Abstract
Neighboring resources can affect insect oviposition behavior when the complexity of sensory information obscures information about host resource availability in heterogeneous resource patches. These effects are referred to as associational effects and are hypothesized to occur through constraints in the sensory processing of the insect during host search, resulting into suboptimal resource use. Because the possibilities to study these constraints on naturally occurring animals are limited, we instead used sensory mutants of Drosophila melanogaster to determine the importance of sensory information in the occurrence of associational effects. We found that oviposition was mainly governed by non-volatile chemical cues and less by volatile cues. Moreover, the loss of gustatory sensilla resulted in random resource selection and eliminated associational effects. In conclusion, our study shows that associational effects do not necessarily depend on constraints in the sensory evaluation of resource quality, but may instead be a direct consequence of distinctive selection behavior between different resources at small scales.
Introduction
Insect oviposition involves several behavioral steps in which females use multiple sensory modalities to evaluate cues indicating the quality of the available resources for progeny development1–4. While numerous studies have shown that the degree to which insects distribute their eggs among hosts can have profound consequences for their reproductive fitness5–7, it has remained difficult to determine how resource heterogeneity affects the selection behavior of insects among alternative hosts. One way to disentangle the consequences of resource heterogeneity on host selection is by looking at associational effects, which allows you to determine whether the likelihood that an insect selects a particular host resource changes due to the presence of neighboring resources8–10. Associational effects have been hypothesized to occur due to limitations in the sensory physiology of insects, which constrain their ability to evaluate resource quality at different levels of host search11–13. It is, for example, possible that the resolution of sensory information used to locate a resource patch will not be sufficient to distinguish between the qualities of the individual resources within the patch14, 15. Consequently, constraints in resource evaluation may cause female insects to under- or overestimate the actual profitability of a patch and change the likelihood of selecting a particular oviposition resource in low and high quality neighborhoods11–13.
The ability to evaluate resource quality is presumably lower from a distance, and previous studies have therefore mainly examined long-range resource selection as a mechanism for associational effect8–10. In this study, we instead examined the possibility that short-range selection may also cause associational effects. Moreover, we also took advantage of the ongoing development of sensory mutants of Drosophila melanogaster Meigen (Diptera: Drosophilidae), to investigate which sensory modalities could be involved in the occurrence of associational effects during short-range resource selection. Many of the molecular studies on sensory mutations have been pivotal in understanding the physiology and evolution of the insect sensory system16–19, but can nowadays also help to gain insight into how sensory information affects insect search behavior20–22. For this reason, we used D. melanogaster as a model organism to study how sensory information can generate associational effects by comparing the resource selection behavior of wild type flies with that of strains with olfactory and gustatory deficiencies (Fig. 1; Supplementary Table S1), using an oviposition assay in which we manipulated the frequency of two alternative oviposition resources.
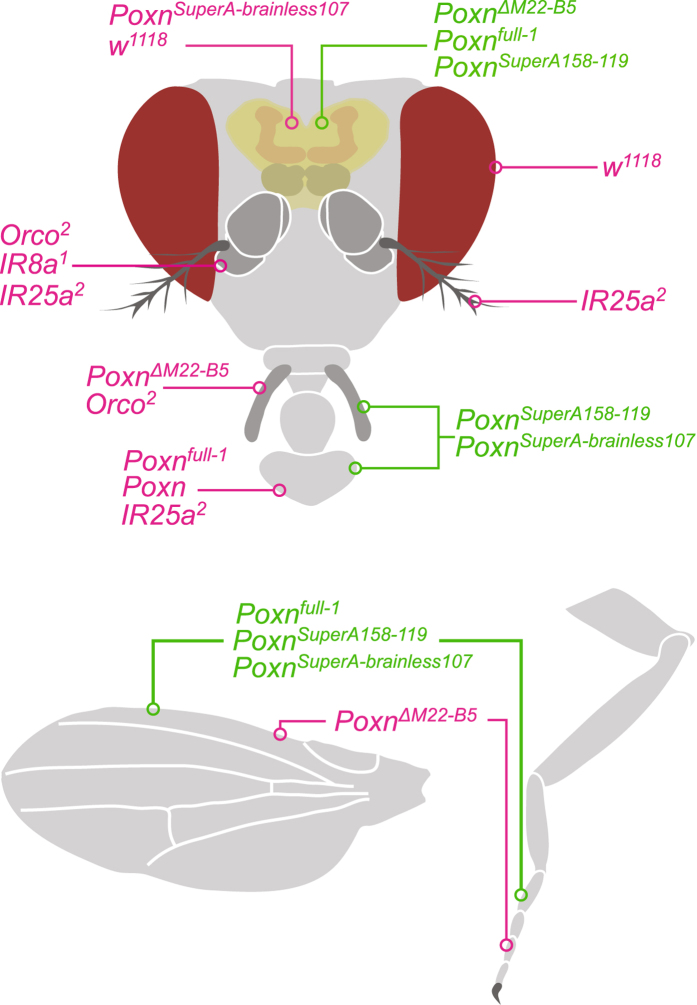
Figure 1.
Representation of the sensory deficiencies in the mutant strains used in our oviposition experiment. The strains in which mutations caused deficiencies in sensory modalities are indicated with magenta, and the strains in which the functioning of sensory modalities were restored are indicated with green. Additional background information and stock numbers can be found in Supplementary Table S1.
With our experimental setup, we studied the behavioral process involved in the short-range evaluation of oviposition resources separately from larger scale behavioral processes preceding the oviposition choice. Consequently, we eliminated the possibility that constraints in the sensory system reduce the ability to evaluate resource quality at the level of a patch or individual resource, and only allowed associational effects to be generated through resource selection at very small scales (for further explanation see Fig. 2). Our results revealed that the occurrence of associational effects does not depend on sensory constraints between the level of a patch and individual resource, but in contrast, may also arise whenever insects distinctively select between two resources at very small scales. Our study also shows that in the case of D. melanogaster, this selection is mainly governed by the gustatory sensory system and less by the olfactory sensory system.
Figure 2.
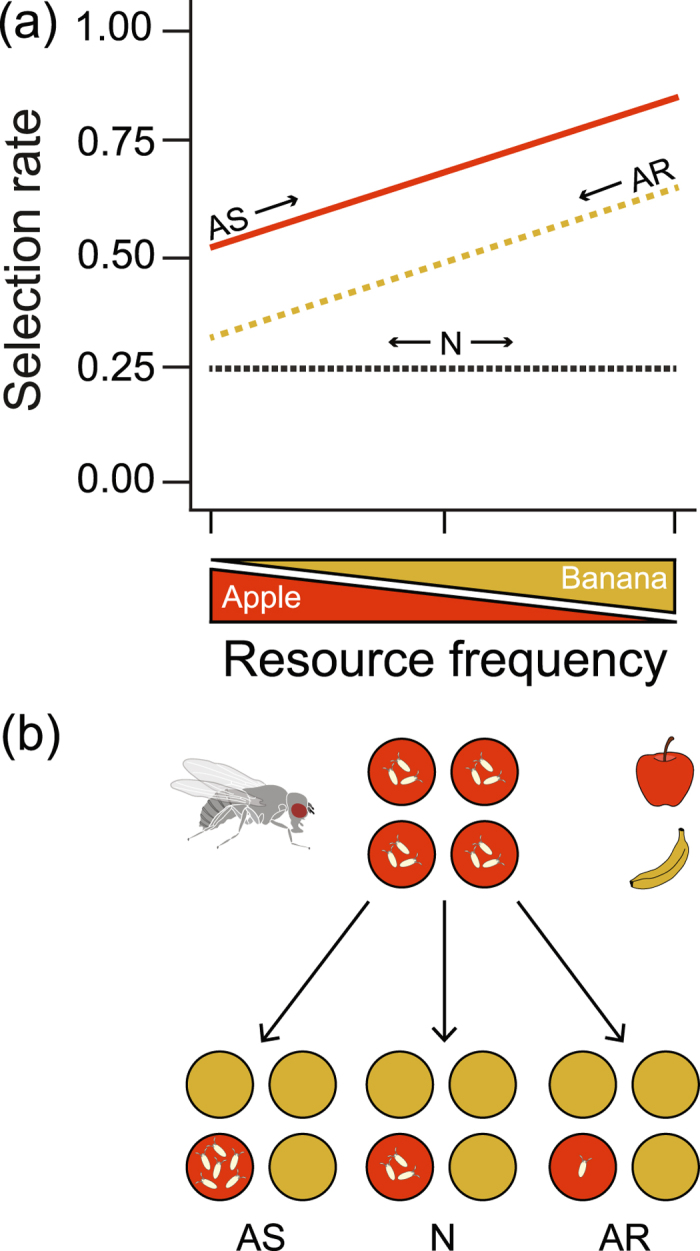
A conceptual framework for the occurrence of associational effects in the oviposition assay. Associational effects occur when the oviposition rate on a resource decreases or increases in the presence of neighboring resources. In (a) apple (red - solid line) experiences associational susceptibility (AS) as the relative selection rate increases with the increasing frequency of banana in the patch. Banana (yellow - dashed line) experiences associational resistance (AR) as the selection rate on banana decreases with the decreasing frequency of banana in the patch. Although the lines have been drawn parallel to each other for visual simplicity, stronger increases in the oviposition rates on one resource type in comparison to the other resource type can result in non-parallel lines, while still representing the same combination of associational effects. When the frequency of neighboring resources has a neutral effect on the oviposition rate (N - grey dashed line) no associational effects occur for that resource. In (b) the different associational effects are illustrated by the number of eggs on apple oviposition resources. When comparing the number of eggs on individual apple resources in pure patches with that in mixed patches, the oviposition rate on apple can either decrease (AR), stay the same (N), or increase (AS). Banana can simultaneously experience associational effects but these effects are not illustrated in this example.
Oviposition behavior of wild type Drosophila
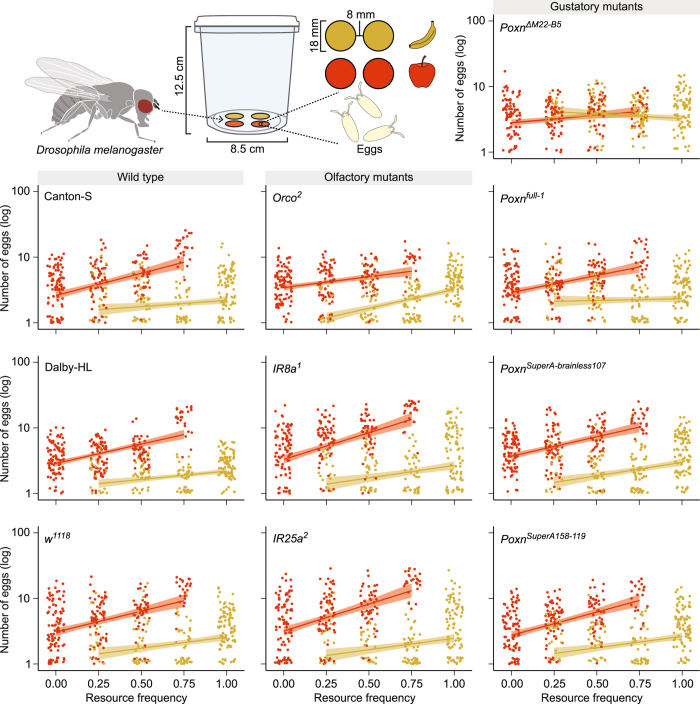
Previous studies on long-range resource selection through olfactory cues showed that when two resources co-occur, the attraction rate of D. melanogaster increases towards preferred resources, and decreases towards less preferred resources, resulting in associational susceptibility (AS) for the former and associational resistance (AR) for the latter23, 24. This combination of AS and AR is generally expected to occur when insects already select resources though long-range olfactory information before entering a resource patch25. However, it is still unknown whether resource selection within a patch, which often depends on sensory information only perceived at much shorter ranges14, 15, generates similar patterns of associational effects between preferred and less preferred resources. To examine this possibility, we performed oviposition experiments in which individual wild type D. melanogaster females (Supplementary Table S1; Canton-S, Dalby-HL and w 1118) were offered four oviposition discs either containing apple or banana fruit pulp (i.e. oviposition discs - Fig. 3). We tested the oviposition behavior across a wide range of resource frequencies, and found that wild type fruit flies strongly preferred to oviposit on apple discs rather than on banana discs (GLM: χ 2 1,1489 = 61.64, P < 0.001), and that the overall oviposition rate is positively affected by the increasing frequency of banana in the patch (GLM: χ 2 1,1489 = 78.84, P < 0.001; Fig. 3). Moreover, we also found that the oviposition rates were affected by an interaction between resource type and resource frequency (GLM: χ 2 1,1489 = 11.35, P < 0.001), which indicates that the relative oviposition rate on apple changed more in the presence of banana than the oviposition rate on banana changed in the presence of apple (Fig. 3). As these effects did not vary between the three strains (GLM: χ 2 2,1489 = 3.91, P = 0.14; Supplementary Tables S2 and S3), we can assume that the observed oviposition patterns are not specific to a particular wild type strain.
Figure 3.
Oviposition behavior of wild type flies and sensory mutants. A graphic representation of the oviposition assay is given at the top of the figure. Each point in the graph represents the number of eggs laid by an individual fruit fly female on either an apple (red) or banana (yellow) oviposition disc (log scale). The x-axis represents the increasing frequency of banana oviposition discs in the patch. In the opposite direction, the frequency of banana also provides the frequency of apple in the patch (i.e. 1.00 minus the frequency of banana). The predicted linear regression lines for oviposition on either resource types are illustrated with their 95% confidence interval. The points representing egg distribution are jittered vertically and horizontally for visualization purposes.
The observed oviposition patterns would translate into associational effects when the oviposition rate on either resource type differs in resource mixtures from the oviposition rate in environments with only one resource type23, 24. Evidently, the observed patterns translated to AS for apple, because the oviposition rate on apple increased with the decreasing frequency of apple in the patch, and AR for banana as the oviposition rate on banana decreased with the decreasing frequency of banana. Because our oviposition assay effectively studies small-scale decisions made during oviposition selection within the patch, our results suggest that associational effects do not necessarily rely on constraints in the ability to interpret sensory information at different levels of search behavior11–13. In contrast, our results suggest that associational effects can also occur when insects distinctively select between two resources at a very small spatial scale. We next investigated which sources of short-range sensory information were used by the fruit flies to select for either of the two oviposition resources within our oviposition assay. We hypothesized that reducing the sensory capabilities of the fruit flies might lower their abilities to distinguish between the two oviposition substrates and alter the associational effects between apple and banana. To validate this hypothesis, we made use of olfactory and gustatory mutants to determine if deficiencies in these sensory modalities influence the oviposition choice of fruit flies (Fig. 1; Supplementary Table S1), and thereby affect the patterns of the associational effects we found in the experiments with the wild type flies.
Oviposition behavior of olfactory mutants
We compared the oviposition behavior of three olfactory mutant strains (Orco 2, IR8a 1 and IR25a 2; Fig. 1; Supplementary Table S1) with that of the wild type w 1118 strain to determine if deficiencies in the olfactory system affects resource selection and could underlie the associational effects observed for the wild type fruit flies. Insect olfactory receptors function as a heteromeric complex formed by ligand-binding odorant receptors (ORs) and a chaperon co-receptor (Orco) that complements the signal transduction pathway26–28. In addition to the ORs, there is a second family of odorant receptors, the ionotropic glutamate-like receptors (IRs), of which several are dependent on either of the two broadly expressed co-receptors IR8a and IR25a29, 30. Due to the ubiquitous expression of Orco in all sensory neurons housing ORs28, 31, Orco 2 mutants can be assumed to have larger sensory deficiencies than IR8a 1 and IR25a 2 mutant flies, which respectively only lack the dedicated ionotropic co-receptors for the reception of certain carboxylic acids and amines29, 30, 32. The reason for comparing the behavior of the mutant strains with that of the w 1118 strain is that all sensory mutants contain the white (w) mutation in their genetic background (Supplementary Table S1), making w 1118 the preferred strain to represent wild type oviposition behavior in pairwise comparisons rather than the two other wild type strains.
These comparisons revealed no differences between the oviposition behavior of the IR8a 1 and IR25a 2 flies and that of the wild type w 1118 flies (Supplementary Table S4). However, when comparing w 1118 and Orco 2 flies we found interactions between resource frequency, resource type and strain affecting the egg distribution patterns (GLM: χ 2 1,990 = 16.45, P < 0.001; Supplementary Table S4). When comparing the three olfactory mutants, we similarly found a three-way interaction between resource frequency, resource type and strain (GLM: χ 2 1,1487 = 8.81, P = 0.01; Supplementary Table S5). These three-way interactions resulted from the presence of an interaction between resource frequency and resource type for the Orco 2 flies (GLM: χ 2 1,495 = 23.77, P < 0.001) and the absence of such an interaction for both the IR8a 1 and IR25a 2 flies (Fig. 3; Supplementary Table S3; Table S5). More specifically, the behavior of the Orco 2 flies separates itself from the behavior of w 1118, IR8a 1 and IR25a 2 flies because the oviposition rate of the Orco 2 flies on apple increased much less with resource frequency (less strong AS) than the oviposition rate on banana (Fig. 3). Similar to the wild type flies, the oviposition rate of Orco 2 flies on apple still increased with the decreasing frequency of apple in the patch (AS). These results suggest that, although olfactory cues might be of importance in long-range resource selection23, 24, the odorants experienced by the flies in our small-scale oviposition assay are not the main source of short-range sensory information used by the fruit flies to distinguish between the two oviposition resources.
Oviposition behavior of gustatory mutants
Because our experiments with the olfactory mutants showed that olfactory mediated selection does not explain the observed associational effects, we next examined the role of non-volatile chemical cues and gustatory receptors in generating these patterns. The most dominantly used receptors in evaluating non-volatile chemical cues are the gustatory receptors (GRs), but broadly tuned IRs and transient receptor potential channels (TRP) have also been found to be of importance33. The GRs are mainly housed in sensilla on the labellum, the anterior wing margins and the tarsi34, 35, but additional sensilla surrounding the ovipositor allow female flies to identify optimal nutritional conditions while selecting oviposition substrates34, 36, 37. We studied the behavior of Pox-neuro mutants (Poxn), with varying degrees of reduced functioning of the gustatory sensilla, to determine the degree to which non-volatile chemical cues are involved in generating associational effects. The Poxn mutation affects the development of the central and peripheral nervous system by turning all poly-innervated gustatory bristles into mono-innervated mechanosensory bristles, eliminating the direct-contact gustatory sensilla in the Poxn ΔM22-B5 mutant flies38–41. We also tested Poxn full-1 flies in which all gustatory sensilla except for those found on the labellum are restored. Finally, we tested the behavior of Poxn SuperA-brainless107 in which all gustatory sensilla are restored but not the Poxn brain development, and Poxn SuperA158-119 in which all gustatory sensilla and the Poxn brain development are restored (Fig. 1; Supplementary Table S1).
The comparisons between w 1118 and Poxn ΔM22-B5, and between w 1118 and Poxn full-1 mutant flies, showed effects of interactions between resource frequency and resource type, resource frequency and strain, and resource type and strain on the egg distribution (Supplementary Table S4). Both Poxn ΔM22-B5 and Poxn full-1 mutant flies differed from the wild type flies by showing a neutral response to the frequency of banana in the patch (see Fig. 2). The egg distribution of the Poxn ΔM22-B5 mutant flies was affected by resource frequency (GLM: χ 2 1,495 = 4.75, P = 0.03; Fig. 3), but only showed a marginally significant difference between the apple and banana substrates (GLM: χ 2 1,495 = 3.78, P = 0.052), and no effect of the interaction between resource type and frequency (GLM: χ 2 1,495 = 3.65, P = 0.06; Fig. 3). As this outcome did not conclusively indicate that the selection between the two resources was affected by the patch arrangements, we removed the non-significant interaction from the analysis and found no effects of resource type (GLM: χ 2 1,495 = 1.56, P = 0.21) or resource frequency (GLM: χ 2 1,495 = 0.44, P = 0.51), indicating random selection between the two oviposition resources. For the Poxn full-1 flies, on the other hand, we found relatively similar egg distributions compared to that of the wild type flies on apple (Fig. 3). More specifically, we found an effect of resource type (GLM: χ 2 1,495 = 5.41, P = 0.02), a strong increase in oviposition with resource frequency (GLM: χ 2 1,495 = 30.63, P < 0.001), and an effect of the interaction between resource type and frequency (GLM: χ 2 1,495 = 11.58, P < 0.001; Supplementary Table S3).
When comparing w 1118 with Poxn SuperA-brainless107 or Poxn SuperA158-119 respectively, there were no effects of any interactions with strain or of strain on its own (Supplementary Table S6), suggesting that the reconstruction of the gustatory sensilla restored wild type oviposition behavior. The absence of interactions in these comparisons with w 1118 also exclude the possibility that neurological deficiencies caused by the white or Poxn mutation had a direct effect on the oviposition behavior of the mutant flies included in our experiments (see Supplementary Table S1). Although the mini-white transgene partially restores white, unforeseen changes in the synthesis of dopamine and serotonin could have affected spatial memory and decision making by the mutants42–44. The absence of interactions between the strains suggests that the patterns found in our oviposition assay can be interpreted as effects of the actual sensory deficiencies rather than consequences of the genetic background in which the strains were constructed. Moreover, this hypothesis was also confirmed by the comparison between all gustatory mutants which indicated significant effects of interactions between resource type, resource frequency and strain on egg distribution (Supplementary Table S2). As expected, these interactions occurred due to apparent differences in the oviposition behavior of Poxn ΔM22-B5 and Poxn full-1 in comparison to that of Poxn SuperA-brainless107 and Poxn SuperA158-119 (Fig. 3; Supplementary Table S6). We can conclude from these results that the oviposition behavior of fruit flies is strongly governed by gustatory input. Entirely or partially losing the ability to evaluate direct contact non-volatile chemical cues resulted in oviposition patterns that deviated from the patterns we observed for wild type flies. This strongly suggests that gustatory mediated resource selection serves as an underlying behavioral mechanism for the occurrence of associational effects between resources for these flies.
Discussion
Our results show that the occurrence of associational effects during oviposition by Drosophila melanogaster mainly relies on the use of non-volatile chemical cues, and less on the use of volatile cues. Our findings contrast with the main hypothesis that associational effects only occur through the misinterpretation of resource patch quality when selecting resources based upon long-range sensory information. These misinterpretations have been suggested to result from constraints in the capacity to evaluate resource availability from a distance, and therefore affect the likelihood of a female selecting a specific oviposition resource when arriving in a patch11–13. Partly due to the intrinsic limitations of testing this hypothesis under natural conditions, it has remained an obstacle for ecologists to empirically demonstrate whether associational effects actually depend on the combination of long- and short-range behavioral processes25, 45. To our knowledge, our study provides the first evidence that search behavior processes occurring at different spatial scales are not necessary in generating associational effects. Our results instead show that these effects can also be generated through sensory preferences solely based on short-range behavioral processes. Moreover, we found that deficiencies of distinct sensory modalities affect oviposition behavior in heterogeneous environments differently, and that such deficiencies either modulate the strength of associational effects between the two resources or eliminate the possibility of associational effects to occur.
We found that wild type fruit flies showed an increasing relative oviposition rate on apple substrates in patches with a higher frequency of banana, and a decreasing relative oviposition rate on banana substrates in patches with an increasing frequency of apple. This behavioral pattern can be translated into associational susceptibility (AS) for apple and associational resistance (AR) for banana. We also found that a complete functional loss of the gustatory sensilla in the Poxn ΔM22-B5 flies entirely eliminated the patterns of the associational effects we observed for wild type fruit flies. We believe that the loss of associational effects for the Poxn ΔM22-B5 flies was because female flies lacking the gustatory sensilla were unable to accurately assess the different substrates before making an oviposition choice, resulting in random resource selection46–48. As the Poxn ΔM22-B5 flies retain the ability to select resources through olfactory cues, our results suggest that the observed associational effects are generated through the use of non-volatile chemical cues. Various studies show that freshly mated D. melanogaster females, as in our study, assess their oviposition environment by probing the available substrates with the proboscis and ovipositor before making any oviposition choice37, 49, 50. This behavior was also observed in our studies, and the inability of Poxn ΔM22-B5 flies to use these sensory cues likely affected the relative egg-laying on the apple and banana substrates.
We also found that the partial olfactory deficiencies of the Orco 2 flies and the partial gustatory deficiencies of the Poxn full-1 flies modulated the associational effects (Fig. 3), but the effects were still largely retained, in contrast to the Poxn ΔM22-B5 flies. Moreover, neither the deficiencies in the acid-sensing (IR8a 1) nor in the amine-sensing pathways (IR25a 2) were able to alter the associational effects observed for wild type flies. Silbering et al.51 showed that Orco 2 flies have a stronger aversion to carboxylic acids (IR8a ligands), and an increased attraction to amines (IR25a ligands) in comparison to wild type flies, which could have influenced their ability to evaluate the resources and thereby affect the associational effects. Similarly, changes in the integration of sensory information by the Poxn full-1 flies, which lack gustatory sensilla on the labellum, could have modulated the strength of associational effects. Gustatory sensilla on the labellum have been shown to be of high importance for substrate selection37, 52–54, making it likely that the absence of the integration of this sensory information during oviposition modulated the strength of associational effects. Interestingly, these results support our hypothesis of the importance of the ability to discriminate between resources as a mechanism underlying associational effects, especially as the rescue of the gustatory sensilla in the Poxn SuperA-brainless107 and Poxn SuperA158-119 flies successfully restored resource preference and associational effects (Fig. 3).
Our current study, showing that associational effects are generated through distinctive selection behavior by the consumer insect, rather than by a sensory misinterpretation as traditionally hypothesized11–13, may have implications for our general understanding of associational effects under natural conditions. Various studies have indicated the difficulty of reaching a consensus on the importance of search behavior by generalist versus specialist insects in the occurrence of associational effects55–57. Our results indicate that we should rather focus on the specific relationships between consumer- and resource traits to further elucidate the mechanisms generating associational effects25, 45. The applicability of our methods can be illustrated by a recent study, which suggested that the invasive agricultural pest species Drosophila suzukii integrates olfactory and gustatory sensory information differently during oviposition than other closely related species58. Hence, the applicability of our methods could lie in improving our understanding of the importance of sensory modalities in insect–host interactions in heterogeneous environments, and help to exploit insect host search behavior in future biological control strategies of pest species.
Material and Methods
Fly husbandry and mating of the flies
All flies were reared under controlled conditions (25 °C, 50% RH, 12:12 Light:Dark) in 28.5 × 95 mm rearing vials on a standard Bloomington diet containing corn syrup (115 mL/L), yeast (26 g/L), soy flour (15 g/L), cornmeal (110 g/L), agar (8.5 g/L) and propionic acid (7 ml/L). We anesthetized newly eclosed flies with CO2 and transferred them separated by sex into 28.5 × 95 mm rearing vials containing the standard diet, where we let them mature for 72 hours. Approximately two hours prior to the behavioral experiments we transferred one female and three males in rearing vials to allow for mating. The vials were checked every five minutes and only those females that had copulated for a minimum of 20 minutes were used in the oviposition experiments. We used Canton S, Dalby-HL and w 1118 to represent wild type oviposition behavior unaffected by sensory deficiencies. To determine the effects of olfactory deficiencies on oviposition behavior we used the IR8a 1 and IR25a 2 (both provided by Anders Enjin and Marcus Stensmyr) and Orco 2 mutant strains. Finally, we used Poxn ΔM22-B5, Poxn full-1 (both provided by Werner Boll) to determine the effect of gustatory deficiencies on oviposition behavior (see Supplementary Table S1).
All sensory mutants used in our experiments contain the white (w) mutation in their genetic background (see Supplementary Table S1 - genetic background), which codes for a transmembrane ABC transporter required for the synthesis of eye color pigment and the synthesis of dopamine and serotonin59, 60. Both dopamine and serotonin have been shown to be involved in spatial memory and decision making in complex environments42–44, and although the white mutation is partially restored by the insertion of the mini-white transgene marker in these strains, they most likely still have some neurological deficiencies caused by the white mutation61, 62. We used the Poxn SuperA-brainless107 and Poxn SuperA158-119 strains (both provided by Werner Boll), in which all gustatory sensilla are restored, as control strains for the behavior of the gustatory mutants and potential neurological deficiencies caused by white or Poxn. In both strains the white mutation is restored by the insertion of the mini-white trans-gene marker, but Poxn SuperA-brainless107 still expresses the Poxn mutation in the central nervous system39, by comparing their behavior we effectively checked for potential effects of neurological deficiencies either caused by the white or Poxn mutation in the oviposition behavior of our flies.
Oviposition experiments
Approximately 30 minutes after copulation we transferred individual females into polypropylene jars containing patches consisting of four oviposition discs placed in two rows separated by 8 mm. The oviposition discs were made of 18 mm filter paper discs (Grade 1003 – 12-15 μm pore size; Munktell Ahlstrom AB, Sweden) loaded with either apple or banana fruit pulp. All experiments were run with four oviposition discs to ensure that we could test the effects of patch heterogeneity along a sequence of different resource frequencies. We used the filter paper discs to ensure a standardized and accessible substrate for the females. The fruit substrates were made by pulverizing ripe apples (variety: Discovery, Sweden) and ripe bananas (Organic Cavendish – Dole, Dominican Republic) into a smooth fruit pulp. To preserve and standardize the quality of the fruit pulp we pulverized single batches of apple and banana, which were frozen in small quantities that could be easily defrosted prior to the experiments. The discs were loaded by slowly dragging the filter paper discs through the pulp, resulting in a thin and accessible layer of apple pulp (383.80 ± 8.69 mg) or banana pulp (331.12 ± 7.07 mg) on each disc. Over the entire time span of the experiments no fruit fluids leaked from the filter paper discs, creating resources with a distinct barrier which did not allow for any chemical diffusion found in similar setups made of agarose.
The jars were closed with airtight transparent lids and covered by an additional darkened lid to eliminate phototaxis of the flies to the top of the jar. We counted the number of eggs laid by Canton S, Dalby-HL, w 1118, IR8a 1 and IR25a 2 flies after 24 hours, but due to low egg laying during the first 24 hours we counted eggs of Orco 2, Poxn ΔM22-B5, Poxn full-1, Poxn SuperA-brainless107 and Poxn SuperA158-119 flies after 48 hours. Each treatment was replicated 25 times and consisted of one of the following patch arrangements; (1) four apple discs; (2) three apple and one banana disc; (3) two apple and two banana discs; (4) one apple and three banana discs; (5) four banana discs. We performed control trials in which Dalby-HL females were only offered a moistened filter paper disc to determine whether the flies would lay eggs on the filter paper discs within 24 hours regardless of the availability of fruit substrates. These tests showed that the females largely restrained themselves from laying eggs on moistened filter paper discs, suggesting that the discs without fruit pulp are not a suitable oviposition substrate. All experiments were conducted under the same controlled conditions as those under which the fly husbandry was maintained (25 °C, 50% RH, 12:12 Light:Dark).
Statistical analysis
We analyzed the egg distribution along the different oviposition disc using Generalized Linear Mixed-effects Models (GLMM) with a negative binomial error distribution and the individual fly as a random factor using the glmmADMB package. We used negative binomial error distributions in our models based upon inspection of the normality of the residuals and the Q-Q plots, and the estimations of over-dispersion of initial models. In this analysis, each individual fly was represented by four separate values accounting for the number of eggs laid on each separate oviposition disc within the oviposition jar. We included the random factor to estimate relative, rather than absolute, oviposition rates by correcting for the number of oviposition discs per resource type in the patch. Consequently, this analysis compares the oviposition rates among single oviposition discs and identifies potential associational effects between individual resources23. The analysis for the individual strains included resource frequency, resource type and an interaction between these two factors as explanatory factors. In each analysis resource frequency was included as a continuous variable accounting for the proportion of banana oviposition discs in the patch, corresponding to values of 0, 0.25, 0.50, 0.75 or 1.
As all sensory mutants contain the white (w) mutation in their genetic background we decided that w 1118 would be the preferred strain to represent the wild type behavior in pairwise comparisons. All analysis that compared the behavior among strains included an additional explanatory factor to account for the strain, and all appropriate two-way interactions and a three-way interaction between strain, resource frequency and resource type. The models were selected using the car package63 for likelihood ratio tests based on χ 2 and Akaike’s information criterion in a step-wise backward selection process. The ggplot2 package was used to visualize the oviposition patterns within the different patch arrangements64. All analyses were carried out in R (v. 3.3.2; R Foundation for Statistical Computing, Vienna, AT).
Data accessibility
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
Electronic supplementary material
Acknowledgements
We thank Werner Boll for providing all Poxn strains and Anders Enjin and Marcus Stensmyr for providing the IR8a 1 and IR25a 2 strains. We also thank Nora Underwood and the anonymous reviewers for constructive comments on the manuscript. This study was supported by grants VR-2009-4943 and VR-2012-3578 from the Swedish Research Council Vetenskapsrådet (to PAH).
Author Contributions
T.A.V., P.A. and P.A.H. conceived the project. T.A.V., M.A.C. and P.A.H. designed the experiments. T.A.V. performed the experiments and the statistical analyses. T.A.V. wrote the first draft of the manuscript and all authors contributed to revisions.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-09728-7
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dweck HKM, et al. Olfactory preference for egg laying on citrus substrates in. Drosophila. Curr. Biol. 2013;23:1–9. doi: 10.1016/j.cub.2012.10.044. [DOI] [PubMed] [Google Scholar]
- 2.Stensmyr MC, et al. A conserved dedicated olfactory circuit for detecting harmful microbes in Drosophila. Cell. 2012;151:1345–1357. doi: 10.1016/j.cell.2012.09.046. [DOI] [PubMed] [Google Scholar]
- 3.Ozaki K, et al. A gustatory receptor involved in host plant recognition for oviposition of a swallowtail butterfly. Nat. Commun. 2011;2 doi: 10.1038/ncomms1548. [DOI] [PubMed] [Google Scholar]
- 4.Bernays EA. Neural limitations in phytophagous insects: implications for diet breadth and evolution of host affiliation. Annu. Rev. Entomol. 2001;46:703–727. doi: 10.1146/annurev.ento.46.1.703. [DOI] [PubMed] [Google Scholar]
- 5.Wetzel WC, Kharouba HM, Robinson M, Holyoak M, Karban R. Variability in plant nutrients reduces insect herbivore performance. Nature. 2016;539:425–427. doi: 10.1038/nature20140. [DOI] [PubMed] [Google Scholar]
- 6.Markow TA, O’Grady P. Reproductive ecology of Drosophila. Funct. Ecol. 2008;22:747–759. doi: 10.1111/j.1365-2435.2008.01457.x. [DOI] [Google Scholar]
- 7.Doak, P., Kareiva, P., Kingsolver, J. Fitness consequences of choosy oviposition for a time-limited butterfly. Ecology87, 395–408, doi:10.1890/05-0647 (2006). [DOI] [PubMed]
- 8.Tahvanainen JO, Root RB. The influence of vegetational diversity on the population ecology of a specialized herbivore, Phyllotreta cruciferae (Coleoptera: Chrysomelidae) Oecologia. 1972;10:321–346. doi: 10.1007/BF00345736. [DOI] [PubMed] [Google Scholar]
- 9.Letourneau DK. Associational susceptibility: effects of cropping pattern and fertilizer on Malawian bean fly levels. Ecol. Appl. 1995;5:823–829. doi: 10.2307/1941990. [DOI] [Google Scholar]
- 10.Pfister CA, Hay ME. Associational plant refuges: convergent patterns in marine and terrestrial communities result from differing mechanisms. Oecologia. 1988;77:118–129. doi: 10.1007/BF00380934. [DOI] [PubMed] [Google Scholar]
- 11.Hambäck PA, Beckerman AP. Herbivory and plant resource competition: a review of two interacting interactions. Oikos. 2003;101:26–37. doi: 10.1034/j.1600-0706.2003.12568.x. [DOI] [Google Scholar]
- 12.Hjältén J, Danell K, Lundberg P. Herbivore avoidance by association: vole and hare utilization of woody plants. Oikos. 1993;68:125–131. doi: 10.2307/3545317. [DOI] [Google Scholar]
- 13.Stephens, D. W., & Krebs, J. R. Foraging Theory. (Princeton University Press, 1986).
- 14.Webster B, Cardé RT. Use of habitat odour by host-seeking insects. Biol. Rev. 2017;92:1241–1249. doi: 10.1111/brv.12281. [DOI] [PubMed] [Google Scholar]
- 15.Murlis J, Elkinton JS, Cardé RT. Odor plumes and how insects use them. Annu. Rev. Entomol. 1992;37:505–532. doi: 10.1146/annurev.en.37.010192.002445. [DOI] [Google Scholar]
- 16.Croset V, et al. Ancient protostome origin of chemosensory ionotropic glutamate receptors and the evolution of insect taste and olfaction. PLOS Genet. 2010;6 doi: 10.1371/journal.pgen.1001064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Missbach C, et al. Evolution of insect olfactory receptors. eLife. 2014;3 doi: 10.7554/eLife.02115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldman-Huertas B, et al. Evolution of herbivory in Drosophilidae linked to loss of behaviors, antennal responses, odorant receptors, and ancestral diet. Proc. Natl. Acad. Sci. USA. 2015;112:3026–3031. doi: 10.1073/pnas.1424656112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McBride CS, Arguello JR. Five Drosophila genomes reveal nonneutral evolution and the signature of host specialization in the chemoreceptor superfamily. Genetics. 2007;177:1395–1416. doi: 10.1534/genetics.107.078683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohr SE, Hu Y, Kim K, Housden BE, Perrimon N. Resources for functional genomics studies in Drosophila melanogaster. Genetics. 2014;197:1–18. doi: 10.1534/genetics.113.154344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Groen SC, Whiteman NK. Using Drosophila to study the evolution of herbivory and diet specialization. Curr. Opin. Insect Sci. 2016;14:66–72. doi: 10.1016/j.cois.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Cande J, Prud’homme B, Gompel N. Smells like evolution: the role of chemoreceptor evolution in behavioral change. Curr. Opin. Neurobiol. 2013;23:152–158. doi: 10.1016/j.conb.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Verschut TA, Becher PG, Anderson P, Hambäck PA. Disentangling associational effects: both resource density and resource frequency affect search behaviour in complex environments. Funct. Ecol. 2016;30:1826–1833. doi: 10.1111/1365-2435.12670. [DOI] [Google Scholar]
- 24.Verschut, T. A., Hambäck, P. A., Anderson, P. Mating affects resource selection through olfactory-guided behaviour and modulates associational effects between neighbouring resources. Oikos, doi:10.1111/oik.04315 (2017).
- 25.Hambäck PA, Inouye BD, Andersson P, Underwood N. Effects of plant neighborhoods on plant-herbivore interactions: resource dilution and associational effects. Ecology. 2014;95:1370–1383. doi: 10.1890/13-0793.1. [DOI] [PubMed] [Google Scholar]
- 26.Larsson MC, et al. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43:703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 27.Vosshall LB, Wong AM, Axel R. An olfactory sensory map in the fly brain. Cell. 2000;102:147–159. doi: 10.1016/S0092-8674(00)00021-0. [DOI] [PubMed] [Google Scholar]
- 28.Sato K, et al. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature. 2008;452:1002–1006. doi: 10.1038/nature06850. [DOI] [PubMed] [Google Scholar]
- 29.Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell. 2009;136:149–162. doi: 10.1016/j.cell.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abuin L, et al. Functional architecture of olfactory ionotropic glutamate receptors. Neuron. 2011;69:44–60. doi: 10.1016/j.neuron.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wicher D, et al. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature. 2008;452:1007–1011. doi: 10.1038/nature06861. [DOI] [PubMed] [Google Scholar]
- 32.Silbering AF, Benton R. Ionotropic and metabotropic mechanisms in chemoreception: ‘chance or design’? EMBO Rep. 2010;11:173–179. doi: 10.1038/embor.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montell C. A taste of the Drosophila gustatory receptors. Curr. Opin. Neurobiol. 2009;19:345–353. doi: 10.1016/j.conb.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stocker RF. The organization of the chemosensory system in Drosophila melanogaster: a review. Cell Tissue Res. 1994;275:3–26. doi: 10.1007/BF00305372. [DOI] [PubMed] [Google Scholar]
- 35.LeDue EE, Chen YC, Jung AY, Dahanukar A, Gordon MD. Pharyngeal sense organs drive robust sugar consumption in. Drosophila. Nat. Commun. 2015;6 doi: 10.1038/ncomms7667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh, R. N. Neurobiology of the gustatory systems of Drosophila and some terrestrial insects. Microsc. Res. Tech. 39, 547–563, doi: 10.1002/(SICI)1097-0029(19971215)39:6<547::AID-JEMT7>3.0.CO;2-A (1997). [DOI] [PubMed]
- 37.Yang CH, Belawat P, Hafen E, Jan LY, Jan YN. Drosophila egg-laying site selection as a system to study simple decision-making processes. Science. 2008;319:1679–1683. doi: 10.1126/science.1151842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Awasaki, T., Kimura, K. pox-neuro is required for development of chemosensory bristles in Drosophila. J. Neurobiol. 32, 707–721, doi:10.1002/(SICI)1097-4695(19970620)32:7<707::AID-NEU6>3.0.CO;2-8 (1997). [DOI] [PubMed]
- 39.Boll W, Noll M. The Drosophila Pox neuro gene: control of male courtship behavior and fertility as revealed by a complete dissection of all enhancers. Development. 2002;129:5667–5681. doi: 10.1242/dev.00157. [DOI] [PubMed] [Google Scholar]
- 40.Clyne PJ, Warr CG, Carlson JR. Candidate taste receptors in Drosophila. Science. 2000;287:1830–1834. doi: 10.1126/science.287.5459.1830. [DOI] [PubMed] [Google Scholar]
- 41.Bopp D, Jamet E, Baumgartner S, Burri M, Noll M. Isolation of two tissue-specific Drosophila paired box genes, Pox meso and Pox neuro. EMBO J. 1989;8:3447–3457. doi: 10.1002/j.1460-2075.1989.tb08509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sitaraman D, et al. Serotonin is necessary for place memory in Drosophila. Proc. Natl. Acad. Sci. USA. 2008;105:5579–5584. doi: 10.1073/pnas.0710168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iwanir S, et al. Serotonin promotes exploitation in complex environments by accelerating decision-making. BMC Biol. 2016;14 doi: 10.1186/s12915-016-0232-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Diegelmann S, Zars M, Zars T. Genetic dissociation of acquisition and memory strength in the heat-box spatial learning paradigm in. Drosophila. Learn. Memory. 2006;13:72–83. doi: 10.1101/lm.45506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Underwood N, Inouye BD, Hambäck PA. A conceptual framework for associational effects: when do neighbors matter and how would we know? Q. Rev. Biol. 2014;89:1–19. doi: 10.1086/674991. [DOI] [PubMed] [Google Scholar]
- 46.Joseph RM, Devineni AV, King IFG, Heberlein U. Oviposition preference for and positional avoidance of acetic acid provide a model for competing behavioral drives in Drosophila. Proc. Natl. Acad. Sci. USA. 2009;106:11352–11357. doi: 10.1073/pnas.0901419106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hussain A, et al. Ionotropic chemosensory receptors mediate the taste and smell of polyamines. PLOS Biol. 2016;14 doi: 10.1371/journal.pbio.1002454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Joseph RM, Heberlein U. Tissue-specific activation of a single gustatory receptor produces opposing behavioral responses in Drosophila. Genetics. 2012;192:521–532. doi: 10.1534/genetics.112.142455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Depetris-Chauvin A, Galagovsky D, Grosjean Y. Chemicals and chemoreceptors: ecologically relevant signals driving behavior in Drosophila. Front. Ecol. Evol. 2015;3 doi: 10.3389/fevo.2015.00041. [DOI] [Google Scholar]
- 50.Schwartz NU, Zhong L, Bellemer A, Tracey WD. Egg laying decisions in Drosophila are consistent with foraging costs of larval progeny. PLOS ONE. 2012;7 doi: 10.1371/journal.pone.0037910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Silbering AF, et al. Complementary function and integrated wiring of the evolutionarily distinct Drosophila ofactory subsystems. J. Neurosci. 2011;31:13357–13375. doi: 10.1523/JNEUROSCI.2360-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jeong YT, et al. Mechanosensory neurons control sweet sensing in. Drosophila. Nat. Commun. 2016;7 doi: 10.1038/ncomms12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sánchez-Alcañiz JA, Zappia G, Marion-Poll F, Benton R. A mechanosensory receptor required for food texture detection in. Drosophila. Nat. Commun. 2017;8 doi: 10.1038/ncomms14192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang YV, Aikin TJ, Li Z, Montell C. The basis of food texture sensation in Drosophila. Neuron. 2016;91:863–877. doi: 10.1016/j.neuron.2016.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jactel H, Brockerhoff EG. Tree diversity reduces herbivory by forest insects. Ecol. Lett. 2007;10:835–848. doi: 10.1111/j.1461-0248.2007.01073.x. [DOI] [PubMed] [Google Scholar]
- 56.Sato Y, Kudoh H. Presence of substitute diets alters plant resistance to specialist and generalist herbivores: a meta-analysis. Ecosphere. 2016;7 doi: 10.1002/ecs2.1446. [DOI] [Google Scholar]
- 57.Castagneyrol B, Giffard B, Péré C, Jactel H. Plant apparency, an overlooked driver of associational resistance to insect herbivory. J. Ecol. 2013;101:418–429. doi: 10.1111/1365-2745.12055. [DOI] [Google Scholar]
- 58.Karageorgi M, et al. Evolution of multiple sensory systems drives novel egg-laying behavior in the fruit pest Drosophila suzukii. Curr. Biol. 2017;27:847–853. doi: 10.1016/j.cub.2017.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Borycz J, Borycz JA, Kubow A, Lloyd V, Meinertzhagen IA. Drosophila ABC transporter mutants white, brown and scarlet have altered contents and distribution of biogenic amines in the brain. J. Exp. Biol. 2008;211:3454–3466. doi: 10.1242/jeb.021162. [DOI] [PubMed] [Google Scholar]
- 60.Campbell JL, Nash HA. Volatile general anesthetics reveal a neurobiological role for the white and brown genes of Drosophila melanogaster. Dev. Neurobiol. 2001;49:339–349. doi: 10.1002/neu.10009. [DOI] [PubMed] [Google Scholar]
- 61.Evans JM, Day JP, Cabrero P, Dow JAT, Davies SA. A new role for a classical gene: white transports cyclic GMP. J. Exp. Biol. 2008;211:890–899. doi: 10.1242/jeb.014837. [DOI] [PubMed] [Google Scholar]
- 62.Minocha S, Boll W, Noll M. Crucial roles of Pox neuro in the developing ellipsoid body and antennal lobes of the Drosophila brain. PLOS ONE. 2017;12 doi: 10.1371/journal.pone.0176002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fox, J., & Weisber, S. An R Companion to Applied Regression. (Sage Publications, 2011).
- 64.Wickham, H. ggplot2: Elegant Graphics for Data Analysis. (Springer, 2016).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.