together, the calcium channel antagonists comprised 17.3% of the agent class, with amlodipine the most common, followed by diltiazem and verapamil. Angiotensin-converting enzyme (ACE) inhibitors comprised only 8.0% of the cardiovascular agent mentions, with lisinopril accounting for >90% of these. Forty-six cardiac glycoside cases were reported, with all involving digoxin except for two digitoxin cases. Other subgroups of cardiovascular agents were less commonly reported.
Table 18 summarizes the 642 antipsychotic agents reported, which accounted for 5.7% of all Registry entries in 2016. Consistent with prior years, quetiapine was by far the most commonly encountered antipsychotic agent. There were more than three times as many quetiapine cases reported (47.8%) as olanzapine (13.6%), the next most common agent. On its own, quetiapine was listed in 3.6% of all Registry cases in 2016. Aripiprazole was also frequently reported (8.1%), similar to past years. Risperidone, haloperidol, and clozapine together accounted for an additional 16.5% of the antipsychotic agent class.
Table 19 shows anticonvulsants and mood stabilizers. Lithium is considered a unique agent class and reported separately in Table 19. Lithium was reported in 176 cases, representing 2.1% of total Registry cases in 2016. Lamotrigine and valproic acid were the two most commonly reported agents in the anticonvulsants and mood stabilizers class. These were followed by phenytoin, carbamazepine, oxcarbazepine, and topiramate.
Table 20 presents information on envenomations and marine poisonings. This class was dominated by snake envenomations in 2016, consistent with prior years. Crotalus spp. were the most commonly reported (34.1%), followed by Agkistrodon spp. (30.9%), and unspecified snakes (20.6%). Spider envenomations (e.g., Loxosceles spp., Latrodectus spp., and unspecified spider) were less common, together making up 9.0% of the class.
Table 21 summarizes the psychoactive agent class, which includes marijuana and other cannabinoid receptor agonists, hallucinogens, and dissociatives. Synthetic cannabinoids were the most frequently entered (32.1%), followed by marijuana, lysergic acid diethylamide (LSD), phencyclidine, and gamma hydroxybutyrate. Amphetamine-like hallucinogens are reported with the psychoactives in Table 21.
Table 22 reports data on diabetic medications. Agent frequencies were similar to previous years, with metformin (41.0%) and insulin (24.8%) the most common. These were followed by the sulfonylurea agents: glipizide, glyburide, and glimepiride.
Table 23 presents the metals agent class which was dominated by lead (30.3%), iron (20.7%), and mercury (13.8%), followed by chromium (7.6%) and cobalt (4.8%). The miscellaneous subclass, comprised of 13 different agents, accounted for 15.9% of entries.
Table 24 presents the gases, irritants, vapors, and dusts class. Carbon monoxide was the predominant agent (43.2%), followed hydrogen sulfide (11.2%), and cyanide and smoke (16.8% combined).
Table 25 reports household product exposures. Sodium hypochlorite ≤6% (household bleach) was the most common (31.3%), followed by other cleaning solutions and disinfectants (20.5%) and laundry detergent pods (13.3%). Caustic agents are described separately in Table S4 in the Supplemental Material.
Table 26 presents the diverse species of the plants and fungi agent class. Consistent with prior years, unspecified mold and unknown mushrooms were the predominant agents, followed by Mitragyna speciosa (kratom), and a miscellaneous category containing 19 different plant and fungi agents.
Additional agent classes presented in the Supplementary Material include cough and cold products (Table S1), herbal products and dietary supplements (Table S2), hydrocarbons (Table S3), caustics (Table S4); antimicrobials (Table S5), other pharmaceutical products (Table S6), other non-pharmaceutical products (Table S7), anticoagulants (Table S8), chemotherapeutic/immunological agents (Table S9), pesticides, including herbicides, insecticides, rodenticides, and fungicides (Table S10), endocrine agents (Table S11), gastrointestinal agents (Table S12), anesthetics (Table S13), anti-parkinsonism drugs (Table S14), pulmonary agents (Table S15), weapons of mass destruction/riot/radiological agents (Table S16), and ingested foreign bodies (Table S17).
Clinical Signs and Symptoms
Table 27 summarizes the 3134 clinical toxidromes reported in 2016. The frequencies of reported toxidromes were similar to past years with sedative-hypnotic the most common (14.9%), followed by anticholinergic (7.4%), sympathomimetic (4.5%), and opioid (4.4%).
Table 27.
ToxIC 2016 cases—toxidromes
| N (%)a | |
|---|---|
| Sedative-hypnotic | 1261 (14.9) |
| Anticholinergic | 629 (7.4) |
| Sympathomimetic | 386 (4.5) |
| Opioid | 378 (4.4) |
| Serotonin syndrome | 259 (3.0) |
| Alcoholic ketoacidosis | 87 (1.0) |
| Sympatholytic | 50 (0.6) |
| Washout syndrome | 36 (0.4) |
| NMS | 19 (0.2) |
| Cholinergic | 16 (0.2) |
| Overlap syndromes (MCS, chronic fatigue, etc.) | 10 (0.1) |
| Miscellaneousb | 8 (0.1) |
| Total | 3134 (36.7) |
NMS neuroleptic malignant syndrome
aPercentage equals number cases reporting specific toxidrome relative to total number of Registry cases in 2016 (N = 8529)
bIncludes anticonvulsant hypersensitivity and fume fever
Table 28 summarizes the 2627 major vital sign abnormalities recorded in 2016. Tachycardia was the most common (12.1%), followed by hypotension (8.0%), bradycardia (4.6%), and several others affecting <4% of total cases. Note that some cases reported more than one major vital sign abnormality. Additionally, cases may include more than one of the other signs/symptom categories described below.
Table 28.
ToxIC 2016 cases—major vital sign abnormalities
| N (%)a | |
|---|---|
| Tachycardia (HR >140) | 1031 (12.1) |
| Hypotension (systolic BP < 80 mmHg) | 684 (8.0) |
| Bradycardia (HR < 50) | 393 (4.6) |
| Bradypnea (RR < 10) | 249 (2.9) |
| Hypertension (systolic BP > 200 mmHg and/or diastolic BP > 120 mmHg) | 224 (2.6) |
| Hyperthermia (temperature > 105 °F) | 46 (0.5) |
| Total | 2627 (30.8)b |
HR heart rate, BP blood pressure, RR respiratory rate
aPercentage equals the number of cases relative to the total number of Registry cases in 2016 (N = 8259)
bTotal reflects cases reporting at least one major vital sign abnormality; cases may be associated with more than one major vital sign abnormality
Table 29 summarizes the neurological signs and symptoms reported in 2016. While the overall numbers of cases with neurological signs and symptoms increased from previous years, the order remained similar, with coma/central nervous system depression the most common (34.7%), followed by agitation (16.7%) and delirium (11.8%).
Table 29.
ToxIC 2016 cases—neurological signs and symptoms
| N (%)a | |
|---|---|
| Coma/CNS depression | 2959 (34.7) |
| Agitation | 1421 (16.7) |
| Delirium | 1005 (11.8) |
| Hyperreflexia/myoclonus/tremor | 635 (7.4) |
| Seizures | 459 (5.4) |
| Hallucinations | 366 (4.3) |
| Weakness/paralysis | 142 (1.7) |
| Dystonia/rigidity/extrapyramidal symptoms | 112 (1.3) |
| Numbness/Paresthesia | 79 (0.9) |
| Peripheral neuropathy | 25 (0.3) |
| Total | 4970 (58.3)a,b |
CNS central nervous system
aPercentage equals number of cases relative to total number of Registry cases in 2016 (N = 8529)
bTotal reflects cases reporting at least one neurological symptom; cases may be associated with more than one neurological symptom
Table 30 summarizes the cardiovascular and pulmonary signs and symptoms reported in 2016. Although the total number of cardiovascular and pulmonary signs reported was higher this year than in previous years, the order remained similar, with prolonged QTc (≥500ms) the most common cardiovascular sign (5.6%), followed by prolonged QRS (≥120 ms) (1.8%). The most common pulmonary sign was respiratory depression (11.1%).
Table 30.
ToxIC 2016 cases—cardiovascular and pulmonary signs
| N (%)a | |
|---|---|
| Cardiovascular | |
| Prolonged QTc (≥500 ms) | 480 (5.6) |
| Prolonged QRS (≥120 ms) | 150 (1.8) |
| Ventricular dysrhythmia | 102 (1.2) |
| Myocardial injury or infarction | 61 (0.7) |
| AV block (>1st degree) | 41 (0.5) |
| Total | 834 (9.8)b |
| Pulmonary | |
| Respiratory depression | 946 (11.1) |
| Aspiration pneumonitis | 182 (2.1) |
| Acute lung injury/ARDS | 117 (1.4) |
| Asthma/reactive airway disease | 60 (0.7) |
| Total | 1305 (15.3)b |
ARDS acute respiratory distress syndrome
aPercentage equals number cases reporting signs of symptoms relative to total number of Registry cases in 2016 (N = 8529)
bTotal reflects cases reporting at least one cardiovascular or pulmonary symptom; cases may be associated with more than one symptom
Table 31 summarizes signs and symptoms involving other organ systems. As in the past years, metabolic signs (e.g., elevated anion gap, metabolic acidosis) were common, reported in 12.2% of cases. Renal and musculoskeletal signs (e.g., rhabdomyolysis, acute kidney injury) were also common (10.3%), followed by hematological (6.2%), gastrointestinal/hepatic (5.3%), and dermal (3.4%) signs and symptoms.
Table 31.
ToxIC 2016 cases—clinical signs—other organ systems
| N (%)a | |
|---|---|
| Metabolic | |
| Elevated anion gap (>20) | 459 (5.4) |
| Metabolic acidosis (pH <7.2) | 387 (4.5) |
| Hypoglycemia (glucose <50 mg/dL) | 126 (1.5) |
| Elevated osmole gap (>20) | 61 (0.7) |
| Total | 1033 (12.1)b |
| Gastrointestinal/hepatic | |
| Hepatotoxicity (AST ≥1000 IU/L) | 296 (3.5) |
| Pancreatitis | 64 (0.8) |
| Gastrointestinal bleeding | 49 (0.6) |
| Corrosive injury | 42 (0.5) |
| Intestinal ischemia | 5 (0.1) |
| Total | 456 (5.3)b |
| Hematological | |
| Coagulopathy (PT >15 s) | 199 (2.3) |
| Leukocytosis (WBC >20 K/μL) | 131 (1.5) |
| Thrombocytopenia (platelets <100 K/μL) | 102 (1.2) |
| Hemolysis (Hgb <10 g/dL) | 56 (0.7) |
| Pancytopenia | 21 (0.2) |
| Methemoglobinemia (MetHgb ≥2%) | 18 (0.2) |
| Total | 527 (6.2)b |
| Renal/musculoskeletal | |
| Rhabdomyolysis (CPK >1000 IU/L) | 489 (5.7) |
| Acute kidney injury (creatinine >2.0 mg/dL) | 386 (4.5) |
| Total | 875 (10.3)b |
| Dermatological | |
| Rash | 165 (1.9) |
| Blister/bullae | 71 (0.8) |
| Necrosis | 31 (0.4) |
| Angioedema | 25 (0.3) |
| Total | 292 (3.4)b |
AST aspartate aminotransferase, PT prothrombin time, WBC white blood cells, Hgb hemoglobin, CPK creatine phosphokinase
aPercentage equals the number of cases reporting specific clinical signs compared to the total number of Registry cases in 2016 (N = 8529)
bTotal reflects cases reporting at least one sign in the category; cases may be associated with more than one symptom
Fatalities
Tables 32 and 33 present data on ToxIC 2016 cases involving exposures which resulted in fatalities, with Table 32 including cases involving single agent exposures and Table 33 including cases involving multiple agents. Table S18 in the Supplementary Information summarizes information on fatality cases with no suspected toxicologic exposure, or an unknown exposure(s).
Table 32.
ToxIC 2016 fatality cases with known toxicological exposure, single agent
| Age/sexa | Agents involved | Clinical findings | Life support withdrawn | Brain death confirmed | Treatmentb |
|---|---|---|---|---|---|
| 40M | Acetaminophen | HT, CNS, MA, AG, HPT, PNC, CPT, PLT, AKI, RBM | No | Unknown | NAC, vitamin K, vasopressors, anticonvulsants, neuromuscular blockers, opioids, continuous renal replacement, intubation, IV fluids |
| 41F | Acetaminophen | TC, RD, AGT, CNS, AG, HPT, PNC, HYS, AKI, RBM | Yes | Unknown | NAC, vitamin K, benzodiazepines, intubation, IV fluids |
| 21F | Heroin | HT, BC, BP, VD, RD, CNS, MA | Yes | Yes | Physostigmine, vasopressors, antiarrhythmics, CPR, intubation, IV fluids, therapeutic hypothermia |
| 41F | Acetaminophen | HT, RD, CNS, MA, AG, HPT, CPT, WBC, RBM | Yes | No | NAC, NaHCO3, vitamin K, vasopressors, anticonvulsants, benzodiazepines, opioids, continuous renal replacement, intubation, IV fluids |
| 43M | Methanol | HT, QRS, RD, CNS, MA, AG, OG, CPT, AKI, RBM | Yes | Yes | Folate, fomepizole, hemodialysis, intubation, IV fluids |
| 29F | Acetaminophen | HT, TC, CNS, HGY, HPT, CPT, AKI | Yes | No | NAC, NaHCO3, vitamin K, benzodiazepines, glucose, neuromuscular blockers, opioids, vasodilators, hemodialysis, continuous renal replacement, intubation, IV fluids, transfusion |
| 52M | Ethanol | HT, TC, MA, AG, HPT, PNC, PLT, AKI | Unknown | Unknown | NAC, vasopressors, continuous renal replacement, cardioversion, intubation, IV fluids |
| 35F | Amitriptyline | HT, TC, BP, VD, QRS, RD, CNS, MA, AG, HPT, AKI | Yes | Yes | Naloxone, NaHCO3, vasopressors, CPR, intubation, IV fluids |
| 54M | Ethanol | CNS | Yes | Yes | Fomepizole, NAC, hemodialysis, intubation, transfusion |
| 80M | Carbon monoxide | HT, AVB, RD, CNS, WKN, MA, OTH1 | Yes | No | Hydroxocobalamin, vasopressors, intubation, IV fluids |
| 77M | Metoprolol | HT, BC, AVB, RD, CNS | Yes | No | Insulin-euglycemic therapy, vasopressors, glucose, intubation, IV fluids |
| 51F | Acetaminophen | HT, TC, BC, BP, VD, ALI, RD, CNS, MA, AG, HPT, HYS, CPT, PLT, WBC, AKI | Yes | No | Lipid resuscitation, methylene blue, NAC, NaHCO3, vasopressors, intubation, transfusion |
| 51F | Heroin | HT, BP, RD, CNS, HPT, WBC, RBM | Yes | Yes | NaHCO3, vasopressors, benzodiazepines, intubation, IV fluids |
| 24F | Acetaminophen | HT, VD, CNS, HGY, MA, AG, HPT, PNC, PLT | Yes | No | NAC, vasopressors, benzodiazepines, glucose, continuous renal replacement, intubation, IV fluids, therapeutic hypothermia |
| 63F | Acetaminophen | HT, BC, BP, VD, ALI, RD, CNS, MA, AG, HPT, PNC, INT, CPT, PLT, WBC | No | No | NAC, vasopressors, CPR, intubation, IV fluids |
| 60M | Colchicine | HT, VD, MA, AG, CPT, PLT, PCT, WBC | No | No | Vasopressors, antiarrhythmics, continuous renal replacement, intubation, IV fluids |
| 51M | Metformin | HT, TC, ALI, MA, AG, RBM | Unknown | Unknown | Hemodialysis, intubation |
| 83F | Diltiazem | HT, BC, CNS, MA, AG, | Unknown | Unknown | Glucagon, insulin-euglycemic therapy, lipid resuscitation, vasopressors, intubation, IV fluids |
| 40F | Heroin | BP, CNS, MA | Yes | Yes | NAC, naloxone, |
| 57M | Bupropion | VD, AP, ALI, CNS, SZ, AKI | No | No | Lipid resuscitation, NaHCO3, vasopressors, benzodiazepines, CPR, intubation, IV fluids |
| 19M | Fentanyl | RD, CNS | Yes | Yes | Naloxone, CPR, intubation |
| 14F | Diphenhydramine | HT, VD, QTc, QRS, CNS, SZ, MA, AG | Yes | No | Vasopressors, antiarrhythmics, anticonvulsants, benzodiazepines, intubation, IV fluids |
| 63F | Doxepin | RD, CNS, AKI | Yes | No | Glucagon, NaHCO3, intubation, IV fluids |
| 22F | Acetaminophen | HT, TC, RD, CNS, SZ, MA, AG, HPT, CPT | Yes | Yes | NAC, vasopressors, anticonvulsants, benzodiazepines, intubation, IV fluids, |
| 14F | Bupropion | HT, QRS, ALI, RD, CNS, SZ, MA, AKI, RBM | Yes | Unknown | Insulin-euglycemic therapy, lipid resuscitation, NaHCO3, vasopressors, urinary alkalinization, CPR, intubation, IV fluids, therapeutic hypothermia |
| 60F | Quinidine | MET | No | No | Calcium, methylene blue, NaHCO3, vitamin K, vasopressors, hypoglycemia, exchange transfusion, IV fluids, transfusion |
| 52M | Sevoflurane | HTN, TC, HYT, VD, QTc, QRS, EPS | Yes | No | Dantrolene, NaHCO3, intubation, IV fluids |
| 32M | Cyanide | HT, TC, CNS, MA | No | No | Hydroxocobalamin, intubation, IV fluids |
| 19F | Acetaminophen | None listed | No | No | NAC, IV fluids |
| 47M | Hydromorphone | AP | No | No | NAC, IV fluids |
| 65F | Digoxin | HT, BC, WKN | Yes | No | Digoxin Fab, vasopressors, IV fluids |
| 60F | Amitriptyline | HT, TC, VD, QRS, RAD, ALI, RD, | Yes | No | NaHCO3, vasopressors, intubation, intubation, IV fluids |
| 17F | Cocaine | TC, RD, AGT, CNS, DLM, SZ, MA, AG, HPT, HYS, CPT, PLT, WBC, AKI | Yes | No | Vasopressors, anticonvulsants, benzodiazepines, glucose, opioids, hemodialysis, CPR, intubation, IV fluids, transfusion |
| 32M | Cyanide | HT, VD, RAD, AGT, CNS, MA | No | No | Hydroxocobalamin, thiosulfate, vasopressors, CPR, intubation, IV fluids |
| 51M | Warfarin | CNS, CPT, WBC | Yes | Yes | Anticoagulant reversal, anticonvulsants, neuromuscular blockers, intubation |
| 33M | Hydrogen sulfide | HT, TC, VD, ALI, RD, CNS, MA | Yes | Unknown | Vasopressors, antiarrhythmics, benzodiazepines, CPR, cardioversion, intubation, IV fluids |
| 61M | Ethanol | HT, DLM, HCN, HPT, PNC, HYS, PLT | Yes | Unknown | Calcium, folate, octreotide, thiamine, benzodiazepines, IV fluids, transfusion |
| 21F | Acetaminophen | HT, BC, VD, ALI, RD, CNS, MA, AG, HPT, CPT, AKI | No | No | NAC, NaHCO3, vasopressors, antiarrhythmics, benzodiazepines, CPR, cardioversion, intubation, IV fluids |
| 45M | Codeine | HT, BC, VD, RD, CNS, MA, AG, | Yes | Yes | NAC, NaHCO3, vasopressors, antiarrhythmics, anticonvulsants, beta blockers, opioids, CPR, cardioversion, intubation, IV fluids, therapeutic hypothermia |
| >89M | Carbon monoxide | HT, VD, RD, CNS, AG | No | No | Thiosulfate, vasopressors, intubation, IV fluids |
| 68M | Digoxin | HT, QRS, RD, AGT, CNS | Yes | Unknown | Digoxin Fab, pacemaker |
| 39F | Gabapentin | HT, VD, QTc, MI, RD, CNS, MA, AG, HPT, MI, WBC, AKI, RBM | Yes | No | Vasopressors, antiarrhythmics, CPR, intubation, therapeutic hypothermia |
| 38F | Acetaminophen | HT, TC, MI, ALI, CNS, MA, AG, HPT, GIB, HYS, CPT, PLT, PCT, AKI, RBM | Yes | Unknown | NAC, NaHCO3, vitamin K, vasopressors, glucose, continuous renal replacement, intubation, IV fluids |
| 68M | Chlorine | HT, QTc, MI, ALI, RD, CNS | No | No | Vasopressors, neuromuscular blockers, intubation, IV fluids |
| 68M | Copper | HT, CRV, GIB, MET, WBC, AKI | No | No | BAL, penicillamine, vasopressors, hemodialysis, continuous renal replacement, CPR, intubation, IV fluids, transfusion |
| 46M | Amlodipine | HT, RAD, CNS, MA, AKI | Yes | No | Lipid resuscitation, methylene blue, NaHCO3, thiamine, vitamin K, vasopressors, bronchodilators, antiarrhythmics, benzodiazepines, neuromuscular blockers, opioids, steroids, intubation, IV fluids |
| 24M | Opioid unspecified | VD, QTc, QRS, MI, AP, RD, CNS, MA, | Yes | Yes | None listed |
| 74M | Ciguatera | PST, WKN | Yes | No | None listed |
| 27M | Morphine | HT, BP, MI, RAD, ALI, RD, CNS, MA, AG. CPT, AKI, RBM | Yes | Yes | Naloxone, vasopressors, CPR, intubation, IV fluids, therapeutic hypothermia |
| 38M | Heroin | HT, TC, MI, ALI, CNS, NP, MA, WBC | Unknown | Unknown | Folate, naloxone, vasopressors, intubation, IV fluids |
| 31M | Heroin | HT, VD, QRS, MI, RAD, RD, CNS, MA, AG, AKI | Yes | Yes | None listed |
| 58M | Amphetamine | QRS, QTc, AGT, MA, AG, OG, INT, PLT, AKI | Yes | Unknown | Fomepizole, vasopressors, hemodialysis, continuous renal replacement, intubation |
| 44F | Heroin | HT, BP, VD, RD, CNS, MA, AG, | No | No | Naloxone, vasopressors, CPR, intubation, IV fluids |
| 34M | Heroin | RD, CNS | Unknown | Unknown | Naloxone, CPR |
| 26M | Heroin | CNS | Yes | Yes | Naloxone, intubation |
| 70M | Diltiazem | HT, CNS | Yes | Yes | Calcium, insulin-euglycemic therapy, vasopressors, IVF |
| 65M | Opioid unspecified | None listed | No | No | Antihypertensives, opioids, |
| 62F | Ethanol | HT, BC, MI, RD, CNS, MA,AG, OG, GIB, WBC, RBM | No | No | Fomepizole, vasopressors, continuous renal replacement, CPR, intubation, IV fluids |
| 60M | Acetaminophen | None listed | No | No | NAC |
| 32F | Aspirin | HT, BC, VD, RD, CNS, | No | No | NaHCO3, vasopressors, CPR, intubation, IV fluids |
| 32M | Cyanide | RD, CNS, AG, MET | Yes | Yes | Hydroxocobalamin, methylene blue, NaHCO3, vasopressors, intubation, IV fluids |
| 44M | Acetaminophen | AP, HPT, MET, MYS, CPT, AKI, RBM | Yes | No | NAC, hemodialysis, continuous renal replacement, intubation, IV fluids, transfusion |
| 43M | Methanol | TC, RD, CNS, SZ, MA, AG, OG | Yes | Yes | Folate, fomepizole, thiamine, vasopressors, anticonvulsants, benzodiazepines, hemodialysis, intubation, IV fluids |
Based on response from medical toxicologist “Did the patient have a toxicological exposure?” equals yes, with known agent(s)
AG anion gap, AGT agitation, AKI acute kidney injury, ALI acute lung injury/ARDS, AP aspiration pneumonia, AVB AV block, BC bradycardia, BP bradypnea, CNS coma/CNS depression, CPT coagulopathy, CRV corrosive injury, DLM delirium, EPS dystonia/rigidity, GIB GI bleeding, HCN hallucinations, HGY hypoglycemia, HPT hepatoxicity, HT hypotension, HTN hypertension, HYS hemolysis, HYT hyperthermia, INT intestinal ischemia, MA metabolic acidosis, MET methemoglobinemia, MI myocardial injury/ischemia, NP neuropathy, OG osmole gap, OTH1 rash, OTH2 skin blisters, necrosis, PCT pancytopenia, PLT thrombocytopenia, PNC pancreatitis, PST paresthesia, QRS QRS prolongation, QTc QTc prolongation, RAD asthma/reactive airway disease, RBM rhabdomyolysis, RD respiratory depression, RFX hyperreflexia/tremor, SZ seizures, TC tachycardia, VD ventricular dysrhythmia, WBC leukocytosis, WKN weakness/paralysis, BAL dimercaprol, CPR cardiopulmonary resuscitation, ECMO extra-corporeal membrane oxygenation, NAC n-acetyl cysteine, NaHCO 3 sodium bicarbonate
aAge in years unless otherwise stated
bPharmcological and non-pharmacological support as reported by medical toxicologist
Table 33.
ToxIC 2016 fatality cases with known toxicological exposure, multiple agents
| Age/sexa | Agents involved | Clinical findings | Life support withdrawn | Brain death confirmed | Treatmentb |
|---|---|---|---|---|---|
| 45F | Carbon monoxide, cyanide | HT, TC, ALI, RD, CNS, MA, AG, | Yes | Yes | Hydroxocobalamin, intubation |
| 30M | Heroin, cocaine, diphenhydramine, dextromethorphan, citalopram | TC, AP, CNS, RFX, MA, HPT, CPT, WBC, AKI, RBM, OTH2 | Yes | No | NaHCO3, antihypertensives, benzodiazepines, neuromuscular blockers, opioids, hemodialysis, intubation, IV fluids |
| 59M | Acetaminophen, ethanol | HT, RD, CNS, HGY, GIB, CPT | Unknown | Unknown | NAC, intubation, IV fluids |
| 16M | Acetaminophen, ethanol | DLM | Unknown | Unknown | None listed |
| 52F | Nicotine, caffeine, dextromethorphan, venlafaxine | VD, QRS, AP, CNS, GIB | Yes | Yes | NAC, NaHCO3, vasopressors |
| 62M | Heroin, cocaine | HT, BC, QTc, ALI, CNS, MA, AG, HPT, AKI, RBM | Yes | Yes | NAC, naloxone, vasopressors, continuous renal replacement, intubation, IV fluids |
| 68F | Nortriptyline, duloxetine, zolpidem, oxycodone | HTN, HT, TC, AP, ALI, RD, AGT, DLM, RFX, MA, PNC, PLT, AKI, RBM | Yes | No | Naloxone, vasopressors, benzodiazepines, neuromuscular blockers, continuous renal replacement, intubation, IV fluids |
| Unknown F | Fentanyl, diazepam | HT, RD, CNS, MA | Yes | Yes | Vasopressors, intubation, IV fluids |
| 15F | Ibuprofen, quetiapine | HT, TC, VD, QRS, RAD, CNS, MA, AG, | Yes | Unknown | Insulin-euglycemic therapy, lipid resuscitation, NaHCO3, vasopressors, antiarrhythmics, activated charcoal, hemodialysis, ECMO |
| 32F | Fentanyl, heroin | BC, BP, RD, CNS, SZ, AKI | Yes | No | Naloxone, intubation, IV fluids, therapeutic hypothermia |
| 28M | Methadone, marijuana | CNS, SZ, MA | Unknown | Unknown | Benzodiazepines, neuromuscular blockers, intubation, IV fluids |
| 65F | Hydrocodone, alprazolam, diazepam | HT, RD, CNS | Yes | No | Calcium, glucagon, insulin-euglycemic therapy, NAC, naloxone, vasopressors, intubation, IV fluids |
| 35M | Olanzapine, valproic acid | HT, TC, AGT, DLM | No | No | NAC, NaHCO3, vasopressors, glucose, hemodialysis, intubation, IV fluids |
| 21F | Dextroamphetamine, lithium | AGT, RBM | Unknown | Unknown | IV fluids |
| 33M | Heroin, valproic acid | HT, BC, VD, AP, AP, ALI, RD, CNS | Yes | No | Carnitine, NaHCO3, intubation, IV fluids |
| 50M | Norepinephrine, argatroban | CPT, PLT, WBC, RBM, OTH2 | Yes | Unknown | Vasopressors, continuous renal replacement, intubation, IV fluids, transfusion |
| 70M | Warfarin, methadone, oxycodone, cocaine, heroin | HT, RD, CNS, CPT, AKI | Yes | Yes | Vitamin K, vasopressors, IV fluids |
| 57M | Acetaminophen, metformin | HT, BC, RD, CNS, SZ, MA, AG, HPT, PNC, PLT, AKI | No | No | NAC, vasopressors, anticonvulsants, steroids, continuous renal replacement, CPR, intubation |
| 38M | Methamphetamine, amphetamine, marijuana, benzodiazepine | HTN, TC | No | No | Antihypertensives, benzodiazepines, CPR, intubation |
| 54M | Ethanol, ibuprofen, acetaminophen | HT, CNS, MA, AG, HPT, PLT, AKI | No | No | Fomepizole, NAC, vitamin K, vasopressors, hemodialysis, continuous renal replacement, IV fluids |
| 47M | Oxycodone, alprazolam, diazepam | HT, TC, BC, BP, VD, MI, RD, CNS, MA, AKI | Unknown | Unknown | None listed |
| Unknown M | Cyanide, carbon monoxide | HT, VD, ALI, RD, CNS, AG | Yes | Yes | Hydroxocobalamin, vasopressors, hyperbaric oxygen, intubation, IV fluids |
| 66F | Fentanyl, hydromorphone | BP, RD, CNS, HGY, MA | No | No | Naloxone, glucose, opioids, intubation, IV fluids |
| 77M | Metoprolol, amlodipine | HT, BC, AVB, QRS, ALI, CNS, MA, HPT | Yes | No | Atropine, calcium, glucagon, insulin-euglycemic therapy, vasopressors, glucose, neuromuscular blockers, intubation, IV fluids |
| 53F | Insulin, zolpidem, venlafaxine | AP, CNS | Yes | Yes | Glucagon, naloxone, glucose, intubation, IV fluids |
| 56M | Cocaine, opioid unspecified | MI, AP, RD, CNS, AKI, RBM | Yes | No | Antihypertensives, benzodiazepines, glucose, opioids, intubation, IV fluids |
| 53F | Amlodipine, toluene, doxepin, carisoprodol, clonazepam | HT, BC, VD, QTc, QRS, RD, CNS, RFX, HGY | No | No | Calcium, methylene blue, naloxone, NaHCO3, methylene blue, naloxone, NaHCO3, thiamine, vasopressors, antiarrhythmics, benzodiazepines, glucose, steroids, intubation, IV fluids |
| 41F | Iron, diphenhydramine | HT, TC, BP, VD, ALI, CNS, MA, AG, HPT | No | No | NaHCO3, deferoxamine, vasopressors, hemodialysis, intubation, IV fluids |
| 21F | Hydroxyzine, fluoxetine, melatonin | HT, BP, QTc, RD, CNS, MA, AG, HPT, CPT, AKI, RBM | No | No | NAC, naloxone, vasopressors, CPR, cardioversion, intubation, IV fluids, therapeutic hypothermia |
| 43F | Heroin, cocaine | VD, RD, CNS | Yes | Yes | NaHCO3, vasopressors, intubation, IV fluids |
| 14M | Bupropion, fluoxetine, meclizine | HT, TC, BC, BP, VD, QTc, AP, AGT, CNS, SZ, RFX, MA, RBM | Yes | No | Lipid resuscitation, vasopressors, antiarrhythmics, anticonvulsants, benzodiazepines, neuromuscular blockers, opioids, CPR, ECMO, intubation |
| 57M | Acetaminophen, metformin | HT, CNS, SZ, HGY, MA, AG, HPT, CPT, WBC, AKI, RBM | No | No | NAC, vitamin K, vasopressors, benzodiazepines, steroids, continuous renal replacement, intubation, transfusion |
| 64M | Cyclobenzaprine, gabapentin, acetaminophen, codeine | CNS, AG | Yes | Yes | Naloxone, physostigmine |
| 53M | Metformin, atenolol | HTN, HT, QTc, QRS, MI, MA, AG, HPT, AKI, RBM | No | No | Calcium, lipid resuscitation, NaHCO3, vasopressors, activated charcoal, hemodialysis, intubation, IV fluids |
Based on response from medical toxicologist “Did the patient have a toxicological exposure?” equals yes, with known agent(s)
AG anion gap, AGT agitation, AKI acute kidney injury, ALI acute lung injury/ARDS, AP aspiration pneumonia, AVB AV block, BC bradycardia, BP bradypnea, CNS coma/CNS depression, CPT coagulopathy, CRV corrosive injury, DLM delirium, EPS dystonia/rigidity, GIB GI bleeding, HCN hallucinations, HGY hypoglycemia, HPT hepatoxicity, HT hypotension, HTN hypertension, HYS hemolysis, HYT hyperthermia, INT intestinal ischemia, MA metabolic acidosis, MET methemoglobinemia, MI myocardial injury/ischemia, NP neuropathy, OG osmole gap, OTH1 rash, OTH2 skin blisters, necrosis, PCT pancytopenia, PLT thrombocytopenia, PNC pancreatitis, PST paresthesia, QRS QRS prolongation, QTc QTc prolongation, RAD asthma/reactive airway disease, RBM rhabdomyolysis, RD respiratory depression, RFX hyperreflexia/tremor, SZ seizures, TC tachycardia, VD ventricular dysrhythmia, WBC leukocytosis, WKN weakness/paralysis, BAL dimercaprol, CPR cardiopulmonary resuscitation, ECMO extra-corporeal membrane oxygenation, NAC n-acetyl cysteine, NaHCO 3 sodium bicarbonate
aAge in years unless otherwise stated
bPharmcological and non-pharmacological support as reported by medical toxicologist
The number of fatalities increased again in 2016 to 126, along with an increased percentage of cases reporting a fatality (1.5%), up from 98 fatalities (1.2%) in 2015, and 89 fatalities (1.0%) in 2014 [2, 7]. Of these 126 fatalities, 63 (0.7%) involved single agent exposures and 35 (0.4%) cases involved multiple agent exposures. The remaining 28 (0.3%) fatalities were categorized as not a toxicological exposure, or unknown with respect to toxicity and presentation and are presented in Table S18.
Six fatalities (four females, two males) involved pediatric (age 0–18 year) patients. All six were adolescents, with ages ranging from 14 to 17 years, constituting 4.8% of fatalities. Five of these were intentional pharmaceutical exposures, with some intent to self-harm, and four were reported as definitive suicide attempts. An additional case reported toxicology involvement to assist with interpretation of lab data and evaluation of malicious or criminal intent. Three pediatric fatalities involved single agents (bupropion, cocaine, and diphenhydramine) and three involved multiple agents (acetaminophen, bupropion, ethanol, fluoxetine, ibuprofen, meclizine, and quetiapine). Life support measures were withdrawn in five of the six pediatric fatality cases. The sixth case is reported as unknown whether life support was withdrawn in a 16-year-old male with an exposure to acetaminophen and ethanol, and no treatment interventions are listed. This entry was categorized as misuse or abuse of a substance by taking higher doses than recommended of an over the counter product without an attempt at self-harm. No further details were provided to describe the lack of interventions or treatments recorded such as pronounced on arrival, or family or religious preference.
Among the single agent fatalities (all ages), there were four deaths attributed to ethanol; of these, three were intubated and mechanically ventilated and received either continuous renal replacement or hemodialysis. Eight single agent fatalities (age range 21–51 years) were attributed to heroin and one to fentanyl (a 19-year-old). Of the multiple agent fatalities, six involved heroin and three involved fentanyl. Fourteen fatalities overall were single agent exposures to opioids, while 15 multiple agent fatalities involved one or more opioids including 4 cases involving a combination of cocaine with heroin or other opioid.
Life support measures were withdrawn in 60 (61.2%) of the fatalities related to a toxicologic exposure (single or multi-agent), with brain death confirmed in 26 (43.3%). The 60 patients ranged in age from 14 to 80 years (including 5 pediatric patients), with two patients of unknown age. The mean and median ages for those with life support withdrawn were 44.6 and 43.5 years, respectively, with a sex distribution of 55.0% male and 45.0% female.
Adverse Drug Reactions
Table 34 presents information on adverse drug reactions (ADRs). In 2016, there were 320 cases (3.8% of Registry cases) that were categorized as ADRs, defined as any unintended response to a medication as used in standard therapeutic dosing. There were 455 total agent entries with 164 unique agents. Some cases reported multiple agents, possibly reflecting drug-drug interactions. The strength of association between the reported agent and the clinical presentation was recorded as definitive by rechallenge in 4.7% of cases, probable in 72.8%, and possible in 22.5%. The most frequently recorded drugs in ADR cases in 2016 were lithium (11.9%) and digoxin (5.9%).
Table 34.
ToxIC 2016—most common drugs associated with ADRs
| N (%)a | |
|---|---|
| Lithium | 38 (11.9) |
| Digoxin | 19 (5.9) |
| Haloperidol | 14 (4.4) |
| Metformin | 13 (4.1) |
| Metoprolol | 12 (3.8) |
| Phenytoin | 12 (3.8) |
| Valproic acid | 12 (3.8) |
| Acetaminophen | 9 (2.8) |
| Olanzapine | 9 (2.8) |
| Sertraline | 8 (2.5) |
ADRs adverse drug reactions
aPercentages are calculated out of the total number of cases reporting an ADR (N = 320)
Treatment
In 2016, there were 3047 cases (35.7% of total Registry cases) with at least one antidote administered, and 3540 antidotes given overall (Table 35). Similar to prior years, N-acetylcysteine was the most common, accounting for more than one quarter of antidotes given in 2016 (27.5%), followed by naloxone/nalmefene (19.9%), and sodium bicarbonate (11.9%). In 2016, 2.7% of Registry cases reported antivenom therapy, with the vast majority of these (96.1%) receiving Crotalidae polyvalent immune fab (ovine), again reflecting the presence of the North American Snake Bite Registry within the overall Registry (Table 36).
Table 35.
Antidotal therapy administered in ToxIC in 2016
| N (%)a | |
|---|---|
| N-Acetylcysteine | 974 (27.5) |
| Naloxone/nalmefene | 705 (19.9) |
| Sodium bicarbonate | 421 (11.9) |
| Thiamine | 250 (7.1) |
| Folate | 201 (5.7) |
| Physostigmine | 147 (4.2) |
| Fomepizole | 119 (3.4) |
| Calcium | 110 (3.1) |
| Glucagon | 98 (2.8) |
| Flumazenil | 63 (1.8) |
| Cyproheptadine | 58 (1.6) |
| Vitamin K | 51 (1.4) |
| Atropine | 47 (1.3) |
| L-Carnitine | 44 (1.3) |
| Insulin-euglycemic therapy | 44 (1.3) |
| Octreotide | 44 (1.3) |
| Fab for digoxin | 34 (1.0) |
| Lipid resuscitation | 25 (0.8) |
| Pyridoxine | 26 (0.7) |
| Hydroxocobalamin | 17 (0.5) |
| Methylene blue | 14 (0.4) |
| Dantrolene | 9 (0.3) |
| 2-PAM | 7 (0.2) |
| Bromocriptine | 6 (0.2) |
| Thiosulfate | 6 (0.2) |
| Anticoagulation reversal | 5 (0.1) |
| Botulinum antitoxin | 3 (0.1) |
| Ethanol | 2 (0.1) |
| Nitrites | 2 (0.1) |
| Coagulation factor replacement | 1 (<0.1) |
| Protamine | 1 (<0.1) |
| Total | 3540 (100) |
aPercentages are out of the total number of antidotes administered (N = 3540); 3047 cases (35.7% of total Registry cases) received at least one antidote; some cases involve multiple antidotes
Table 36.
Antivenom therapy administered in ToxIC in 2016
| N (%)a | |
|---|---|
| Crotalidae polyvalent immune fab (ovine) | 222 (96.1) |
| Other snake antivenom | 5 (2.2) |
| Scorpion antivenom | 3 (1.3) |
| Spider antivenom | 1 (0.4) |
| Total | 231 (100) |
aPercentages are out of the total number of antivenom treatments administered (N = 231)
Tables 37 and 38, respectively, summarize the pharmacological and non-pharmacological supportive care intervention frequencies in 2016. A total of 3830 pharmacological and 4522 non-pharmacological supportive care interventions were reported. There were 2741 cases (32.1% of total Registry cases) reporting at least one form of pharmacologic treatment and 3508 cases (41.1% of total Registry cases) reporting at least one non-pharmacological intervention. Some cases involved more than one form of treatment. Similar to past years, benzodiazepines were used in half of the pharmacological interventions, followed by opioids (10.8%), vasopressors (10.3%), and antipsychotics (7.2%). Of the non-pharmacological interventions, intravenous fluid resuscitation was the most common (70.3%), followed by intubation and ventilatory management (25.1%).
Table 37.
Supportive care interventions administered in ToxIC in 2016—pharmacological
| N (%)a | |
|---|---|
| Benzodiazepines | 1917 (50.1) |
| Opioids | 415 (10.8) |
| Vasopressors | 393 (10.3) |
| Antipsychotics | 277 (7.2) |
| Neuromuscular blockers | 172 (4.5) |
| Antihypertensives | 160 (4.2) |
| Anticonvulsants | 140 (3.7) |
| Glucose (concentration > 5%) | 134 (3.5) |
| Corticosteroids | 70 (1.8) |
| Albuterol (or other bronchodilator) | 66 (1.7) |
| Antiarrhythmics | 49 (1.3) |
| Beta blockers | 27 (0.7) |
| Vasodilators | 10 (0.3) |
| Total | 3830 (100) |
aPercentages are out of the total number of treatments administered (3830); 2741 Registry cases (31.8%) received at least one form of pharmacological treatment; cases may have involved multiple forms of treatment
Table 38.
Supportive care interventions administered in ToxIC in 2016—non-pharmacological
| N (%)a | |
|---|---|
| IV fluid resuscitation | 3179 (70.3) |
| Intubation/ventilatory management | 1133 (25.1) |
| CPR | 81 (1.8) |
| Transfusion | 41 (1.0) |
| Hyperbaric oxygen | 19 (0.4) |
| Cardioversion | 18 (0.4) |
| Therapeutic hypothermia | 18 (0.4) |
| Pacemaker | 16 (0.4) |
| ECMO | 11 (0.2) |
| Aortic balloon pump | 1 (0.02) |
| Organ transplantation | 1 (0.02) |
| Total | 4522 (100) |
CPR cardiopulmonary resuscitation, ECMO extracorporeal membrane oxygenation
aPercentages are out of the total number of treatments administered (N = 4522); 3508 cases (41.1% of total Registry cases) received at least one form of non-pharmacological treatment; cases may have involved multiple forms of treatment
Table 39 summarizes the 31 chelation therapy interventions, which accounted for 0.4% of total Registry cases in 2016. Deferoxamine and dimercaptosuccinic acid (DMSA) were reported in equal numbers.
Table 39.
Chelation therapy administered in ToxIC in 2016
| N (%)a | |
|---|---|
| Deferoxamine | 11 (35.5) |
| DMSA | 11 (35.5) |
| Dimercaprol | 4 (12.9) |
| EDTA | 4 (12.9) |
| Pencillamine | 1 (3.2) |
| Total | 31 (100) |
DMSA dimercaptosuccinic acid, EDTA ethylenediamine-tetraacetic acid
aPercentages are out of the total number of chelation treatments administered (N = 31); 28 cases (0.3% of total Registry cases) received at least one form of chelation treatment
Table 40 summarizes the 334 decontamination procedures reported in the Registry in 2016. There were 318 cases (3.7% of total Registry cases) in which at least one decontamination procedure was performed. Activated charcoal was most common (79.3%), followed by whole bowel irrigation (9.9%), and external irrigation (8.7%). Gastric lavage was uncommon with only seven cases (2.1%) reported.
Table 40.
Decontamination interventions administered in ToxIC in 2016
| N (%)a | |
|---|---|
| Activated charcoal | 265 (79.3) |
| Whole bowel irrigation | 33 (9.9) |
| External irrigation | 29 (8.7) |
| Gastric lavage | 7 (2.1) |
| Total | 334 (100) |
aPercentages are out of the total number of treatments administered (N = 334); 318 cases (3.7% of Registry total) received at least one form of decontamination
Table 41 reports enhanced elimination procedures. Enhanced elimination was performed in 280 cases (3.3% of total cases), with some cases reporting more than one, for a total of 321 reported treatments. Hemodialysis for toxin removal (32.1%) and urinary alkalinization (29.3%) were the most common. Hemodialysis for other indications comprised an additional 19.0% of enhanced elimination procedures.
Table 41.
Enhanced elimination interventions administered in ToxIC in 2016
| N (%)a | |
|---|---|
| Hemodialysis (toxin removal) | 103 (32.1) |
| Urinary alkalinization | 94 (29.3) |
| Hemodialysis (other indication) | 61 (19.0) |
| Continuous renal replacement therapy | 55 (17.1) |
| Multiple-dose activated charcoal | 6 (1.9) |
| Exchange Transfusion | 2 (0.6) |
| Total | 321 (100) |
aPercentages are out of the total number of treatments administered (N = 321); 280 cases (3.3% of total Registry cases) received at least one form of enhanced elimination
Discussion
This report summarizes the seventh year of data collected in ACMT’s ToxIC Case Registry. Although the Registry is not population based, it is a large source of information that can be used in conjunction with data from health agencies, poison centers, and other sources to produce a more detailed picture of poisoning trends and their public health implications. Novel exposure surveillance data from the Registry is not reported in this Annual Report, but is being analyzed, with results to be published separately. The most common agent classes, types of encounters, toxidromes, and treatments were similar to prior years. Some broad trends, both within the Registry itself and within the larger national context, are discussed.
Opioids
Opioid fatalities reported in the ToxIC Registry over the past few years mirror the national trends of steadily increasing age-adjusted death rates (per 100,000 population) from heroin and synthetic opioids other than methadone (e.g., fentanyl, tramadol) according to National Vital Statistics System data [12, 13]. In 2016, heroin-related deaths in ToxIC were the highest reported since the Registry’s inception, increasing both in absolute number and in percentage of all fatalities cases—11.1% of fatalities in 2016 versus 4.1% in 2015 and 6.7% in 2014 [2, 7]. Fentanyl-related deaths also increased in 2016 with four reported cases (3.2%), up from 0.0% in 2015 and 1.1% in 2014 [2, 7]. The total number of opioid-related fatalities (all agents) in ToxIC increased to 29 cases (23.0% of total fatalities) in 2016, compared to 14.2% in 2015 and 18.0% in 2014 [2, 7].
Deaths related to illicit street opioid use may be more complicated to ascribe definitively to heroin or to fentanyl and newer fentanyl derivatives since testing for these agents is not routinely conducted. Thus, it is possible that some of the deaths attributed to heroin in the ToxIC Registry may be related to newer synthetic opioids. Of note, the number of ToxIC cases (fatality and non-fatality) involving an “unspecified opioid” increased to 8.1% of total opioid cases in 2016, compared to 6.5, 4.1, and 0.6% in 2015, 2014, and 2013, respectively [2, 7, 8]. In addition, two fatalities were attributed to unspecified opioids as single agents and one to unspecified opioids with cocaine in 2016, in contrast to zero deaths from unspecified opioids in 2014 and 2015 [2, 7]. One possible explanation for the rise in unspecified opioid case reporting may be the difficulty in clinically identifying the specific illicit opioid responsible.
The increase in ToxIC opioid cases (both fatal and non-fatal) in 2016 likewise reflects the national opioid crisis. The percentage of opioid cases vs. total Registry cases increased from 8.8% in 2015 to 9.8% in 2016 [2]. As in 2015, heroin, oxycodone, and methadone were the most commonly reported opioids in 2016, each up slightly from 2015 in terms of absolute numbers and percent of total opioid cases [2]. In addition, several designer opioids—3-methylfentanyl, remifentanil, and U-47700—were reported for the first time in ToxIC in 2016. Of all the opioids reported, two decreased notably from 2015 to 2016: buprenorphine, down to 4.2% from 7.5% of opioid cases in 2015, and tramadol, down to 7.8% from 10.5% of opioid cases in 2015 [2].
Benzodiazepines
Benzodiazepines were again the most commonly reported type of agent in the sedative-hypnotics/muscle relaxants class in 2016. For the first time since the start of the Registry, alprazolam was the most commonly reported benzodiazepine with 276 cases (20.6% of the sedative-hypnotics/muscle relaxants agent class). Clonazepam had previously been the most commonly reported, but was second this year with 207 cases (15.5% of the class). In 2016, the mean age reported for alprazolam ingestions was 33.4 years with 50.7% male. For clonazepam, the mean age reported was 34.5 years with 38.2% males. Lorazepam, the third most common benzodiazepine, had a mean age of 35.3 years in reported cases with 31.3% males. Coingestants were common in benzodiazepine exposures, being reported in 191 (69.2%) alprazolam cases, 165 (79.7%) clonazepam cases, and 76 (76.8%) lorazepam cases. Opioids were commonly reported coingestants for all three benzodiazepines. Eighty-five (30.8%) of alprazolam exposures involved at least one opioid. Cases could involve multiple opioid coingestants. Heroin was the most common opioid reported in alprazolam exposures with 25 cases (9.1%), followed by oxycodone 20 cases (7.2%) and methadone 11 cases (4.0%). Forty-three (20.8%) of the clonazepam exposures involved at least one opioid. As with alprazolam, cases could involve multiple opioid coingestants. Among clonazepam exposures, oxycodone was most commonly reported with 16 cases (7.7%), followed by heroin with 8 cases (3.9%) and hydrocodone with 6 cases (2.9%). Lorazepam exposures involved an opioid in only seven cases (7.1%).
Laundry Detergent Pods
Liquid laundry detergent pods have been responsible for a number of pediatric poisonings, both mild and severe, in the USA and several European countries, with young children (i.e., age 0–6 years) disproportionately affected [14–24]. In response to public concern and warnings from the US Consumer Product Safety Commission (CPSC), the American Association of Poison Control Centers (AAPCC), and others, several manufacturers (including P&G, responsible for 80% of the share of the US market) recently agreed to adopt a new safety standard for detergent pod packaging released in 2015 by the American Society of Testing and Materials [25–28]. Among other things, the standard mandates using opaque instead of clear packaging, adding a bittering agent to the outer membrane, and other features designed to make the pods less attractive to young children [27, 28].
Although it is unclear whether or not these changes have succeeded in reducing accidental pediatric ingestions, the early numbers are promising. Detergent pod cases reported annually to AAPCC/US poison control centers declined in absolute number from 2015 to 2016 (12,616 vs. 11,545 cases, respectively), and monthly case numbers reported in the first half of 2017 were consistently lower than the corresponding months of 2015 and 2016 [26]. Similarly, since the first detergent pod cases were recorded in the ToxIC Registry in 2012, cases involving young children (i.e., age 0–6 years) have declined both in absolute and relative terms, from a peak in 2013 of 32 cases (4.2% of total cases age 0–6 years) to 9 cases (1.4% of total cases age 0–6 years) in 2016 [8, 9]. In 2016, one ToxIC pediatric case involved a 9-year-old female, and all others were age ≤3 years. Among the pediatric cases, central nervous system depression was reported in four, bradypnea with respiratory depression in one, corrosive injury in two, and reactive airway disease and aspiration pneumonitis requiring intubation in one, with no fatalities reported. It is unclear what effect the new safety standards have had on cases of laundry pod exposures as reported to the Registry, though the continued decline is reassuring.
Adverse Drug Reactions
Lithium has been the most commonly reported ADR agent in ToxIC each year since the field was added in 2014 and was responsible for 11.9% of ADRs in 2016 [2, 7]. This is out of proportion to its overall Registry representation, with only 176 total cases involving lithium reported in 2016. Quetiapine was one of the most common medications involved in ADRs in 2014 and 2015 but did not top the list in 2016, despite being the most commonly reported antipsychotic drug in the Registry [2, 7]. Instead, haloperidol and olanzapine were both among the top 10 ADR-related drugs in 2016, together comprising 7.2% of reported ADRs. Digoxin was the second most commonly reported ADR-related drug this year, despite being infrequently reported in the Registry overall; 43.2% of digoxin cases in 2016 were categorized as ADRs. In 2016 acetaminophen was also one of the most common agents associated with an ADR, although ADRs were only implicated in 0.9% of all acetaminophen cases in the Registry. In five ADR cases the outcomes were fatal, with the strength of the association between the exposure and clinical presentation reported as probable in all five. Among those five ADRs which had a fatal outcome, the ages ranged from 41 to 76. Two of the cases were also recorded as medication errors.
Limitations
ToxIC contains data as reported by medical toxicologists treating patients at the bedside, with reliable associations between clinical signs and symptoms and the toxic agents responsible. Nonetheless, there are some inherent limitations of the Registry from a descriptive epidemiological perspective, as well as structural and data quality limitations that continue to be addressed through an ongoing quality improvement process. One inherent limitation is that the central case inclusion criterion—consultation by a medical toxicologist—likely creates a reporting bias toward more severe or unusual cases. At the site level, bias may be introduced through either the decision to consult and/or by the reporting medical toxicologist her/himself. We attempt to control this bias through written agreements with the participating sites that every case seen by the medical toxicologist will be logged in the Registry. In addition, ToxIC cases represent those presenting for clinical care, thus are likely biased toward severe/unusual exposures compared to sources such as Poison Control Centers.
Another inherent bias is the limited number of medical toxicologists in practice, currently estimated to be only a few hundred nationwide. This restricts the Registry cases to those geographic areas with actively practicing medical toxicologists, who have also agreed to ToxIC’s participation rules and guidelines. Indeed, the Registry has grown to include sites from 22 states across the country and thus includes a large, diverse collection of cases and practice patterns, which for some agents/exposures may reasonably be considered representative of national trends. But some areas, such as the southeastern and western USA, are underrepresented in ToxIC compared to others, so trends unique to those areas may not be fully reflected in the database. Limited case entries required that two of the international sites not be included in the Registry in 2016, thus limiting the scope of data collected largely to the USA.
In 2014, centralized, ongoing quality assurance procedures were initiated, designed to improve the accuracy of data entry and coding, and to minimize the number of missing entries. Year 2016 is also the second complete year of data collection since several data fields were made mandatory including include race, ethnicity, reason for encounter, presence of suicidal intent, signs and symptoms, treatments, other interventions, and fatalities. Prior to this, it was unknown if items left blank were not applicable or simply not recorded. This improvement has nearly eliminated missing responses in these fields, and future research will benefit from the improved clarity of responses.
Despite the fact that the race and ethnicity questions were made mandatory in 2014, approximately one quarter of cases were reported as unknown/uncertain race and/or ethnicity in both 2015 and 2016. These data are not self-reported and may not routinely be reflected in patient charts, requiring the examiner to specifically recall this information. For some participating clinicians, race and ethnicity may not be a routine part of their information gathering. Additionally, with the Registry’s potential bias toward more complicated and critically ill exposures, there may be a larger than expected number of patients unable to report this information to the examiner. Quality improvement efforts continue, with a goal of minimizing the number of cases reported as unknown or uncertain race and ethnicity.
Significant physical examination findings, vital sign abnormalities, supportive interventions, and some clinical laboratory data are collected; however, the severity of illness is not directly recorded. Although the completeness of the clinical data has improved since creating the mandatory fields, there remain some cases, such as fatalities, where multiple interventions might be expected but are not recorded. In addition, the timeline of events and cause of death are not described. Multiple agents may be reported, but unless free text fields are used, it may be unclear to what degree each agent contributed.
Additional limitations include those inherent in any database. Each case is unique, and some details may be too complicated to be adequately described in a series of programmed data fields. Free text fields are available to enter additional information at the medical toxicologist’s discretion, but the free text fields are not easily searchable and must be reviewed manually.
Conclusions
Since its inception in 2010, the ToxIC Registry has continued to grow and is the only database of its kind logging all cases encountered by participating medical toxicologists. While agent frequencies may vary from year to year, and new trends may emerge, overall the most common exposures, toxidromes, clinical abnormalities, and antidotes recorded in ToxIC represent the routine practice of the subspecialty of medical toxicology. It is thus potentially useful as an instructional tool for trainees in toxicology, valuable for toxicosurveillance and research, and as a resource for the practice of medical toxicologists.
Electronic supplementary material
(DOCX 28 kb)
(DOCX 53 kb)
(DOCX 42 kb)
(DOCX 45 kb)
(DOCX 44 kb)
(DOCX 40 kb)
(DOCX 45 kb)
(DOCX 31 kb)
(DOCX 34 kb)
(DOCX 50 kb)
(DOCX 34 kb)
(DOCX 34 kb)
(DOCX 30 kb)
(DOCX 30 kb)
(DOCX 28 kb)
(DOCX 30 kb)
(DOCX 28 kb)
(DOCX 77 kb)
Acknowledgments
Toxicology Investigators Consortium (ToxIC) Study Group Collaborators:
Aks SE, Algren DA, Alwasiyah D, Beauchamp G, Bentur Y, Beuhler MC, Boyle KL, Bruccoleri RE, Burns, MM, Cahana A, Cannon RD, Caravati EM, Carey JL, Chhabra N, Chomin J, Christian MR, Conner K, Cook MD, Cumpston KL, Dissanayake V, Dribben WH, Eisenga BH, Engebretsen KM, Falkowitz D, Farkas A, Fenton A, Froberg BA, Furmaga JF, Ganetsky M, Garlich F, Geib AJ, Gorodetsky R, Greene S, Greller HA, Gummin DD, Hart K, Hendrickson RG, Hernandez S, Hoyte CO, Judge BS, Kazzi Z, Kerns W, Kessler BD, King J, Kirschner R, Kleinschmidt KC, Kostic MA, Kusin S, Leikin, JB,LeRoy JM, Levine M, Lo CY, Lowry JA, Lung D, Lurie Y, Maddry J, Majlesi N, Manini AF, Marlin MB, McKay C, McKeever RG, McKeown NJ, Meggs WJ, Miller SN, Minns A, Moore E, Morgan BW, Mullins ME, Nappe TM, Nogar JN, Oakley E, Olmedo R, Othong R, Parker-Cote J,Regina A, Riley BD, Rowden A, Ruha AM, Rusyniak DE, Schult, R, Schwarz ES, Seifert SA, Sessions D, Shulman J, Smolinske SC, Smollin C, Spyres MB, Steck A, Stellpflug SJ, Sullivan R, Toce MS, Troendle MM, Vearrier D, Warrick BJ, Watts DC, Wills BK, Wolk BJ, Zosel AE.
We also wish to thank study coordinators AB Adefeso, Mary Connie Aubin, Anita Kurt, Julie Licata, Misti Marshall, Maureen Morgan, Tammy Phan, Andrea Ramirez, and Melissa VandenBerg.
Compliance with Ethical Standards
Funding
ToxIC was supported by a continuation of a grant from the US National Institutes of Health on cardiovascular drug toxicity, a new contract with the US Food and Drug Administration, and the continuation of unrestricted grant support from BTG International, which was used to support the North American Snakebite Registry.
Conflicts of Interest
None
Previous Presentation of Data
None
Funding Sources
This study received funding from the NIH National Institute on Drug Abuse 1R56DA38366, and 1R01DA037317-01, a data sharing contract with the U.S. Food and Drug Administration, and BTG International Inc.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s13181-017-0627-3) contains supplementary material, which is available to authorized users.
Contributor Information
Lynn A. Farrugia, Phone: 860-972-0001, Email: Lynn.Farrugia@hhchealth.org
On behalf of the Toxicology Investigators Consortium Study Group:
S. E. Aks, D. A. Algren, D. Alwasiyah, G. Beauchamp, Y. Bentur, M. C. Beuhler, K. L. Boyle, R. E. Bruccoleri, M. M. Burns, A. Cahana, R. D. Cannon, E. M. Caravati, J. L. Carey, N. Chhabra, J. Chomin, M. R. Christian, K. Conner, M. D. Cook, K. L. Cumpston, V. Dissanayake, W. H. Dribben, B. H. Eisenga, K. M. Engebretsen, D. Falkowitz, A. Farkas, A. Fenton, B. A. Froberg, J. F. Furmaga, M. Ganetsky, F. Garlich, A. J. Geib, R. Gorodetsky, S. Greene, H. A. Greller, D. D. Gummin, K. Hart, R. G. Hendrickson, S. Hernandez, C. O. Hoyte, B. S. Judge, Z. Kazzi, W. Kerns, B. D. Kessler, J. King, R. Kirschner, K. C. Kleinschmidt, M. A. Kostic, S. Kusin, J. B. Leikin, J. M. LeRoy, M. Levine, C. Y. Lo, J. A. Lowry, D. Lung, Y. Lurie, J. Maddry, N. Majlesi, A. F. Manini, M. B. Marlin, C. McKay, R. G. McKeever, N. J. McKeown, W. J. Meggs, S. N. Miller, A. Minns, E. Moore, B. W. Morgan, M. E. Mullins, T. M. Nappe, J. N. Nogar, E. Oakley, R. Olmedo, R. Othong, J. Parker-Cote, A. Regina, B. D. Riley, A. Rowden, A. M. Ruha, D. E. Rusyniak, R. Schult, E. S. Schwarz, S. A. Seifert, D. Sessions, J. Shulman, S. C. Smolinske, C. Smollin, M. B. Spyres, A. Steck, S. J. Stellpflug, R. Sullivan, M. S. Toce, M. M. Troendle, D. Vearrier, B. J. Warrick, D. C. Watts, B. K. Wills, B. J. Wolk, and A. E. Zosel
References
- 1.Wax PM, Kleinschmidt KC, Brent J. ACMT ToxIC Case Registry Investigators. The Toxicology Investigators Consortium (ToxIC) Registry. J Med Toxicol. 2011;7(4):259–265. doi: 10.1007/s13181-011-0177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farrugia L, Rhyee SH, Campleman SL, et al. The Toxicology Investigators Consortium Case Registry—the 2015 experience. J Med Toxicol. 2016;12(3):224–247. doi: 10.1007/s13181-016-0580-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beauchamp GA, Hendrickson RG, Hatten BW, Toxicology Investigators Consortium Endotracheal intubation for toxicologic exposures: a retrospective review of Toxicology Investigators Consortium (ToxIC) cases. J Emerg Med. 2016;51(4):382–388. doi: 10.1016/j.jemermed.2016.05.056. [DOI] [PubMed] [Google Scholar]
- 4.Judge BS, Ouellette LM, VandenBerg M, Riley BD, Wax PM, On behalf of the ACMT Toxicology Investigators Consortium (ToxIC) Utilization of observation units for the care of poisoned patients: trends from the Toxicology Investigators Consortium Case Registry. J Med Toxicol. 2016;12:111–120. doi: 10.1007/s13181-015-0498-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riederer AM, Campleman SI, Carlson RG, Toxicology Investigators Consortium (ToxIC) et al. Acute poisonings from synthetic cannabinoids—50 US Toxicology Investigators Consortium registry sites, 2010–2015. MMWR. Morb Mortal Wkly Rep. 2016;65(27):692–695. doi: 10.15585/mmwr.mm6527a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang GS, Levitan R, Wiegand TJ, et al. Extracorporeal membrane oxygenation (ECMO) for severe toxicological exposures: review of the Toxicology Investigators Consortium (ToxIC) J Med Toxicol. 2016;12(1):95–99. doi: 10.1007/s13181-015-0486-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhyee SH, Farrugia L, Campleman SL, et al. The Toxicology Investigators Consortium Case Registry—the 2014 experience. J Med Toxicol. 2015;11(4):388–409. doi: 10.1007/s13181-015-0507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rhyee SH, Farrugia L, Wiegand T, et al. The Toxicology Investigators Consortium Case Registry—the 2013 experience. J Med Toxicol. 2014;10(4):342–359. doi: 10.1007/s13181-014-0417-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wiegand T, Wax P, Smith E, et al. The Toxicology Investigators Consortium Case Registry—the 2012 experience. J Med Toxicol. 2013;9(4):380–404. doi: 10.1007/s13181-013-0352-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiegand TJ, Wax PM, Schwartz T, et al. The Toxicology Investigators Consortium Case Registry—the 2011 experience. J Med Toxicol. 2012;8(4):360–377. doi: 10.1007/s13181-012-0264-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brent J, Wax PM, Schwartz T, et al. The toxicology investigators consortium case registry—The 2010 experience. J Med Toxicol. 2011;7(4):266–276. doi: 10.1007/s13181-011-0185-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hedegaard H, Warner M, Minino AM. Drug overdose deaths in the United States, 1999-2015. NCHS Data Brief. 2017;273:1–8. [PubMed] [Google Scholar]
- 13.Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths - United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(5051):1445–1452. doi: 10.15585/mmwr.mm655051e1. [DOI] [PubMed] [Google Scholar]
- 14.Sjogren PP, Skarda DE, Park AH. Upper aerodigestive injuries from detergent ingestion in children. Laryngoscope. 2017;127(2):509–512. doi: 10.1002/lary.26184. [DOI] [PubMed] [Google Scholar]
- 15.Swain TA, McGwin G, Jr, Griffin R. Laundry pod and non-pod detergent related emergency department visits occurring in children in the USA. Inj Prev. 2016;22(6):396–399. doi: 10.1136/injuryprev-2016-041997. [DOI] [PubMed] [Google Scholar]
- 16.Stromberg PE, Burt MH, Rose SR, Cumpston KL, Emswiler MP, Wills BK. Airway compromise in children exposed to single-use laundry detergent pods: a poison center observational case series. Am J Emerg Med. 2015;33(3):349–351. doi: 10.1016/j.ajem.2014.11.044. [DOI] [PubMed] [Google Scholar]
- 17.Gray ME, West CE. Corneal injuries from liquid detergent pods. J AAPOS. 2014;18(5):494–495. doi: 10.1016/j.jaapos.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Huntington S, Heppner J, Vohra R, Mallios R, Geller RJ. Serious adverse effects from single-use detergent sacs: report from a U.S. statewide poison control system. Clin Toxicol (Phila). 2014 Mar;52(3):220–5. [DOI] [PubMed]
- 19.Valdez AL, Casavant MJ, Spiller HA, Chounthirath T, Xiang H, Smith GA. Pediatric exposure to laundry detergent pods. Pediatrics. 2014;134(6):1127–1135. doi: 10.1542/peds.2014-0057. [DOI] [PubMed] [Google Scholar]
- 20.Beuhler MC, Gala PK, Wolfe HA, Meaney PA, Henretig FM. Laundry detergent "pod" ingestions: a case series and discussion of recent literature. Pediatr Emerg Care. 2013;29(6):743–747. doi: 10.1097/PEC.0b013e318294f3db. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention (CDC). Health hazards associated with laundry detergent pods - United States, May–June 2012. MMWR Morb Mortal Wkly Rep. 2012 Oct 19;61(41):825–9. [PubMed]
- 22.Settimi L, Giordano F, Lauria L, Celentano A, Sesana F, Davanzo F. Surveillance of paediatric exposures to liquid laundry detergent pods in Italy. Inj Prev. 2017 Feb 10. pii: injuryprev-2016-042263. [DOI] [PMC free article] [PubMed]
- 23.Villa A, Médernach C, Arropetian N, Lagrange F, Langrand J, Garnier R. Exposure to liquid detergent capsules: a study of the cases reported to the Paris Poison Center, 2011–2012. Arch Pediatr. 2014;21(6):608–613. doi: 10.1016/j.arcped.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 24.Williams H, Jones S, Wood K, Scott RA, Eddleston M, Thomas SH, Thompson JP, Vale JA. Reported toxicity in 1486 liquid detergent capsule exposures to the UK National Poisons Information Service 2009-2012, including their ophthalmic and CNS effects. Clin Toxicol (Phila) 2014;52(2):136–140. doi: 10.3109/15563650.2013.855315. [DOI] [PubMed] [Google Scholar]
- 25.CPSC. 2013. CPSC and ACCC warn of poison dangers with liquid laundry packets. Release date: March 21, 2013; Available: https://www.cpsc.gov/content/cpsc-and-accc-warn-of-poison-dangers-with-liquid-laundry-packets, Accessed 18 June 2017.
- 26.AAPCC. 2017. Laundry detergent packets (unit dose liquid) Data, May 31, 2017. Available: http://www.aapcc.org/alerts/laundry-detergent-packets/, Accessed 18 June 2017.
- 27.ASTM International. New releases: new ASTM International standard will help improve safety of liquid detergent laundry packets. Release #9900, September 4, 2015. Available: http://www.astmnewsroom.org/default.aspx?pageid=3813, Accessed 18 June 2017.
- 28.P&G (Proctor & Gamble). P&G announces change to laundry pacs and taps safe kids worldwide to help promote safety among parents. June 30, 2015; Available: http://news.pg.com/press-release/pg-corporate-announcements/pg-announces-change-laundry-pacs-and-taps-safe-kids-worldwi, Accessed 18 June 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 28 kb)
(DOCX 53 kb)
(DOCX 42 kb)
(DOCX 45 kb)
(DOCX 44 kb)
(DOCX 40 kb)
(DOCX 45 kb)
(DOCX 31 kb)
(DOCX 34 kb)
(DOCX 50 kb)
(DOCX 34 kb)
(DOCX 34 kb)
(DOCX 30 kb)
(DOCX 30 kb)
(DOCX 28 kb)
(DOCX 30 kb)
(DOCX 28 kb)
(DOCX 77 kb)