Abstract
Brassinosteroids are phytohormones involved in plant development and physiological processes. Brassinosteroids Insensitive 1 (BRI1) is required for BR perception and initiation of subsequent signal transduction in Arabidopsis. In this study, the orthologue of BRI1 in the model legume species Medicago truncatula, MtBRI1, was identified and characterised. Three allelic Tnt1 insertion mutants, mtbri1-1, mtbri1-2, and mtbri1-3, were obtained from the M. truncatula Tnt1 insertion population. mtbri1 mutants displayed characteristic bri1 mutant phenotypes: extreme dwarfness, dark green curled leaves, short primary roots, less lateral roots, and insensitive to exogenous brassinolide (BL). Moreover, mtbri1 mutants show decreased total nodule number and defects in nitrogen fixation. MtBRI1 is able to complement an Arabidopsis BRI1 mutant, bri1-5. Similar to the interaction of BRI1 and BAK1 in Arabidopsis, MtBRI1 interacts with MtSERK1 in vivo. Global gene expression profiling revealed that the expression of BR biosynthesis genes and SAUR genes are significantly altered in mtbri1 mutants. MapMan analysis indicated that genes involved in signaling, hormone, cell wall, and biotic stress responses are over-represented in differentially expressed genes. Taken together, the results indicate that MtBRI1 is the BR receptor in M. truncatula and that BR signaling may play a conserved role in balancing plant growth and defenses.
Introduction
Brassinosteroids (BRs) are phytohormones that play important roles in regulating plant growth, development, and stress responses. It is crucial to adjust and maintain appropriate levels of endogenous BRs for normal plant growth and development1, 2. A combination of genetics and analytical biochemistry approaches has helped elucidate the BR biosynthetic pathways in Arabidopsis1. BR biosynthesis is regulated by two major mechanisms. First, the level of endogenous BRs is regulated by modulating the transcriptional activity of the biosynthetic genes, such as De-etiolated-2 (DET2)3, Constitutive Photomorphogenesis and Dwarfism (CPD)4, and DWARF4 (DWF4)5. Second, BRs are inactivated, resulting in reduced levels of bioactive BRs1. The BR biosynthetic pathway is subject to feedback regulation at multiple points to ensure the homeostasis of endogenous BRs6, 7. When the endogenous BR level is high, accumulated Brassinazole-Resistant 1 (BZR1) and BRI1-EMS-Suppressor 1 (BES1)/BZR2 repress the expression of BR biosynthetic genes and activate genes for BR degradation as a feedback inhibition of BR function; when the endogenous BR level is low, the expression of BR biosynthetic genes is increased7. Besides the regulation of BR biosynthesis, inactivation of BRs is also pivotal in maintaining the hormonal homeostasis. PhyB Activation-tagged Suppressor1 (BAS1) is the first yet the most important BR-inactivating gene identified8. Impairment or mutation of genes in BR biosynthesis leads to characteristic phenotypes including dwarfism, curled leaves, male sterility, and light-grown morphology in the dark1, 2.
In addition to biosynthesis, BR perception and subsequent signal transduction are also key components of BR functions in plant growth and development. Mutants of BR perception and signaling show similar phenotypes as BR biosynthesis mutants. While mutants impaired in BR biosynthesis can be rescued by application of exogenous BRs4, 9–12, BR signaling mutants are insensitive to BR treatment13, 14. With the help of genome sequencing and establishment of large scale mutant populations in model plant Arabidopsis, the BR signal transduction pathway and the downstream transcription regulatory network have been well elucidated by molecular, biochemical, genetics, and proteomics studies15–20. BR is perceived by the leucine rich repeat receptor protein kinase (LRR-RLK) BRI1 (Brassinosteroids Insensitive 1) located on the cell surface. Binding of BRs to BRI1 induces the association and transphosphorylation between BRI1 and BRI1-Associated Kinase 1 (BAK1), the co-receptor of BRs21, 22. The activated BRI1 phosphorylates BRI1 Kinase Inhibitor 1 (BKI1), resulting in the dissociation of BKI1 from BRI1 and thus from the plasma membrane23. Dissociated BKI1 phosphorylates Constitutive Differential Growth 1 (CDG1) and BR-Signaling Kinase 1 (BSK1) to promote their binding to the Phosphatase BRI1 Suppressor 1 (BSU1), which subsequently dephosphorylates BR-Insensitive 2 (BIN2) to inactivate its kinase activity24. When the BR level is low, BIN2 is phosphorylated and is in the active form. The phosphorylated BIN2 inactivates the transcription factors BZR1 and BES1/BZR2 to suppress the transcriptional activity. In contrast, when the BR level is high, BIN2 is dephosphorylated and inactivated by BSU1, such that BZR1 and BES1/BZR2 are dephosphorylated by PP2A and translocated into the nucleus to promote the BR-regulated downstream transcriptional network, thus altering the expression of genes and changing cellular events14, 25, 26. BZR1 and BES1/BZR2 share 88% overall amino acid identity and 97% identity in the DNA-binding domain. Both BZR1 and BES1/BZR2 bind to BR responsive elements (BRRE) and E-box that are present in promoters of BR responsive genes, directly or indirectly interacting with other transcription factors and functioning as transcription repressors or activators to coordinate the BR signaling, biosynthesis, and growth responses in the BR signaling pathway27–30.
BRI1 is a leucine-rich repeat (LRR) transmembrane receptor-like kinase and functions as the BR receptor. Binding of BR to the LRR domain activates BRI1 and initiates the BR-mediated signal transduction. Mutation of BRI1 in Arabidopsis leads to dwarf plants with small curled dark green leaves, photomorphogenesis in dark, insensitiveness to exogenous BL treatment, accumulation of endogenous BRs, and feedback regulation of BR biosynthesis gene expression7, 13, 31, 32. BRI1 orthologous genes have been isolated and characterised from several species, including dicots such as tomato (Lycopersicon esculentum) and pea (Pisum sativum), and monocots such as rice (Oryza sativa), barley (Hordeum vulgare) and Brachypodium distachyon. Mutations in BRI1 orthologues cause similar pleiotropic phenotypes in pea, tomato, rice and barley33–38. The similarity in both gene sequences and mutant phenotypes indicates functional conservation among BRI1 genes in different species.
To get insights of BR functions in the model legume species M. truncatula, we isolated BRI1 orthologue, MtBRI1, and obtained three independent Tnt1 insertion mutants of MtBRI1. Characterization of the mutants and the gene expression analysis indicated that MtBRI1 is the BR receptor and plays important and conserved roles in the BR signaling pathway for plant growth and defense in M. truncatula.
Results
MtBRI1 is the orthologue of BRI1 in M. truncatula
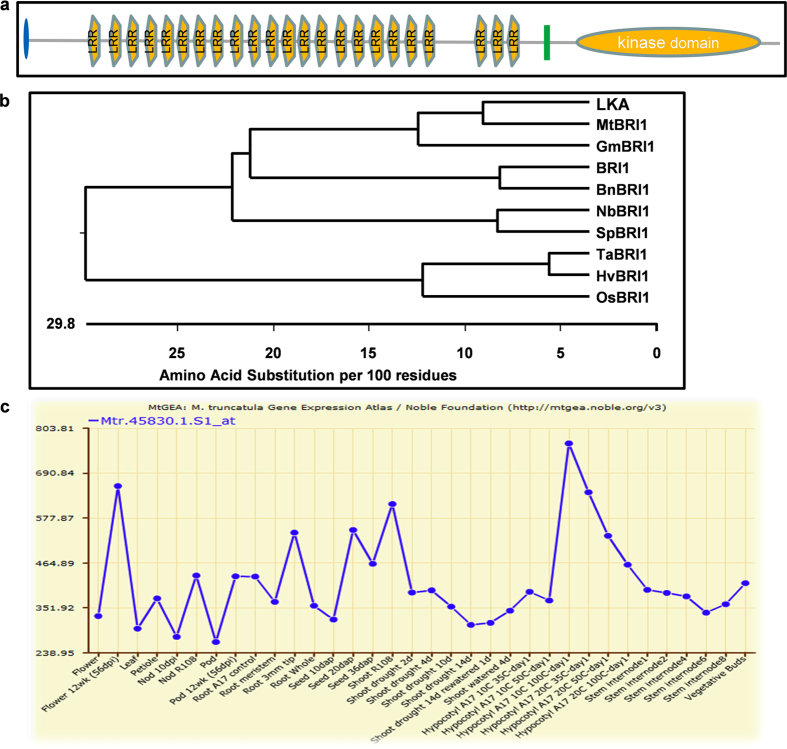
By searching the genome sequence database of M. truncatula, an LRR receptor-like protein kinase (MTR3g095100) that has high homology with Arabidopsis BRI1, was identified and designated MtBRI1. The genomic sequence of MtBRI1 is 3564 base pairs without introns and the deduced protein consists of 1188 amino acids, containing a signal peptide, 23 extracellular leucine-rich repeats, a transmembrane domain, and a cytoplasmic serine/threonine kinase domain at its C-terminus (Fig. 1a). MtBRI1 shares 67% and 88% identity at the amino acid level with Arabidopsis BRI1 and pea orthologue LKA, respectively. Phylogenetic analysis of known BRI1 proteins indicated that MtBRI1 falls into the same clade with orthologues from two other legume species pea (LKA) and soybean (GmBRI1) (Fig. 1b). According to Medicago truncatula Gene Expression Atlas (MtGEA), MtBRI1 is expressed in all tissue types (Fig. 1c).
Figure 1.
(a) MtBRI1 protein structure showing leucine-rich domains (LRR) and the kinase domain analysed in the Expysa-Prosite website.  Represents the signal peptide;
Represents the signal peptide;  represents the transmembrane domain. (b) Phylogenetic tree of MtBRI1and other known BRI proteins analysed using the MegAlign software (DNAstar). LKA, pea, AB104529.1; GmBRI1: soybean, FJ014768.1; BRI1: Arabidopsis, NM120100.2; BnBRI1: Brasssica napus, JX868513.1; NbBRI1: Nicotiana benthamiana, EF471738.1; SpBRI1: Solanum pimpinellifolium, EF471736.1; TaBRI1: Triticum aestivum, DQ655711.1; HvBRI1: Hordeum vulgare, AB088206.1; OsBRI1: Oryza sativa (Japonica), NM_001050612.1. (c) MtBRI expression patterns from the Medicago Gene Expression Atlas, which is represent by probe set Mtr.45830.1.S1_at.
represents the transmembrane domain. (b) Phylogenetic tree of MtBRI1and other known BRI proteins analysed using the MegAlign software (DNAstar). LKA, pea, AB104529.1; GmBRI1: soybean, FJ014768.1; BRI1: Arabidopsis, NM120100.2; BnBRI1: Brasssica napus, JX868513.1; NbBRI1: Nicotiana benthamiana, EF471738.1; SpBRI1: Solanum pimpinellifolium, EF471736.1; TaBRI1: Triticum aestivum, DQ655711.1; HvBRI1: Hordeum vulgare, AB088206.1; OsBRI1: Oryza sativa (Japonica), NM_001050612.1. (c) MtBRI expression patterns from the Medicago Gene Expression Atlas, which is represent by probe set Mtr.45830.1.S1_at.
Isolation of mtbri1 mutants from the Tnt1 insertion population in M. truncatula
To better understand the functions of MtBRI1, we took advantage of the M. truncatula Tnt1 insertion mutant population generated at the Samuel Roberts Noble Foundation to identify MtBRI1 mutants. Based on the MtBRI1 genomic sequence, primers were designed and PCR-based reverse screening was performed in pooled genomic DNAs from 21,000 Tnt1 insertion lines following the standard screening protocol39, 40. Three independent Tnt1 insertion mutant lines, mtbri1-1, mtbri1-2, and mtbri1-3, were identified for MtBRI1. Sequence alignment indicated that Tnt1 inserts at 709th bp, 15th bp and 468th bp downstream of the start codon in the three mutants, respectively (see Supplementary Fig. S1). Homozygosity of Tnt1 insertions was confirmed by genotyping (see Supplementary Fig. S1) and by semi-quantitative RT-PCR analysis with gene-specific primer pairs flanking Tnt1 insertion sites (see Supplementary Fig. S1). From the results of RT-PCR, no MtBRI1 transcript was detected by using the primer pairs flanking the Tnt1 insertions (see Supplementary Fig. S1).
Phenotypes of mtbri1 mutants
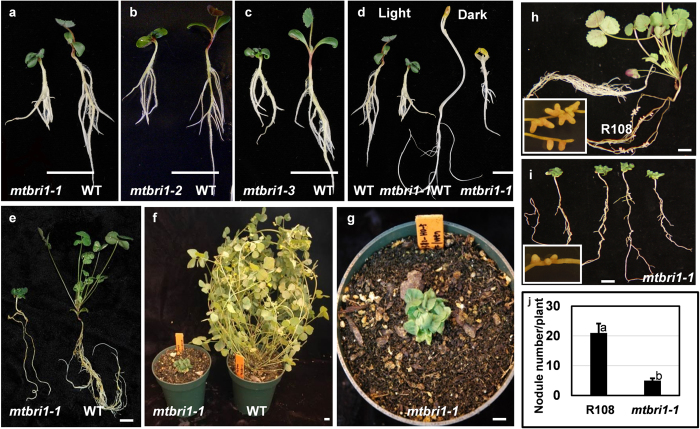
Though Tnt1 inserts are at different locations of MtBRI1, all three mtbri1 mutant alleles display similar defective phenotypes: extreme dwarfism and compact shoots with curly dark green leaves, shortened leaf blades and petioles, and reduced lateral roots (Fig. 2a–c). No elongated stems are formed (Fig. 2e,g). Flowers are rarely observed and no pods are produced in all mutants (Fig. 2f). The dark grown mtbri1 seedlings exhibit typical photomorphogenesis with short hypocotyls and open cotyledons, in contrast to the etiolated morphology with elongated hypocotyls and closed cotyledons in wild type seedlings (Fig. 2d). Interestingly, mtbri1 mutants have significantly reduced nodule numbers compared to wild type plants (Fig. 2h–j). In addition, unlike pink nodules in wild type plants, nodules in mtbri1 mutants are small and mostly white, which are defective in nitrogen fixation (Fig. 2h,i, insets). This phenotype is different from the results observed by Ferguson et al.41 in another legume species, pea. Ferguson et al. only observed reduced nodule number but not reduced nodule size and the nitrogen fixation activity in brassinosteroid responsive mutant lka. In mtbri1 mutants, both total nodule number and nodule size are reduced. The small nodules in mtbri1 mutants are also defective in nitrogen fixation.
Figure 2.
Phenotypes of mtbri1 mutants. (a–c) 8-day-old seedlings grown on ½ MS media under light. (a) mtbri1-1 & wild type (WT); (b) mtbri1-2 & WT; (c) mtbri1-3 & WT. (d) Comparison of 8-day-old seedlings between wild type and mutant seedlings grown under light or dark. (e) One-month-old mtbri1-1 and wild type plants grown in soil. (f,g) Two-month-old mtbri1-1 and wild type plants grown in soil. (h) Wild type R108 plants at 21 days post-inoculation with nodules shown in the inset. (i) mtbri1-1 plants at 21 days post-inoculation with nodules shown in the inset. (j) Nodule numbers per plant in wild type R108 and mtbri1-1. Error bars represent the standard error of the means (n ≥ 10). Means with different letters are significantly different (P < 0.005; Tukey’s test). Scale bars: 10 mm.
mtbri1 seedlings are insensitive to brassinolide and brassinazole treatment
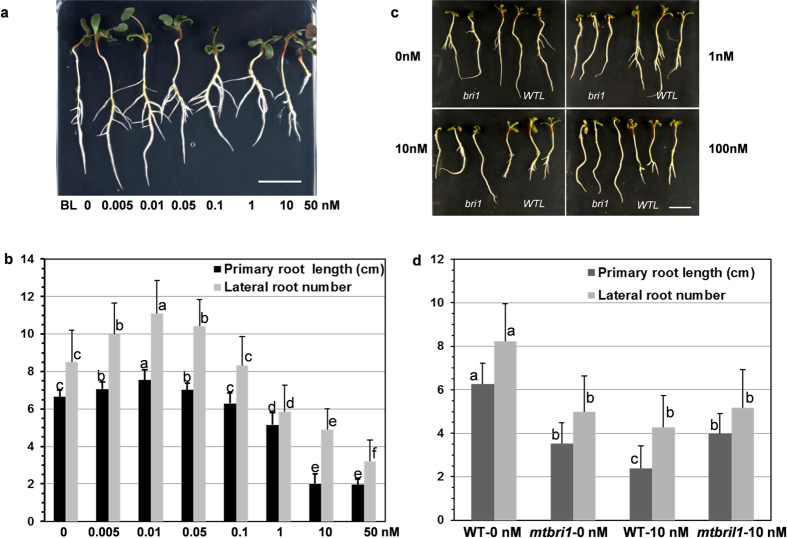
Exogenous application of certain amount of bioactive brassinolide (BL) promotes plant growth. To examine the responsiveness of wild type R108 seedlings to exogenous BL application, we grew germinated seeds on the ½ MS media supplemented with BL at a series of concentrations (0, 0.005, 0.01, 0.05, 0.1, 1.0, 10, and 50 nM) for seven days and measured the primary root length and counted lateral root numbers. Seedlings grown on media containing 0.01 to 0.05 nM of BL showed longer primary roots and more lateral roots than the control seedlings (no BL), whereas the primary root length and the lateral root number gradually decreased with further increase of BL concentrations. Seedlings grown on MS media containing 10 nM BL exhibited very short primary roots and only a few lateral roots, resembling the root phenotypes of mtbri1 mutants (Fig. 3a,b). The results indicated that BL at low concentrations (≤0.05 nM) promotes the primary root elongation and lateral root formation, whereas at high concentrations BL inhibits both primary root elongation and lateral root formation in M. truncatula.
Figure 3.
Effects of exogenous BL on the primary root length and lateral root number of wild type (WT) and mtbri1 seedlings. (a) Germinated wild type seeds were grown on ½ MS media supplemented with 0, 0.005, 0.01, 0.05, 0.1, 1.0, 10, or 50 nM BL for 7 days (one representative plant is shown for each concentration); (b) The primary root length and the lateral root number of wild type seedlings grown on the media with a series concentration of BL. Error bars represent the standard error of the means (n ≥ 20). Means with different letters are significantly different (P < 0.005; Tukey’s test). (c) mtbri1-1 and wild type-like (WTL) seedlings grown on ½ MS media supplemented with 0, 1.0, 10, or 100 nM BL for 7 days; (d) Comparison of the primary root length and lateral root number in mtbri1-1 and wild type-like seedlings grown on ½ MS media with 0 and 10 nM BL for 7 days. Error bars represent the standard error of the means (n ≥ 20). Means with different letters are significantly different (P < 0.005; Tukey’s test). Scale bars: 20 mm.
To understand how mtbri1 mutants respond to exogenous BL application, we also grew mtbri1 mutant seedlings on MS media with a series of BL concentrations. In contrast to the inhibited primary root elongation in wild type seedlings, no apparent difference was observed in both primary root length and lateral root number of mtbri1 mutants grown on various BL concentrations (Fig. 3c,d), indicating that mtbri1 mutants are insensitive to exogenous BL treatment.
Brassinazole (BRZ) is a specific inhibitor of BR biosynthesis and inhibits the hypocotyl elongation in both light and dark in Arabidopsis7, 27, 42. To confirm the responses of M. truncatula wild type and mtbri1 mutants to BRZ, we grew germinated seeds on ½ MS media containing different concentrations of BRZ in light or in the dark for five days. Similarly as observed in Arabidopsis, BRZ decreases the hypocotyl length of wild type seedlings by 60% and 56% under light and dark conditions, respectively (see Supplementary Fig. S2). The hypocotyl length of mtbri1 mutants is very short and show no significant difference with or without BRZ treatment (see Supplementary Fig. S2). In addition, under dark conditions, in contrary to the closed cotyledons of the control seedlings (no BRZ), the BRZ-treated wild type seedlings have opened cotyledons, resembling the phenotype of mtbri1 mutants (see Supplementary Fig. S2). Under both light and dark conditions, mtbri1 mutants are insensitive to the BRZ treatment (see Supplementary Fig. S2).
MtBRI1 complements Arabidopsis BRI1 mutant bri1-5
bri1-5 is a weak mutant allele of Arabidopsis BRI1, containing a point mutation resulting in a C69Y amino acid substitution in the extracellular domain of BRI131. Compared to null alleles of bri1 mutants, bri1-5 is semi-dwarf and sets nearly normal amount of seeds, making it an ideal material for BRI1 complementation studies. We transformed bri1-5 plants with the MtBRI1 cDNA and the transgenic plants showed nearly complete complementation of the bri1-5 mutant phenotypes (Fig. 4). The MtBRI1 complemented transformants resembled those of BRI1 complemented transgenic lines with elongated petioles of rosette leaves (Fig. 4a), indicating the conserved functionality of MtBRI1. We also cloned the orthologue of Arabidopsis BAK1, MtSERK1, from M. truncatula and introduced it into bri1-5 plants. The transgenic plants harboring MtSERK1 partially suppressed the bri1-5 mutant phenotypes (Fig. 4a), similar to the partial complementation of BAK1 21.
Figure 4.
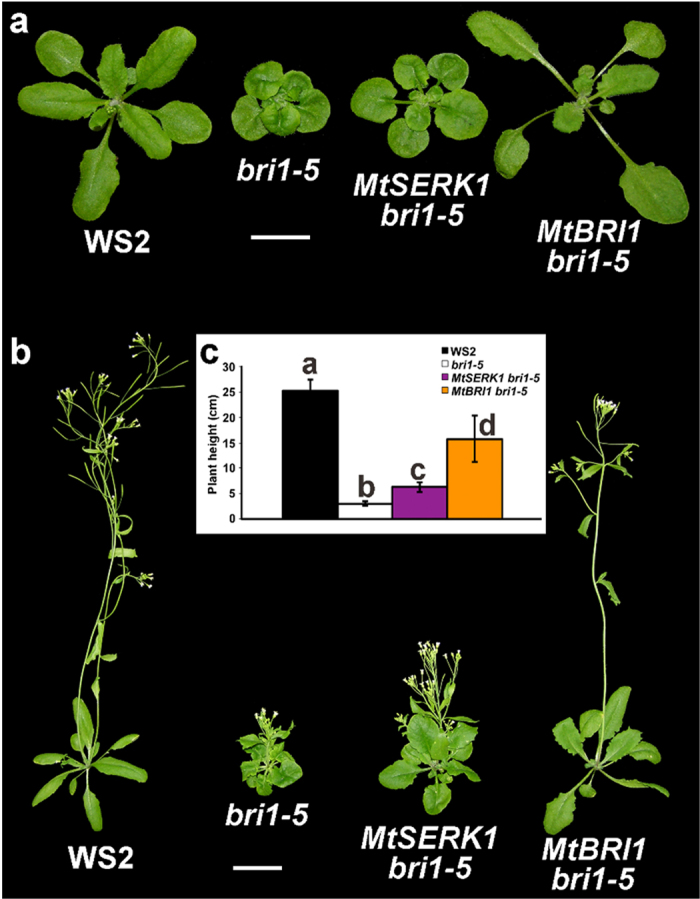
Overexpression of MtSERK1 and MtBRI1 suppresses the defective phenotypes of bri1-5. (a) 18-day-old plants. (b) 30-day-old plants. (c) Measurements of the plants shown in b. Error bars represent standard error (n > 20). Means with different letters are significantly different (P < 0.005; Tukey’s test). Scale bars: 2 cm.
MtBRI1 interacts with MtSERK1 in vivo
It has been well established that BAK1 is a co-receptor of BRs in Arabidopsis and interacts with BRI1 to initiate the BR signal transduction. To confirm whether MtBRI1 interacts with MtSERK1 in vivo, we separately transformed GFP-tagged MtBRI1 and FLAG-tagged MtSERK1 into Arabidopsis mutant bri1-5. Transformants of either MtBRI1-GFP or MtSERK1-FLAG suppresses bri1-5 phenotypes (Fig. 4a,b). Transgenic MtBRI1-GFP bri1-5 and MtSERK1-FLAG bri1-5 plants were crossed to generate a double transgenic plant harboring both MtBRI1-GFP and MtSERK1-FLAG. MtSERK1-FLAG and MtBRI1-GFP in the membrane fraction were immunoprecipitated with either agarose-linked α-FLAG antibody or α-GFP antibody. The immunoprecipitated proteins were detected by both α-FLAG antibody and α-GFP antibody (Fig. 5), indicating in vivo interaction of MtBRI1 and MtSERK1 in Arabidopsis. However, the interaction was not enhanced by BL treatment, though the phosphorylation of MtBRI1 was elevated by BL application (Fig. 5b).
Figure 5.
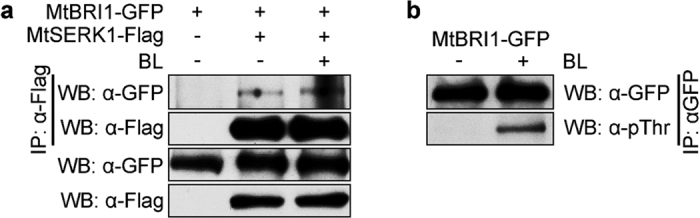
MtSERK1 interacts with MtBRI1 and MtBRI1 responds to exogenously applied BR. (a) Co-immunoprecipitation result indicates that MtSERK1 interact with MtBRI1 in vivo. (b) The phosphorylation of MtBRI1 is elevated in response to BR treatment. Blot images were cropped for better display and comparison. For the full-length western blots see Supplementary Figure S6.
Global gene expression changes in mtbri1 mutants by Affymetrix microarray and MapMan analysis
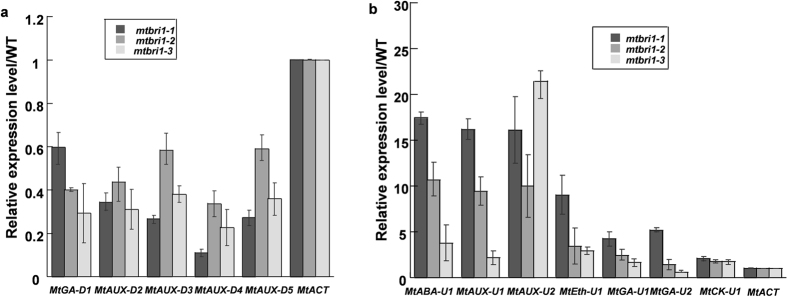
The mutation of MtBRI1 leads to dramatic pleiotropic phenotypes in M. truncatula. To further decipher the underlying molecular mechanism, we analysed the overall gene expression in 10-day-old seedlings of three mtbri1 mutants relative to the corresponding wild type-like seedlings from the same segregating progeny by Affymetrix microarray. Totally, 603 genes were up-regulated and 312 genes were down-regulated by at least 2-fold changes in all the three mutant alleles (see Supplementary Table S1). The microarray results were validated by qRT-PCR in 12 out of 15 genes related to hormone metabolism, while the other three genes were expressed at very low levels (Fig. 6).
Figure 6.
qRT-PCR verification of gene expression levels in three mtbri1 mutants. (a) Expression levels of five down-regulated genes; (b) Expression levels of seven up-regulated genes. MtACT was used as the internal control.
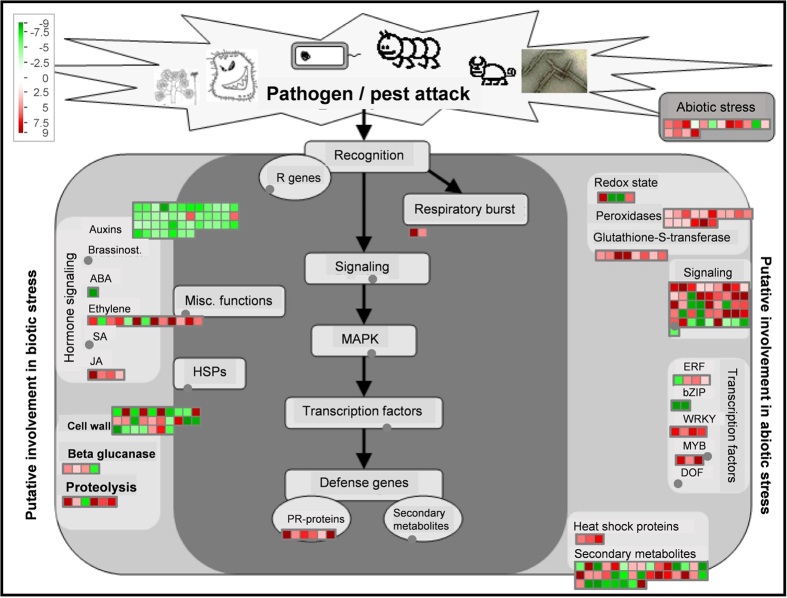
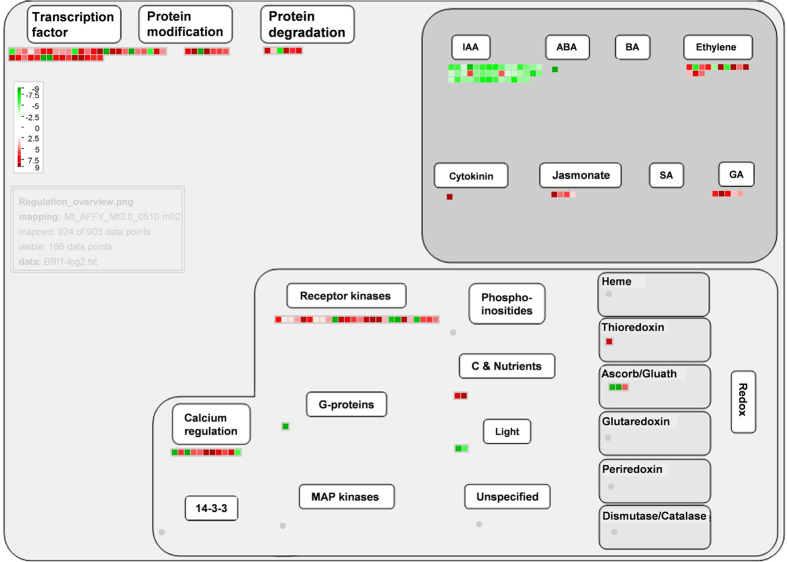
To better visualize and understand the 915 regulated genes in mtbri1 mutants, the fold-change values (≥2 or ≤0.5, P ≤ 0.05) from the microarray analysis were converted into log2 values and input to the MapMan software program. The overview pathway by MapMan analysis revealed that 903 out of the 915 regulated genes, which show 924 data points, are mapped and classified to various functional categories and half of them are grouped into unknown functions (see Supplementary Table S2). Among the functionally classified genes, except for a large group of miscellaneous, hormone metabolism, RNA regulation, stress, secondary metabolism, cell wall, and signaling genes are over-represented (see Supplementary Fig. S3). When these genes are mapped into specific pathways by MapMan, two pathways are standout: one is the biotic stress pathway, where 250 out of the 924 data points are mapped (Fig. 7); the other is the regulation pathway, where 190 points are mapped (Fig. 8). Interestingly, a group of 43 auxin-responsive genes, bin 17.2, are significantly regulated in both biotic stress and regulation pathways (Figs 7, 8). Among them, two P450 reductase genes are up-regulated but the other 41 genes, including one auxin-amino acid transferase gene GH3.3 and 40 SAUR (small auxin-upregulated RNA) genes, are down-regulated. The SAURs share more than 80% sequence identity among each other and share some similarity to SAUR14 and SAUR-AC1. Nine out of 40 SAUR genes are located between Medtr3g084150 to Medtr3g084250; 30 are clustered between Medtr4g072220.1 to Medtr4g072980.1. Based on MtGEA, most of these SAUR genes show similar expression patterns and are detectable in shoots, buds, leaves, flowers, and pods. They are highly expressed in hypocotyls but lowly expressed in roots. They all respond to drought treatments (see Supplementary Fig. S4).
Figure 7.
Visualization of differentially expressed genes mapped into the biotic stress pathway with MapMan3.5.
Figure 8.
Visualization of differentially expressed genes mapped into the regulation pathway with MapMan3.5.
Expression of BR signaling pathway genes in mtbri1 mutants
The BR signaling cascade is initiated by BRs binding to BRI1, followed by sequential phosphorylation to BR transcription factors BZR1/2. To understand the response of the gene expression to the impairment of BR signal initiation in M. truncatula, we searched the Medicago genome database and obtained the following BR signaling pathway orthologous genes in M. truncatula: MtBSK1, MtCDG1, MtBSU1, MtBIN2, MtPP2A, MtBZR1 and MtBZR2. All these genes have represented probe sets in Medicago Affychip, but none of the probe sets was present in the 2-fold up or down change list in the microarray analysis result. By searching the individual gene expression results, MtBSK1, MtCDG1, MtPP2A, MtBIN2 and MtBSU1, which are in the upstream of the signaling pathway, show the expression ratio value (mtbri1/WT) close to 1 (see Supplementary Table S3). However, the transcription regulators, MtBZR1 and MtBZR2, show changes in expression levels. MtBZR1, which is represented by 3 probe sets, is down-regulated by ~0.75 fold, whereas MtBZR2 is up-regulated by ~1.5 fold in mtbri1 mutants (see Supplementary Table S3). The microarray results were further confirmed by qRT-PCR (see Supplementary Fig. S5). In summary, mutations in MtBRI1 have no effects on the expression of most genes involved in BR signaling pathway, whereas the expression of two core transcription regulators MtBZR1/2 is regulated in opposite directions.
Regulation of BR metabolism genes in mtbri1 mutants
The BR biosynthesis pathway is subject to feedback regulation at multiple points to ensure homeostasis of endogenous BRs. To reveal the expression of BR metabolic genes in mtbri1 mutants, we searched Medicago genome database for orthologues of known genes involved in the BR biosynthetic pathway. Six biosynthetic genes that are orthologous to corresponding Arabidopsis genes, MtBrox1, MtBrox2, MtCPD, MtDWF4, MtDET2, and MtDET2-1, were retrieved. In addition, we also identified MtBAS1, the orthologue of Arabidopsis BAS1, which is involved in BR inactivation and photomorphogenesis. Microarray analysis results showed that the expression of MtDWF4 is increased by 5.6 fold; the expression of MtDET2 is slightly decreased to 0.88 fold; whereas the expression of MtBAS1 is decreased to ~0.36 fold in mtbri1 mutants (see Supplementary Table S3). No probe sets corresponding to MtBrox1, MtBrox2 and MtCPD in Medicago Affychip were found and the expression of these genes were analysed by qRT-PCR. The results showed that the expression of these three genes is increased in mtbri1 mutants. Furthermore, microarray results of MtDWF4, MtDET2, and MtBAS1 was also confirmed by qRT-PCR (see Supplementary Fig. S5). The expression pattern analysis indicated that the BR biosynthesis pathway is enhanced, whereas the BR inactivation by MtBAS1 is inhibited in mtbri1 mutants.
E-box is the potential common DNA binding domain for BR signaling targets
BZR1/2 are two core transcription factors in the BR signaling pathway and BZR1 directly or indirectly regulates the expression of about 80% of the genes downstream of BRI1. Both BZR1 and BZR2 bind to BR responsive element (BRRE) (CGTGT/CG) and E-box (CANNTG) in promoters of BR responsive genes43, 44. In this study, gene expression analysis of mtbri1 mutants revealed the genes directly or indirectly regulated by BR signaling. To get more insights of potential BR targeted genes, we scanned 1 kb genomic sequences upstream of ATG of well-known BZR1/2 target genes for BRRE and E-box elements. First, we scanned for BRRE and E-box in promoter regions of BR down-regulated SAUR genes. Two to eleven E-boxes are located in the promoter regions of all 40 SAUR genes, with most of the E-boxes inside 200 bp upstream of ATG. For three other auxin-responsive down-regulated genes, one contains two E-boxes, while the other two, including GH3.3, contains no E-box in the 1 kb promoter region. No BRRE was detected in the 1 kb promoter region of all genes examined (see Supplementary Table S4).
We further scanned for BRRE and E-box in the promoter region of up-regulated BR biosynthetic genes and other hormone related genes. Two or more E-boxes are located in the promoter region for BR biosynthetic genes. E-boxes are enriched in all auxin responsive genes. We also found E-boxes in the promoters of three gibberellin-related genes and ethylene-related genes (see Supplementary Table S4). Again, BRRE was not found in the scanned promoter regions of all examined genes, except that one BRRE motif in the MtCPD promoter. Therefore, E-boxes are enriched in the promoter regions of BR-regulated genes, and it is the potential common DNA binding element in MtBZR1/2 targets.
Discussion
In this study, we demonstrated that MtBRI1 shares high sequence and protein structure similarity with BR receptors from other plant species; mutations in MtBRI1 impair the BR signaling pathway and lead to the characteristic developmentally defective phenotypes of bri1 mutants. Moreover, mutations in MtBRI1 cause defective nodule development and nitrogen fixation, which is a novel observation in bri1 mutants. MtBRI1 not only suppresses the defects of Arabidopsis mutant bri1-5, but interacts with MtSERK1 in vivo as well. In agreement with previous microarray reports, typical BR signaling target genes, such as SAUR genes, and BR biosynthetic genes are differentially regulated in mtbri1 mutants. Over-represented hormone related genes imply that extensive crosstalk exists among different hormone pathways, which is consistent with the dramatic pleiotropic phenotypes of mtbri1 mutants. Collectively, the results indicated that MtBRI1 is a BR receptor in M. truncatula and has conserved functions as other BRI1 genes; and the BR signaling plays important roles in plant growth, development, and defense in M. truncatula.
BZR1 and BES1/BZR2 are two core transcription factors in the BR signaling pathway. Activation of either BZR1 or BES1/BZR2 by dominant mutation suppresses the phenotypes of bri1 in Arabidopsis. Microarray analysis of bzr1-1D and bri1 mutants indicated that BZR1 regulates directly or indirectly the expression of 80% of the genes downstream of BRI1 27, 28, 45, 46. BZR1 and BES1/BZR2 share 88% overall amino acid identity and 97% identity in the DNA-binding domain. Both BZR1 and BES1/BZR2 bind to BR responsive element (BRRE) and E-box present in promoters of BR responsive genes, directly or indirectly interacting with other transcription factors and functioning as transcription repressors and activators to regulate multiple developmental events17, 47. The exclusive existence of E-box, but not BRRE, in the promoter regions of both up- and down-regulated genes suggests that E-box is potentially the common DNA-binding motif for both MtBZR1 and MtBZR2 to activate or repress the expression of downstream BR signaling genes in M. truncatula. Enriched E-box motif in the promoter region of known BR regulated genes indicates that these genes are also the potential targets of MtBZR1/2. In mtbri1 mutants, the expression of MtBZR1 and MtBZR2, is altered in opposite ways. If E-box is the common DNA binding motif, MtBZR1 and MtBZR2 may have different regulatory mechanisms. The decreased expression of MtBZR1 in mtbri1 may relieve the repression of BR feedback regulation, which supports that BZR1 plays a role as a transcriptional repressor. The increased expression of MtBZR2 promotes BR signaling in mtbri1 mutants, supporting that BZR2 functions as a transcriptional activator.
In nature, plants constantly encounter changing external and internal environmental cues. It is crucial for plants to adjust and maintain the balance between growth and defense in response to diverse and complex signals. In the crosstalk between defense signaling and growth hormones, BR, salicylic acid, and auxin play important roles in the plant growth-defense tradeoff48, 49. It is suggested that BR-mediated growth directly antagonizes innate immune signaling. On one hand, the competition between BRI1 and the flagellin receptor FLS2 for the association of co-receptor BAK1 may facilitate BR-mediated suppression of PR1-mediated defense50, 51; on the other hand, BZR1 targets a group of downstream WRKY genes to inhibit PTI (pathogen-associated molecular pattern triggered immunity) and interacts with other hormone signaling transcription factors to mediate plant growth48. In mtbri1 mutants, a large number of genes potentially involved in the biotic stress pathway are differentially regulated. On the defense side, when MtBRI1 is disrupted, most defense related genes are up-regulated, including genes in defense hormone ethylene/JA pathways, such as signaling components, ERF, WKRY and MYB transcription factors, respiratory burst genes, PR proteins, and ROS reaction genes. Some putative chitinases, glucosidases and glucanases, which were reported to act synergistically to degrade fungal cell walls52, are also up-regulated. The results suggest that the entire defense system from hormone signaling, signal transduction, transcription to metabolism is enhanced in mtbri1 mutants. On the growth side, the auxin-responsive gene GH3.3 is upregulated and a group of SAUR genes are down-regulated in mtbri1 mutants. GH3 enzymes inactivate IAA by forming conjugates with amino acids. Overexpression of several GH3 genes retards plant growth. It is suggested that the GH3-mediated growth suppression directs re-allocation of metabolic resources to resistance establishment and represents the fitness costs of induced resistance48, 53. SAURs are members of a large multigene family in the Arabidopsis genome comprising of 72 members and are early auxin responsive genes with unclear functions. The expression of SAURs correlates well with both auxin-induced and BR-mediated cell elongation. It has been reported that SAUR19 has functions in cell expansion, SAUR63 promotes hypocotyl and stamen filament elongation, and AAM1 is related to apical hook development54–57. In M. truncatula, SAURs are highly expressed in elongating tissues, indicating they may also have functions in cell elongation or expansion to mediate plant growth. Combining with the dwarf phenotype, it is indicated that plant growth is compromised in mtbri1 mutants. Taken together, we concluded that the repression of plant growth caused by BR signaling deficiency triggers the plant biotic defense system and turns the plant from growth into the defense mode. Recently, it is reported that mutation of BRI enhances plant resistance to fungal and viral pathogens in both barley and B. distachyon 38, 58. However, how mtbri1 mutants respond to pathogen attack needs to be tested in future experiments.
Nodulation is a unique feature for legume species. In pea, there is no difference in nodule size and nitrogen fixation activity between wild type and BR response mutant lka. Disruption of BR signaling only reduces the nodule number. However, in M. truncatula, mutation in MtBRI1 leads to reductions in nodule number, nodule size and nitrogen fixation activity. The mechanism of MtBRI1 involvement in nodulation will be further investigated in the future. Since all the mtbri1 mutants are extremely small, weak mutant alleles from the BR signaling pathway could be better to study the function of BRI1 in legume plant development.
Material and Method
Seed treatment and seedling growth
Seeds of wild type M. truncatula R108 and mtbri1 heterozygous lines were scarified with concentrated sulfuric acid for 8 minutes and rinsed with water, followed by sterilization in 30% bleach for 10 minutes, rinse with ddH2O, germinated for 2 days in dark at room temperature, and then transferred the germinated seeds onto ½ MS medium and grown in a growth chamber with 18-h-light/25 °C and 6-h-dark/ 22 °C photoperiod. For microarray experiments, shoots of 7-day-old seedlings of wild type like (including wild type and heterozygous plants in the same segregating progeny) and mutants were sampled. After 2 weeks, seedlings were transferred into Metro-Mix 350 (Scotts) composite soil and grown in the greenhouse until maturation.
All Arabidopsis plants including WS2, Col, bri1-5, and corresponding transgenic plants were grown at 22 °C in a long-day condition (16 h of light and 8 h of dark) in a greenhouse.
Nodulation of M. truncatula plants
Germinated wild type R108 and mtbri1 seedlings were transferred to plastic cones containing a 2:1 ratio of turface/vermiculite. Plants were cultivated under a 16-h/8-h light/dark regime with 200 μE m−2 s−1 light irradiance at 21 °C and 40% relative humidity. After seven days of growth under normal nitrogen condition, plants in each cone were inoculated with 50 mL of Sinorhizobium meliloti strains Sm1021. Rhizobia S. meliloti were grown overnight in TY (tryptone yeast extract) liquid medium, with shaking at 250 rpm, to OD600 approximately 1.0, pelleted by centrifugation, and resuspended in half-strength Broughton and Dilworth (BD) solution with 0.5 mM KNO3 at OD600 approximately 0.02 for inoculation. After inoculation, plants were watered once a week with half-strength BD solution with 0.5 mM KNO3 for three weeks. At 21 days post-inoculation, plant roots were washed in water for nodule phenotyping.
PCR-based reverse screening for mtbri1 mutants in Tnt1 insertion population in M. truncatula
MtBRI1 genomic sequence was retrieved by searching the M. truncatula genome database using the Arabidopsis BRI1 sequence. Two pairs of primers (MtBRI1-F, MtBRI1-F1, MtBRI1-R and MtBRI1-R1, see sequences in Supplementary Table S5) were designed at 5′ and 3′ end of the MtBRI1 genomic sequence. PCR-based reverse screening was carried out in Tnt1 insertion population59 as described previously39, 40.
Brassinolide and brassinazole treatment
After sterilization, the seeds of wild type M. truncatula R108 and mutant lines were germinated on filter paper in dark for two days. The germinated seeds were transferred to solidified ½ MS medium supplemented with various concentrations of BL (0, 0.005, 0.01, 0.05, 0.1, 1, 10, 50, and 100 nM) or BRZ (0, 1, 5 μM). Seedlings were grown in a growth chamber with a regime of 18-h-light/25 °C and 6-h-dark/ 22 °C photoperiod for seven (for BL) or five (for BRZ) days. For BRZ treatment in dark, seedlings (wild type and mtbri1 mutant) were vertically grown on plates covered with aluminum foil for five days in the same growth chamber as for light treatment. The primary root length of the seedlings was measured and the lateral root number was counted for BL treatment. For BRZ-treated seedlings, the hypocotyl length was measured.
Complementary DNA cloning and plant transformation
The coding sequences of M. truncatula SERK1 (MtSERK1) and BRI1 (MtBRI1) were cloned with the Gateway technology for overexpression in bri1-5 and Col using primers MtBRI1PB1, MtBRI1PB2, MtSERK1PB1, and MtSERK1PB2. The amplified CDS sequences were introduced into the destination vectors pB35GWF and pH35GWG with the help of Gateway technology60. The cloned sequences were confirmed by DNA sequencing and Arabidopsis plants were transformed by the floral dip method.
Membrane protein extraction, co-immunoprecipitation and western blot analysis
Nine-day-old liquid-cultured seedlings of transgenic plants harboring 35 S::MtSERK1-FLAG, or 35 S::MtSERK1-FLAG and 35 S::MtBRI1-GFP transgenes were treated with or without 24-epiBL for 90 min and ground to fine powder in liquid nitrogen for total protein preparation. MtSERK1-FLAG was immunoprecipitated with agarose-linked α-FLAG antibody (Sigma, St. Louis, MO). For phosphorylation analysis, transgenic plants of 35 S::MtBRI1-GFP were treated with mock solution or 24-epiBL for 90 min, and ground to fine powder in liquid nitrogen for membrane protein isolation21. MtBRI1-GFP was immunoprecipitated with the α-GFP antibody (Invitrogen, Carlsbad, CA) and protein G beads (Roche, Indianapolis, IN). The immunoprecipitated proteins were separated on 7.5% SDS polyacrylamide gel for western blot analyses with α-GFP, α-FLAG or α-phosphothreonine antibodies as previously described60.
Microarray analysis and qRT-PCR
For gene expression analysis, control (wild type-like) and mutant shoot samples were harvested from 10-day-old seedlings. Three biological replicates were performed for control plants and each of the homozygous mtbri1-1, mtbri1-2 and mtbri1-3 plants. Total RNA was extracted using RNeasy Plant Mini Kit (Qiagen). Purified RNA was treated with Turbo DNase I (Ambion). For microarray analysis, 10 µg of total RNA from 3 control samples and 3 mutant samples of mtbri1-1, mtbri1-2 and mtbri1-3 were used for probe labeling. Hybridization and scanning for microarray analysis were conducted according to the manufacturer’s instructions (Affymetrix). Differentially expressed genes between wild type-like plants and homozygous mtbri1 mutants were selected using associative analysis as previously described61. For RT-PCR and qRT-PCR, three µg of total RNA of each samples were used for reverse transcription using SuperScript III Reverse Transcriptase (Invitrogen) with oligo (dT)20 primer. Two µl of 1:20 diluted cDNA were used as templates. Gene-specific primers used for RT-PCR and qRT-PCR were listed in Supplementary Table S5. All qRT-PCR was carried out using a 7900HT Fast Real-Time PCR System (Applied Biosystems) and the data were analyzed using SDS 2.2.1 software (Applied Biosystems). The transcript levels were determined by relative quantification using the M. truncatula actin gene (tentative consensus no. 107326) as the reference gene.
Electronic supplementary material
Acknowledgements
The work was supported by National Natural Science Foundation of China (31471402), and Ministry of Education (113058A) to X.G., and by the Fundamental Research Funds for the Central Universities (Grant No. lzujbky-2015-250 to H.Y.). Generation of M. truncatula Tnt1 mutants was partly funded by NSF Plant Genome Grants (DBI-0703285, IOS-1127155). We thank Dr. Yuhong Tang at Noble Research Institute, LLC for microarray analysis, Kuihua Zhang for plant care, and Ivone Torres-Jerez for plant nodulation analysis. We are grateful to Mr. Chong Hu for his technique assistance in western blot assays.
Author Contributions
X.C., X.P.G. and H.J.Y. carried out all experiments and data analysis. K.S.M. provided materials and involved in manuscript revision; J.L. and J.W. designed experiments and wrote the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Xiaofei Cheng and Xiaoping Gou contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-09297-9
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jia Li, Email: lijia@lzu.edu.cn.
Jiangqi Wen, Email: jwen@noble.org.
References
- 1.Chung Y, Choe S. The regulation of brassinosteroid biosynthesis in Arabidopsis. Crit. Rev. Plant Sci. 2013;32:396–410. doi: 10.1080/07352689.2013.797856. [DOI] [Google Scholar]
- 2.Zhao B, Li J. Regulation of brassinosteroid biosynthesis and inactivation. J. Integr. Plant Biol. 2012;54:746–759. doi: 10.1111/j.1744-7909.2012.01168.x. [DOI] [PubMed] [Google Scholar]
- 3.Li J, Nagpal P, Vitart V, McMorris TC, Chory J. A role for brassinosteroids in light-dependent development of Arabidopsis. Science. 1996;272:398–401. doi: 10.1126/science.272.5260.398. [DOI] [PubMed] [Google Scholar]
- 4.Szekeres M, et al. Brassinosteroids rescue the deficiency of CYP90, a cytochrome P450, controlling cell elongation and de-etiolation in Arabidopsis. Cell. 1996;85:171–182. doi: 10.1016/S0092-8674(00)81094-6. [DOI] [PubMed] [Google Scholar]
- 5.Choe S, et al. The DWF4 gene of Arabidopsis encodes a cytochrome P450 that mediates multiple 22alpha-hydroxylation steps in brassinosteroid biosynthesis. Plant Cell. 1998;10:231–243. doi: 10.1105/tpc.10.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noguchi T, et al. Biosynthetic pathways of brassinolide in Arabidopsis. Plant Physiol. 2000;124:201–209. doi: 10.1104/pp.124.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanaka K, et al. Brassinosteroid homeostasis in Arabidopsis is ensured by feedback expressions of multiple genes involved in its metabolism. Plant Physiol. 2005;138:1117–1125. doi: 10.1104/pp.104.058040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neff MM, et al. BAS1: A gene regulating brassinosteroid levels and light responsiveness in Arabidopsis. PNAS. 1999;96:15316–15323. doi: 10.1073/pnas.96.26.15316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathur JT, et al. Transcription of the Arabidopsis CPD gene, encoding a steroidogenic cytochrome P450, is negatively controlled by brassinosteroids. Plant J. 1998;14:593–602. doi: 10.1046/j.1365-313X.1998.00158.x. [DOI] [PubMed] [Google Scholar]
- 10.Choe S, et al. The Arabidopsis dwarf1 mutant is defective in the conversion of 2,4-methylenecholesterol to campesterol in brassinosteroid biosynthesis. Plant Physiol. 1999;119:897–907. doi: 10.1104/pp.119.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schumacher K, et al. The Arabidopsis det3 mutant reveals a central role for the vacuolar H(+)-ATPase in plant growth and development. Genes Dev. 1999;13:3259–3270. doi: 10.1101/gad.13.24.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim TW, et al. Arabidopsis CYP85A2, a cytochrome P450, mediates the Baeyer-Villiger oxidation of castasterone to brassinolide in brassinosteroid biosynthesis. Plant Cell. 2005;17:2397–2412. doi: 10.1105/tpc.105.033738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clouse SD, Langford M, McMorris TC. A brassinosteroid-insensitive mutant in Arabidopsis thaliana exhibits multiple defects in growth and development. Plant Physiol. 1996;111:671–678. doi: 10.1104/pp.111.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J, Nam KH, Vafeados D, Chory J. BIN2, a new brassinosteroid-insensitive locus in Arabidopsis. Plant Physiol. 2001;127:14–22. doi: 10.1104/pp.127.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clouse SD. Molecular genetic studies confirm the role of brassinosteroids in plant growth and development. Plant J. 1996;10:1–8. doi: 10.1046/j.1365-313X.1996.10010001.x. [DOI] [PubMed] [Google Scholar]
- 16.Ecker JR. BRI-ghtening the pathway to steroid hormone signaling events in plants. Cell. 1997;90:825–827. doi: 10.1016/S0092-8674(00)80346-3. [DOI] [PubMed] [Google Scholar]
- 17.Sun Y, et al. Integration of brassinosteroid signal transduction with the transcription network for plant growth regulation in Arabidopsis. Dev. Cell. 2010;19:765–777. doi: 10.1016/j.devcel.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang W, Deng Z, Wang ZY. Proteomics shed light on the brassinosteroid signaling mechanisms. Curr. Opin. Plant Biol. 2010;13:27–33. doi: 10.1016/j.pbi.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang C, et al. Identification of BZR1-interacting proteins as potential components of the brassinosteroid signaling pathway in Arabidopsis through tandem affinity purification. Mol. Cell. Proteomics. 2013;12:3653–3665. doi: 10.1074/mcp.M113.029256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu JY, Sae-Seaw J, Wang ZY. Brassinosteroid signalling. Development. 2013;140:1615–1620. doi: 10.1242/dev.060590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J, et al. BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell. 2002;110:213–222. doi: 10.1016/S0092-8674(02)00812-7. [DOI] [PubMed] [Google Scholar]
- 22.Nam KH, Li JM. BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell. 2002;110:203–212. doi: 10.1016/S0092-8674(02)00814-0. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Chory J. Brassinosteroids regulate dissociation of BKI1, a negative regulator of BRI1 signaling, from the plasma membrane. Science. 2006;313:1118–1122. doi: 10.1126/science.1127593. [DOI] [PubMed] [Google Scholar]
- 24.Kim TW, Guan S, Burlingame AL, Wang ZY. The CDG1 kinase mediates brassinosteroid signal transduction from BRI1 receptor kinase to BSU1 phosphatase and GSK3-like kinase BIN2. Mol. Cell. 2011;43:561–571. doi: 10.1016/j.molcel.2011.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J, Jin H. Regulation of brassinosteroid signaling. Trends Plant Sci. 2007;12:37–41. doi: 10.1016/j.tplants.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Kim TW, Wang ZY. Brassinosteroid Signal Transduction from Receptor Kinases to Transcription Factors. Ann. Rev. Plant Biol. 2010;61:681–704. doi: 10.1146/annurev.arplant.043008.092057. [DOI] [PubMed] [Google Scholar]
- 27.Yin Y, et al. BES1 accumulates in the nucleus in response to brassinosteroids to regulate gene expression and promote stem elongation. Cell. 2002;109:181–191. doi: 10.1016/S0092-8674(02)00721-3. [DOI] [PubMed] [Google Scholar]
- 28.He JX, et al. BZR1 is a transcriptional repressor with dual roles in brassinosteroid homeostasis and growth responses. Science. 2005;307:1634–1638. doi: 10.1126/science.1107580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li L, Deng XW. It runs in the family: regulation of brassinosteroid signaling by the BZR1-BES1 class of transcription factors. Trends Plant Sci. 2005;10:266–268. doi: 10.1016/j.tplants.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Vert G, Chory J. Downstream nuclear events in brassinosteroid signalling. Nature. 2006;441:96–100. doi: 10.1038/nature04681. [DOI] [PubMed] [Google Scholar]
- 31.Noguchi T, et al. Brassinosteroid-insensitive dwarf mutants of Arabidopsis accumulate brassinosteroids. Plant Physiol. 1999;121:743–752. doi: 10.1104/pp.121.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friedrichsen DM, Joazeiro CA, Li J, Hunter T, Chory J. Brassinosteroid-insensitive-1 is a ubiquitously expressed leucine-rich repeat receptor serine/threonine kinase. Plant Physiol. 2000;123:1247–1256. doi: 10.1104/pp.123.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamamuro C, et al. Loss of function of a rice brassinosteroid insensitive1 homolog prevents internode elongation and bending of the lamina joint. Plant Cell. 2000;12:1591–1606. doi: 10.1105/tpc.12.9.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montoya T, et al. Cloning the tomato curl3 gene highlights the putative dual role of the leucine-rich repeat receptor kinase tBRI1/SR160 in plant steroid hormone and peptide hormone signaling. Plant Cell. 2002;14:3163–3176. doi: 10.1105/tpc.006379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chono M, et al. A semidwarf phenotype of barley uzu results from a nucleotide substitution in the gene encoding a putative brassinosteroid receptor. Plant Physiol. 2003;133:1209–1219. doi: 10.1104/pp.103.026195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nomura T, et al. The LKA gene is a BRASSINOSTEROID INSENSITIVE 1 homolog of pea. Plant J. 2003;36:291–300. doi: 10.1046/j.1365-313X.2003.01863.x. [DOI] [PubMed] [Google Scholar]
- 37.Holton N, Harrison K, Yokota T, Bishop GJ. Tomato BRI1 and systemin wound signalling. Plant Signal. Behav. 2008;3:54–55. doi: 10.4161/psb.3.1.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goddard R, Peraldi A, Ridout C, Nicholson P. Enhanced disease resistance caused by BRI1 mutation is conserved between Brachypodium distachyon and barley (Hordeum vulgare) MPMI. 2014;27:1095–1106. doi: 10.1094/MPMI-03-14-0069-R. [DOI] [PubMed] [Google Scholar]
- 39.Cheng X, Wen J, Tadege M, Ratet P, Mysore KS. Reverse genetics in medicago truncatula using Tnt1 insertion mutants. Methods Mol. Biol. 2011;678:179–190. doi: 10.1007/978-1-60761-682-5_13. [DOI] [PubMed] [Google Scholar]
- 40.Cheng X, et al. An efficient reverse genetics platform in the model legume Medicago truncatula. New Phytol. 2014;201:1065–1076. doi: 10.1111/nph.12575. [DOI] [PubMed] [Google Scholar]
- 41.Ferguson BJ, Ross JJ, Reid JB. Nodulation phenotypes of gibberellin and brassinosteroid mutants of pea. Plant Physiol. 2005;138:2396–2405. doi: 10.1104/pp.105.062414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Asami T, et al. Characterization of brassinazole, a triazole-type brassinosteroid biosynthesis inhibitor. Plant Physiol. 2000;123:93–100. doi: 10.1104/pp.123.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goda H, Shimada Y, Asami T, Fujioka S, Yoshida S. Microarray analysis of brassinosteroid-regulated genes in Arabidopsis. Plant Physiol. 2002;130:1319–1334. doi: 10.1104/pp.011254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li J. Regulation of the nuclear activities of brassinosteroid signaling. Curr. Opin. Plant Biol. 2010;13:540–547. doi: 10.1016/j.pbi.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang ZY, et al. Nuclear-localized BZR1 mediates brassinosteroid-induced growth and feedback suppression of brassinosteroid biosynthesis. Dev. Cell. 2002;2:505–513. doi: 10.1016/S1534-5807(02)00153-3. [DOI] [PubMed] [Google Scholar]
- 46.Yin Y, et al. A new class of transcription factors mediates brassinosteroid-regulated gene expression in Arabidopsis. Cell. 2005;120:249–259. doi: 10.1016/j.cell.2004.11.044. [DOI] [PubMed] [Google Scholar]
- 47.Guo H, Li L, Aluru M, Aluru S, Yin Y. Mechanisms and networks for brassinosteroid regulated gene expression. Curr. Opin. Plant Biol. 2013;16:545–553. doi: 10.1016/j.pbi.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 48.Huot B, Yao J, Montgomery BL, He SY. Growth-defense tradeoffs in plants: A balancing act to optimize fitness. Mol. Plant. 2014;7:1267–1287. doi: 10.1093/mp/ssu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saini S, Sharma I, Pati PK. Versatile roles of brassinosteroid in plants in the context of its homoeostasis, signaling and crosstalks. Front Plant Sci. 2015;6 doi: 10.3389/fpls.2015.00950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Albrecht C, et al. Brassinosteroids inhibit pathogen-associated molecular pattern-triggered immune signaling independent of the receptor kinase BAK1. PNAS. 2012;109:303–308. doi: 10.1073/pnas.1109921108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Belkhadir Y, et al. Brassinosteroids modulate the efficiency of plant immune responses to microbe-associated molecular patterns. PNAS. 2012;109:297–302. doi: 10.1073/pnas.1112840108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jongedijk E, et al. Synergistic activity of chitinases and β-1, 3-glucanases enhances fungal resistance in transgenic tomato plants. Euphytica. 1995;85:173–180. doi: 10.1007/BF00023946. [DOI] [Google Scholar]
- 53.Park JE, et al. GH3-mediated auxin homeostasis links growth regulation with stress adaptation response in Arabidopsis. J. Biol. Chem. 2007;282:10036–10046. doi: 10.1074/jbc.M610524200. [DOI] [PubMed] [Google Scholar]
- 54.Nakamura A, et al. An early auxin inducible gene, SAUR-AC1 is rapidly induced by brassinosteroid. Plant Cell Physiol. 2002;43 doi: 10.1093/pcp/pcf091. [DOI] [Google Scholar]
- 55.Park JE, Kim YS, Yoon HK, Park CM. Functional characterization of a small auxin-up RNA gene in apical hook development in Arabidopsis. Plant Sci. 2007;172:150–157. doi: 10.1016/j.plantsci.2006.08.005. [DOI] [Google Scholar]
- 56.Chae K, et al. Arabidopsis SMALL AUXIN UP RNA63 promotes hypocotyl and stamen filament elongation. Plant J. 2012;71:684–697. doi: 10.1111/j.1365-313X.2012.05024.x. [DOI] [PubMed] [Google Scholar]
- 57.Spartz AK, et al. The SAUR19 subfamily of SMALL AUXIN UP RNA genes promote cell expansion. Plant J. 2012;70:978–990. doi: 10.1111/j.1365-313X.2012.04946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ali SS, et al. Plant disease resistance is augmented in uzu barley lines modified in the brassinosteroid receptor BRI1. BMC Plant Biol. 2014;14 doi: 10.1186/s12870-014-0227-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tadege M, et al. Large-scale insertional mutagenesis using the Tnt1 retrotransposon in the model legume Medicago truncatula. Plant J. 2008;54:335–347. doi: 10.1111/j.1365-313X.2008.03418.x. [DOI] [PubMed] [Google Scholar]
- 60.Gou X, et al. Genome-wide cloning and sequence analysis of leucine-rich repeat receptor-like protein kinase genes in Arabidopsis thaliana. BMC Genomics. 2010;11 doi: 10.1186/1471-2164-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Benedito VA, et al. A gene expression atlas of the model legume. Plant J. 2008;55:504–513. doi: 10.1111/j.1365-313X.2008.03519.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.